Abstract
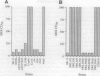
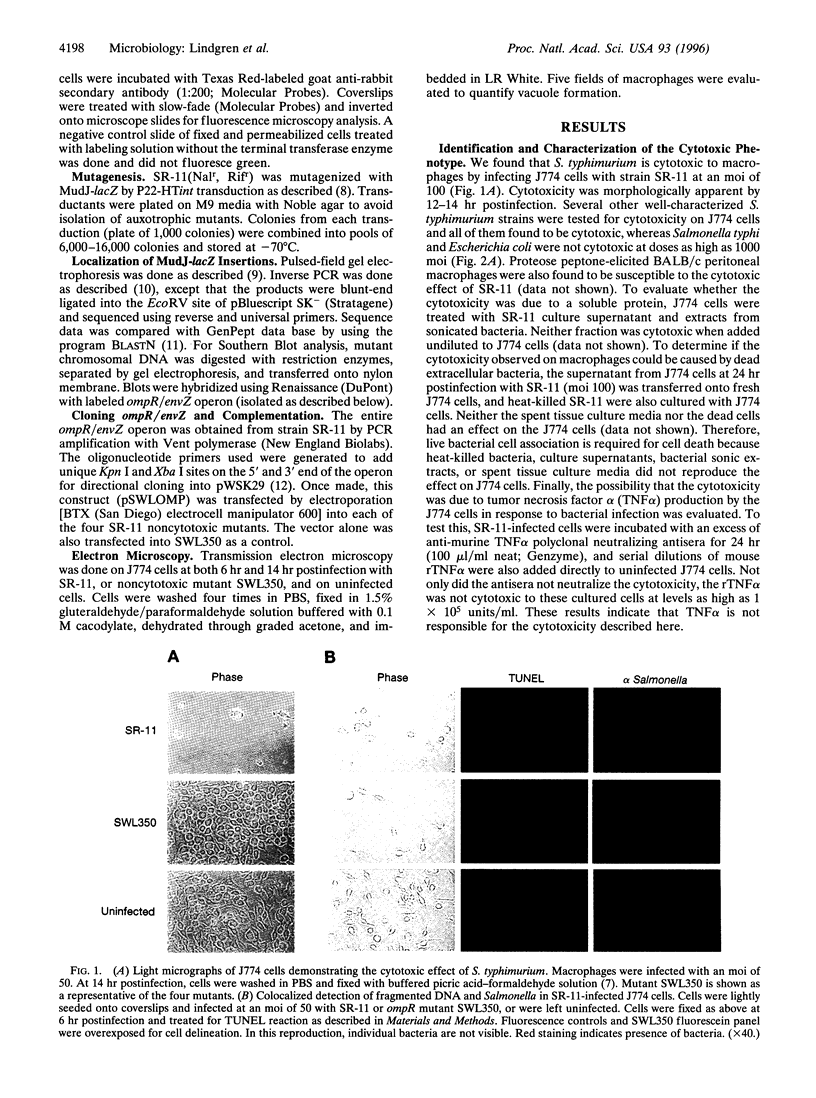
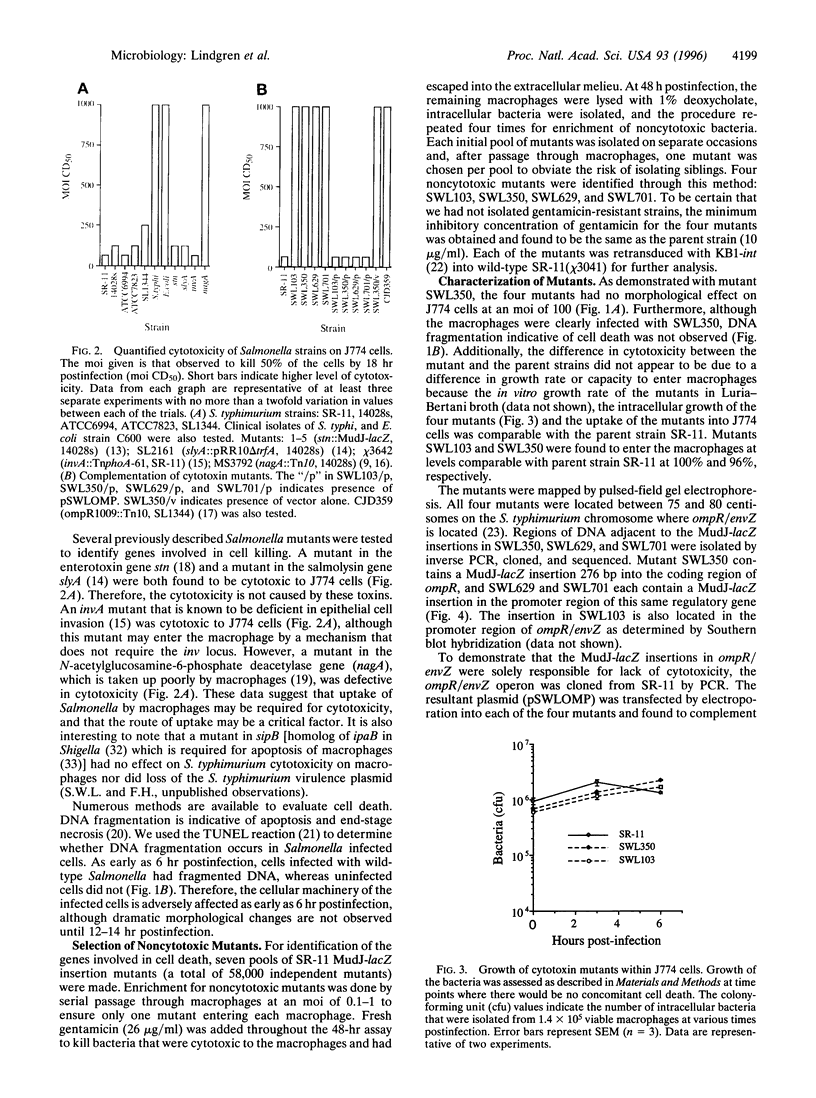
Phagocytic cells are a critical line of defense against infection. The ability of a pathogen to survive and even replicate within phagocytic cells is a potent method of evading the defense mechanisms of the host. A number of pathogens survive within macrophages after phagocytosis and this contributes to their virulence. Salmonella is one of these pathogens. Here we report that 6-14 hr after Salmonella enters the macrophage and replicates, it resides in large vacuoles and causes the destruction of these cells. Furthermore, we identified four independently isolated MudJ-lacZ insertion mutants that no longer cause the formation of these vacuoles or kill the macrophages. All four insertions were located in the ompR/envZ regulon. These findings suggest that killing and escape from macrophages may be as important steps in Salmonella pathogenesis as are survival and replication in these host cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpuche-Aranda C. M., Racoosin E. L., Swanson J. A., Miller S. I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994 Feb 1;179(2):601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bernardini M. L., Fontaine A., Sansonetti P. J. The two-component regulatory system ompR-envZ controls the virulence of Shigella flexneri. J Bacteriol. 1990 Nov;172(11):6274–6281. doi: 10.1128/jb.172.11.6274-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler A. J., Kusters J. G., Stojiljkovic I., Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994 May;62(5):1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumler A. J., Tsolis R. M., Heffron F. The lpf fimbrial operon mediates adhesion of Salmonella typhimurium to murine Peyer's patches. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):279–283. doi: 10.1073/pnas.93.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield S. N., Dorman C. J., Hayward C., Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both ompC and ompF are attenuated in vivo. Infect Immun. 1991 Jan;59(1):449–452. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A. K., Peterson J. W., Chary P., Prasad R. Molecular characterization of an enterotoxin from Salmonella typhimurium. Microb Pathog. 1994 Feb;16(2):85–98. doi: 10.1006/mpat.1994.1010. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Chatfield S., Higgins C. F., Hayward C., Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989 Jul;57(7):2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990 Nov;162(5):1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- Forst S. A., Roberts D. L. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res Microbiol. 1994 Jun-Aug;145(5-6):363–373. doi: 10.1016/0923-2508(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Francis C. L., Starnbach M. N., Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low-oxygen conditions. Mol Microbiol. 1992 Nov;6(21):3077–3087. doi: 10.1111/j.1365-2958.1992.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Gahring L. C., Heffron F., Finlay B. B., Falkow S. Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun. 1990 Feb;58(2):443–448. doi: 10.1128/iai.58.2.443-448.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson M. M., Ellis E. M., Graeme-Cook K. A., Higgins C. F. OmpR and EnvZ are pleiotropic regulatory proteins: positive regulation of the tripeptide permease (tppB) of Salmonella typhimurium. Mol Gen Genet. 1987 Apr;207(1):120–129. doi: 10.1007/BF00331499. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Transitory cis complementation: a method for providing transposition functions to defective transposons. Genetics. 1988 May;119(1):9–12. doi: 10.1093/genetics/119.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaniga K., Tucker S., Trollinger D., Galán J. E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995 Jul;177(14):3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Gobé G. C., Winterford C. M., Harmon B. V. Anatomical methods in cell death. Methods Cell Biol. 1995;46:1–27. doi: 10.1016/s0091-679x(08)61921-4. [DOI] [PubMed] [Google Scholar]
- Kohbata S., Yokoyama H., Yabuuchi E. Cytopathogenic effect of Salmonella typhi GIFU 10007 on M cells of murine ileal Peyer's patches in ligated ileal loops: an ultrastructural study. Microbiol Immunol. 1986;30(12):1225–1237. doi: 10.1111/j.1348-0421.1986.tb03055.x. [DOI] [PubMed] [Google Scholar]
- Libby S. J., Goebel W., Ludwig A., Buchmeier N., Bowe F., Fang F. C., Guiney D. G., Songer J. G., Heffron F. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeström P., Laamanen I., Palva E. T. Structure and expression of the ompB operon, the regulatory locus for the outer membrane porin regulon in Salmonella typhimurium LT-2. J Mol Biol. 1988 Jun 20;201(4):663–673. doi: 10.1016/0022-2836(88)90465-2. [DOI] [PubMed] [Google Scholar]
- McIntire S. A. Transduction with integration-defective mutants of Salmonella typhimurium bacteriophage KB1. J Bacteriol. 1974 Feb;117(2):907–908. doi: 10.1128/jb.117.2.907-908.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L. M cells--entryways of opportunity for enteropathogens. J Exp Med. 1994 Jul 1;180(1):7–9. doi: 10.1084/jem.180.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J., Hayman M. J., Galán J. E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993 Feb 26;72(4):505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- Pickard D., Li J., Roberts M., Maskell D., Hone D., Levine M., Dougan G., Chatfield S. Characterization of defined ompR mutants of Salmonella typhi: ompR is involved in the regulation of Vi polysaccharide expression. Infect Immun. 1994 Sep;62(9):3984–3993. doi: 10.1128/iai.62.9.3984-3993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hessel A., Rudd K. E. Genetic map of Salmonella typhimurium, edition VIII. Microbiol Rev. 1995 Jun;59(2):241–303. doi: 10.1128/mr.59.2.241-303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolis R. M., Bäumler A. J., Stojiljkovic I., Heffron F. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 1995 Aug;177(16):4628–4637. doi: 10.1128/jb.177.16.4628-4637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. F., Kushner S. R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991 Apr;100:195–199. [PubMed] [Google Scholar]
- Zychlinsky A., Kenny B., Ménard R., Prévost M. C., Holland I. B., Sansonetti P. J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994 Feb;11(4):619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]