Abstract
Background
Acute i.v. treatment for pediatric headache varies widely.
Objectives
Our aim was to describe our experience with i.v. magnesium for acute treatment of pediatric headache.
Methods
We reviewed the electronic medical records of all patients ages 5 to 18 years old treated with a standard dose of i.v. magnesium for headache at our institution from January 2008 to July 2010. Charts were assessed for headache diagnosis, prior medications given, side effects, tolerability, and response to treatment. Individuals were excluded if they had an underlying unstable medical condition or a secondary etiology for headache. Only first encounters were included if the patient had multiple encounters.
Results
There were 34 episodes of children who received i.v. magnesium in the emergency department (ED) or hospital. Of these, 14 were excluded because the patients had complex medical conditions (n = 6), they were repeat encounters (n = 7), or known secondary etiology for the headache (n = 1). Of the 20 included charts (range 13–18 years old), 5 had migraine, 4 had tension-type headache, and 11 had status migrainosus. Thirteen were treated in the ED and seven as an inpatient with a standard i.v. dose of magnesium. Ten of thirteen adolescents receiving i.v. magnesium in the ED were admitted for further headache treatment but not for side effects, and three were discharged home. Side effects of treatment included pain (1 of 20), redness (1 of 20), burning (1 of 20), and decreased respiratory rate without change in oxygenation (1 of 20).
Conclusions
In our case series, adolescents given i.v. magnesium as an abortive therapy for headache experienced minimal side effects and further studies should evaluate for effectiveness.
Keywords: pediatric, alternative therapy, headache, pain, CAM, migraine
INTRODUCTION
Migraine disorders are present in childhood with an increasing prevalence through adolescence (1). When home treatments for an acute migraine fail, children are often referred to the emergency department (ED) for i.v. therapies. Common i.v. therapies used in the ED to abort migraines include anti-dopaminergic nausea medications, such as prochlorperazine and metoclopramide; anticonvulsant medications such as valproic acid; anti-inflammatory medications, such as ketorolac; or i.v. magnesium (2). Magnesium has been implicated in a number of mechanisms that may play a role in the pathogenesis of migraines (3). Although i.v. magnesium has been widely used in the pediatric population for the treatment of other childhood illnesses, there is limited clinical evidence regarding its use an abortive therapy for headaches in children (4,5). Our objective was to describe the use of a standard dose of i.v. magnesium for pediatric headache abortive treatment. Additionally, we describe the side effects and clinical response to i.v. magnesium.
METHODS
We performed a retrospective medical record review for all patients ages 5 to 18 years old who received i.v. magnesium in the ED or hospital for a headache diagnosis at our institution from January 2008 to July 2010. The study was approved by our Institutional Review Board. In 2007, our institution began using a standard dose for i.v. magnesium sulfate of 30 mg/kg with a maximum dose of 2000 mg infused over 30 min (1000-mg dose was diluted in 50–100 mL of 5% dextrose water or normal saline) while on cardiorespiratory monitor. Serum magnesium level and, in females, urine pregnancy test were recommended before administration. Intravenous magnesium administration was not recommended if the serum magnesium level was >3 mg/dL (reference range 1.7–2.4 mg/dL). The dose could be repeated in 2 h if the first dose was tolerated and serum magnesium level remained < 3 mg/dL. Individuals were excluded if they had an underlying unstable medical condition or a secondary etiology for headache. Only first encounters were included if the patient had multiple encounters.
We reviewed records for any symptoms specifically identified as side effects related to administration of magnesium. In addition, the following objective measures were reviewed: changes in blood pressure > 15 mm Hg systolic or 10 mm Hg diastolic, heart rate increase or decrease by 20 beats/min, respiratory rate increase or decrease by 6 breaths/min, pulse oxygenation decrease < 92% on room air, and temperature during or after administration for 2 h.
Responders were identified as those with moderate to significant improvement in qualitative or numeric pain scores. Pain improvement was defined as a decrease in pain severity from severe to moderate or less pain or a decrease of 3 points or more on a 0–10 pain rating scale. Additional information collected included other abortive medications given during ED or hospital care, discharge or admission to hospital, headache diagnosis as determined by neurologist review of history and documentation. Fisher’s exact test was performed to compare the favorable response to i.v. magnesium among different headache types.
RESULTS
There were 34 episodes of children who received i.v. magnesium in the ED or hospital. Of these, 14 were excluded because the patients had complex medical conditions (n = 6), they were repeat encounters (n = 7), or known secondary etiology for the headache (n = 1). The 20 children meeting inclusion criteria had an average age of 15.7 years (standard deviation 1.7 years; range 13–18 years) and were predominately female (80%) (Table 1). Thirteen (65%) received magnesium in the ED and seven (35%) as an inpatient. Five (25%) had migraine, four (20%) had tension-type headache (TTH), and 11 (55%) had status migrainosus. Median number of medications given before i.v. magnesium was 5 (interquartile range 3–6). Medications given most often before administration of i.v. magnesium were i.v. ketorolac, diphenhydramine, and prochlorperazine or ondansetron given simultaneously with i.v. fluids, commonly followed by i.v. valproic acid. Twelve children had serum magnesium levels obtained before i.v. magnesium administration and levels ranged from 1.5 mg/dL to 2.6 mg/dL (mean 2.01 mg/dL).
Table 1.
Characteristics of Intravenous Magnesium Cases (N = 20)
| Age (years), Mean ± SD | 15.7 ± 1.7 |
| Female sex, n (%) | 16 (80) |
| ED administration, n (%) | 13 (65) |
| Inpatient administration, n (%) | 7 (35) |
| Migraine, n (%) | 5 (25) |
| Tension-type headache, n (%) | 4 (20) |
| Status migrainosus, n (%) | 11 (55) |
ED = emergency department; SD = standard deviation.
No major side effects were noted. Minor side effects included mild transient pain (1 of 20), redness or burning at the injection site (2 of 20), which resolved after infusion and decreased respiratory rate of 9 breaths/min during sleep without change in oxygenation (1 of 20).
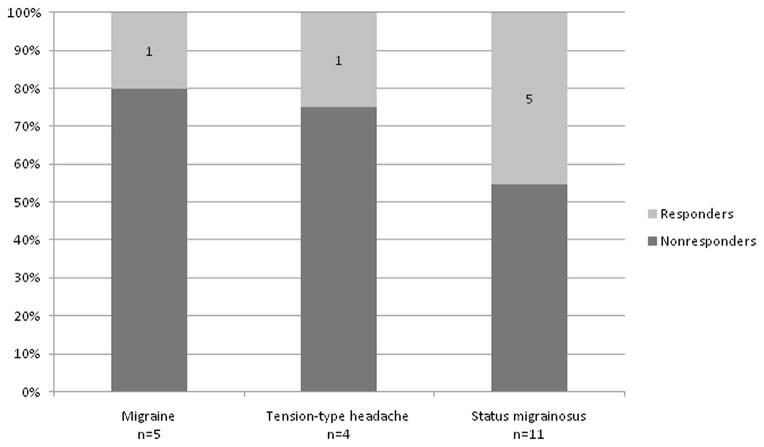
Seven (35%) patients showed favorable response, one with migraine, one with TTH, and five with status migrainosus (p = 0.57) (Figure 1). None of the patients in our cohort had worsened headache after magnesium administration. Ten of thirteen (77%) children receiving i.v. magnesium in the ED were admitted for further treatment, and three were discharged home. Three (15%) patients received a second dose of i.v. magnesium, with one patient having headache resolution after two doses, another showing minimal response after the first dose and no improvement after the second, and one patient having no response to either dose.
Figure 1.
Percentage of responders and non-responders in the children and adolescents with migraine, tension-type headache, and status migrainosus (p=0.57).
DISCUSSION
We show no serious adverse events and good tolerability with i.v. administration of magnesium for acute treatment of headache in adolescents. Intravenous magnesium has been widely used in the pediatric population for the treatment of other childhood illnesses, such as asthma and cardiac dysrhythmias, and has been found to be safe in these illnesses (4,5). However, there is limited clinical evidence regarding safety and efficacy of using i.v. magnesium for headaches in children or adolescents. Although adult headache studies report the common side effects with i.v. magnesium, including flushing from 8% to almost all patients in another cohort, lightheadedness, and burning at the site of infusion in up to 25%, these were not as common in our sample of adolescents and could be partly attributed by our slow infusion rate (6).
Magnesium has been implicated in a number of mechanisms that may play a role in the pathogenesis of migraines (7). These include a role in both neuronal and vascular theories for migraine pathogenesis and a possible relationship between intracellular magnesium concentrations and migraine attacks (8). Thirteen of the 20 individuals had serum magnesium checked before magnesium administration and there was no association with serum concentration and response (data not shown). Ionized magnesium levels are a better indicator of intra-cellular stores of magnesium and may need to be explored as a possible predictor to response (9).
In adults, evidence of i.v. magnesium efficacy is conflicting. Some studies have shown i.v. magnesium to be effective for migraines and associated features, and others have shown no improvement compared with placebo (5,6,9,10). To our knowledge, there are no controlled studies in the pediatric population. Although, in our case series magnesium overall did not appear to perform well, we cannot make any conclusions regarding efficacy, given the small sample size, multiple potential confounders, and lack of controls. Individuals were generally given magnesium after they already failed a medication cocktail and valproic acid, therefore, our case series represents a more severe spectrum of disease. The marked response among children with status migrainosus is an intriguing finding that should be examined further in future studies along with i.v. magnesium given as first-line treatment.
Limitations
Our descriptive retrospective study was limited by the inherent difficulties in retrospective studies, including the inability to examine recurrence rate of headache symptoms within 24 h, nonstandardized pain assessments, and potential reporter bias. Although we reviewed charts of children ages 5 to 18 years, only 13- to 18-year-olds met inclusion criteria; therefore, our findings cannot be extrapolated to younger children. Magnesium was generally given after several medications had already been administered and clinical response may reflect the additive effect of magnesium or delayed response from other medications. In addition, there was also no formal assessment of side effects during and after magnesium infusions. Therefore, some minor or common side effects might not have been captured, such as flushing or burning, due to lack of patient reporting or provider documentation.
CONCLUSIONS
In our case series, adolescents with acute headache who were given a standard dose of i.v. magnesium experienced minimal side effects. Larger prospective studies are needed to further establish the efficacy and role of i.v. magnesium for abortive treatment of headaches in the pediatric population ED population.
ARTICLE SUMMARY.
1. Why is this topic important?
IV magnesium is increasingly being used in Emergency Departments acutely for the treatment of pediatric headache, however there is a paucity of evidence for its use in children with headache.
2. What does this study attempt to show?
We describe our experience using a standard protocol for IV magnesium at our institution for treatment of pediatric headache.
3. What are the key findings?
Children with acute headaches experienced minimal side-effects when infused with 30 mg/kg of IV magnesium sulphate with maximum dose of 2000 mg. Thirty five percent of children showed favorable response to IV magnesium.
4. How is patient care impacted?
IV magnesium needs to be further investigated in larger double blinded studies to understand efficacy for acute pediatric headache treatment.
Acknowledgments
SK was supported by a Health Resources and Service Administration (HRSA) Faculty Development Research Fellowship. Statistical analysis was supported in part by National Institutes of Health (NIH)/National Center for Research Resources (NCRR) Colorado Clinical & Translational Sciences Institute (CCTSI) Grant Number UL1 RR025780.
References
- 1.Lewis DW. Pediatric migraine. Neurol Clin. 2009;27:481–501. doi: 10.1016/j.ncl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Kabbouche MA, Cleves C. Evaluation and management of children and adolescents presenting with an acute setting. Semin Pediatr Neurol. 2010;17:105–8. doi: 10.1016/j.spen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Mauskop A, Altura BM. Role of magnesium in the pathogenesis and treatment of migraines. Clin Neurosci. 1998;5:24–7. [PubMed] [Google Scholar]
- 4.Ciarallo L, Sauer A, Shannon M. Intravenous magnesium therapy for moderate to severe pediatric asthma: results of a randomized, placebo-controlled trial. J Pediatr. 1996;129:809–14. doi: 10.1016/s0022-3476(96)70023-9. [DOI] [PubMed] [Google Scholar]
- 5.Hoshino K, Ogawa K, Hishitani T, et al. Influence of heart surgery on magnesium concentrations in pediatric patients. Pediatr Int. 2003;45:39–44. doi: 10.1046/j.1442-200x.2003.01667.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelley N, Tepper D. Rescue therapy for acute migraine, part 1:triptans, dihydroergotamine, and magnesium. Headache. 2012;52:114–28. doi: 10.1111/j.1526-4610.2011.02062.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469–83. doi: 10.1111/j.1526-4610.2011.01846.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramadan N. Low brain magnesium in migraine. Headache. 1989;29:590–3. doi: 10.1111/j.1526-4610.1989.hed2909590.x. [DOI] [PubMed] [Google Scholar]
- 9.Corbo J, Esses D, Bijur PE, et al. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann Emerg Med. 2001;38:621–7. doi: 10.1067/mem.2001.119424. [DOI] [PubMed] [Google Scholar]
- 10.Cete Y, Dora B, Ertan C, et al. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs. metoclopramide in the management of acute migraine attacks in the Emergency Department. Cephalalgia. 2005;25:199–204. doi: 10.1111/j.1468-2982.2004.00840.x. [DOI] [PubMed] [Google Scholar]