Abstract
The objective of this study was to investigate the effects of the combined RF radiation (837 MHz CDMA plus 1950 MHz WCDMA) signal on levels of intracellular reactive oxygen species (ROS) in neuronal cells. Exposure of the combined RF signal was conducted at specific absorption rate values of 2 W/kg of CDMA plus 2 W/kg of WCDMA for 2 h. Co-exposure to combined RF radiation with either H2O2 or menadione was also performed. The experimental exposure groups were incubator control, sham-exposed, combined RF radiation-exposed with or without either H2O2 or menadione groups. The intracellular ROS level was measured by flow cytometry using the fluorescent probe dichlorofluorescein diacetate. Intracellular ROS levels were not consistently affected by combined RF radiation exposure alone in a time-dependent manner in U87, PC12 or SH-SY5Y cells. In neuronal cells exposed to combined RF radiation with either H2O2 or menadione, intracellular ROS levels showed no statically significant alteration compared with exposure to menadione or H2O2 alone. These findings indicate that neither combined RF radiation alone nor combined RF radiation with menadione or H2O2 influences the intracellular ROS level in neuronal cells such as U87, PC12 or SH-SY5Y.
Keywords: combined RF radiation, menadione, H2O2, reactive oxygen species, neuronal cells
INTRODUCTION
As a large number of individuals have been exposed to the radiofrequency (RF) signals from cellular phones, social concerns regarding the possible biological effects of RF radiation emitted from cellular phones on human health have been increasing. While a large part of the world, including Europe, Australia and East Asia, use wideband code division multiple access (WCDMA), several countries use the code division multiple access (CDMA), and some countries, such as Korea, use both [1]. However, information about the biological effects of combined RF radiation exposure has not been well established. Due to humans often being simultaneously exposed to multi-signal RF radiation, we have recently focused our research on the possible biological effects of exposure to multi-signal RF radiation [1–3].
Incomplete one-electron reduction of oxygen, such as singlet oxygen, superoxides, peroxides, hydroxyl radical and hypochlorous acid, form the reactive oxygen species (ROS). ROS causes oxidative modification of DNA, proteins, lipids and small intracellular molecules, which are associated with cell or tissue damage, and are the contributing factors for cellular events including gene expression, cell proliferation, apoptosis, differentiation and senescence [4]. Furthermore, overproduction of ROS plays an important role in several diseases, including cancer, diabetes, cardiovascular diseases, aging and neurodegenerative diseases such as Alzheimer's and Parkinson's diseases under both physiologic and pathologic conditions [5, 6]. Therefore, balancing of the intracellular ROS level plays an important role in preventing thes pathophysiological conditions.
A number of studies have been made into possible adverse biological effects of RF fields on oxidative stress. While most studies have shown no effects of RF radiation on ROS formation [7–11], some studies have recently reported that RF radiation emitted from mobile phones could promote ROS production in some cell types including neuronal cell models. Due to the proximity of mobile phones to the head and the electrical activity of the brain, the nervous system may be a preferential target for the study of RF radiation effects on biological substrates. Several studies have explored the effects of exposure on nervous systems including the SH-SY5Y neuroblastoma and SN56 cholinergic cell lines, as well as rat primary cortical neurons [12–20]. Enhancement of chemically induced ROS production in neuroblastoma cells has been demonstrated after 872 MHz CW RF radiation exposure, but not after GSM-modulated RF radiation exposure [18]. Höyto et al. (2008) [19] also reported that the GSM-modulated signal enhanced chemically induced oxidative stress, but that RF radiation alone did not enhance oxidative stress in neuroblastoma cells. Another study reported that the neurotoxic effects of hydrogen peroxide were exacerbated by RF radiation exposure in SN56 cells but not in primary cortical neurons, suggesting that the RF signal acts as a co-stressor for the oxidative damage of neural cells under particular circumstances [16]. Xu et al. (2009) [20] reported that RF radiation alone significantly increased ROS production, thereby leading to further oxidative damage to mitochondria DNA in primary cultured neurons.
Information related to the biological effects of multiple RF radiation is still very minimal, and studies related to the RF radiation effect on oxidative stress are still controversial. In order to further investigate this issue, we examined the possible effects of combined (837 MHz and 1950 MHz) RF radiation on the intracellular ROS level in neuronal cell culture systems. The present study focused on co-exposures, i.e. combining RF radiation with chemicals that induce oxidative stress, in U87, PC12 and SH-SY5Y cells as neuronal cell models.
MATERIALS AND METHODS
RF radiation exposure system
The Radial Transmission Line (RTL) exposure system was used as an in vitro multi-frequency radiation exposure system for this study. The details about this exposure system have been described previously [3]. A typical CDMA signal of 837 MHz and a WCDMA signal of 1950 MHz were applied to the RTL after amplification.
RF radiation exposure protocol
For these experiments, cells were plated in plastic 65-mm cell culture dishes ∼ 16 h prior to each exposure. The cell count (2 × 106) seeded per dish was selected from preliminary experiments to obtain a subconfluent culture at the end of each exposure. The exposure system was then warmed up for 2 h to equilibrate it prior to RF exposure. The petri dishes (8 dishes/exposure) were placed within the exposure chamber. Exposure of RF radiation for combined (CDMA at 2 W/kg plus WCDMA at 2 W/kg) signals was performed for 2 h. During the exposure period, the temperature in the chamber was maintained within a range of 37 ± 0.3°C by circulating water within the cavity. The temperature of the culture media was monitored twice per second throughout the exposure period in the sham- and the RF radiation-exposed groups. A temperature-controlled mixture of air and CO2 (5% CO2 inside the chambers) was provided by ventilation from a modified cell culture incubator. For the sham exposure, the cells were kept in the RF radiation exposure device, but were not exposed to RF radiation. The cells were exposed in six groups: (i) incubator control group, (ii) sham exposure group, (iii) RF radiation exposure group, (iv) ROS inducers (in the form of hydrogen peroxide (H2O2) (Sigma Chemical Co., St Louis, USA) or menadione (Sigma Chemical Co., St Louis, USA)) exposure group, (v) sham + ROS inducers co-exposure group, (vi) RF radiation + ROS inducers co-exposure group. After 2 h RF radiation exposure, the cells were immediately transferred to a cell culture incubator and further analyzed at 1, 3, 6 and 12 h after H2O2 treatment, or at 0.5, 1 and 3 h after menadione treatment.
Cell cultures
The NIH3T3 mouse fibroblast cells, U87 human glioma cells, PC12 rat pheochromocytoma cells and SH-SY5Y human neuroblastoma cells were purchased from American Type Culture Collections (ATCC) (Manassas, VA, USA). NIH3T3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Paisley, Scotland, UK) and 25 U/ml penicillin/streptomycin. U87 cells were cultured in DMEM supplemented with 10% FBS (WelGENE, Daegu, Korea) and 25 U/ml penicillin/streptomycin. PC12 cells were maintained in DMEM medium supplemented with 10% horse serum (Invitrogen, Paisley, Scotland, UK), 5% FBS, and 25 U/ml penicillin/streptomycin. SH-SY5Y cells were cultured in a 1:1 mixture of ATCC-formulated Eagle's Minimum Essential Medium (ATCC, Manassas, VA, USA) and Ham's-F12 medium (Invitrogen, Cergy Pontoise, France) supplemented with 15% FBS and 25 U/ml penicillin/streptomycin. Cells were kept at 37°C in a cell culture incubator with a humidified atmosphere of 5% CO2.
Analysis of intracellular ROS levels
To quantify intracellular ROS production the fluorescent probe 2′7′-dichlorofluorescein-diacetate (DCFH-DA) was employed. It is a non-polar compound that easily penetrates the cell membrane and is hydrolyzed by intracellular esterases to its nonfluorescent polar derivate DCFH. In the presence of ROS, DCFH is oxidized to the fluorescent dichlorofluorescein (DCF) [21]. After exposure to either RF radiation alone or RF radiation with ROS inducers, the cells were washed twice with chilled PBS and incubated with 10 µM DCFH-DA at 37°C for 15 min in the dark, followed by washing twice with chilled PBS. Cells were trypsinized and analyzed through a Flow Cytometer (Beckton Dickinson, Franklin Lakes, NJ, USA) with excitation and emission at 490 and 530 nm respectively.
Statistical analysis
All values were expressed as the mean value of duplicates of at least six independent experiments ± SD. The results were subjected to an analysis of the variance (ANOVA) using the Tukey post hoc test for the multiple comparisons (SigmaStat 3.1; Systat Software Inc., Chicago, IL, USA) in the incubator control, sham-exposed and the RF-exposed groups with or without ROS inducers. Values of P < 0.05 were considered as significantly different from either sham-exposed or incubator control, respectively.
RESULTS
Effect of combined RF radiation exposure on intracellular ROS levels in neuronal cells
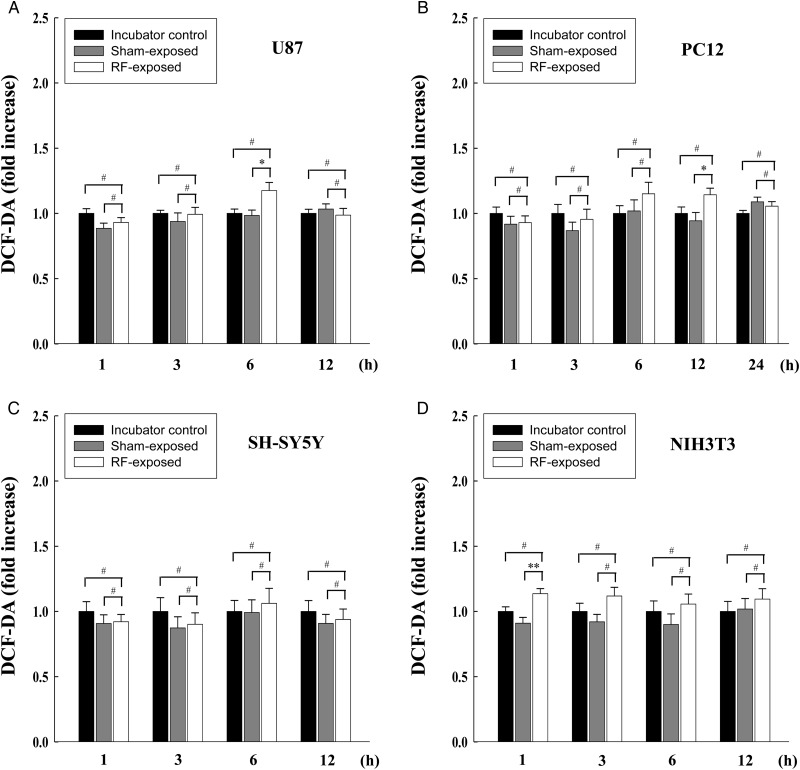
To examine whether combined RF radiation (CDMA at specific absorption rate (SAR) of 2 W/kg plus WCDMA at SAR of 2 W/kg for 2 h) affects the intracellular ROS levels in neuronal cells, we measured ROS levels by DCF-DA staining in three different neuronal cell lines (U87, PC12 and SH-SY5Y) at various time-points (1, 3, 6 and 12 h) after combined RF exposure (Fig. 1). ROS levels in U87, PC12 and SH-SY5Ycells did not show any changes with the exceptions of 6 h post RF-exposure in U87 cells and 12 h post RF-exposure in PC12 cells (Fig. 1A–C). The increased ROS level at 12 h post RF-exposure was not sustained until 24 h post RF-exposure in the PC12 cells. NIH3T3 mouse embryonic fibroblasts were included in every experiment for this study as an alternative type of control cells. In NIH3T3 cells, ROS levels after combined RF exposures were unchanged except for 1 h post RF-exposure (Fig. 1D). Every ROS level was analyzed by ANOVA using the Tukey post hoc test for the multiple comparisons. Our results indicated that the ROS level was neither consistently nor sustainably affected by the combined RF radiation exposure, with statistical significance in four different types of cell lines.
Fig. 1.
Intracellular ROS levels after exposure of neuronal cells to combined RF radiation alone (CDMA at 2 W/kg plus WCDMA at 2 W/kg for 2 h). Intracellular ROS levels were measured by DCF-DA staining in (A) U87, (B) PC12, (C) SH-SY5Y, and (D) NIH3T3 cells at 1, 3, 6 and 12 h (additional 24 h in the case of PC12 cells) after exposure. The data are expressed as the means of six independent experiments together with the standard deviations of the means (M ± SD). Statistical calculations were performed by ANOVA via the Tukey post hoc test. Statistical significance values were *P < 0.05 and **P < 0.01, compared with either the incubator control or sham-exposed groups. # indicates non-significance (P > 0.05).
Effect of H2O2 on ROS levels and cell viability in neuronal cells
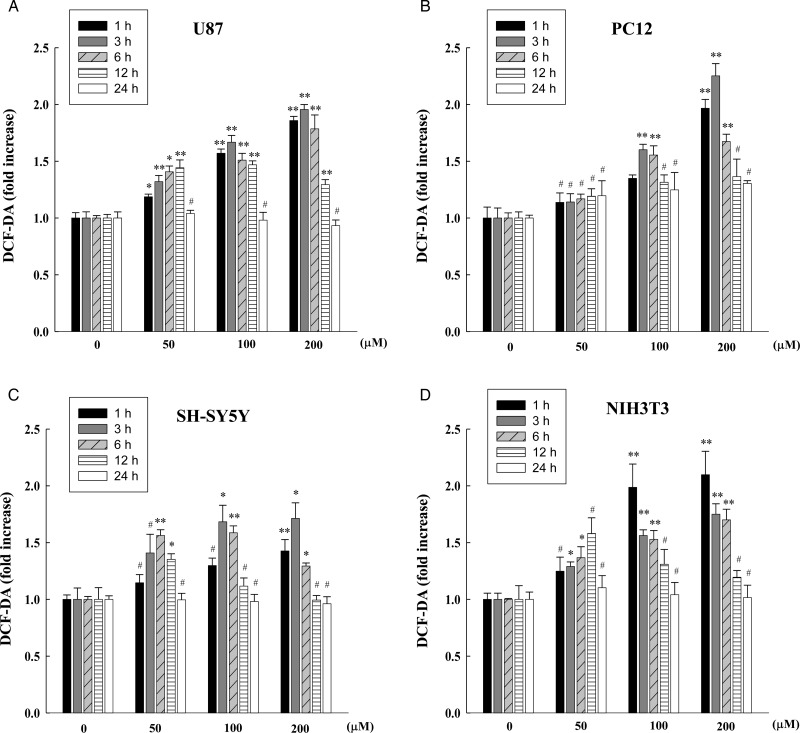
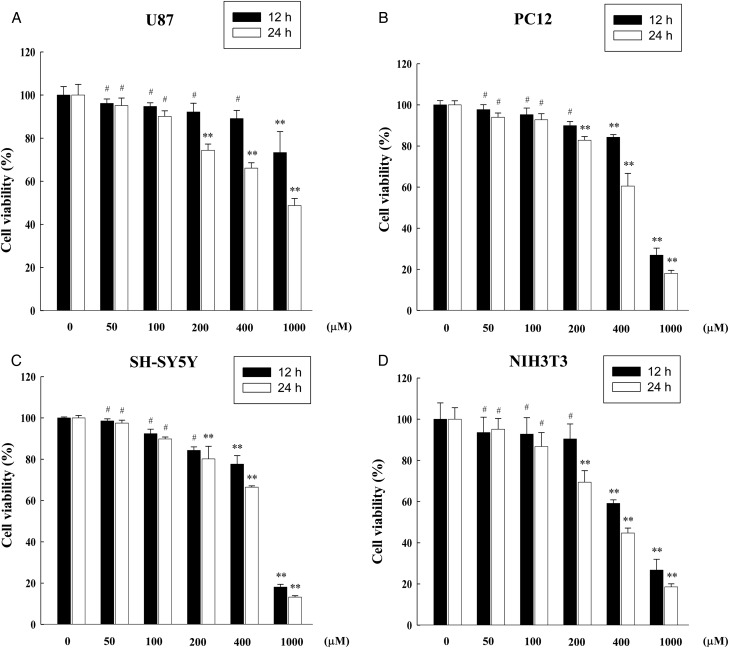
To investigate the effect of co-exposure with combined RF radiation and the ROS inducer H2O2, we first wanted to determine the optimal H2O2 concentration for the co-exposure. ROS levels were determined by DCF-DA staining at 1, 3, 6, 12 and 24 h in U87, PC12 and NIH3T3 cells after treatment with 0, 50, 100 and 200 µM H2O2 (Fig. 2). ROS levels in H2O2-treated U87, PC12 and NIH3T3 cells were increased in time- and dose-dependent manners up to a certain level of H2O2 concentration (Fig. 2A, B and D). Since SH-SY5Y cells are more resistant to oxidative stress compared with the other three cell lines, we treated SH-SY5Y cells with a range of doses of H2O2 including 0, 100, 200 and 400 µM then measured intracellular ROS levels (Fig. 2C). After treatment with various concentrations of H2O2 (0, 50, 100, 200, 400 and 1000 µM), cell viability was also measured by MTT assay in U87, PC12, SH-SY5Y and NIH3T3 cells 12 and 24 h post exposure (Fig. 3). From the statistical analyses by ANOVA, treatment with 100 µM H2O2 resulted in a statistically significant increase in the ROS level >1.5-fold and, at the same time, maintained cell viability above 80% up to 24 h post exposure (without statistical significance). Therefore, we selected 100 µM H2O2 concentration for the co-exposure of H2O2 and combined RF radiation.
Fig. 2.
Measurement of ROS levels in H2O2-treated neuronal cells. ROS levels were measured by DCF-DA staining in 0, 50, 100 and 200 µM of H2O2-treated U87 (A), PC12 (B), and NIH3T3 cells (D), or in 0, 100, 200 and 400 µM of H2O2-treated SH-SY5Y cells (C) at indicated times after exposure. The data are expressed as the means of six independent experiments together with the standard deviations of the means (M ± SD). Statistical calculations were performed by ANOVA via the Tukey post hoc test. Statistical significance values were *P < 0.05 and **P < 0.01, compared with the untreated control at the same time-point. # indicates non-significance (P > 0.05). The presented significances were selected from multiple comparisons.
Fig. 3.
Cell viabilities of H2O2-treated neuronal cells. Cell viability was measured by MTT assay in U87 (A), PC12 (B), SH-SY5Y (C), and NIH3T3 cells (D) at 12 and 24 h after different doses of H2O2 treatment (0, 50, 100, 200, 400 and 1000 µM). The data are shown as the means of six independent experiments together with the standard deviations of the means (M ± SD). Statistical calculations were performed by ANOVA via the Tukey post hoc test. The statistical significance value was **P < 0.01, compared with the untreated control at the same time-point. # indicates non-significance (P > 0.05). The presented significances were selected from multiple comparisons.
Effect of combined RF and H2O2 co-exposure on ROS levels in neuronal cells
We investigated the effects of co-exposure to combined RF radiation and H2O2 on ROS levels in three different neuronal cell lines (U87, PC12 and SH-SY5Y) and additionally in NIH3T3 mouse fibroblast cells. Cells were exposed to combined RF radiation (CDMA at SAR of 2 W/kg plus WCDMA at SAR of 2 W/kg) for 2 h prior to 100 µM H2O2 treatment, and then incubated for the indicated periods for the ROS measurements. ROS levels in cells exposed to combined RF radiation with H2O2 showed no statistically significant changes compared with incubator control or sham-exposed groups of 100 µM H2O2-treated cells (Fig. 4A–C). We could only detect a statistically significant change at 1 h co-exposure of RF radiation and 100 µM H2O2 in NIH3T3 cells (Fig. 4D). Conclusively, from our statistical analyses using ANOVA, we observed that combined RF exposure provided no significant additive effects on the ROS level changes induced by treatment with H2O2 in four different kinds of cells, with the exception of 1 h post co-exposure to RF radiation and H2O2 of NIH3T3 cells.
Fig. 4.
Measurement of ROS levels in neuronal cells after co-exposure to multiple RF signals (CDMA at 2 W/kg plus WCDMA at 2 W/kg for 2 h) and 100 µM H2O2. ROS were measured by DCF-DA staining in U87 (A), PC12 (B), SH-SY5Y (C), and NIH3T3 cells (D) at indicated periods after exposure. The data are shown as the means of six independent experiments together with the standard deviations of the means (M ± SD). Statistical calculations were performed by ANOVA via the Tukey post hoc test. Statistical significance values were *P < 0.05 and **P < 0.01, compared with either the incubator control group with or without H2O2, or the sham-exposed group with H2O2 treatment at each time-point. # indicates non-significance (P > 0.05).
Effect of menadione treatment on ROS level and cell viability in neuronal cells
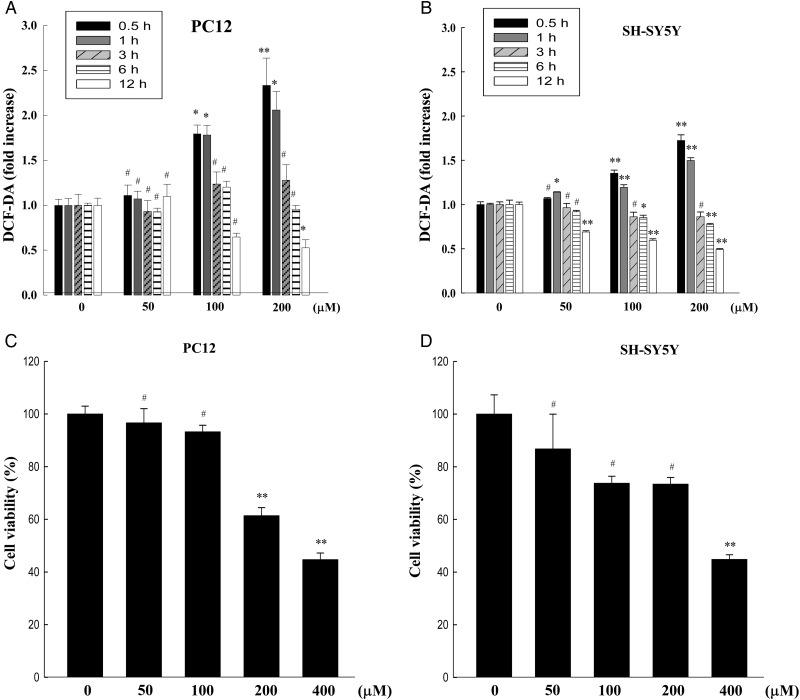
ROS levels and cell viability were assessed in four different cell lines (U87, PC12, SH-SY5Y and NIH3T3 cells) after menadione treatment. Since U87 and NIH3T3 cells did not show detectable changes in ROS levels up to 12 h after menadione treatment up to a concentration of 400 µM (data not shown), we measured the ROS level and cell viability only in PC12 and SH-SY5Y cells. In PC12 cells, from our statistical analyses using ANOVA, a statistically significant increase in the ROS level (>1.5-fold) was detected at 0.5 h and 1 h, but cell viability did not show a statistically significant change (still above 80%) at 24 h after 100 µM of menadione treatment (Fig. 5A and C). In SH-SY5Y cells, differences in cell viabilities did not reach statistically significant levels with 100 or 200 µM menadione treatments, but ROS levels were more evidently increased (>1.5-fold) with statistical significance after 200 µM menadione treatment than 100 µM treatment (Fig. 5B and D). Therefore, we selected 100 and 200 µM concentrations of menadione for the co-exposure experiments on PC12 and SH-SY5Y cells, respectively.
Fig. 5.
ROS levels and cell viabilities in menadione-treated neuronal cells. ROS levels were measured by DCF-DA staining in PC12 (A) and SH-SY5Y cells (B) after a range of concentrations of menadione treatment at indicated time-points. Cell viabilities were measured by MTT assay in PC12 (C) and SH-SY5Y cells (D) after different concentrations of menadione treatment for 24 h. The data are expressed as the means of six independent experiments together with the standard deviations of the means (M ± SD). Statistical calculations were performed by ANOVA via the Tukey post hoc test. Statistical significance values were *P < 0.05 and **P < 0.01, compared with untreated the control group at the same time-point. # indicates non-significance (P > 0.05). The presented significances were selected from multiple comparisons.
Effects of combined RF radiation and menadione co-exposure on ROS levels in neuronal cells
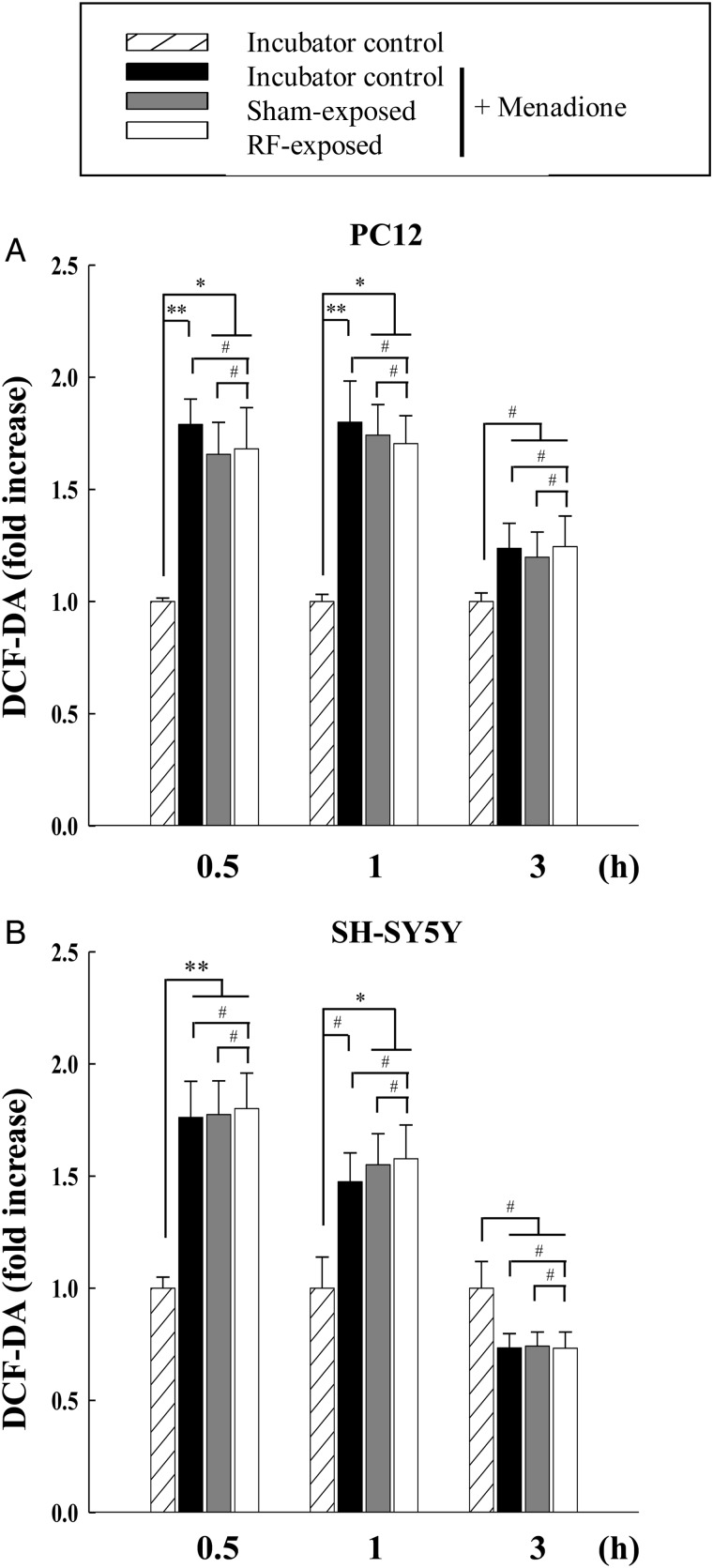
To determine the effect of combined RF radiation and menadione co-exposure on ROS levels, we measured the ROS levels in PC12 and SH-SY5Y cells at 0.5, 1 and 3 h after treatment with 100 and 200 µM menadione in combination with combined RF radiation, respectively. From our statistical analyses using ANOVA, ROS were increased with statistical significance in incubator control and sham-exposed cells for 0.5 and 1 h after menadione treatment, however we could not detect any synergistic or additive effect when menadione was treated with combined RF radiation in both cell lines (Fig. 6A and B).
Fig. 6.
Measurement of intracellular ROS levels in PC12 and SH-SY5Y cells which were co-exposed to multiple RF signals (CDMA at 2 W/kg plus WCDMA at 2 W/kg for 2 h) and menadione. ROS were measured by DCF-DA staining in PC12 (A) and SH-SY5Y cells (B) at 0.5, 1 and 3 h after co-exposure. The data are expressed as the means of six independent experiments together with the standard deviations of the means (M ± SD). Statistical calculations were performed by ANOVA via the Tukey post hoc test. Statistical significance values were *P < 0.05 and **P < 0.01, compared with either the incubator control group with or without menadione, or the sham-exposed group with menadione treatment at each time-point. # indicates non-significance (P > 0.05).
DISCUSSION
Even though a considerable number of epidemiological, in vivo and in vitro studies have been conducted to provide scientific evidence for a health risk assessment of mobile phone radiation [22, 23], apart from our group's recent papers no reports regarding the biological effects of exposure to multiple RF radiation signals are available. We reported that combined exposure of CDMA and WCDMA RF radiation did not cause any observable adverse effects on mouse fetuses [24]. We demonstrated that combinations of CDMA signal at 837 MHz and WCDMA signal at 1950 MHz did not affect cell cycle progression in vitro [3]. We also observed no effect of combined RF radiation on ROS level, superoxide dismutase activity, or ratio of reduced/oxidized glutathione in human mammary epithelial MCF10A cells at 10, 24 or 48 h after exposure [25]. As an extension of our efforts to elucidate the biological effects of multiple RF radiation signals on oxidative stress, in this study we investigated whether co-exposure to multi-RF radiation signals and ROS inducers could modify ROS production in neuronal cell models.
The possibility that RF radiation might act as a co-stressor with well-known neurotoxic challenges, thus playing a role in neurodegenerative diseases, has so far been poorly explored. This could be potentially important, since no details are available on the possible role of RF radiation as an environmental factor affecting brain aging quality and the risk of neurodegenerative diseases. In particular, Del Vecchio and colleagues [16] tested the influence of RF radiation on glutamate toxicity, which is a final molecular mechanism in many neurodegenerative diseases including stroke, epilepsy, and perhaps Alzheimer's disease. They concluded that exposure to an RF signal acts as a co-stressor for the oxidative damage of neural cells but only under particular circumstances. They also found that RF radiation reduced the amount of neuritis generated by neuronal cells [26]. Hossman and Hermann [13] observed that most of the positive results reported so far could be attributed to thermal effects from the experimental conditions described in their review paper about the effects of RF radiation of mobile phones on the central nervous system.
Recently, oxidative stress has been suggested to be the underlying mechanism responsible for the reported cellular effects of RF radiation, because ROS affects a large number of physiological functions via damage of cell lipids, proteins, DNA and other intracellular macromolecules [27–30]. Oxidative stress-mediated molecular damage, as a result of excessive accumulation of ROS, contributes to the pathogenesis of several chronic diseases including cancer, atherosclerosis, stroke, rheumatoid arthritis, neurodegeneration and diabetes. DNA oxidation has been given more attention because DNA damage directly results in mutagenesis and carcinogenesis [31]. Oxidative damage to not only nuclear DNA but also mitochondrial DNA could contribute to human diseases. The most often measured index of oxidative DNA damage is 8-hydroxyguanine (8-OHG) or 8-hydroxy deoxyguanosine (8-OHdG) [32]. Some studies have demonstrated that mobile phone radiation induce ROS production or oxidative stress in human spermatozoa in vitro, in rat lymphocytes, in rat cornea and lens, and in human lens epithelial cells [33–36]. On the contrary, in other studies no significant difference in ROS production and no oxidative stress induction was observed after RF radiation exposures in fibrosarcoma cells, immune cells or spermatozoa [7–9, 11, 37, 38]. However, there are very few peer-reviewed scientific publications addressing RF radiation effects on ROS production, and there is a lack of consistent results in neuronal cells. Luukkonen et al. [18] exposed SH-SY5Y neuroblastoma cells to CW GSM 900 signals at 5 W/kg for 1 h (alone or in combination with menadione), which induced intracellular ROS production and DNA damage 30 and 60 min after the end of exposure. De Gannes et al. [30] indicated that exposure to EDGE signal RF radiation at 1800 MHz (2 and 10 W/kg for 1 and 24 h) did not induce ROS production in any of three human brain cell lines. The question as to the potential hazard posed by combined RF radiation has not yet been definitively answered for neuronal cells. In the light of these considerations, our study chose to investigate the ROS parameter as one of the most important cellular determinants known to affect a large number of physiological functions.
In this study, we present the results of a study aimed at assessing the effects of combined RF radiation (CDMA at 2 W/kg plus WCDMA at 2 W/kg for 2 h) on the intracellular ROS level in neuronal cell models: U87 human glioma cells, PC12 rat pheochromcytoma cells, and SH-SY5Y human neuroblastoma cells. Three experimental systems were used to test the hypothesis that multiple RF signals might act as promoters of ROS formation: combined (837 MHz and 1950 MHz) RF radiation alone, and combined RF radiation in combination with either H2O2 or menadione. H2O2 is a relatively stable ROS and is capable of diffusing through the cellular membrane [39]. H2O2 can generate the more detrimental hydroxyl radical (OH˙) or degrade into H2O and O2 via the enzymatic reaction of catalase and glutathione peroxidase [40, 41]. Thus, the combined action of these two enzymes provides a protective mechanism against oxidation of various intracellular components. Menadione (2-methyl-1,4-naphthoquinone: vitamin K3) has two major mechanisms for cytotoxic action in a variety of biological systems. First, menadione undergoes one-electron reduction by microsomal NADPH-cytochrome P-450 reductase and mitochondrial NADH ubiquinone oxidoreductase, yielding the corresponding semiquinone radicals. Under aerobic conditions, the semiquinone radicals participate in redox cycling to generate ROS such as the superoxide anion (O2−) and H2O2. Second, menadione is capable of reacting with the thiol groups of proteins and glutathione (GSH) [42–44]. In this study, we tested whether exposure to ROS inducers such as H2O2 and menadione as well as to combined RF produces additive or synergistic effects on ROS production. We observed that none of the responses to either combined RF alone or combined RF with ROS inducers showed additive or synergistic effects on ROS production. In our experiments, only a few conditions presented a slight increase in ROS in comparison with sham exposure, and those slight increases were not consistent or sustained. Living organisms possess natural defense systems of complex antioxidant mechanisms to detoxify intracellular ROS [45]. ROS is controlled through the activity of intracellular antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione reductase, and catalase (CAT) [46, 47]. Non-enzymatic antioxidants including carotenoids, flavonoids and related polyphenols, vitamins, and glutathione are also capable of neutralizing ROS and their actions. Antioxidants act at different stages and by different mechanisms for balancing of the intracellular ROS level [48]. Since various antioxidant defense mechanisms could help to maintain homeostasis in the face of a sudden burst of ROS production, we measured ROS accumulation at 0.5, 1, 3, 6 and 12 h after the RF radiation exposures, instead of measuring acute ROS generation. Although we currently cannot fully explain our results, they might reflect differences in the cell culture systems used, or could suggest that exposure to RF signals acts as a co-stressor for oxidative damage of neural cells only under particular circumstances, as suggested by Del Vecchio et al. [16].
CONCLUSION
In conclusion, we found a transient small increase in the ROS level after exposure of neuronal cells to multiple RF signals (Fig. 1). However, we observed no prolonged or further increase in the ROS level. In addition, we observed no evident synergistic or additive effect of multiple RF signals on ROS generation when combined with additional treatment with ROS inducers. This was consistent with our previous report which demonstrated that single or combined RF radiation exposure alone did not elicit oxidative stress in MCF10A cells [25].
FUNDING
This study was supported by the Korea Communications Commission (2012) grant funded by the Korean government.
ACKNOWLEDGEMENTS
This data was presented at the BioEM conference in Greece, 10 June 2013.
REFERENCES
- 1.Jin YB, Lee HJ, Seon Lee J, et al. One-year, simultaneous combined exposure of CDMA and WCDMA radiofrequency electromagnetic fields to rats. Int J Radiat Biol. 2011;87:416–23. doi: 10.3109/09553002.2010.537428. [DOI] [PubMed] [Google Scholar]
- 2.Lee HJ, Jin YB, Kim TH, et al. The effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic fields on rat testicular function. Bioelectromagnetics. 2011;33:356–64. doi: 10.1002/bem.20715. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, Kim BC, Han NK, et al. Effects of combined radiofrequency radiation exposure on the cell cycle and its regulatory proteins. Bioelectromagnetics. 2011;32:169–78. doi: 10.1002/bem.20618. [DOI] [PubMed] [Google Scholar]
- 4.Pan JS, Hong MZ, Ren JL. Reactive oxygen species: a double-edged sword in oncogenesis. World J Gastroenterol. 2009;15:1702–7. doi: 10.3748/wjg.15.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 6.Roessner A, Kuester D, Malfertheiner P, et al. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–24. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Hook GJ, Spitz DR, Sim JE, et al. Evaluation of parameters of oxidative stress after in vitro exposure to FMCW- and CDMA-modulated radiofrequency radiation fields. Radiat Res. 2004;162:497–504. doi: 10.1667/rr3251. [DOI] [PubMed] [Google Scholar]
- 8.Lantow M, Lupke M, Frahm J, et al. ROS release and Hsp70 expression after exposure to 1,800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat Environ Biophys. 2006;45:55–62. doi: 10.1007/s00411-006-0038-3. [DOI] [PubMed] [Google Scholar]
- 9.Lantow M, Schuderer J, Hartwig C, et al. Free radical release and HSP70 expression in two human immune-relevant cell lines after exposure to 1800 MHz radiofrequency radiation. Radiat Res. 2006;165:88–94. doi: 10.1667/rr3476.1. [DOI] [PubMed] [Google Scholar]
- 10.Simkó M, Hartwig C, Lantow M, et al. Hsp70 expression and free radical release after exposure to non-thermal radio-frequency electromagnetic fields and ultrafine particles in human Mono Mac 6 cells. Toxicol Lett. 2006;161:73–82. doi: 10.1016/j.toxlet.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Zeni O, Di Pietro R, d'Ambrosio G, et al. Formation of reactive oxygen species in L929 cells after exposure to 900 MHz RF radiation with and without co-exposure to 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone. Radiat Res. 2007;167:306–11. doi: 10.1667/RR0595.1. [DOI] [PubMed] [Google Scholar]
- 12.Stagg RB, Thomas WJ, Jones RA, et al. DNA synthesis and cell proliferation in C6 glioma and primary glial cells exposed to 836.55 MHz modulated radiofrequency field. Bioelectromagnetics. 1997;18:230–6. doi: 10.1002/(sici)1521-186x(1997)18:3<230::aid-bem5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Hossmann KA, Hermann DM. Effects of electromagnetic radiation of mobile phones on the central nervous system. Bioelectromagnetics. 2003;24:49–62. doi: 10.1002/bem.10068. [DOI] [PubMed] [Google Scholar]
- 14.Merola P, Marino P, Lovisolo GA, et al. Proliferation and apoptosis in a neuroblastoma cell line exposed to 900 MHz modulated radiofrequency field. Bioelectromagnetics. 2006;27:164–71. doi: 10.1002/bem.20201. [DOI] [PubMed] [Google Scholar]
- 15.Meral I, Mert H, Mert N, et al. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. 2007;1169:120–4. doi: 10.1016/j.brainres.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Del Vecchio G, Giuliani A, Fernandez M, et al. Effect of radiofrequency electromagnetic field exposure on in vitro models of neurodegenerative disease. Bioelectromagnetics. 2009;30:564–72. doi: 10.1002/bem.20507. [DOI] [PubMed] [Google Scholar]
- 17.Poulletier de Gannes F, Haro E, Hurtier A et al. Effect of exposure to the edge signal on oxidative stress in brain cell models. Radiat Res. 2011;175:225–30. doi: 10.1667/rr2320.1. [DOI] [PubMed] [Google Scholar]
- 18.Luukkonen J, Hakulinen P, Mäki-Paakkanen J, et al. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat Res. 2009;662:54–8. doi: 10.1016/j.mrfmmm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Höytö A, Luukkonen J, Juutilainen J, et al. Proliferation, oxidative stress and cell death in cells exposed to 872 MHz radiofrequency radiation and oxidants. Radiat Res. 2008;170:235–43. doi: 10.1667/RR1322.1. [DOI] [PubMed] [Google Scholar]
- 20.Xu S, Zhou Z, Zhang L, et al. Exposure to 1800 MHz radiofrequency radiation induces oxidative damage to mitochondrial DNA in primary cultured neurons. Brain Res. 2010;1311:189–96. doi: 10.1016/j.brainres.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Le Bel CP, Ali SF, McKee M, et al. Organometal-induced increases in oxygen reactive species: the potential of 2',7'-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol Appl Pharmacol. 1990;104:17–24. doi: 10.1016/0041-008x(90)90278-3. [DOI] [PubMed] [Google Scholar]
- 22.Morrissey JJ. Radio frequency exposure in mobile phone users: implications for exposure assessment in epidemiological studies. Radiat Prot Dosimetry. 2007;123:490–7. doi: 10.1093/rpd/ncl547. [DOI] [PubMed] [Google Scholar]
- 23.Susa M, Pavicić I. Effects of radiofrequency electromagnetic fields on mammalian spermatogenesis. Arh Hig Rada Toksikol. 2007;58:449–59. doi: 10.2478/v10004-007-0038-y. (in Croatian) [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Lee JS, Pack JK, et al. Lack of teratogenicity after combined exposure of pregnant mice to CDMA and WCDMA radiofrequency electromagnetic fields. Radiat Res. 2009;172:648–52. doi: 10.1667/RR1771.1. [DOI] [PubMed] [Google Scholar]
- 25.Hong MN, Kim BC, Ko YG, et al. Effects of 837 and 1950 MHz radiofrequency radiation exposure alone or combined on oxidative stress in MCF10A cells. Bioelectromagnetics. 2012;33:604–11. doi: 10.1002/bem.21731. [DOI] [PubMed] [Google Scholar]
- 26.Del Vecchio G, Giuliani A, Fernandez M, et al. Continuous exposure to 900MHz GSM-modulated EMF alters morphological maturation of neural cells. Neurosci Lett. 2009;455:173–7. doi: 10.1016/j.neulet.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 27.Friedman J, Kraus S, Hauptman Y, et al. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem J. 2007;405:559–68. doi: 10.1042/BJ20061653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao K, Wu W, Wang K, et al. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol Vis. 2008;14:964–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Esmekaya MA, Ozer C, Seyhan N. 900 MHz pulse-modulated radiofrequency radiation induces oxidative stress on heart, lung, testis and liver tissues. Gen Physiol Biophys. 2011;30:84–9. doi: 10.4149/gpb_2011_01_84. [DOI] [PubMed] [Google Scholar]
- 30.Poulletier de Gannes F, Haro E, Hurtier A et al. Effect of exposure to the edge signal on oxidative stress in brain cell models. Radiat Res. 2011;175:225–30. doi: 10.1667/rr2320.1. [DOI] [PubMed] [Google Scholar]
- 31.Shaikh S, Nicholson LF. Advanced glycation end products induce in vitro cross-linking of alpha-synuclein and accelerate the process of intracellular inclusion body formation. J Neurosci Res. 2008;86:2071–82. doi: 10.1002/jnr.21644. [DOI] [PubMed] [Google Scholar]
- 32.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Iuliis GN, Newey RJ, King BV, et al. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLOS ONE. 2009;4 doi: 10.1371/journal.pone.0006446. e6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zmyslony M, Politanski P, Rajkowska E, et al. Acute exposure to 930 MHz CW electromagnetic radiation in vitro affects reactive oxygen species level in rat lymphocytes treated by iron ions. Bioelectromagnetics. 2004;25:324–8. doi: 10.1002/bem.10191. [DOI] [PubMed] [Google Scholar]
- 35.Balci M, Devrim E, Durak I. Effects of mobile phones on oxidant/antioxidant balance in cornea and lens of rats. Curr Eye Res. 2007;32:21–5. doi: 10.1080/02713680601114948. [DOI] [PubMed] [Google Scholar]
- 36.Yao K, Wu W, Wang K, et al. Electromagnetic noise inhibits radiofrequency radiation-induced DNA damage and reactive oxygen species increase in human lens epithelial cells. Mol Vis. 2008;14:964–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Brescia F, Sarti M, Massa R, et al. Reactive oxygen species formation is not enhanced by exposure to UMTS 1950 MHz radiation and co-exposure to ferrous ions in Jurkat cells. Bioelectromagnetics. 2009;30:525–35. doi: 10.1002/bem.20502. [DOI] [PubMed] [Google Scholar]
- 38.Falzone N, Huyser C, Franken DR, et al. Mobile phone radiation does not induce pro-apoptosis effects in human spermatozoa. Radiat Res. 2010;174:169–76. doi: 10.1667/RR2091.1. [DOI] [PubMed] [Google Scholar]
- 39.Ohno Y, Gallin JI. Diffusion of extracellular hydrogen peroxide into intracellular compartments of human neutrophils. Studies utilizing the inactivation of myeloperoxidase by hydrogen peroxide and azide. J Biol Chem. 1985;260:8438–46. [PubMed] [Google Scholar]
- 40.Götz ME, Künig G, Riederer P, et al. Oxidative stress: free radical production in neuronal degeneration. Pharmacol Therapeut. 1994;63:37–122. doi: 10.1016/0163-7258(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 41.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–8. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 42.Monks TJ, Jones DC. The metabolism and toxicity of quinones, quinonimines, quinine methides, and quinone-thioethers. Curr Drug Metabol. 2002;3:425–38. doi: 10.2174/1389200023337388. [DOI] [PubMed] [Google Scholar]
- 43.Chung SH, Chung SM, Lee JY, et al. The biological significance of non-enzymatic reaction of menadione with plasma thiols: enhancement of menadione-induced cytotoxicity to platelets by the presence of blood plasma. FEBS Lett. 1999;23:235–40. doi: 10.1016/s0014-5793(99)00452-4. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez CE, Shinyashiki M, Froines J, et al. An examination of quinone toxicity using the yeast Saccharomyces cerevisiae model system. Toxicology. 2004;201:185–96. doi: 10.1016/j.tox.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Abbas SR, Ahmad SD, Sabir SM, et al. Antioxidant activity, repair and tolerance of oxidative DNA damage in different cultivars of Sugarcane (Saccharum officinarum) leaves. Aust J Crop Sci. 2013;7:40–5. [Google Scholar]
- 46.Marjanović AM, Pavičić I, Trošić I. Biological indicators in response to radiofrequency/microwave exposure. Arh Hig Rada Toksikol. 2012;63:407–16. doi: 10.2478/10004-1254-63-2012-2215. (in Croatian) [DOI] [PubMed] [Google Scholar]
- 47.Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–50. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 48.Devasagayam TPA, Tilak JC, Boloor KK, et al. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Phys of India. 2004;52:794–804. [PubMed] [Google Scholar]