Abstract
The influence of a host defense protein, lactoferrin (LF), contained in exocrine secretions such as milk, on radiation disorder was investigated. A total of 25 C3H/He mice in each of two groups were maintained with 0.1% LF-added and LF-free diets, respectively, for one month. The mice were then treated with single whole-body X-ray irradiation at a sublethal dose (6.8 Gy), and the survival rate after irradiation was investigated. The survival rate at 30 d after irradiation was relatively higher in the LF group than in the control group (LF-free), (85 and 62%, respectively). The body weight 15 d after X-ray irradiation was also significantly greater in the LF group than in the control group. The hemoglobin level and hematocrit value were higher in the LF group at 5 d before X-ray irradiation. Another 52 mice underwent whole-body X-ray irradiation at the sublethal dose (6.8 Gy), and then LF was intraperitoneally injected once at 4 mg/animal to half of them. The survival rate in LF-treated mice 30 d after irradiation was 92%, significantly higher than in mice treated with saline (50%) (P = 0.0012). In addition, LF showed hydroxyl radical scavenger activity in vitro. These findings suggest that LF may inhibit radiation damage.
Keywords: lactoferrin, radioprotection, sub-lethal X-ray irradiation, mice
INTRODUCTION
Radiation damage is basically due to DNA injury caused by the ionization effect of radiation. This process involves hyperoxidation by the radicals produced. Radiation injures DNA, even at a very low level, and the body has mechanisms for repairing it. However, when DNA injury cannot be repaired, such as that caused mainly by exposure to very high-level radiation, cell death and mutation occur, and various malfunctions develop. With the progression of studies in the radiation medicine and biology fields, the developmental mechanisms of radiation damage have been gradually elucidated, and radioprotectors for inhibiting these processes in radiotherapy have been increasingly investigated and developed. Radiation exposure is divided into external and internal types, and various compounds, such as cytokines, cystine, and aminothiol drugs, are known radioprotectors [1]. Ginseng extract, chitosan, alcohol, zinc and oligoelements and Lachesis mut venom (O-LM) have all been reported as radioprotective materials [2–5].
Lactoferrin (LF) is an iron-binding protein contained in exocrine secretions, such as milk and saliva, that is also found in neutrophils, and it has diverse biological activities, acting as an antimicrobial, immunomodulator, and an antioxidant, suggesting it has a role in host protection [6]. Since LF chelates iron ions, it inhibits the hyperoxidation of lipids by inhibiting the iron ion-catalyzed Fenton reaction [7]. It has also been reported that lactoferricin (LFcin), a peptide produced by the digestion of LF with pepsin, exhibits antioxidant activity similar to that of LF, despite the iron ion-chelating region being absent [8]. It has recently been shown that LF purified from cow's milk exhibits various beneficial effects in humans and animals, including protection of the body from external and internal impairments, such as protection from infection and inhibition of carcinogenesis and drug-induced impairment [9]. Regarding the inhibitory effect on drug-induced impairment, inhibition of NSAID-induced intestinal impairment in humans [10] and small intestinal injury in rats [11], improvement of drug-induced colitis in rats [12], and prevention of chemotherapy-induced ovarian disorder in mice [13] have been reported. In addition, an increase in salivary flow and improvement of bacterial flora has been reported associated with gel and dentifrice containing LF in xerostomia induced by radiotherapy for oral cancer [14]. However, it is not known whether LF exerts protective effects against sublethal X-ray radiation. In the present study, mortality and physiological properties were investigated in LF-fed and LF-injected mice in an X-ray radiation experiment. A radical-scavenging ability of LF was also evaluated by electron spin resonance spectrometry.
MATERIALS AND METHODS
Animal experiment by oral administration of LF
Male C3H/He mice were purchased from SLC Inc. (Hamamatsu, Japan) and kept in a conventional animal room before and after X-ray irradiation. Animals were treated under the Guide for the Care and Use of Laboratory Animals in the National Institute of Radiological Sciences. For the oral LF administration experiment, 50 mice were divided into two groups. One group was fed a completely purified diet, AIN-93G containing 0.1% bovine LF (Morinaga Milk Industry, Co. Ltd, Tokyo, Japan), and the control group was fed AIN-93G without LF (Table 1). The animals were maintained for one month on these diets in a conventional animal experiment facility. The mice were then treated with single whole-body X-ray irradiation (Pantak HF-320: Shimazu, Kyoto, Japan; 200 kV, 20 mA; filter: Al 0.5 mm + Cu 0.5 mm; dose rate: 0.865 Gy/min) at 6.8 Gy, and the survival rates and body weights were investigated for 30 d after irradiation. The animals were maintained on the same diets after irradiation. Both groups for observation of changes in the body weight and blood composition was separately established, and five animals were randomly selected 5 d before and 15 and 30 d after irradiation and subjected to the experiment.
Table 1.
Composition of completely purified diets
| Composition (g/kg diet) |
||
|---|---|---|
| Control diet | Lactoferrin diet | |
| Corn starch | 532 | 531 |
| Casein | 200 | 200 |
| Granulated sugar | 100 | 100 |
| Soybean oil | 70 | 70 |
| Cellulose powder | 50 | 50 |
| Mineral mix (AIN93G) | 35 | 35 |
| Vitamin mix (AIN93) | 10 | 10 |
| L-cystine | 3 | 3 |
| LF | 0 | 1 |
Hematological analysis
Blood was collected by cardiac puncture under ether anesthesia 5 d before and 15 d after irradiation. The leukocyte count, red blood cell count, hemoglobin concentration, and hematocrit value were evaluated on an automated hematology analyzer F-820 (Sysmex Co., Kobe, Japan).
Animal experiment using intraperitoneal injection of LF
To perform an experiment using intraperitoneal LF administration, 52 male C3H/He mice aged 6 weeks were treated with whole-body X-ray irradiation at 6.8 Gy. Immediately after irradiation, 0.3 ml of bovine LF dissolved in saline to adjust the concentration to 4 mg/animal was intraperitoneally injected into 26 mice. Saline was intraperitoneally injected into the remaining 26 mice as a control group. After irradiation, both groups were maintained with commercial pellets, MF (Oriental Yeast Co. Ltd, Tokyo, Japan), and the survival rate was observed for 30 d in a conventional animal experiment facility.
Electron spin resonance
LF was dissolved in water to provide a 1% w/w solution. Glutathione (GSH) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were used as the reference materials. The radical scavenging ability of LF was evaluated by the electron spin resonance (ESR) spin trapping method, in which 5,5-dimethyl-1-pyrroline-N-oxide (DMPO, Labotec, Tokyo, Japan) was used as a spin trap [4]. Superoxide anions (O2-) were generated with the hypoxanthine–xanthine oxidase system. Hydroxyl radicals (·OH) were generated with the Cu (en)2 or H2O2/ultraviolet (UV)-ray system. For measuring superoxide anion and hydroxyl radical scavenging, a free radical monitor, JES-FR30S (JEOL, Tokyo, Japan), was used. Normalization of all spectra for accurate calculations was done using manganese oxide (MnO) as an internal standard. Manganese oxide provided a constant signal with which all peak heights were compared. A sample peak height was divided by the MnO peak height to give the relative peak height. ESR measurements were made under the following conditions: magnetic field, 335.8 mT; power, 4 mW; modulation frequency, 9.4 GHz; modulation amplitude, 0.079 mT; response time, 0.03 s; sweep time, 2 min. ESR spectra were measured at 23°C. Data analysis was performed using a computer program (version 5.2 for JES-FR30) connected to the free radical monitor.
Statistical analysis
Data are expressed as means ± standard deviation (SD). The survival rate of mice between the two groups was analyzed by the Kaplan–Meier test (log-rank), using the computer program JMP version 5 (SAS Institute Japan, Tokyo, Japan). Otherwise, statistical analysis was conducted employing the two-tailed Student's t-test. P-values of <0.05 were considered to indicate a significant difference.
RESULTS AND DISCUSSION
Radioprotective effect of orally administered LF
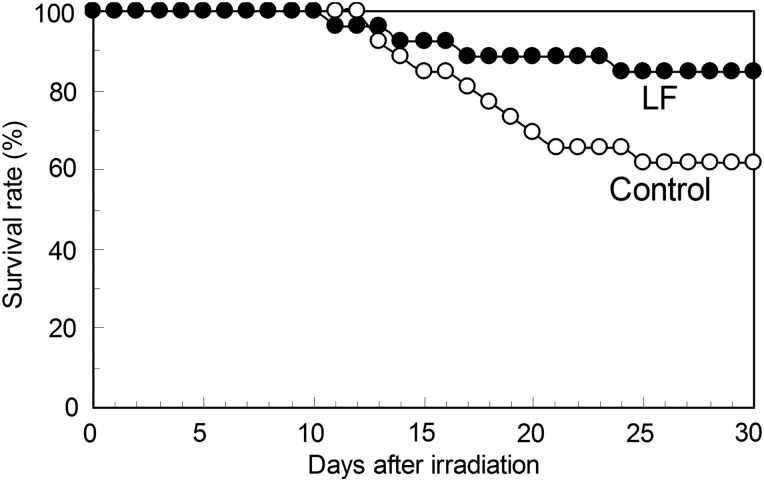
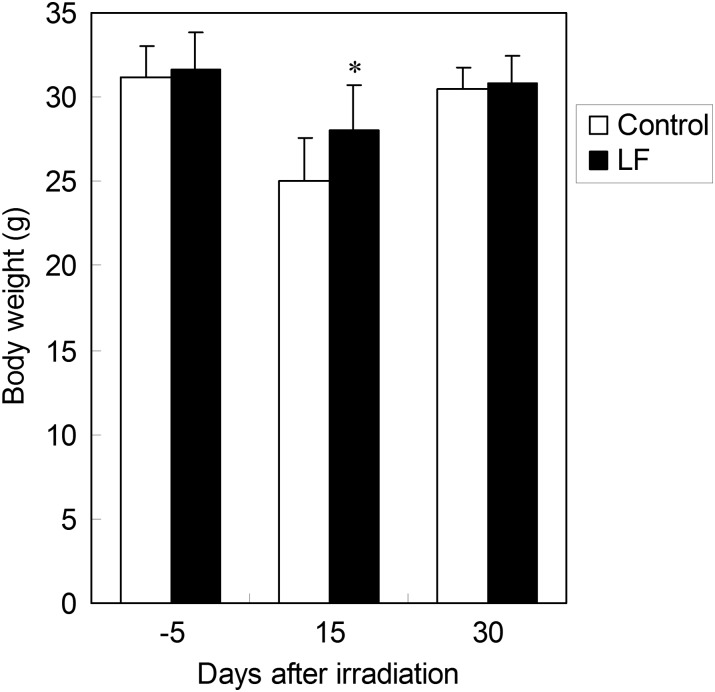
After maintenance with either the 0.1% LF-containing or LF-free diet for 30 d, the animals were treated with whole-body X-ray irradiation at 6.8 Gy. Changes in the survival rate after the irradiation are shown in Fig. 1. Mice died 10–25 d after irradiation, but the survival rate at 30 d was higher in the LF group (84.6%) than in the control group (61.5%) (P = 0.0704). The body weights at 5 d before (control group: n = 47, LF group: n = 47), 15 d after (control group: n = 9, LF group: n = 10) and 30 d after irradiation (control group: n = 6, LF group: n = 5) are shown in Fig. 2. Compared with before irradiation, the body weight slightly decreased at 15 d, but it was significantly higher in the LF group than in the control group. At 30 d after irradiation, the body weights of both groups were restored to a level similar to that before irradiation.
Fig. 1.
A survival curve for mice fed either the LF-supplemented or the control diet after whole-body X-ray irradiation. After feeding on the LF-supplemented (n = 25) or LF-free (control) diet (n = 25) for 30 d, the mice were treated with whole-body X-ray irradiation at 6.8 Gy. The survival rates (%) during the 30-d period after irradiation are presented. The P-value on comparison of the two groups was 0.0704.
Fig. 2.
The body weight of mice treated with X-ray irradiation, with or without oral LF. The body weights at 5 d before and 15 d after irradiation are shown. *P < 0.05 of significant difference between the control and LF groups.
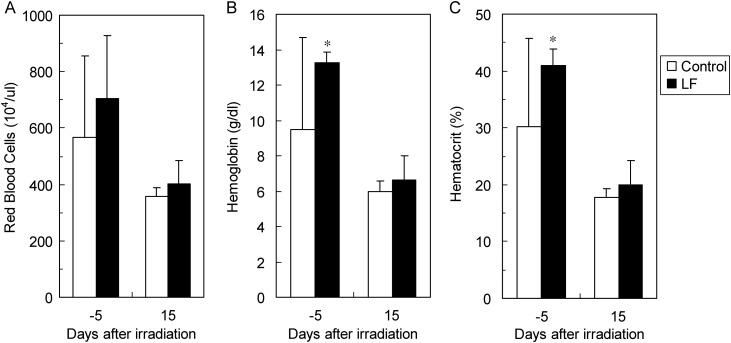
The red blood cell count (A), hemoglobin level (B), and hematocrit value (C) measured 5 d before (control group: n = 8, LF group: n = 5) and 15 d after irradiation (control group: n = 5, LF group: n = 7) are shown as peripheral red blood cell-related indices in Fig. 3. All of these were decreased at 15 d after irradiation, compared with those before irradiation. The hemoglobin level and hematocrit value were significantly higher in the LF group than in the control group at 5 d before irradiation, and similar situation was present 15 d after irradiation. Regarding the white blood cell-related indices, the values at 15 d after irradiation were low, and no stable data could be obtained.
Fig. 3.
Red blood cell-related indices in mice treated with X-ray irradiation, with or without oral LF. The peripheral blood red blood cell count (A), hemoglobin level (B), and hematocrit value (C) at 5 d before and 15 d after irradiation are shown.
In the experiment in which mice maintained on the LF-containing diet were irradiated with X-rays at half the lethal dose, the survival rate was apparently higher in the LF group than in the control group, although the difference was not significant. Since the absence of a significant difference may have been due to the small number of animals, continuation of the experiment is necessary. Body weight in the LF group was significantly higher than that in the control group 15 d after X-ray irradiation, and the hemoglobin level and hematocrit value were significantly higher in the LF than in the control group 5 d before irradiation.
Although the mechanism of radioprotection by LF has not been fully clarified, oral LF elevated the hemoglobin level and hematocrit value before irradiation. On the other hand, hemoglobin level and hematocrit value were not significantly different between the control and LF groups after irradiation. It was reported that, when infants were fed an LF-added powdered infant formula, the hematocrit value was higher compared with that in the group fed the conventional powdered infant formula [15]. Increases in the mean corpuscular volume (MCV) and hemoglobin (MCH) after LF ingestion compared with those before ingestion were observed in female marathon runners [16]. These findings show that LF increases red blood cell-related indices. We previously reported that oral chitosan administration increased the survival rate after irradiation [4]. In this system, the red blood cell count, hemoglobin level, and hematocrit value were higher in the chitosan-treated than in the non-treated group at 14 d after irradiation, suggesting its relation to the radioprotective effect. It has also been reported that sodium tungstate administration improved hematocrit value reduced by irradiation and also the survival rate in rats following irradiation [17]. Oral LF-induced increases in the hemoglobin level and hematocrit value before irradiation may have contributed to host protection after irradiation. The difference in the body weight was observed after irradiation, whereas the changes in hemoglobin level and hematocrit value were observed before irradiation. These results suggest that the difference in body weight may be associated with radioprotective effects of LF, and that the difference in the hemoglobin level and the hematocrit value may reflect the general nutritional effects of LF.
Radioprotective effect of intraperitoneally injected LF
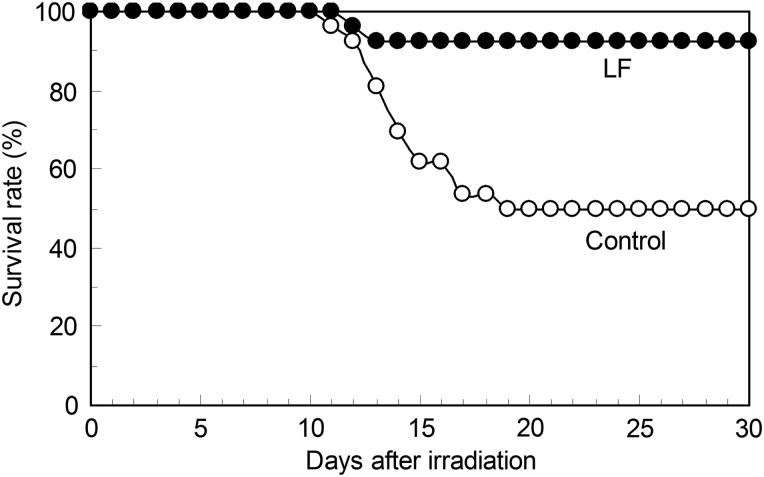
Mice were treated with whole-body X-ray irradiation at 6.8 Gy followed by the intraperitoneal administration of LF (experimental group) or saline (control group). The survival rates in these two groups are shown in Fig. 4. Animals started to die about 10 d after irradiation. The survival rate at 30 d after irradiation was 50.0% in the control group, but it was significantly higher (92.3%) in the LF group (P = 0.0012).
Fig. 4.
A survival curve for mice treated with whole-body X-ray irradiation, with or without intraperitoneal LF administration. Mice were treated with whole-body irradiation at 6.8 Gy, and LF was intraperitoneally administered immediately after X-ray irradiation (n = 26). Saline was intraperitoneally administered to the control group (n = 26). The survival rates (%) during the 30-d period after X-ray irradiation are presented. The P-value is 0.0012 between the two groups.
When LF was intraperitoneally injected once into mice irradiated with X-rays at a sublethal dose, the survival rate was significantly higher than that in the control group. There have been several reported cases in which an intraperitoneally administered substance exhibited a marked radioprotective effect [18–20]. Although the mechanism of this event is unclear, a higher ratio of the substance may have entered the portal system compared with that after oral administration. Elucidation of this mechanism may facilitate prevention of adverse effects and increase the efficacy of radiotherapy. Detailed investigation of the route of administration is necessary.
Radical-scavenging activity of LF
The radical-scavenging activity of LF was measured. In the two ·OH generation systems, the Cu(en)2 and H2O2/UV systems, LF demonstrated radical-scavenging activity (Table 2). The molar-base IC50 value of LF was about 100–1000 times lower than that of GSH, and several thousand times lower than that of Trolox. LF did not show radical-scavenging activity in a system producing O2· − (data not shown).
Table 2.
Scavenging abilities of LF against ·OH
| Compound | IC50 against ·OH formation (mM) |
|
|---|---|---|
| in Cu(en)2 system | in H2O2/UV system | |
| LF | 0.0035 | 0.0025 |
| GSH | 0.17 | 2.8 |
| Trolox | 15 | ND |
ND = not determined.
Generally, radiation resistance is considered to be due to the inhibition of reactive oxygen by antioxidative activity and activation of the immune function. About 70% of the body is comprised of water. When radiation hits water, free radicals are produced. Many of the actions of radiation on the body are derived from reactive oxygen and free radicals produced by the radiolysis of water. Irradiation of water produces two free radicals: superoxide anions (O2·−) and hydroxyl radicals (·OH). The body has a potent chemical protective system scavenging active oxygen and free radicals and preventing hyperoxidation of cell membranes, and this free radical scavenging action is essential for maintaining life. The following mechanisms to protect the body from impairment by these are considered: (i) inhibition of hydroxyl radical-producing resources, such as by superoxide dismutase and catalase, (ii) trapping of metals, such as iron and copper, to inhibit hydroxyl radical production, and (iii) trapping of generated hydroxyl radicals to prevent the injury of biological components.
In the experiment using ESR, LF had no superoxide-scavenging ability but showed hydroxyl radical-scavenging activity. Since LF exhibits antioxidative activity by inhibiting the Fenton reaction through chelating iron [7], the activity may have been due to the mechanism described in (ii). This action may particularly contribute to the radioprotective effect of intraperitoneally administered LF. Although we did not perform measurements using ESR, LFcin, a peptide produced by digestion of LF by the gastric digestive enzyme pepsin, is also known to show antioxidative activity [8], suggesting an involvement of LFcin as a part of the radioprotection mechanism of orally administered LF.
Another considered mechanism of the antiradiation effect of LF is improvement of the intestinal bacterial flora and protection against infection by an immunomodulatory function. Oral LF administration to mice decreased the intestinal Enterobacteriaceae and Clostridium [21], and inhibited bacterial translocation from the intestine to lymph nodes [22]. Decreases in the incidence of sepsis and mortality from sepsis by LF administration to extremely low-birth-weight infants have also been reported [23]. One cause of death from irradiation may be infection due to a reduced immune function. It is also possible that the LF-induced improvement in intestinal bacterial flora and protection against infection can inhibit the development and aggravation of infectious diseases, improving the survival rate.
It was suggested that LF could be used as a radioprotector to reduce the adverse effects of radiotherapy, in addition to its utilization in food form. We are planning to prepare an experimental system in which low-dose irradiation induces sublethal disorders, and another system that induces radiation cancers, and investigate the efficacy of LF at reducing the effects of accidental exposure at nuclear power plants and in radiotherapy.
REFERENCES
- 1.Nair CKK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 2.Yonezawa M. Restoration of radiation injury by intraperitoneal injection of ginseng extract in mice. J Radiat Res. 1976;17:111–3. doi: 10.1269/jrr.17.111. [DOI] [PubMed] [Google Scholar]
- 3.Crescenti EJ, Medina VA, Croci M, et al. Radioprotection of sensitive rat tissue by oligoelements Se, Zn, Mn plus Lachesis muta venom. J Radiat Res. 2011;52:557–67. doi: 10.1269/jrr.11031. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura Y, Kim HS, Ikota N, et al. Radioprotective effect of chitosan in sub-lethally X-ray irradiated mice. J Radiat Res. 2003;44:53–8. doi: 10.1269/jrr.44.53. [DOI] [PubMed] [Google Scholar]
- 5.Matsubara J, Shibat T, Ishioka K, et al. Protective effect of zinc against lethality in irradiated mice. Environ Res. 1986;41:558–67. doi: 10.1016/s0013-9351(86)80150-5. [DOI] [PubMed] [Google Scholar]
- 6.Lönnerdal B. Nutritional roles of lactoferrin. Curr Opin Clin Nutr Metab Care. 2009;12:293–7. doi: 10.1097/MCO.0b013e328328d13e. [DOI] [PubMed] [Google Scholar]
- 7.Gutteridge J, Paterson SK, Segal AW, et al. Inhibition of lipid peroxidation by the iron-binding protein lactoferrin. Biochem J. 1981;199:259–61. doi: 10.1042/bj1990259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakabayashi H, Matsumoto H, Hashimoto K, et al. Inhibition of iron/ascorbate-induced lipid peroxidation by an N-terminal peptide of bovine lactoferrin and its acylated derivatives. Biosci Biotechnol Biochem. 1999;63:955–7. doi: 10.1271/bbb.63.955. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi H, Yamauchi K, Takase M. Lactoferrin research, technology and applications. Int Dairy J. 2006;16:1241–51. [Google Scholar]
- 10.Dial EJ, Dohman AJ, Romero JJ, et al. Recombinant human lactoferrin prevents NSAID-induced intestinal bleeding in rodents. J Pharm Pharmacol. 2005;57:93–9. doi: 10.1211/0022357055191. [DOI] [PubMed] [Google Scholar]
- 11.Van't Land B, van Beek NM, van den Berg JJ, et al. Lactoferrin reduces methotrexate-induced small intestinal damage, possibly through inhibition of GLP-2-mediated epithelial cell proliferation. Digest Dis Sci. 2004;49:425–33. doi: 10.1023/b:ddas.0000020497.35250.93. [DOI] [PubMed] [Google Scholar]
- 12.Togawa J, Nagase H, Tanaka K, et al. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am J Physiol Gastrointest Liver Physiol. 2002;283:G187–95. doi: 10.1152/ajpgi.00331.2001. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi Y, Higashi T, Tatsumi K, et al. Lactoferrin is associated with a decrease in oocyte depletion in mice. Fertil Steril. 2009;91:2069–78. doi: 10.1016/j.fertnstert.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Nagy K, Urban E, Fazekas O, et al. Controlled study of lactoperoxidase gel on oral flora and saliva in irradiated patients with oral cancer. J Craniofac Surg. 2007;18:1157–64. doi: 10.1097/scs.0b013e3180de6311. [DOI] [PubMed] [Google Scholar]
- 15.King JC, Cummings GE, Guo N, et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J Pediatr Gastroenterol Nutr. 2007;44:245–51. doi: 10.1097/01.mpg.0000243435.54958.68. [DOI] [PubMed] [Google Scholar]
- 16.Koikawa N, Nagaoka I, Yamaguchi N, et al. Preventive effect of lactoferrin intake on anemia in female long distance runners. Biosci Biotechnol Biochem. 2008;72:931–5. doi: 10.1271/bbb.70383. [DOI] [PubMed] [Google Scholar]
- 17.Sato K, Ichimasa M, Miyahara K, et al. Radioprotective effects of sodium tungstate on hematopoietic injury by exposure to 60Co γ-rays in wistar rats. J Radiat Res. 1999;40:101–13. doi: 10.1269/jrr.40.101. [DOI] [PubMed] [Google Scholar]
- 18.Takeda A, Yonezawa M, Katoh N. Restoration of radiation injury by ginseng. I. Responses of X-irradiated mice to ginseng extract. J Radiat Res. 1981;22:323–35. doi: 10.1269/jrr.22.323. [DOI] [PubMed] [Google Scholar]
- 19.Jagetia GC, Baliga MS, Venkatesh P. Influence of seed extract of Syzygium cumini (Jamun) on mice exposed to different doses of gamma-radiation. J Radiat Res. 2005;46:59–65. doi: 10.1269/jrr.46.59. [DOI] [PubMed] [Google Scholar]
- 20.Morita A, Yamamoto S, Wang B, et al. Sodium orthovanadate inhibits p53–mediated apoptosis. Cancer Res. 2010;70:257–65. doi: 10.1158/0008-5472.CAN-08-3771. [DOI] [PubMed] [Google Scholar]
- 21.Teraguchi S, Shin K, Ozawa K, et al. Bacteriostatic effect of orally administered bovine lactoferrin on proliferation of Clostridium species in the gut of mice fed bovine milk. Appl Environ Microbiol. 1995;61:501–6. doi: 10.1128/aem.61.2.501-506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teraguchi S, Shin K, Ogata T, et al. Orally administered bovine lactoferrin inhibits bacterial translocation in mice fed bovine milk. Appl Environ Microbiol. 1995;61:4131–4. doi: 10.1128/aem.61.11.4131-4134.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzoni P, Rinaidi M, Cattani S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–8. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]