Figure 4.
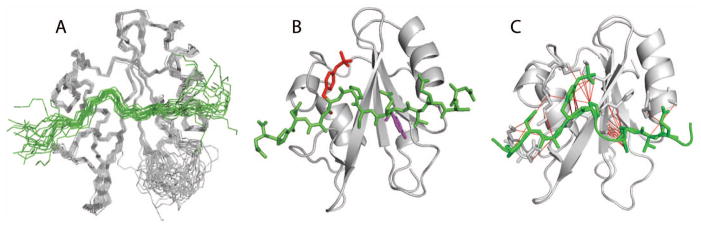
A: set of twenty lowest-energy structures determined from NMR-restrained MD of the Vav1 SH2-YpY complex. Structures are represented by a main chain trace of SH2 (grey) and SykLB-YpY (green), and superimposed based on main chain atoms of the central β-sheet. B: Ribbon drawing of the average structure obtained by averaging coordinates from the set of twenty structures, followed by energy minimization. The SykLB-YpY peptide is green. Y342 (purple) occupies the pTyr pocket, and pY346 (red) the specificity pocket. C: Intermolecular nOe interactions visualized with lines between the SykLB-YpY protons and Vav1 SH2 protons.
