Abstract
Morphologically mixed chemical/electrical synapses at axon terminals, with the electrical component formed by gap junctions, is common in the CNS of lower vertebrates. In mammalian CNS, evidence for morphologically mixed synapses has been obtained in only a few locations. Here, we used immunofluorescence approaches to examine the localization of the neuronally expressed gap junction forming protein connexin36 (Cx36) in relation to the axon terminal marker vesicular glutamate transporter1 (vglut1) in spinal cord and trigeminal motor nucleus (Mo5) of rat and mouse. In adult rodents, immunolabelling for Cx36 appeared exclusively as Cx36-puncta, and was widely distributed at all rostro-caudal levels in most spinal cord laminae and in the Mo5. A high proportion of Cx36-puncta was co-localized with vglut1, forming morphologically mixed synapses on motoneurons, in intermediate spinal cord lamina, and in regions of medial lamina VII, where vglut1-containing terminals associated with Cx36 converged on neurons adjacent to the central canal. Unilateral transection of lumbar dorsal roots reduced immunolabelling of both vglut1 and Cx36 in intermediate laminae and lamina IX. Further, vglut1-terminals displaying Cx36-puncta were contacted by terminals labelled for glutamic acid decarboxylase65, which is known to be contained in presynaptic terminals on large diameter primary afferents. Developmentally, mixed synapses begin to emerge in the spinal cord only after the second to third postnatal week and thereafter increase to adult levels. Our findings demonstrate that axon terminals of primary afferent origin form morphologically mixed synapses containing Cx36 in broadly distributed areas of adult rodent spinal cord and Mo5.
Keywords: gap junctions, vesicular glutamate transporter1, motoneurons, interneurons
INTRODUCTION
A surge of reports over the past decade on electrical synapses formed by gap junctions between neurons in mammalian brain was fueled in part by the discovery of the first gap junction forming protein expressed in neurons, namely, connexin (Cx36) (Condorelli et al., 1998; Söhl et al., 1998). Subsequent findings of widespread neuronal expression of Cx36, (Condorelli et al., 2000), demonstrations of Cx36 in ultrastructurally-identified neuronal gap junctions in adult rodent brain (Nagy et al., 2004; Rash et al., 2000,2001a,b,2007a,b), and indications of the physiological relevance of these junctions, partly deduced from functional deficits in Cx36 knockout (ko) mice, all contributed to the general acceptance of the prevalence and importance of electrical transmission in most major regions of mammalian CNS (Bennett and Zukin, 2004; Connors and Long, 2004; Hormuzdi et al., 2004; Söhl et al., 2005; Meier and Dermietzel, 2006). Specifically, electrical coupling is considered to generate synchrony of subthreshold membrane oscillations and to promote synchronous recruitment of rhythmic firing (Bennett and Zukin, 2004; Connors and Long, 2004), which is emerging as a key feature of information processing in neuronal networks (Singer, 1999; Whittington and Traub, 2003; Senkowski et al., 2008). Nearly all electrical synapses so far studied in mammalian brain occur between dendrites and/or somata of specific classes of neurons. However, gap junctions can also be found at axon terminals, as in many systems of lower vertebrates (Bennett and Goodenough, 1978; Bennett, 1997), creating “mixed synapses” that provide for dual chemical and electrical neurotransmission. Mixed synapses in rodent CNS have been described in only a few CNS areas, including the lateral vestibular nucleus (LVN) (Sotelo and Palay, 1970; Korn et al., 1973; Nagy et al., 2013), hippocampus (Vivar et al., 2012; Hamzei-Sichani et al., 2012; Nagy, 2012) and spinal cord (Rash et al., 1996).
Electrical synapses and neuronal gap junctions in mammalian spinal cord have received far less attention than those in brain. In developing and juvenile rodent spinal cord, there is gap junction-mediated coupling between distinct subsets of interneurons (Hinckley and Ziskind-Conhaim, 2006; Wilson et al., 2007; Bautista et al., 2012) as well as between motoneurons, with progressive loss of motoneuronal coupling during the second week of life (Arasaki et al., 1984; Fulton et al., 1980; Walton and Navarette, 1991; Bou-Flores and Berger, 2001). Electrical coupling between developing motoneurons was suggested to support synchronous neuronal activity prior to the maturation of chemical synaptic connectivity (Kiehn et al., 2000; Kiehn and Tresch, 2002; Tresch and Kiehn, 2000,2002). Analysis of connexin mRNA and/or protein expression has suggested that multiple connexins are expressed in spinal and trigeminal motoneurons, including Cx26, Cx32, Cx36, Cx37, Cx40, Cx43 and Cx45 (Chang et al., 1999; Chang and Balice-Gordon, 2000; Chang et al., 2000; Personius and Balice-Gordon, 2000, 2001; Personius et al., 2001, 2007; Micevych and Abelson, 1991; Matsumoto et al., 1991,1992), raising the possibility that each of these connexins could form functional gap junctions between motoneurons. However, our recent analysis of connexin expression among trigeminal and spinal cord motoneurons in postnatal and adult rodent, using antibodies specific for each connexin, found labelling of only Cx36 in these neurons (Bautista et al., 2013a,b). Our results suggests that Cx36 is the major connexin mediating the electrical and dye-coupling observed between motoneurons at early postnatal ages.
In spinal cord as in brain, neuronal gap junctions and/or functional neuronal coupling persist among specific neuronal populations in adult animals (Matsumoto et al., 1988,1989; van der Want et al., 1998; Logan et al., 1996; Coleman and Sengelaub, 2002). Recently, we reported the expression of Cx36 in ventral and intermediate regions of the lumbar spinal cord of adult mice, and the loss of sensory-evoked presynaptic inhibition in mice lacking Cx36 (Bautista et al., 2012). The present study was motivated in part by our detection of abundant labelling of Cx36 in most spinal cord laminae, including extensive immunolabelling for Cx36 among motoneurons in lamina IX in adult rat and mouse (Bautista et al., 2012; Bautista et al., 2013a,b). The persistence of Cx36-puncta in adult spinal cord, particularly those associated with motoneurons, and the presence of ultrastructurally-identified gap junctions associated with some populations of motoneurons in adult rodent spinal cord (Matsumoto et al., 1988,1989; van der Want et al., 1998; Rash et al., 1996) appears to be at odds with the developmental loss of electrical coupling and dye-coupling between motoneurons (Fulton et al., 1980; Walton and Navarette, 1991; Chang et al., 1999). To reconcile this disparity, the present study provides a more comprehensive analysis of the immunofluorescence localization of Cx36 in the ventral horn and deep dorsal horn laminae as well as the trigeminal motor nucleus of mouse and rat spinal cord. In addition, we considered subcellular locations at which Cx36-containing neuronal gap junctions occur. We investigated the association of Cx36-containing gap junctions with axon terminals; an association that would suggest the presence of mixed synapses capable of dual chemical-electrical transmission in the adult spinal cord. Specifically, we examined the localization of Cx36 in relation to a marker of excitatory axon terminals of myelinated lumbar sensory afferents, namely vesicular glutamate transporter-1 (vglut1; Alvarez et al., 2004), in relation to vesicular glutamate transporter-2 (vglut2), a marker of other excitatory intraspinal axon terminals (Persson et al., 2006) and in relation to a marker of axon terminals containing glutamic acid decarboxylase65 (GAD65) that are in part associated with inhibitory synapses on sensory afferents (Hughes et al., 2005).
EXPERIMENTAL PROCEDURES
Animals and antibodies
Animals used included twenty-two adult (30 to 50 days old) male Sprague-Dawley rats, and eighteen adult wild-type mice and four Cx36 knockout mice from colonies of C57BL/6-129SvEv mice (Deans et al., 2001) that were established at the University of Manitoba through generous provision of breeding pairs of these mice from Dr. David Paul (Harvard). In addition, four male rats and four male wild-type mice were used at each of various developmental ages, including postnatal day (PD) 5, 10, 15, 20, 25 and 30. Tissues from some of these animals were taken for use in parallel unrelated studies. Animals were utilized according to approved protocols by the Central Animal Care Committee of University of Manitoba, with minimization of the numbers animals used.
Three different antibodies against Cx36 were obtained from Life Technologies Corporation (Grand Island, NY, USA) (formerly Invitrogen/Zymed Laboratories), and included two rabbit polyclonal antibodies (Cat. No. 36-4600 and Cat. No. 51-6300) and one mouse monoclonal antibody (Cat. No. 39-4200), each used in incubations of tissue sections at a concentration of 1–2 µg/ml. The monoclonal anti-Cx36 was used for most of the studies involving double or triple immunolabelling with two or three different primary antibodies. Specificity characteristics of Cx36 detection by the anti-Cx36 antibodies in various regions of rodent brain have been previously reported (Li et al., 2004; Rash et al., 2007a,b; Curti et al., 2012). Additional antibodies included: guinea pig polyclonal antibodies against vglut1 and vglut2 obtained from Millipore (Temecula, CA, USA), and used at a dilution of 1:1000; and an anti-GAD65 obtained from BD Biosciences (Birmingham, UK) and used at a dilution of 1:100. In addition, we used a chicken polyclonal anti-peripherin antibody (obtained from Millipore and diluted of 1:500) to identify motoneurons and primary afferent fibers in the spinal cord. Peripherin is an intermediate filament protein highly expressed in neurons of the peripheral nervous system, but is also found in central neurons that have projections to peripheral structures, including motoneurons which display intense labelling for this protein (Clarke et al., 2010). Various secondary antibodies included Cy3-conjugated goat or donkey anti-mouse and anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), Alexa Flour 488-conjugated goat or donkey anti-rabbit and anti-mouse IgG (Molecular Probes, Eugene, OR, USA), AlexaFluor-647 conjugated goat anti-chicken IgG (Life Technologies Corporation) and Cy3-conjugated goat anti-chicken (Jackson ImmunoResearch Laboratories). All secondary antibodies were used at a dilution of 1:600, and all primary and secondary antibodies were diluted in 50 mM Tris-HCl, pH 7.4, containing 1.5% sodium chloride (TBS), 0.3% Triton X-100 (TBSTr) and 10% normal goat or normal donkey serum.
Dorsal rhizotomy
Primary afferent input to the lumbar spinal cord of rats was unilaterally disrupted by dorsal rhizotomy. Four animals weighing from 250–280 grams were anesthetized with isofluorane delivered via a nose cone, and monitored for anesthesia (heart rate, respiratory rate) using an infrared oximeter placed on a forepaw. Temperature was maintained at 37°C with a heating blanket and monitored through a rectal thermometer. After shaving the hind lumbar dorsal skin area and sanitizing with betadine, the lumbar segments L1 to L5 were exposed and a laminectomy was performed, with care to prevent injury to the spinal cord. The dura was then opened to allow access to the right L1 to L4 dorsal roots, which were then transected approximately 3 mm proximal to the dorsal root ganglia. Lidocaine (2%) was applied locally to the dorsal roots before section to prevent an afferent barrage to the spinal cord. After rhizotomy, the exposed roots were extensively rinsed with sterile saline solution to remove surplus lidocaine, the exposed spinal cord was covered with sterile gel foam, the muscle layers were closed using 4-0 silk sutures, and the skin wound was closed with stainless steel skin clips. During recovery over the first two days post-surgery, animals were treated every 12 h with buprenorphine as an analgesic. Rats were taken for experiments after a survival period of seven days.
Tissue preparation
Adult animals and those at PD15 to PD30 were deeply anesthetized with equithesin (3 ml/kg), placed on a bed of ice, and perfused transcardially with cold (4°C) pre-fixative consisting of 50 mM sodium phosphate buffer, pH 7.4, 0.1% sodium nitrite, 0.9% NaCl and 1 unit/ml of heparin, followed by perfusion with fixative solution containing cold 0.16 M sodium phosphate buffer, pH 7.4, 0.2% picric acid and either 1% or 2% formaldehyde prepared from freshly depolymerized paraformaldehyde. Animals were then perfused with a cold solution containing 10% sucrose and 25 mM sodium phosphate buffer, pH 7.4, to wash out excess fixative. Spinal cords at PD5 and PD10, and some at PD15, were removed and taken for immersion fixation with 1–2% formaldehyde prepared as above. No major differences in immunolabelling quality were observed in tissues from PD15 animals fixed by perfusion vs. immersion.
Animals taken for dorsal rhizotomy as described above were perfused with fixative prepared as above but containing either 1% or 4% formaldehyde. Use of the two different fixatives was necessary because weak tissue fixation was required for optimum detection of Cx36 (e.g., 1–2% formaldehyde) and stronger fixative was required for optimum detection of vglut1 (e.g., 2–4% formaldehyde). As a control procedure to insure that double labelling for vglut1 and Cx36 with weaker fixations, chosen as a compromise for detection of these proteins, reflected a representative proportion of vglut1-terminals on the control vs. rhizotomy side, vglut1 was examined in some animals prepared with the stronger fixative. Some detailed consideration regarding fixation conditions required for optimum immunohistochemical detection of Cx36 and vglut1, alone or in combination, are described elsewhere (Nagy et al., 2012).
After fixation, spinal cords were stored at 4°C for 24–48 h in cryoprotectant containing 25 mM sodium phosphate buffer, pH 7.4, 10% sucrose, and 0.04% sodium azide. Transverse or horizontal sections of spinal cord were cut at a thickness of 10–15 µm using a cryostat and collected on gelatinized glass slides. Slide-mounted sections could be stored at −35 °C for several months before use.
Immunofluorescence procedures
Slide mounted sections removed from storage were air dried for 10 min, washed for 20 min in TBSTr, and processed for immunofluorescence staining, as previously described (Bautista et al., 2012; Curti et al., 2012). For single, double, or triple immunolabelling, sections were incubated with a single primary antibody, or simultaneously with two or three primary antibodies for 24 h at 4°C in TBSTr. The sections were then washed for 1 h in TBSTr and incubated with a single or appropriate combinations of secondary antibodies for 1.5 h at room temperature. Some sections processed by single or double immunolabelling were counterstained with Blue Nissl NeuroTrace (stain N21479) (Molecular Probes, Eugene, OR, USA). All sections were coverslipped with the antifade medium Fluoromount-G (SouthernBiotech, Birmingham, AB, USA). Control procedures involving omission of one of the primary antibodies with inclusion of the secondary antibodies used for double and triple labelling indicated absence of inappropriate cross-reactions between primary and secondary antibodies for all of the combinations used in this study.
Immunofluorescence was examined on a Zeiss Axioskop2 fluorescence microscope and a Zeiss 710 laser scanning confocal microscope, using Axiovision 3.0 software or Zeiss ZEN image capture and analysis software (Carl Zeiss Canada, Toronto, Ontario, Canada). Data from wide field and confocal microscopes were collected either as single scan images or z-stack images with multiple optical scans capturing a thickness of 2 to 14 µm of tissue at z scanning intervals of 0.4 to 0.6 µm. Images of immunolabelling obtained with Cy5 fluorochrome were pseudo colored blue. Final images were assembled using CorelDraw Graphics (Corel Corp., Ottawa, Canada) and Adobe Photoshop CS software (Adobe Systems, San Jose, CA, USA). Movie files included as supplementary data were constructed using Zeiss ZEN software.
Quantitative analyses
Sections of spinal cords from adult rats and mice at various postnatal ages (PD 5, 10, 15, 20, 25, 30) were double-labelled for Cx36 and vglut1 and taken for quantitative analysis of the proportion of Cx36-immunopositive puncta associated with vglut1-positive axon terminals in selected regions of spinal cord. In each of four animals at each age, confocal immunofluorescence images ranging from 17 to 19 fields of spinal cord in the regions of interest were collected at sufficient magnification, using a x60 objective lens, to resolve and visualize individual Cx36-puncta. Single confocal scans rather than z-stack images were used to avoid false-positive overlap of Cx36-puncta with vglut1-positive terminals, which may be encountered in stacked images. Puncta with and without vglut1 association were then counted manually, and averaged over the total fields examined per animal. The data from each animal was then normalized by calculating the percentage of total Cx36-puncta associated with vglut1-positive terminals, and an average percentage was then obtained from the number of animals examined and expressed as mean ± s.e.m.
Lumbar spinal cord sections from animals receiving dorsal rhizotomy were triple-labelled for Cx36, vglut1 and peripherin, and taken for quantitative analysis of Cx36-puncta and vglut1-positive terminals remaining among peripherin-positive motoneurons in lamina IX and lamina VII on the rhizotomy vs. intact contralateral side. Similar procedures for analysis were used as described above, except that one or two images of labelling were acquired on the control intact side, and images of corresponding areas were obtained on the rhizotomy side. Counts of Cx36-puncta and vglut1-positive terminals was conducted using Zeiss ZEN software (Zeiss Canada), with appropriate thresholding for signal-to-noise and for size of objects to be counted. In a few images, the reliability of automated counting was compared to manual counting, and the two methods were found to yeild similar results.
RESULTS
Cx36 in adult spinal cord
Immunofluorescence labelling of Cx36 in gray matter of mouse and rat spinal cord is heterogeneously distributed in areas of both dorsal and ventral horn. As in all other areas of the CNS we have examined, immunolabelling is exclusively punctate (Cx36-puncta) in appearance, with absence of diffuse or punctate intracellular immunofluorescence (Fig. 1), indicating either inaccessibility of anti-Cx36 antibody to cytoplasmic Cx36, or rapid trafficking of Cx36 with insufficient cytoplasmic levels to be detectable by immunofluorescence. Absence of Cx36 immunoreactivity throughout the interior of cells precludes immediate identification of cell-types that express Cx36, but Cx36-puncta are often localized to the surface of neuronal somata and dendrites, which allows cell identification when labelling for Cx36 is combined with labelling of a neuron specific marker.
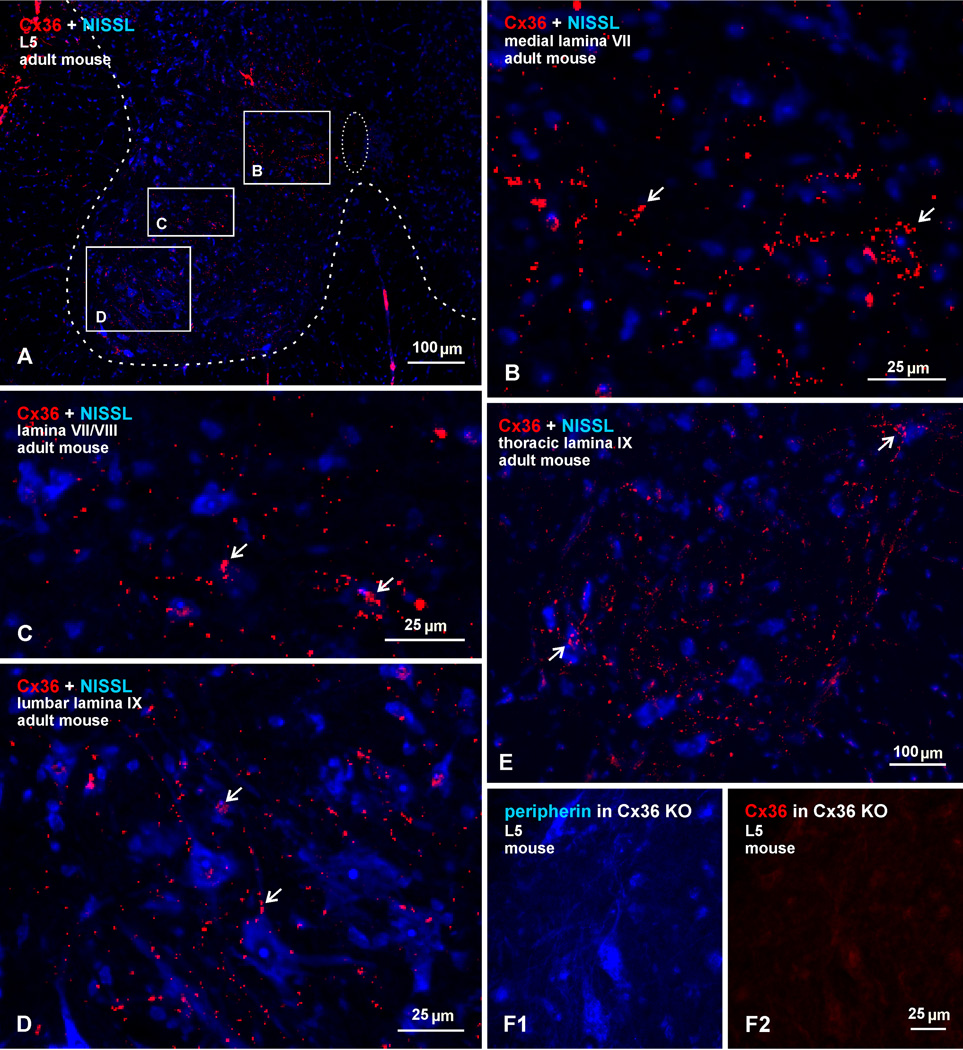
Fig. 1.
Overview of widely distributed immunofluorescence labelling of Cx36 in transverse sections of adult mouse spinal cord, counterstained by blue fluorescence Nissl or labelled for peripherin. (A) Low magnification at an L5 level, with border between gray and white matter outlined by coarse dotted line, and the central canal outlined by fine dotted line. Boxed areas indicate regions examined in greater detail in subsequent Figures. (B-D) Higher magnifications of the boxed areas in (A), showing dense collections of Cx36-puncta in medial regions of lamina VII near the central canal (B, arrows), a moderate distribution of Cx36-puncta in intermediate lamina VII and VIII (C, arrows), and Cx36-puncta localized to somata and dendrites of motoneurons in lamina IX (D, arrows). (E) Lamina IX at a thoracic level, showing Cx36-puncta (arrows) distributed among motoneurons. (F) Lamina IX at L5 level from spinal cord of a Cx36 knockout mouse, showing peripherin-positive motoneurons (F1) and, in the same field, an absence of labelling for Cx36 (F2).
In the overview of labelling for Cx36 in deep dorsal horn and ventral horn laminae in adult mouse spinal cord at an L5 level shown in Figure 1A, with blue Nissl counterstaining, Cx36-puncta are less visible at the low magnification presented, but become evident with application of the zoom function in the electronic version of the image. Although Cx36-puncta are scattered throughout these laminae, they are most concentrated in four spinal cord regions, three of which are indicated by the boxed areas in Figure 1A, with the corresponding boxed areas magnified in Figures 1B-D. These regions include: i) the medial portion of lamina VII, specifically encompassing an area just lateral to the central canal (Fig. 1A,B); ii) the intermediate and ventral horn lamina VII and VIII (Fig. 1A,C), where Cx36-puncta are associated with many small and a few large neurons; and iii) the ventral horn lamina IX, where Cx36-puncta are often localized to motoneurons at all spinal levels, as shown at a lumbar level (Fig. 1A,D) and at a thoracic level (Fig. 1E). A fourth region containing a relatively high density of Cx36-puncta, as we noted in an earlier report (Bautista et al., 2012), encompasses medial regions of deep dorsal horn lamina and will be the subject of a separate report. Immunolabelling for Cx36 in all these regions of adult mouse is absent in Cx36 knockout mice, shown by comparison of a field in lamina IX from a wild-type mouse (Fig. 1D) with a corresponding field in lamina IX from a Cx36 knockout mouse, where motoneurons are labelled for peripherin (Fig. 1F1) and where the same field shows an absence of labelling for Cx36 (Fig. 1F2), indicating specificity of anti-Cx36 antibody. As there is some variation in the literature on the designation of boundaries between spinal cord lamina, we use the mouse and rat spinal cord atlas of Watson et al. (2009) for lamina and spinal nuclear locations.
Cx36 at vglut1-containing axon terminals in lamina IX
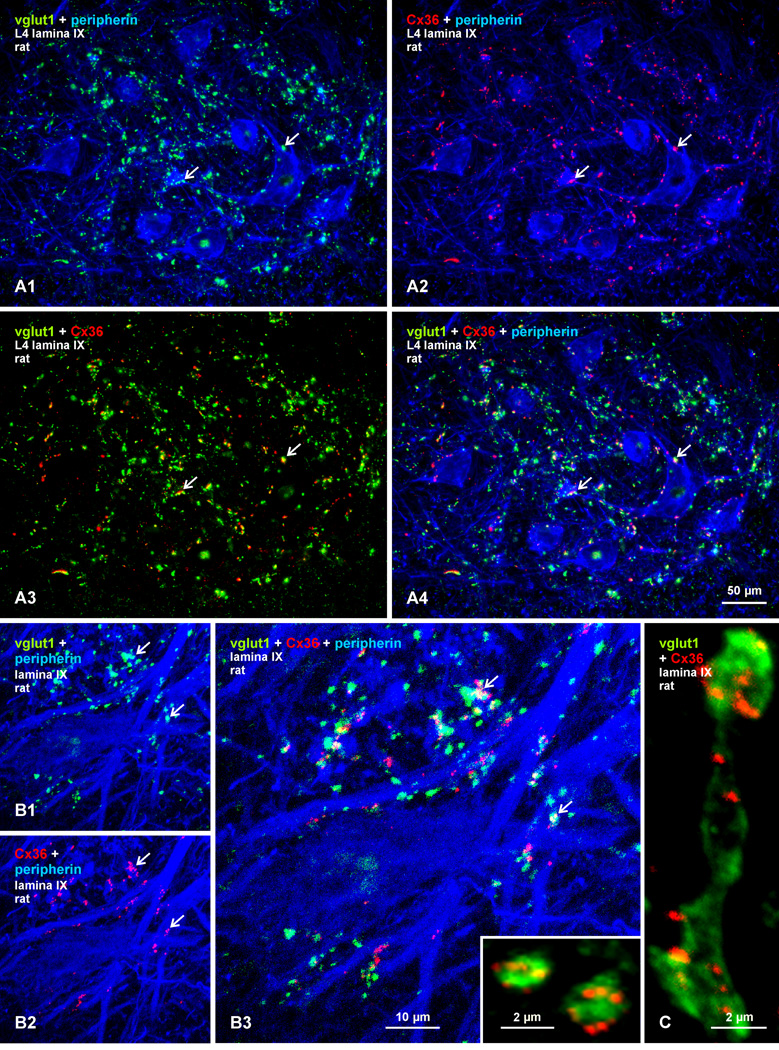
We examined the localization of Cx36 in relation to vglut1, which is a marker of excitatory axon terminals and is known to be associated with large diameter sensory afferents and the terminals of some descending systems, particularly the corticospinal system (Alvarez et al., 2004; Persson et al., 2006). Triple immunofluorescence labelling of Cx36, vglut1, and the motoneuron marker peripherin (Clarke et al., 2010) in lamina IX of adult rat spinal cord is shown at the L4 level (Fig. 2). The set of four images in Figures 2A1-4 show the same field, with different combinations of image overlay. In overlay of labels for vglut1 (green) and peripherin (blue), most vglut1-positive terminals are seen contacting peripherin-positive motoneuronal somata or dendrites (Fig. 7A1). Many of these terminals are relatively large, with the largest reaching approximately 4 µm in diameter, and likely represent terminations of low threshold, large diameter primary afferents. In overlay of labels for Cx36 (red) and peripherin (blue), many though not all Cx36-puncta are localized to the surface of peripherin-positive motoneuron somata and along their proximal dendrites (Fig. 2A2). Overlay of green and red labels reveals that many Cx36-puncta are localized to vglut1-positive terminals (Fig. 2A3), as further shown by overlay of all three labels (Fig. 2A4). Higher confocal magnification of Cx36/vglut1 association is shown in Figure 2B, where many vglut1-positive terminals (Fig. 2B1) and Cx36-puncta in the same field (Fig. 2B2) are seen to overlap along large, peripherin-positive dendrites (Fig. 2B3, overlay). Individual terminals often display several Cx36-puncta (Fig. 2B3, inset), as do extended axonal segments appearing to form en passant terminals (Fig. 2C), which are characteristic of primary afferents ending on motoneurons (Brown and Fyffe, 1978).
Fig. 2.
Triple immunofluorescence labelling for Cx36, vglut1 and peripherin in lamina IX at the L4 level in adult rat spinal cord. (A1-A4) Images showing the same field, with overlay of labelling for peripherin and vglut1 (A1), peripherin and Cx36 (A2), Cx36 and vglut1 (A3), and for all three proteins (A4). Red/green overlay appears as yellow, and red/green/blue overlay appears as white. Peripherin-positive motoneurons receiving a moderate innervation by vglut1-containing terminals (A1, arrows) display Cx36-puncta localized on their somata or dendrites (A2, arrows). Most though not all of these Cx36-puncta are co-localized with vglut1-positive terminals (A3 and A4, arrows). (B1-B3) Higher magnification confocal triple immunofluorescence showing immunolabels (arrows) for vglut1 (B1) and Cx36 (B2) associated with initial dendrites of peripherin-positive motoneurons. A proportion of Cx36-puncta are seen co-localized with vglut1-positive terminals, as seen in overlay (B3, arrows), but not all of these terminals harbor Cx36-puncta. (C) Individual vglut1-positive boutons or fibers appearing to form en passant terminal contacts display several Cx36-puncta; also shown in inset in (B3).
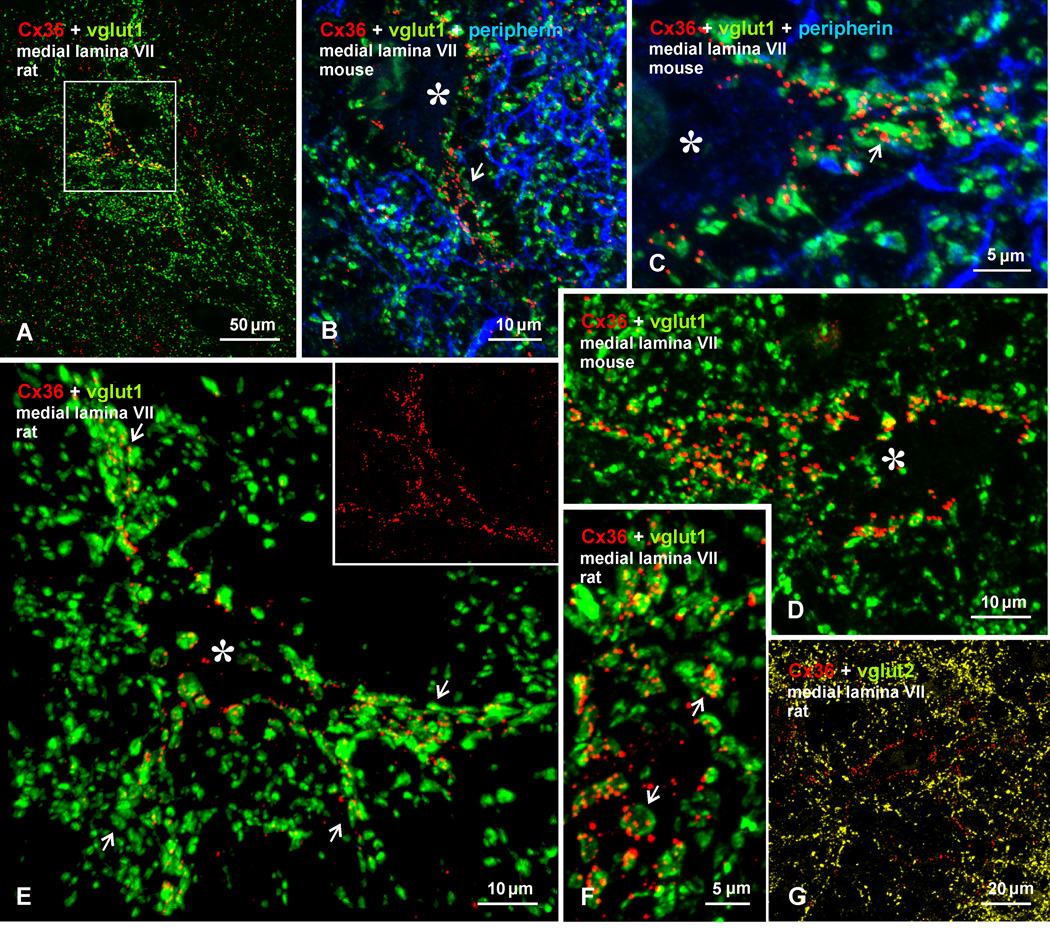
Fig. 7.
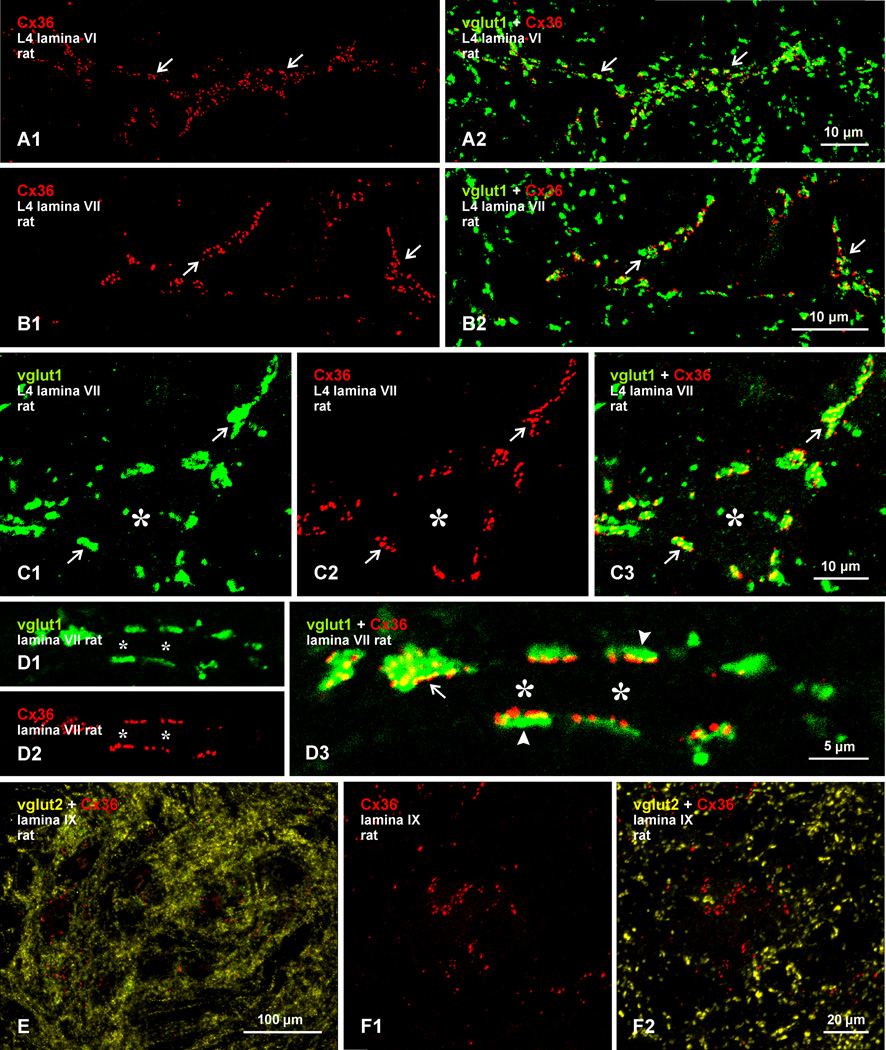
Confocal immunofluorescence of vglut1 and Cx36 in medial lamina VII of adult rat and mouse lumbar spinal cord. (A) Image from rat cord, showing a vglut1-positive patch of terminals outlining a neuron decorated with Cx36-puncta (boxed area). (B-D) Higher magnification immunofluorescence images from medial lamina VII of mouse cord, showing a dense meshwork of peripherin-positive primary afferent fibers (blue) within a vglut1-positive patch, and individual neurons (marked by asterisks) with Cx36-puncta concentrated along their initial dendritic segments (B, arrows), where many are associated with vglut1-terminals (C, arrows), or with Cx36-puncta associated with vglut1-terminals distributed around the entire neuronal somata (D). (E) Higher magnification of the neuron (marked by asterisk) in the boxed area in (A), showing Cx36-puncta co-distributed with vglut1-terminals contacting the neuronal somata and several initial dendrites (arrows). The inset shows the same field as in (E), with labelling for Cx36 alone delineating the entire neuronal somata. (F) Magnification showing multiple Cx36-puncta localized to most vglut1-terminals (arrows). (G) Double immunofluorescence of Cx36 and vglut2 in a field similar to that in (A), showing lack of Cx36-puncta co-localization with vglut2-positive terminals.
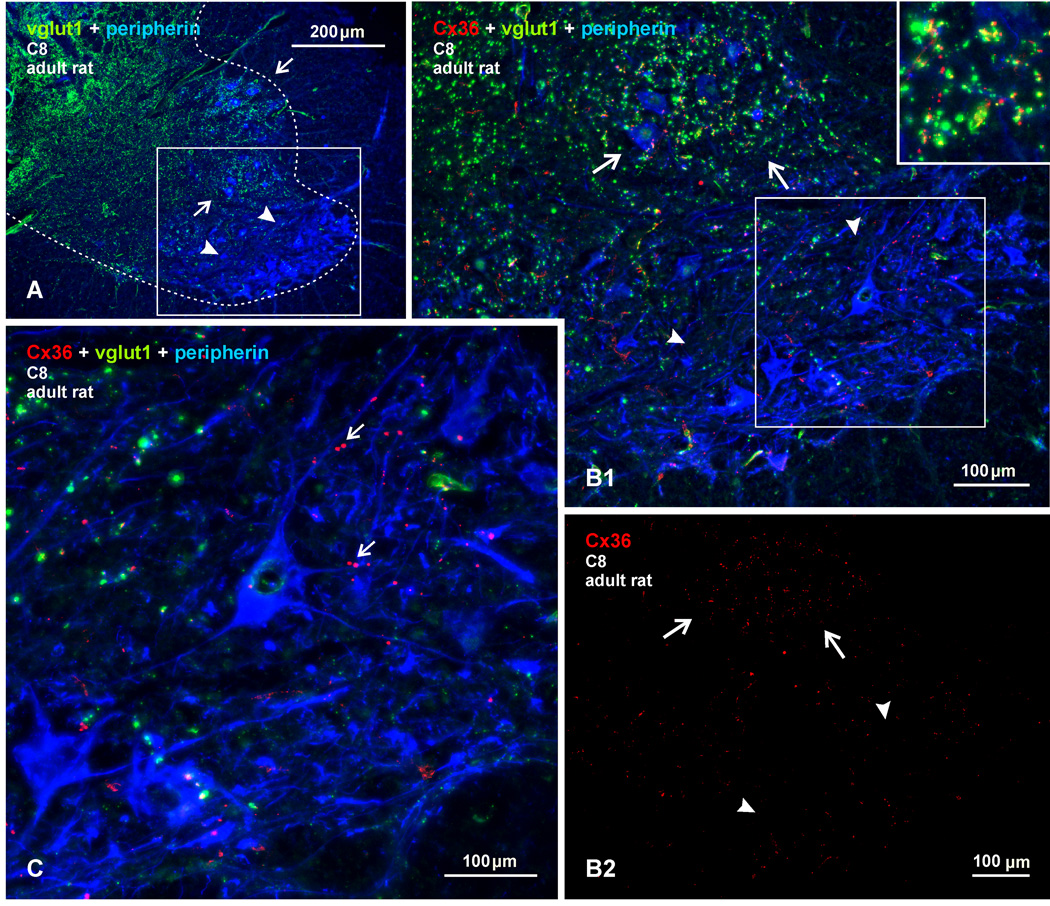
These results suggest that vglut1-terminals in lamina IX may form morphologically mixed chemical/electrical synapses. However, as evident in Figures 2A and B, not all vglut1-terminals display labelling for Cx36, suggesting mixed synapse formation by select afferent terminals. Conversely, some Cx36-puncta lack overlap with vglut1-terminals, and these may represent purely electrical synapses between as yet unidentified neuronal elements. Similar results were obtained in motoneuronal pools at cervical, thoracic, sacral and other lumbar levels in both rat and mouse, except among sexually dimorphic motor nuclei where abundant Cx36-puncta localized to motoneurons lack association with vglut1 (Bautista et al., 2013b). One other exception was encountered at a lower cervical level (C8). At this level, ventrolaterally located motor nuclei, corresponding to the location containing motoneurons likely innervating deltoid and pectoral muscles (Watson et al., 2009), were conspicuous in their paucity of vglut1-terminals (Fig. 3A, B1) and their sparse scattering of Cx36-puncta (Fig. 3C, 3B2). Adjacent dorsally located motor nuclei contained an abundance of both Cx36-puncta and vglut1-terminals that displayed co-localization similar to that observed at other spinal levels (Fig. 3B1).
Fig. 3.
Triple immunofluorescence labelling of Cx36, vglut1 and peripherin at a lower cervical level (C8) in adult rat spinal cord. (A) Low magnification showing the distribution of vglut1-terminals in spinal cord gray matter (outlined by dotted line), and the heterogeneous density of these terminals among groups of motor nuclei in lamina IX (arrows, high levels; arrowheads, low levels). (B1) Higher magnification of boxed area in (A), showing a moderate density of vglut1-terminals among a dorsally located group of motoneurons (arrows), and few vglut1-terminals in a ventrally located motoneuron group (arrowheads). Cx36-puncta in the upper group of motoneurons show extensive co-localization with vglut1-terminals (shown in inset). (B2) The same field as in (B1), with labelling of Cx36 alone, showing a high density of Cx36-puncta in the upper motoneuronal group (arrows) and sparse Cx36-puncta in the lower group (arrowheads). (C) Magnification of the boxed area in (B2), showing only a few Cx36-puncta (arrows) associated with peripherin-positive motoneurons in the lower group and their lack of co-localization with vglut1.
Cx36 at vglut1-terminals in intermediate lamina VI, VII and VIII
Immunolabelling for vglut1 and Cx36 is present at moderate density in lamina VI, VII and VIII and is unevenly distributed among small, medium-sized, and large neurons. Cx36-puncta and vglut1-terminals tend to be concentrated in arrays sweeping horizontally or vertically across lamina VI (Fig. 4A) and VII (Fig. 4B), or are seen heavily decorating the somata and initial dendrites of a few, relatively large neurons per section in lamina VII (Fig. 4C) and lamina VIII (not shown). Immunolabelling of Cx36 appears as isolated puncta, or more often as an assembly of 5 to 10 closely-clustered puncta (Fig. 4A1, B1 and C2). These clusters were invariably co-localized with vglut1-terminals, as seen in overlay images (Fig. 4A2, B2 and C3). As in lamina IX, not all vglut1-terminals display Cx36-puncta, but nearly all such terminals surrounding infrequently encountered neurons in lamina VII (Fig. 4C; neurons not counterstained, but marked by asterisk), were decorated with these puncta. Immunolabelled terminal boutons, having a somewhat flattened shape against their postsynaptic elements, could be viewed en face (Fig. 4C1, D1), in which case Cx36-puncta are seen distributed largely within the confines of the terminal (Fig. 4C3, 2D3), giving the impression that these puncta are contained within the terminal interior. Alternatively, vglut1-positive terminals could be viewed on edge, such as those contacting a large dendrite in Figure 4D (dendrite marked by asterisks). In these views, Cx36-puncta are seen aligned along bouton surfaces that were in contact with the dendrite (Fig. 4D2, 4D3), as might be expected given the plasma membrane localization of Cx36-containing gap junctions, and presumptive gap junction formation at points of apposition between the terminals and the dendrite.
Fig. 4.
Confocal double immunofluorescence labelling of Cx36 with vglut1 or vglut2 in adult rat lumbar spinal cord. (A,B) Images show the same field in lamina VI (A1,A2) and in lamina VII (B1,B2), with labelling for Cx36 alone (A1,B1) and after overlay with labelling for vglut1 (A2,B2). Arrays of Cx36-puncta are seen streaming across mid regions of these lamina (A1,B1, arrows). Labelling for vglut1 follows similar trajectories as the Cx36-puncta, and nearly all Cx36-puncta are either co-localized with, or lie close to, vglut1-terminals (A2,B2, arrows). (C1-C3) Images show the same field of a single medium sized neuron in lamina VII (not counterstained, but marked with asterisk). The neuron is contacted by vglut1-terminals (C1, arrows) displaying clusters of Cx36-puncta (C2 and C3, arrows). (D1-D3) Images of the same field in lamina VII, showing label for vglut1 (D1) and Cx36 (D2) on a large initial dendrite (not counterstained, but marked with asterisks). Terminals labelled for vglut1 are viewed on edge flattened against the dendrite (D3, arrowheads) or en face (D3, arrow). In edge views, Cx36-puncta are localized to the side of the terminal contacting the dendrite, and straddle sites of terminal/dendrite apposition. (E,F) Double immunofluorescence labelling of Cx36 (red) and vglut2 (yellow) in lamina IX at low (E) and higher confocal magnification (F), showing clusters of Cx36-puncta (F1) that in the same field lack association with vglut2-positive terminals (F2).
Lack of co-localization of Cx36-puncta with vglut2-containing terminals
We tested whether another class of glutamatergic terminals containing vglut2 shared a similar co-localization relationship with Cx36. Nerve terminals containing vglut2 are distributed throughout spinal cord gray matter, far out number terminals containing vglut1 (Fig. 4E), and only very rarely contain both vglut1 and vglut2. Terminals containing vglut2 are considered to arise largely from other than primary afferent neurons (Persson et al., 2006), and include some arising from spinal interneurons and various descending systems (Alvarez et al., 2004; Persson et al., 2006). Double labelling revealed a total lack of Cx36 co-localization with terminals containing vglut2 in all spinal cord areas examined, as shown by example in lamina IX (Fig. 4F1, 4F2).
Cx36 at vglut1-terminals in medial regions of lamina VII
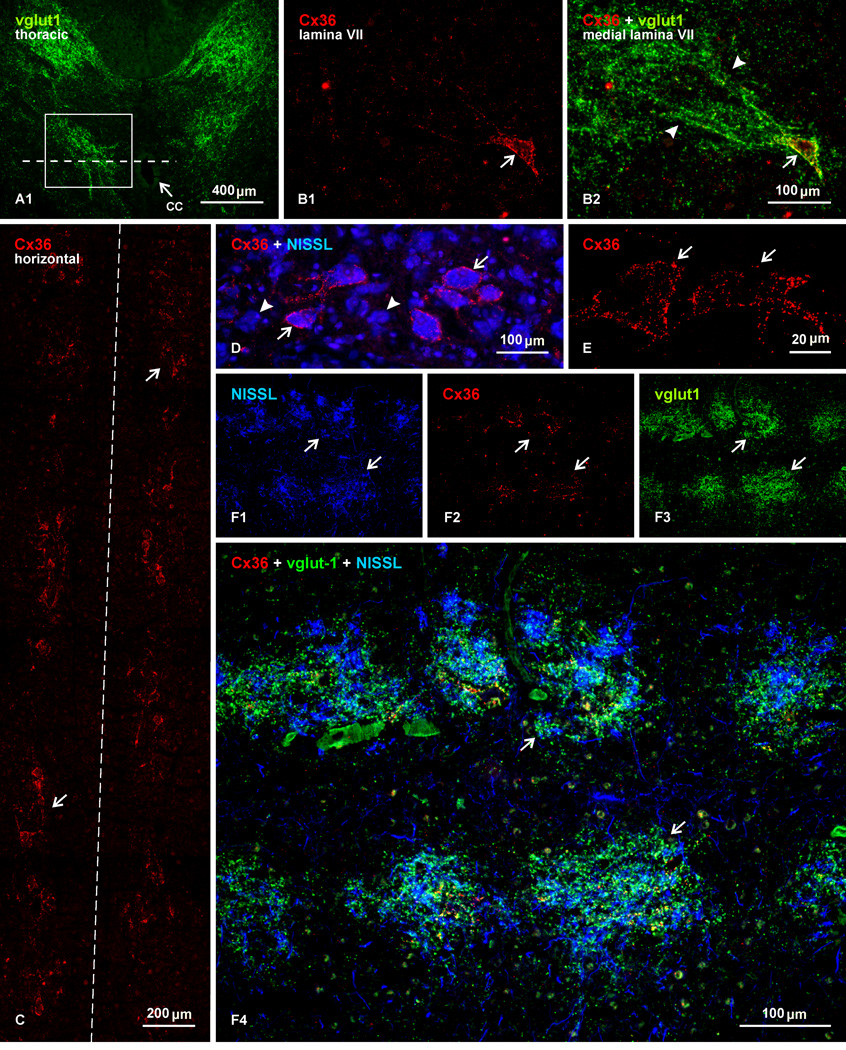
Particularly striking is the organization and Cx36-labelling relationships of vglut1-immunopositive fibers and terminals in medial portions of lamina VII immediately adjacent to the central canal at cervical, thoracic, lumbar and sacral levels. In adult rat, dense collections of fibers labelled for vglut1 along their length course in a ventro-medial direction, producing a confluence of terminals in a region adjacent and dorsolateral to the central canal (Fig. 5A). This region contains a mix of small and medium-sized neurons, with diameters ranging from 25 to 50 µm. Some of these neurons are heavily laden with Cx36-puncta distributed mostly on their somata and initial dendrites (Fig. 5B1). Most of these neurons are targets of dense innervation by vglut1-positive afferents (Fig. 5B2). In transverse sections, these neurons were erratically encountered, making it difficult to appreciate their relative numbers and distribution in a single section. Examination of horizontal sections, however, reveals a clear pattern to their organization. Figure 5C shows a photomontage illustrating Cx36 immunolabelling in a 2 mm length of spinal cord at a T9 level in the horizontal plane of the central canal. Although the section is not counterstained, locations of neurons displaying Cx36-puncta are readily discernible by densely distributed puncta around their somata. Only in this view did it became evident that these neurons occur in clusters of 2 to 4 cells and occasionally up to 6 cells, that the clusters are distributed intermittently along the length of the spinal cord, and often appear to be in register between the left and right side of the cord. At higher magnification with blue fluorescence Nissl counterstaining, neurons decorated with Cx36-puncta are seen intermingled with neurons of similar size lacking these puncta (Fig. 5D). Confocal analysis showed that the labelling was exclusively punctate, and through focus analysis indicated that Cx36 immunofluorescence was localized to the neuronal surface (Fig. 5E). Double immunolabelling for Cx36 and vglut1, with fluorescence Nissl counterstaining, further revealed that vglut1-terminals are concentrated in intermittent patches among the clusters of Cx36-positive and negative neurons, and that these terminals target both neurons displaying or lacking Cx36-puncta (Fig. 5F1-F4, showing the same field). The patches of vglut1-terminals, together with their clusters of neurons decorated with Cx36-puncta, were roughly round or oval, and sometimes appeared to merge with adjacent patches. On average, there were about 8–9 patches per segment at cervical, thoracic and lumbar levels. The diameters of the vglut1-patches were not significantly different at these various levels (190 ± 7.9 µm, 235 ± 13 µm, 223 ± 18 µm, respectively). However, the distances from the center of one patch to the center of an adjacent patch were less at cervical (206 ± 12 µm) and lumbar (266 ± 24 µm) than at thoracic (338 ± 9 µm) levels, indicating that they were more closely spaced at the two former levels.
Fig. 5.
Immunofluorescence localization of Cx36 in relation to vglut1-terminals in medial lamina VII at a thoracic level in adult rat spinal cord. (A) Low magnification transverse section, showing vglut1-positive fibers and terminals (outlined by boxed area) lateral to the central canal (cc, arrow). (B) Magnification of a similar area as the boxed region in (A), showing a single neuron decorated with Cx36-puncta (B1, arrow) and, in the same field, a confluence of vglut1-positive terminals (arrowheads) terminating on that neuron (B2, arrow). (C) Photomontage of a field 2 mm in length, showing immunolabelling for Cx36 in a horizontal section taken at a dorso-ventral plane indicated by the dotted line in (A), with the central canal lying at midline (dashed line). Cx36-puncta are sufficiently concentrated on neurons to reveal intermittent clusters of Cx36 laden cells (arrows) flanking the central canal. (D,E) Higher magnifications of one of these clusters in a section counterstained with blue fluorescence Nissl, showing Cx36-puncta on some neurons (D, arrows) intermingled with those lacking Cx36-puncta (D, arrowheads). A confocal image shows the punctate appearance of labelling associated with and delineating two neurons within a cluster (E, arrows). (F1-F4) Horizontal section showing the same field double-labelled and blue Nissl counterstained, revealing that clusters of these neurons (F1, arrows), with their associated Cx36-puncta (F2, arrows), are all targeted by vglut1-terminals (F3, arrows), producing patches of labelling for vglut1 that demarcate locations of the clusters (F4, arrows).
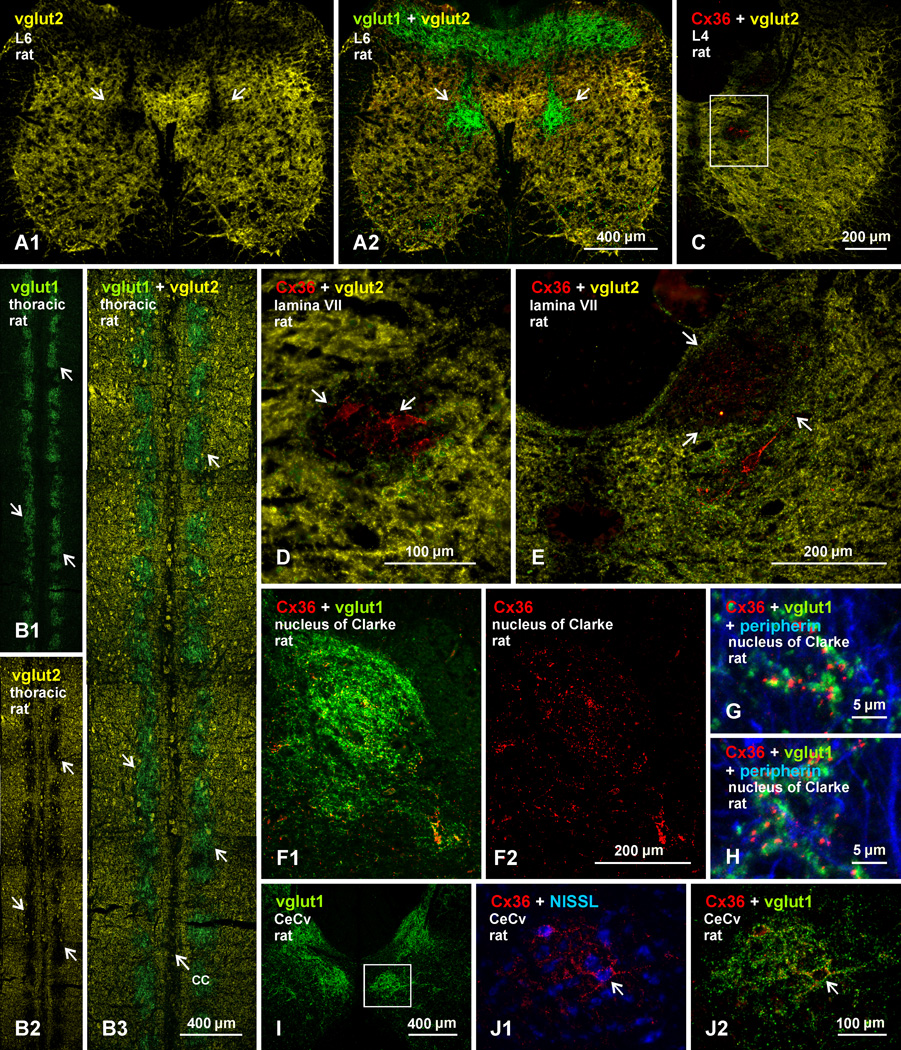
Immunolabelling for vglut1 and Cx36 was compared with labelling for vglut2 among neurons in medial lamina VII. From a global view in transverse sections, shown at a lower lumbar level, patches of dense vglut1 labelling occupy regions almost devoid of labelling for vglut2 (Fig. 6A1; and overlay with labelling for vglut1 in 6A2). These regions without vglut2 are remarkable considering that nearly the entire remainder of the spinal cord gray matter contains a dense distribution of vglut2. Similarly, horizontal sections reveal a nearly exact correspondence between patches of dense vglut1-containing terminals in regions adjacent to the central canal (Fig. 6B1) and patches devoid of vglut2 labelling in these regions (Fig. 6B2 and overlay in 6B3). At a higher lumbar level, two distinct medially located regions are seen to be devoid of labelling for vglut2; a ventral region adjacent to the central canal, containing clusters of neurons decorated with Cx36-puncta (Fig. 6C, shown magnified in 6D), and a dorsal region corresponding to the dorsal nucleus of Clarke (Fig. 6E), which was previously found to contain low levels of vglut2 and high levels of vglut1 (Shrestha et al., 2012). Although not examined in detail here, the nucleus of Clarke was delineated by dense labeling for vglut1 (Fig. 6F1) and was also rich in Cx36-puncta that were randomly distributed (Fig. 6F2), rather than concentrated on individual neuronal somata. These Cx36-puncta were extensively co-localized with vglut1-terminals (Fig. 6G,H).
Fig. 6.
Comparison of immunofluorescence labelling for vglut1 and vglut2 in relation to Cx36-puncta on neurons in medial lamina VII in adult rat spinal cord. (A1-A2) The same field of a double-labelled transverse section at L6, showing immunofluorescence for vglut2 (yellow) nearly throughout spinal cord gray matter, except in a restricted region of medial lamina VII devoid of vglut2-terminals (A1, arrows), which corresponds to an area rich in vglut1-terminals (A2, arrows). (B1-B3) Horizontal thoracic section at the level of the central canal (cc), showing more broadly the correspondence between patches of dense vglut1-terminals (B1, arrows) and voids in labelling for vglut2 (B, arrows), as seen in overlay (B3, arrows). (C-E) Transverse sections at mid lumbar levels, showing two voids in labelling for vglut2 in medial lamina VII: one adjacent to the central canal (C, boxed area), containing neurons densely decorated with Cx36-puncta (D, arrows; magnification of box in C); and the other corresponding to the nucleus of Clarke (E, arrows). (F-H) Images showing nucleus of Clarke outlined by dense labelling for vglut1 (F1) and containing a moderate distribution of scattered Cx36-puncta (F2) that display co-localization with vglut1, as shown by examples in two regions of Clark’s nucleus (G,H). (I) Image of adult rat spinal cord at C8, showing dense labelling for vglut1 in the central cervical nucleus (CeCv) (boxed area). (J) Higher magnification of CeCv showing neurons decorated with Cx36-puncta (J1, arrow) display co-localization with vglut1-terminals (J2, arrow), similar to those seen in medial lamina VII at other spinal levels.
Previous studies have described a moderate concentration of vglut1-terminals in the medial region of lamina VII (Alvarez et al., 2004; Persson et al., 2006), which at lower thoracic levels was reported to consist of a continuous column of vglut1-labelling and was considered to represent Clark’s Column (Alvarez et al., 2004). Our results at this spinal level distinguish the more dorsal location of Clark’s Column from the region in medial lamina VII that contained the intermittent patches of vglut1-labelling and clusters of neurons labelled with Cx36-puncta. Likewise, at cervical levels, vglut1-terminals are concentrated in two medially located regions; one immediately adjacent to ventral portions of the dorsal column, and the other corresponding to the central cervical nucleus (CeCv) located adjacent to the central canal (Fig. 6I). The CeCv contained clusters of neurons decorated with Cx36-puncta (Fig. 6J1), and these puncta displayed co-localization with vglut1-terminals (Fig. 6J2). Thus, the CeCv appears to represent a rostral extension of the patches of vglut1-terminals that contain neurons bearing Cx36-puncta at more caudal spinal levels.
Confocal examination of labelling for Cx36 in patches of vglut1-terminals in medial lamina VII of rat spinal cord is presented in Figure 7, where a single patch is shown in a transverse section at an upper lumbar level (Fig. 7A). Triple labelling shows the patches to be rich in peripherin-positive primary afferent fibers (Fig. 7B,C), consistent with the primary afferent origin of vglut1-containing terminals in the patches (see below). Among terminals and fibers labelled for vglut1 and peripherin, neurons are seen outlined by Cx36-puncta overlapping or located near vglut1-terminals along their initial dendritic segments (Fig. 7B,C). Examination of mouse spinal cord shows a similar association of Cx36-puncta with vglut1-terminals surrounding neurons in medial areas of lamina VII (Fig. 7D). The image in Figure 7E is a magnification of the boxed area in Figure 7A, and shows a z-stack of a single neuron (somata indicated by asterisk), where many vglut1-terminals and their associated Cx36-puncta are seen en face covering several initial dendrites and the soma surface. Rotation of a 3D version of this image (Supplementary movie Fig. S1), reveals Cx36-puncta localized at vglut1-terminals at all angles of rotation. This indicates that this association is not an artifact of z-stack imaging, where labels for Cx36 and vglut1 separated in the z-axis could result in false-positive overlap. As in lamina IX, vglut1-terminals viewed en face display multiple Cx36-puncta on their surface (Fig. 7F). Confocal examination of double labelling for vglut2 and Cx36 in medial regions of lamina VII showed a negligible overlap of Cx36-puncta with vglut2-terminals (Fig. 7G).
Cx36 at vglut1-terminals in the trigeminal motor nucleus
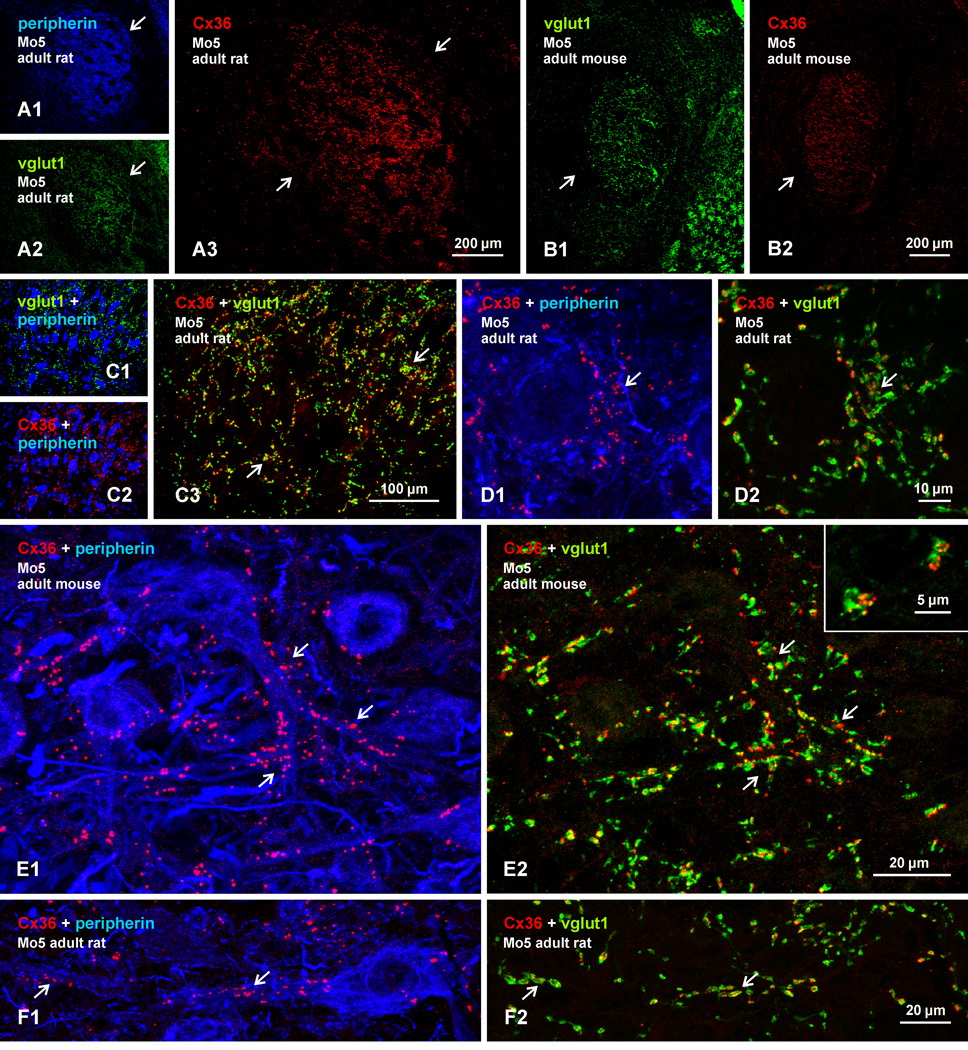
We next examined one other location where Cx3-puncta are densely distributed among motoneurons, namely the trigeminal motor nucleus (Mo5) in rat and mouse. Motoneurons in Mo5 are tighly packed (8A1) and receive a greater density of vglut1-containing terminals compared with their spinal counterparts (Fig. 8A2). In both rat and mouse, labelling for Cx36 is remarkably dense throughout the Mo5 (Fig. 8A3, 8B2). The Cx36 labelling effectively delineates the nucleus as there is very little detectable Cx36 in immediately surrounding regions. Nearly all labelling for vglut1 (Fig. 8C1) and Cx36 (8C2) appears to be associated with peripherin-positive motoneurons, and nearly all Cx36 appears to be associated with vglut1-terminals (Fig. 8C3). As in spinal cord, labelling for Cx36 has a punctate appearance in Mo5, and Cx36-puncta are seen localized to individual vglut1-positive terminals (8D-F). The Cx36-puncta are seen in association with vglut1-terminals contacting motoneuronal somata (Fig. 8D), but many more are seen along initial dendritic segments (Fig. 8E), as well as more distal portions of dendrites (Fig. 8F). Although not examined quantitatively, vglut1-terminals in Mo5 are somewhat smaller and displayed fewer Cx36-puncta (Fig. 8E2, inset) than those in lamina IX of spinal cord.
Fig. 8.
Triple immunofluorescence labelling of Cx36, vglut1 and peripherin in the trigeminal motor nucleus (Mo5) of adult rat and mouse brainstem. (A,B) Low magnification images of the same field of the Mo5 in rat (A1-A3) and in mouse (B1,B2), showing peripherin-positive motoneurons (A1, arrow), dense labelling for vglut1 (A2,B1, arrows) and Cx36 (A3,B2, arrows) throughout the nucleus, and sparse labelling for these proteins outside the nucleus. (C) Higher magnification showing the distribution of labelling for Cx36 (C1) and vglut1 (C2) among peripherin-positive motoneurons and, in the same field, co-localization of labelling for Cx36 with vglut1-terminals (C3, arrows). (D,E) Higher magnification confocal images, where the fields in D1 and D2 are the same and those in E1 and E2 are the same. Images show punctate labelling of Cx36, distribution of Cx36-puncta around a motoneuronal soma (D1, arrow), association of Cx36-puncta with initial dendrite segments (E1, arrows) as well as more distally along dendrites (F1, arrow), and overlap of the vast majority of Cx36-puncta with vglut1-terminals (D2,E2,F2, arrows). Inset in E2 shows multiple Cx36-puncta localized to individual vglut1-terminals.
The source of vglut1-containing axon terminal in the Mo5 has, to our knowledge, not been determined, but the trigeminal sensory ganglion and/or mesencephalic nucleus of the trigeminal nerve are likely candidates based on the primary afferent origin of similar terminals at spinal levels. The Mo5 appears to be unique among cranial motor nuclei in displaying such a rich content of Cx36. In the same brains taken for Mo5 examination, the facial motor nucleus was nearly devoid of labelling for Cx36, the hypoglossal nucleus has sparse Cx36-puncta associated with only a few small neurons, and the remaining nuclei contain only a scattering of these puncta (not shown).
Primary afferent origin of terminals associated with Cx36-puncta
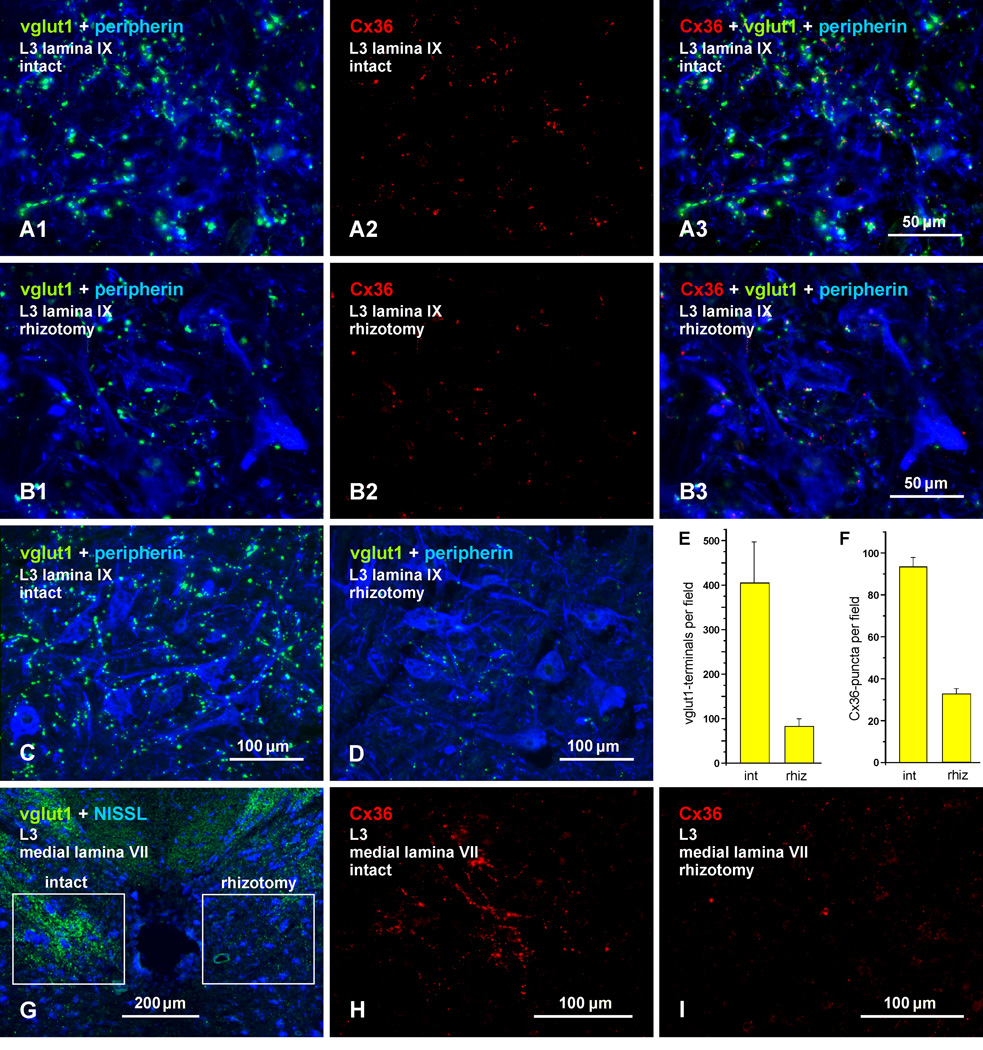
We next examined the source of vglut1-terminals at which Cx36-puncta were localized in the lumbar spinal cord. There is evidence that vglut1 in spinal cord gray matter is contained largely, though not exclusively, in terminals of primary afferent origin (Oliveira et al., 2003; Alvarez et al., 2004; Wu et al., 2004; Persson et al., 2006). The association of Cx36 with nerve terminals containing vglut1 suggests that some of these afferents form morphologically mixed synapses. This possibility would require transport of Cx36 from dorsal root ganglion neurons to the presynaptic terminal membrane for gap junction formation by docking of connexons in hemiplaques contributed by the pre- and postsynaptic elements. Because we have found Cx36 only in intact gap junctions (i.e., never in a gap junctional hemiplaque without an apposing hemiplaque) (Rash et al., 2000, 2001a, 2007a,b), we predict that elimination of terminals bearing Cx36 would result in the loss of gap junctions at these terminals (i.e., loss of both pre and postsynaptic hemiplaques). To test this and to confirm primary afferent localization of Cx36 in the spinal cord regions examined above, we performed unilateral dorsal rhizotomies, transecting the L1 to L4 dorsal roots in adult rats. On the intact side of the cord, typical labelling for vglut1 is seen among peripherin-positive motoneurons in lamina IX, as shown at an L3 spinal level (Fig. 9A1). Rhizotomy on the other side of the cord resulted in a large loss of labelling in lamina IX (Fig. 9B1). The same fields show comparisons of Cx36 labelling on the control vs. rhizotomy side at the L3 level, with labelling for Cx36 alone (Fig. 9A2 vs. 9B2), and after overlay with labelling for vglut1 and peripherin (Fig. 9A3 and 9B3). There was a large reduction in the density of Cx36-puncta, and some of those remaining on the rhizotomy side displayed the typical co-localization with vglut1 (Fig. 9B3) seen on the unoperated control side (9A3). In comparisons of animals prepared with weak and strong tissue fixatives (see Methods), the depletion of vglut1 on the rhizotomy vs. the intact side in animals fixed with 4% formaldehyde (Fig. 9C, D) was similar to that seen in animals prepared with the weaker fixative (Fig. 9B1), indicating that less than optimal fixation for vglut1 did not qualitatively confound the results.
Fig. 9.
Association of Cx36 with vglut1-containing axon terminals of primary afferent origin in lamina IX and VII of adult rat. (A,B) Triple immunofluorescence labelling for vglut1, peripherin and Cx36 in lamina IX after unilateral dorsal rhizotomy at the L1-L4 levels. Images at L3 on the intact side (A1-A3, same field) and rhizotomy side (B1-B3, same field) show vglut1/peripherin overlay (A1,B1), Cx36 alone (A2,B2) and overlay of labelling for all three proteins (A3,B3). The intact side shows normal labelling of vglut1 (A1) and Cx36 (A2). The rhizotomy side shows reduced labelling of vglut1 (B1) and Cx36 (B2). Overlay shows vglut1/Cx36 co-localization at some of the remaining vglut1-terminals on the rhizotomy side (B3). (C,D) Labelling for vglut1 and peripherin under optimum fixation conditions for vglut1 (4% formaldehyde), showing vglut1 on the intact side (C) and extensive loss of vglut1-terminals on the rhizotomy side (D). (E,F) Quantitation of labelling in fields of lamina IX on the intact and rhizotomy side, showing an 80% reduction of vglut1-terminals and a 65% reduction of Cx36-puncta on the rhizotomy side. (G) Low magnification blue fluorescence Nissl counterstained transverse section showing areas adjacent to the central canal after unilateral dorsal rhizotomy. Dense labelling for vglut1 is seen on the intact side (boxed region at left), and depletion of labelling is seen on the rhizotomy side (boxed region at right). (H,I) Immunolabelling for Cx36 in the boxed areas shown in (G), with the left box magnified in (H) showing clusters of Cx36-puncta on the intact side, and the right box magnified in (I) showing only a few puncta remaining on the rhizotomy side.
Quantitative analyses (Fig. 9E,F) showed that the number of vglut1-terminals in lamina IX on the deafferented side of the lumbar cord was reduced by 80 ± 1.2% (n = 4), and Cx36-puncta were reduced by 65 ± 3% (n = 4). Because many afferents give off both acending and descending branches and numerous collaterals upon enerting the cord (Hongo et al., 1978, 1987) it is likely that at least some of the remaining terminals were from primary afferents entering above or below the segments with rhizotomy (L1-L4). The extent of vglut1 and Cx36 depletions in other lamina of spinal cord were not examined quantitatively, but it appeared from visual inspection that there was less reduction in vglut1 terminals and Cx36-puncta in dorsal horn lamina than in intermediate lamina and other areas of the ventral horn. As shown in Fig. 9G, the loss of the patches of vglut1 labelling adjacent to the central canal was particularly striking. This depletion was accompanied by an equally extensive loss of Cx36-puncta, as shown by images of labelling for Cx36 alone on the intact side [(Fig. 9H, magnified from field in left box of (G)] vs. rhizotomy side [(Fig. 9I, magnified from field in right box of (G)].
GAD65 and mixed synapses
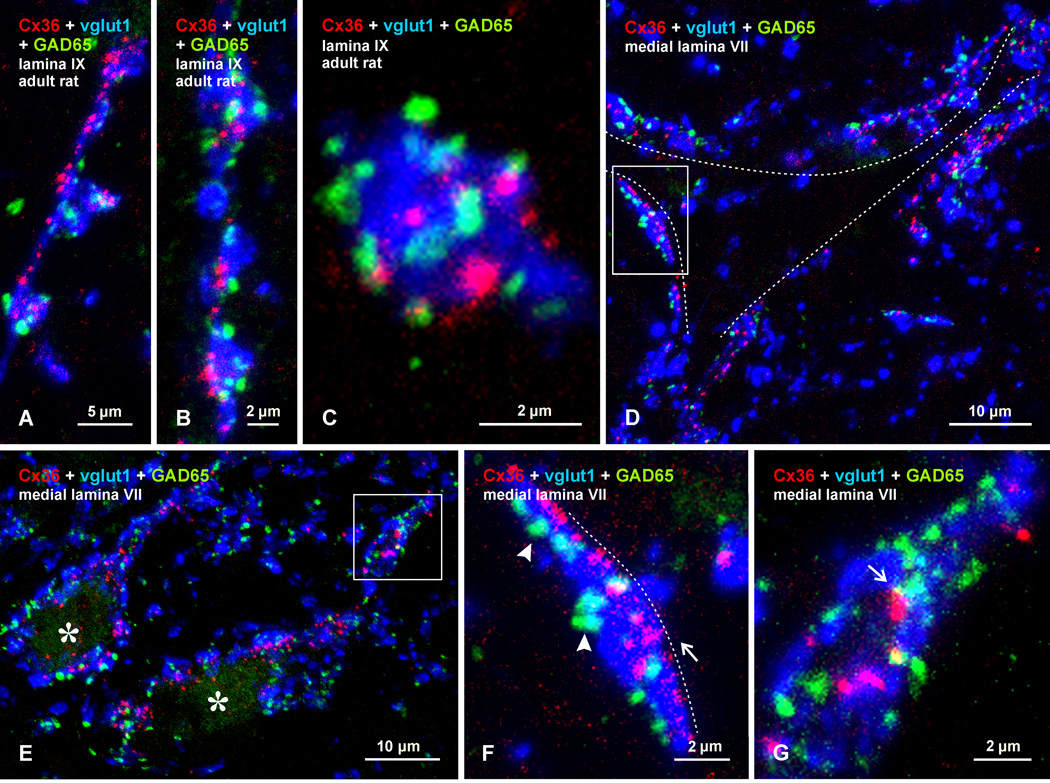
It is well known that myelinated muscle and cuntaneous afferents are subject to presynaptic regulation of transmitter release mediated by gamma-aminobutyric acid (GABAergic) contacts on primary afferent fiber terminals (Rudomin and Schmidt, 1999). These GABAergic terminals can be identified by the presence of a specific glutamic acid decarboxylase isomer, GAD65, (Hughes et al., 2005). In view of the above results, indicating association of Cx36-puncta with primary afferent terminals, we explored the localization of labelling for vglut1 and Cx36 in relation to that of terminals labelled for GAD65. Specifically, we determined whether those terminals bearing Cx36-puncta in lamina IX and medial lamina VII are among afferent terminals contacted by presynaptic GAD65-containing P boutons (Hughes et al., 2005). In lamina IX, GAD65-positive terminals were frequently seen closely apposed to vglut1-terminals displaying co-localization with Cx36-puncta (Fig. 10A-C). In these images, vglut1 is pseudo-colored blue rather than green, to avoid obscuring fluorochrome labels for Cx36 and GAD65. As shown at successively higher magnifications, these relationships were found at vglut1-terminals forming what appeared to be en passant type contacts with motoneurons (Fig. 10A), at series of clustered terminals (Fig. 10B), and at individual isolated terminals (Fig. 10C). Similar results were obtained in medial lamina VII, where terminals labelled for GAD65 were invariably seen in apposition to vglut1-terminals that overlap with Cx36-puncta. These associations of the three labels were seen on neuronal somata (Fig. 10D) and were especially prominent at large initial dendrites encircled by vglut1-terminals (Fig. 10E). As shown by high magnification at vglut1-terminals viewed on edge and in apposition to neuronal somata, Cx36-puncta appeared at sites on these terminals closest to the somata, whereas GAD65-positive terminals were more distally located on these terminals (Fig. 10F). Also seen are cases of close proximity of Cx36-puncta to GAD65-positive P boutons (Fig. 10G). Although requiring ultrastructural confirmation, this result suggests that primary afferent terminals subject to presynaptic inhibition are among those afferents that form (morphologically) mixed synapses.
Fig. 10.
Confocal triple immunofluorescence labelling for Cx36 (red), vglut1 (blue) and GAD65 (green), showing localization of GAD65-positive P boutons on vglut1-terminals displaying Cx36-puncta in lamina IX and VII of adult rat. Terminals labelled for vglut1 are pseudo colored blue to avoid obscuring GAD65 P boutons and Cx36-puncta. (A-C) Images from lamina IX, showing GAD65-positive P boutons associated with what appear to be en passant type vglut1-terminals (A) or clusters of these terminals (B), with often multiple P boutons and Cx36-puncta associated with individual vglut1-terminals (C). (D-G) Images from lamina VII showing a single neuron (D, outlined by dotted line), and two tangentially cut dendrites (E, asterisks). In each image, Cx36-puncta are seen co-localized with large vglut1-terminals that contact neuronal somata and dendrites, and that are themselves contacted by GAD65-positive P boutons. (F,G) Boxed areas in (D) and (E) (magnified in F and G, respectively), showing Cx36-puncta (F, arrow) localized along portions of vglut1-terminals nearest the neuronal soma (F, dotted line), and GAD65-positive P boutons (F, arrowheads) at more distal portions of the vglut1-terminals. Cx36-puncta are occasionally in close proximity to GAD65-positive P boutons (G, arrow).
Developmental profile of mixed synapses in the spinal cord
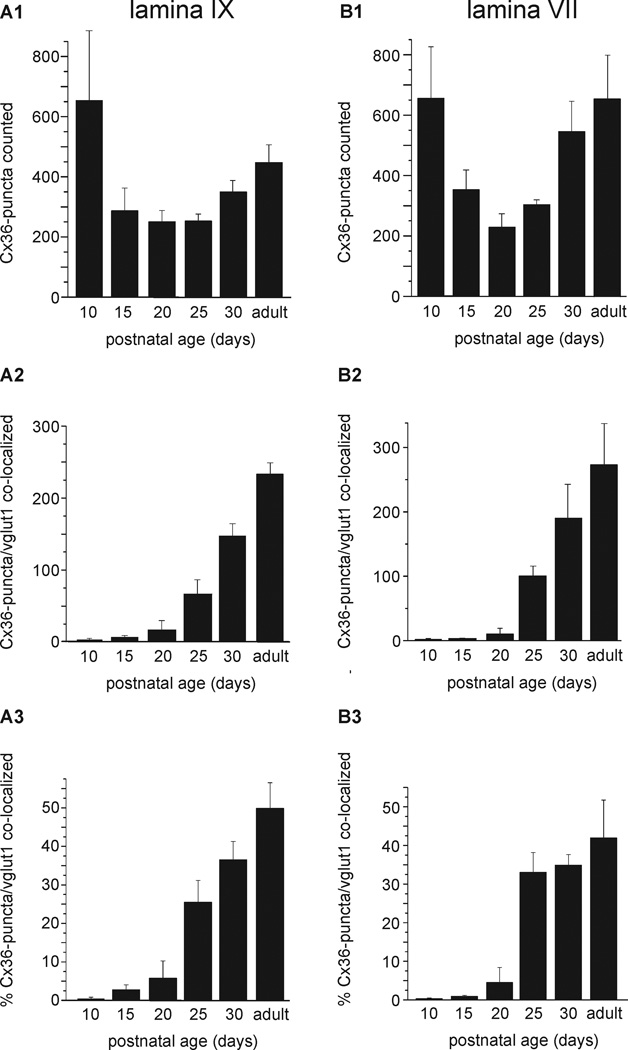
We next determined the developmental profile of Cx36 association with vglut1-terminals in rodent spinal cord. At each age examined, fields in lamina IX and VII containing numerous Cx36-puncta and vglut1-labelled terminals were photographed at medium confocal magnification and taken for counts of total Cx36-puncta. The data are presented as the average of total Cx36-puncta counted in each of the two spinal cord areas from four animals (Fig. 11A1 and 11B1), the average number of Cx36-puncta co-localized (i.e., clear overlap between labelling for Cx36 and vglut1) with vglut1 in these animals (Fig. 11A2 and 11B2), and the average percentage of Cx36-puncta/vglut1-terminal co-localization (Fig. 11A3 and 11B3). Surprisingly, in both lamina IX and VII, Cx36-puncta were rarely co-localized with labelling for vglut1 prior to the end of the second postnatal week, and minimally even up to the end of the third postnatal week. Co-localization thereafter increased, reaching values of 49% in lamina IX and 41% in lamina VII of adults rats. Mice were examined in less detail, but showed a similar paucity of Cx36/vlgut-1 co-localization during early development, with co-localization reaching 28% in lamina IX at PD30 and 38% in adults.
Fig. 11.
Developmental profile of Cx36 association with vglut1-terminals in lamina IX and VII in rat lumbar spinal cord. (A,B) Histograms show the average of total Cx36-puncta counted in fields of lamina IX and VII from four animals are each age indicated (A1,B1), the total Cx36-puncta colocalized with vglut1-terminals averaged from the four animals (A2,B2), and the average percentage of Cx36/vglut1 co-localization (A3,B3). The values at different ages in (A1,B1) reflect differences in the number of fields/per animal taken for counts and/or heterogeneities in numbers of Cx36-puncta in fields chosen for counts, rather than absolute differences in density of Cx36-puncta at the ages examined. In lamina XI, the average percentage of Cx36-puncta/vglut1 co-localization was 0.4% at PD10, 2.7% at PD15, 5.7% at PD20, 25% at PD25, 36% at PD30 and 49% in adult. In lamina VII, the values were 0.3% at PD10, 0.9% at PD15, 4% at PD20, 33% at PD25, 34% at PD30 and 41% in adult. Values are means ± s.e.m. (n = 4).
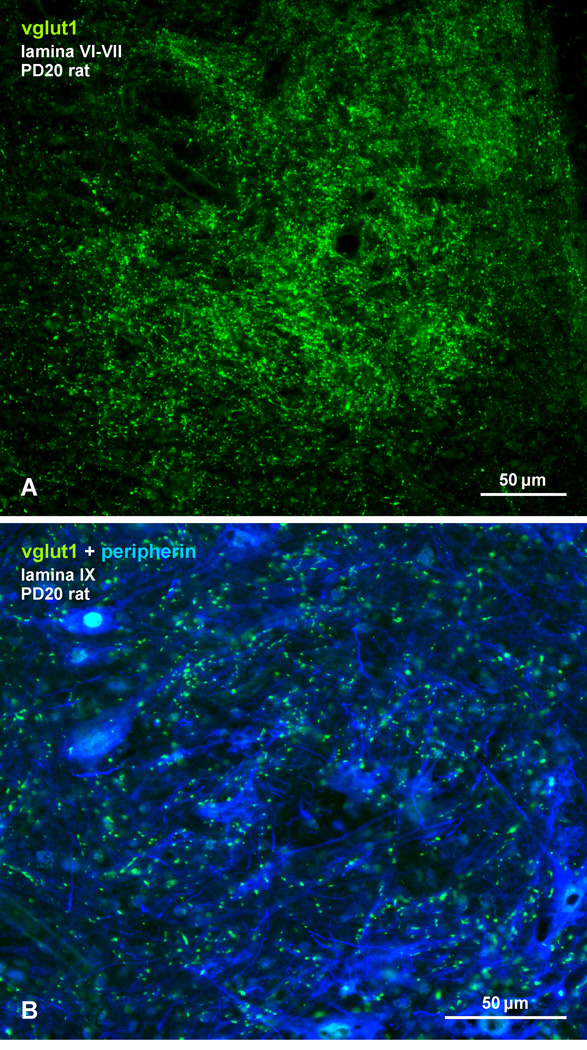
To exclude the possibility that the low level of co-localization seen during early development was due to a relatively slow maturation of vglut1-containing primary afferent terminals in the spinal cord or a delayed appearance of vglut1 in these terminals, we examined labelling for vglut1 in rat spinal cord at an age (PD20) when the percentage of Cx36-puncta co-localization with vglut1 was still relatively low in lamina VII (4%) and lamina IX (5.7%). As shown in Figure 12, lamina VI and VII contained a very high density of vglut1-positive terminals at PD20 (Fig. 12A), and lamina IX was well populated by these terminals among peripherin-positive motoneurons (Fig. 12B). This is consistent with observations of the rapid maturation of vglut1-containing terminals in spinal cord up to PD15 (Mentis et al., 2006), when Cx36/vglut1 co-localization was minimal (0.9% in lamina VII; 2.7% in lamina IX). Thus, the developmentally delayed association of Cx36 with primary afferent terminals appears to be unrelated to the morphological maturation state of these terminals.
Fig. 12.
(A,B) Immunofluorescence labelling of vglut1 (green) in laminae VI - VII (A) and in lamina IX (B) at the L4 level of rat spinal cord at PD20. Motoneurons in (B) are immunolabelled for peripherin (blue). A high density of axon terminals are labelled for vglut1 in lamina VI and VII, and abundant vlgut1-positive terminals are present among motoneurons in lamina IX at this postnatal age when Cx36-puncta/vglut1 co-localization in these lamina was a small fraction of that seen in these lamina of adult spinal cord.
DISCUSSION
The present study extends our previous observations on Cx36 localization in brainstem and spinal cord (Bautista et al 2012; 2013b; Nagy et al., 2013) by demonstrating: 1. That immunolabelling for Cx36 is extensive in the trigeminal motor nucleus and in multiple areas of the adult rat and mouse spinal cord; and 2. That Cx36 is frequently co-localized with vglut-1, which appears around the third postnatal week and persists in adults. The association between vglut1 and Cx36 is discussed as the anatomical substrate of morphologically mixed (electrical/chemical) excitatory synapses arising from large diameter sensory afferents in the spinal cord.
Cx36/vglut1 co-localization in motor nuclei
In both spinal lamina IX and the trigeminal motor nucleus of adult rat and mouse, Cx36 was often localized either at vglut1-containing axon terminals or in very close proximity (i.e., <1 µm) to these terminals. While most spinal motor nuclei displayed Cx36/vglut1 co-localization, some motor nuclei at the lower cervical level (C8) were nearly devoid of both vglut1 and Cx36. Based on the spinal cord atlas of Watson et al. (2009), these motor nuclei most likely innervate deltoid and pectoral muscles. In addition, we report elsewhere (Bautista et al., 2013b) that a separate class of rodent motoneurons in sexually dimorphic dorsomedial and dorsolateral motor nuclei found at lower lumbosacral levels exhibit dense Cx36-puncta that is not co-localized with vglut1. Cx36-puncta are found at somatic locations and dendritic appositions of these motoneurons, and the motor nuclei are largely devoid of vglut1-containing terminals. Thus, we distinguish three classes of spinal motor nuclei in the rodent: i) the majority where Cx36 is affiliated with vglut1-containing nerve terminals, forming morphologically mixed synapses (discussed below); ii) certain cervical motor nuclei without vglut1 and Cx36; and iii) sexually dimorphic nuclei where Cx36-puncta occur in the absence of vglut1, and almost certainly represent what we define as “purely electrical synapses”. Among cranial motor nuclei, only the Mo5 displayed an abundance of Cx36 co-localization with vglut1.
Cx36/vglut1 co-localization in medial intermediate lamina
In spinal cord, extensive co-localization of vglut1 with Cx36 was also found in areas outside of motor nuclei. The almost continuous rings of Cx36 and vglut1 on the soma of some neurons in medial lamina VII were particularly striking as was the longitudinal grouping of these neurons in clusters along the cord. Other areas rich in co-localized Cx36 and vglut1 included deeper regions of the intermediate spinal lamina (laminae V-V1) and regions corresponding to Clarke’s nucleus.
Clusters of Cx36-laden neurons located in medial lamina VII near the central canal displayed a complex organization, with characteristic features distinguishing these clusters from surrounding regions, including: i) the regularity and high density of vglut1-containing primary afferent (“PA”) convergence on these clusters ; ii) their intermittent rostro-caudal occurrence in separated domains at all spinal levels; iii) the presence of neurons receiving Cx36-containing (“Cx”) primary afferent terminals (i.e., Cx36 co-localized with vglut1) intermingled with those lacking these terminals; and iv) the paucity of innervation of these neurons by vglut2-containing terminals. Based on these features and for ease of reference, we term these clusters, together with their contingent of vglut1-containing primary afferents, as medial lamina VII PACx (M-VII PACx) domains. A coalescence of primary afferents and/or their collaterals with intermittent fields of arborizations generating a modular pattern has been described in this area of the spinal cord (Scheibel & Scheibel, 1969; Szentagothai 1966; Smith, 1983). Studies of terminal degeneration following dorsal rhizotomy have also identified plexuses of primary afferent terminations lateral and ventrolateral to the central canal that were distributed intermittently (Imai & Kusama, 1969), at similar distances between the PACx domains measured here. Further, at lower thoracic and lumbar levels, distinct sets of primary afferent fibers entering Clark’s column were distinguished from those with deeper trajectories terminating in the medial area of lamina VII (Rethelyi 1968), corresponding to the area of the MVII PACx domains identified here.
Importantly, the PACx domains are distinct from the nearby intermediomedial nucleus, which is known to contain ChAT-positive neurons (Stepien et al 2010), whereas our results show these domains to contain only a few neuronal somata immunopositive of ChAT (unpublished observations), and these ChAT-positive somata did not correspond to neuronal somata heavily laden with Cx36-puncta and vglut1-terminals. Similarly, these PACx domains are distinct from Clarke’s nucleus at lower thoracic and upper lumbar levels. However, at cervical levels, the PACx domains appear to correspond to the central cervical nucleus (CeVe), which also occupies medial regions of lamina VII. Neurons in the M-VII PACx domains were somewhat smaller and more medially located in thoracic segments than those in lumbar levels. Their dendrites almost certainly extend beyond the domains containing their somata, where they are contacted by fewer vglut1-containing terminals. Consequently, while the extent of excitatory input to their dendrites from vglut2-terminals is uncertain, it is likely that excitatory vglut1-containing afferents concentrated on their somata and initial dendrites could exert a powerful influence on their activity. The sensory information conveyed by these afferents is unclear, but likely does not include group Ia fibers that lack terminations in medial areas of lamina VII (Brown and Fyffe, 1978; Hongo et al., 1978, 1987).
Mixed synapses and their structural/functional correlate
As described in the Introduction, the presence of gap junctions at nerve terminals is the anatomical substrate for mixed synapses employing both chemical and electrical modes of neuronal communication. Functional mixed synapses formed by primary afferents on motoneurons have been described in lower vertebrates (Shapovalov et al., 1980), and morphologically mixed synapses have been demonstrated ultrastructurally in rodent spinal cord (Rash et al., 1996). By thin-section electron microscopy (EM), terminals forming these synapses in rat spinal cord were large (3 µm in diameter) and in older animals were more often encountered than gap junctions at dendro-dendritic contacts (Motorina, 1989, 1991). By freeze-fracture EM, gap junctions at mixed synapses in rat spinal cord: i) were found throughout the rostro-caudal spinal cord axis; ii) detected in lamina III-IX at various spinal levels; iii) were located on interneurons as well as motoneurons; iv) were distributed often on neuronal somata and initial dendrites; v) occurred in clusters at axon terminals, with up to six gap junctions per cluster; and vi) were estimated to be present at a frequency of about 300 per spinal neuron (Rash et al., 1996; 1997,1998). These series of findings are remarkably harmonious with our results on the overall distribution and cellular localization of Cx36-puncta associated with axon terminals in adult rodent spinal cord. In particular, the relatively large number of mixed synapses estimated to contact spinal neurons is easily envisioned when considering what the projected density of Cx36-puncta associated with terminals would be on for example motoneurons, such as those shown in Fig. 2, if these neurons were subject to full 3D reconstruction. It is of note that while spinal neurons have been considered to express other connexins in addition to Cx36 (Chang et al., 1999), the above accordance between our results and earlier ultrastructural work further supports our observations reported elsewhere that neuronal gap junctions in adult spinal cord are formed primarily by Cx36 (Bautista et al. 2013a).
Instances were encountered where Cx36-puncta were seen without an obvious association with vglut1 in areas otherwise rich in co-localized Cx36 and vglut1. We suggest that some of these instances were also examples of co-localization that escaped classification as such for a number of reasons. First, given the requirement of Cx36/vglut1 overlap in labelling as criteria for co-localization, false-negatives could result when Cx36 and vglut1 staining occurred in different focal planes or in adjacent sections. Second, synaptic vesicles in primary afferent terminals are not always immediately adjacent or crowded against the presynaptic plasma membrane. In such cases, they may not appear to be co-localized with Cx36 puncta located at the terminal periphery. Indeed, Cx36-puncta were often seen very near vglut1-positive terminals, but could not be disignated as colocalized with these terminals. And third, the differences in fixation conditions for optimal detection of Cx36 vs. vglut1 require a compromise in fixation to allow double labelling for these proteins. This likely resulted in some reduction of labelling for vglut1. We estimate that these factors together may have resulted in an underestimate of Cx36-puncta association with vglut1-terminals by as much as 25–35%, as judged from the large number of puncta that were very near but not co-localized with vglut1 terminals, and from a few instances in individual adult animals, where co-localization in areas of the ventral horn reached as high as 80–90%. The remaining percentage of Cx36-puncta seen without an obvious association with vglut1 in dorsal and ventral horn lamina likely represent purely electrical synapses between either motoneurons or between other types of neurons, and will require further studies to determine the identity of neurons to which they confer electrical coupling. In addition, we cannot exclude the possibility that some Cx36-puncta are associated with axon terminals containing other transmitters. Given the presence of gap junction appositions in adult rat motoneurons (van Der Want et al., 1998) and the extensive distribution of Cx36 in the cord, it would seem likely that gap junctions could be responsible for interactions previously ascribed to ephaptic (i.e. electrical field) interactions between motoneuron dendrites (Matthews et al., 1971).
Considering the relatively high density of Cx36-puncta in rodent spinal cord, it may be surprising that mixed synapses have not been more frequently encountered in nearly a half century of ultrastructural studies of spinal cord synaptology. There are several possible reasons for this. One is that it is generally recognized that neuronal gap junctions in the CNS are difficult to find and could be overlooked at EM magnifications typically used for studies of synaptic connectivity. This is especially the case with technical limitations imposed by thin-section EM identification of the typically small gap junctions that occur between neurons, though some of these limitations are mitigated by freeze-fracture EM (Rash et al., 1998). Another is that tissue preparation has not always been optimal for gap junction detection, especially in early EM work, where less than optimal conditions sometimes precluded distinguishing between gap junctions, tight junctions and desmosomes in mammalian CNS (Rash et al., 1998). Furthermore, many of the early classic EM studies of spinal cord synaptology were conducted using cat. While we have succeeded in detecting Cx36 by immunofluorescence in some areas of cat brain (Nagy et al., 2013), we do not yet have sufficient confidence in labelling of this connexin in adult cat spinal cord to consider the likelihood of Cx36-containing mixed synapses in the feline cord.
Mixed synapses on type Ia and other afferent fiber types
It was previously shown that a substantial proportion of vglut1-containing terminals in the spinal cord are endings of primary afferent fibers (Alvarez et al., 2004; Persson et al., 2006). The extensive co-localization of Cx36 with vglut1 in rodent spinal cord thus indicates a strong association of Cx36 with primary afferent terminals, and hence the existence of mixed synapses between these afferents and their spinal targets. Our finding of a large reduction in labelling for both Cx36 and vglut1 following dorsal rhizotomy supports this possibility. Although we did not determine the proportion of vglut1-terminals displaying Cx36-puncta, it is clear that Cx36 is associated with only select populations of afferent fibers. In this respect, it is well known that in the lumbar spinal cord, individual Ia spindle afferents make multiple monosynaptic connections throughout the motor pool (Brown and Fyffe 1978), while group II spindle afferents make monosynaptic connections with smaller EPSPs and in only a portion of the motoneuron pool (Sypert et al., 1980). Because motoneuron excitation from tendon organ afferents (Eccles et al., 1957) and cutaneous afferents (see Labella and McCrea 1990) occurs at longer than monosynaptic latencies, these afferents do not form monosynaptic connections to motoneurons and therefore, do not form mixed synapses in lamina IX. Thus, the majority of mixed Cx36/vglut1 synapses at least in lamina IX are likely terminals of monosynaptic connections between Ia muscle spindle afferents and motoneurons. On the other hand, the mixed synapses in medial lamina VII (Fig. 7) are more likely from other afferent types since Ia afferents neurons do not project to this area (Brown and Fyffe 1978; Hongo et al., 1987). The extensive distribution of co-localized vglut1 and Cx36 in the deep dorsal horn and Clarke’s nucleus could be associated with either Ia spindle or Ib golgi tendon organ afferents since both afferent classes project to these locations (Hongo et al., 1987; Tracey and Walmsley 1984). Because P-boutons are involved in the presynaptic inhibition of large diameter muscle afferents (Hughes et al., 2005), our finding of P-boutons in association with vglut1-containing afferents forming morphologically mixed synapses further supports the spindle or tendon origin of some afferents with mixed synapses.
The presence of the anatomical substrate for combined electrical and chemical transmission at afferent fiber synapses does not of course indicate that the gap junction component of these synapses are functional. To our knowledge, mixed synapses on motoneurons have not been identified electrophysiologically in late adolescent rodents when these synapses become abundant or in adult rodents. In cat, however, the notion that there is a direct electrical component to the arguably most studied synapse in mammals, the monosynaptic Ia connection to lumbar motoneurons, was examined decades ago and remains the subject of controversy.
It has been pointed out that electrical transmission is not necessarily faster than chemical transmission in all cases (Bennett, 1997). Nevertheless, in support of the possibility of an electrical component to the synapse between motoneurons and Ia afferents, these afferents can be excited or their thresholds to electrical activation lowered at a very short latency when an antidromic action potential occurs in the target motoneuron (Decima and Goldberg, 1973, 1976; Curtis et al., 1979). Gogan et al. (1977) suggested electrical coupling as one explanation for their findings of short latency excitation between some motoneurons in the cat. The observations of Esplin et al. (1972) are of particular interest as they found that systemic administration of a cholinesterase inhibitor unmasked strong, presumable electrical, interactions between action potentials in the motoneuron and the Ia afferents. It thus remains a possibility that functional mixed synapses are regulated and expressed only under specific conditions.
Besides the mediation of coupling by purely electrical synapses, mixed synapses might provide another means of electrical communication between neurons. In the lateral vestibular nucleus of adult rat, it has been deduced that electrical coupling occurs between neurons that themselves appear not to be linked by gap junctions. Rather, coupling was proposed to be mediated by way of mixed synapses formed by presynaptic fibers, where collaterals of individual fibers forming mixed synapses with separate neurons provide a pathway for electrical coupling of those neurons (Korn et al., 1973). Neuronal coupling mediated by presynaptic fibers has been extensively documented in lower vertebrates (see Bennett and Goodenough, 1978). In the spinal cord, individual Ia afferent fibers are known to form many collaterals that innervate multiple motoneurons (Brown and Fyffe, 1978, 1981; Hongo et al., 1978, 1987). Assuming that fibers with such collateral terminations include those at which we find morphologically mixed synapses, these fibers potentially could mediate coupling between spinal neurons in adult rodents.
Development of mixed synapses in ventral horn laminae
At early postnatal ages, the widespread presence of Cx36-puncta observed in spinal cord is consistent with reports of electrical- and dye-coupling between developing spinal neurons, particularly motoneurons (see Introduction). In contrast, morphologically mixed synapses in each of the spinal cord gray matter regions examined had a surprisingly late developmental appearance and were first observed, in for example lamina IX, at a time (i.e. PD8-13) when motoneurons cease to exhibit direct intercellular electrical coupling (Walton & Navarrete, 1991). Equally remarkable was the continued increase in the incidence of these synapses even after the third postnatal week to adulthood. Notwithstanding that the incidence of Cx36/vglut1 co-localization may have been underestimated based on technical considerations (discussed above) that should apply to animals of all ages, we nevertheless believe the relatively low incidence of Cx36/vglut1 co-localization at early ages reflects a delayed development of morphologically mixed synapses that begins around the third postnatal week and increases towards adulthood. We do not exclude the possibility that some motoneurons in addition to those in the sexually dimorphic nuclei remain coupled in adults by gap junctions (e.g. soleus motoneurons; van der Want et al., 1998). However, our results indicate that most motoneurons transition from an early state of coupling with each other mediated by Cx36 (Bautista et al., 2012) to a later state of mixed synapse formation with afferent terminals. The functional relevance of the late onset of mixed synapses remains to be determined, as does elucidation of the physiological processes for which they would presumably be required. Further insight into these issues will require the use of adult or late adoslescent rodent preparations when mixed synapses are developed and not the early postnatal in vitro preparations commonly used for electrophysiological investigations in the spinal cord.
Supplementary Material
Supplementary Fig. 1. Movie file of the image in Fig. 7E (z-stack of 9 µm), showing maintained association of Cx36-puncta (red) with vglut-1-positive nerve terminals (green) at all angles of rotation shown. Immunolabelled terminals are located on the initial dendrites and somata of a non-counterstained neurons (marked by asterisks) located in the medial area of lamina VII near the central canal.
Highlights.
Gap junctions formed by connexin36 are widely distributed in adult rodent spinal cord
Connexin36 is found at axon terminals in brainstem and spinal cord
Axon terminals bearing connexin36 in spinal cord are of primary afferent origin
Primary afferent terminals form morphologically mixed chemical/electrical synapses
Muscle spindle Ia afferents potentially form mixed synapses on motoneurons
Acknowledgements
This work was supported by a grant from the Canadian Institutes of Health Research to J.I.N. (MOP 106598) and to DM (MOP 37756), and by grants from the National Institutes of Health (NS31027, NS44010, NS44295) to JE Rash with sub-award to JIN. We thank B. McLean for excellent technical assistance, Dr. B. Lynn for genotyping of transgenic mice and Dr. D. Paul (Harvard University) for providing breeding pairs of Cx36 knockout and wild-type mice.
Abbreviations
- CeVe
central cervical nucleus
- CNS
Central nervous system
- C
cervical
- Cx36
connexin36
- GAD65
glutamic acid decarboxylase65
- L
lumbar
- Mo5
trigeminal motor nucleus
- PBS
phosphate-buffered saline
- PD
postnatal day
- TBS
50 mM Tris-HCl, pH 7.4, 1.5% NaCl
- TBSTr
TBS containing 0.3% Triton X-100
- T
thoracic
- vglut1
vesicular glutamate transporter-1
- vglut2
vesicular glutamate transporter-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Arasaki K, Kudo N, Nakanishi T. Firing of spinal motoneurons due to electrical interactions in the rat: an in vitro study. Exp Brain Res. 1984;54:437–445. doi: 10.1007/BF00235469. [DOI] [PubMed] [Google Scholar]
- Bautista W, Nagy JI, Dai Y, McCrea DA. Requirement of neuronal connexin36 in pathways mediating presynaptic inhibition of primary afferents in functionally mature mouse spinal cord. J Physiol. 2012;590:3821–3839. doi: 10.1113/jphysiol.2011.225987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista W, Rash JE, Vanderpool KG, Yasumura T, Nagy JI. Re-evaluation of connexin (Cx26, Cx32, Cx36, Cx37, Cx40, Cx43, Cx45) association with motoneurons in rodent spinal cord, sexually dimorphic motor nuclei and trigeminal motor nucleus. 2013a doi: 10.1111/ejn.12450. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista W, Nagy JI. Connexin36 forms purely electrical synapses in sexually dimorphic lumbosacral motor nuclei in spinal cord of developing and adult rat and mouse. 2013b doi: 10.1111/ejn.12439. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MVL, Goodenough DA. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Prog Bull. 1978;16:373–485. [PubMed] [Google Scholar]
- Bennett MVL. Gap junctions as electrical synapses. J Neurocytol. 1997;26:349–366. doi: 10.1023/a:1018560803261. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Zukin SR. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Berger AJ. Gap junctions and inhibitory synapses modulate inspiratory motoneuron synchronization. J Neurophysiol. 2001;85:1543–1551. doi: 10.1152/jn.2001.85.4.1543. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Fyffe RE. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Balice-Gordon RJ. Gap junctional communication among developing and injured motor neurons. Brain Res Rev. 2000;3:242–249. doi: 10.1016/s0165-0173(99)00085-5. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gonzalez M, Pinter MJ, Balice-Gordon RJ. Gap junctional coupling and patterns of connexin expression among neonatal rat lumbar spinal neurons. J Neurosci. 1999;19:10813–10828. doi: 10.1523/JNEUROSCI.19-24-10813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Pereda A, Pinter MJ, Balice-Gordon RJ. Nerve injury induces gap junctional coupling among axotomized adult motor neurons. J Neurosci. 2000;20:674–684. doi: 10.1523/JNEUROSCI.20-02-00674.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WT, Edwards B, McCullagh KJ, Kemp MW, Moorwood C, Sherman DL, Burgess M, Davies KE. Syncoilin modulates peripherin filament networks and is necessary for largecalibre motor neurons. J Cell Sci. 2010;123:2543–2552. doi: 10.1242/jcs.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman AM, Sengelaub DR. Patterns of dye coupling in lumbar motor nuclei of the rat. J Comp Neurol. 2002;454:34–41. doi: 10.1002/cne.10438. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Parenti R, Spinella F, Salinaro AT, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudo G. Expression of Cx36 in mammalian neurons. Brain Res Rev. 2000;32:72–85. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Lodge D, Headley PM. Electrical interaction between motoneurons and afferent terminals in cat spinal cord. J Neurophysiol. 1979;3:635–641. doi: 10.1152/jn.1979.42.3.635. [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Decima EE, Goldberg LJ. Antidromic Electrical Interaction Between Alpha Motoneurons and Presynaptic Terminals. Brain Research. 1973;57:1–14. doi: 10.1016/0006-8993(73)90563-5. [DOI] [PubMed] [Google Scholar]
- Decima EE, Goldberg LJ. Motoneuron-presynaptic interaction in the spinal cord of the cat: rebuttal to a denial. Brain Research. 1976;110:387–391. doi: 10.1016/0006-8993(76)90413-3. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957;138:227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplin DW, Capek R, Esplin BA. Pharmacological studies on dorsal root responses produced by ventral root stimulation in the cat. Can J Physiol Pharmacol. 1972;50:119–122. doi: 10.1139/y72-018. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Miledi R, Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc R Soc London Ser B. 1980;206:115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Gogan P, Gueritaud JP, Horcholle-Bossavit G, Tyc-Dumont S. Direct excitatory interactions between spinal motoneurones of the cat. J Physiol. 1977;272:755–767. doi: 10.1113/jphysiol.1977.sp012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei-Sichani F, Davidson KGV, Yasumura T, Janssen WGM, Wearne SL, Hof PR, Traub RD, Gutierrez R, Ottersen OP, Rash JE. Mixed electrical-chemical synapses in adult rat hippocampus are primarily glutamatergic and coupled by connexin36. Front Neuroanat. 2012;6:1–26. doi: 10.3389/fnana.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley CA, Ziskind-Conhaim L. Electrical coupling between locomotor-related excitatory interneurons in the mammalian spinal cord. J Neurosci. 2006;26:8477–8483. doi: 10.1523/JNEUROSCI.0395-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi SG, Filippov MA, Mitropoulon G, Monyer H, Bruzzone R. Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks. Biochem Biophys Acta. 2004;1662:113–137. doi: 10.1016/j.bbamem.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Hongo T, Ishizuka N, Mannen H, Sasaki S. Axonal trajectory of single group Ia and Ib fibers in the cat spinal cord. Neurosci Lett. 1978;8:321–328. doi: 10.1016/0304-3940(78)90143-x. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kudo N, Sasaki S, Yamashita M, Yoshida K, Ishizuka N, Mannen H. Trajectory of group Ia and Ib fibers from the hind-limb muscles at the L3 and L4 segments of the spinal cord of the cat. J Comp Neurol. 1987;262:159–194. doi: 10.1002/cne.902620202. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Mackie M, Nagy GG, Riddell JS, Maxwell DJ, Szabo G, Erdelyi F, Veress G, Szucs P, Antal M, Todd AJ. P boutons in lamina IX of the rodent spinal cord express high levels of glutamic acid decarboxylase-65 and originate from cells in deep medial dorsal horn. Proc Natl Acad Sci. 2005;102:9038–9043. doi: 10.1073/pnas.0503646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Kusama T. Distribution of the dorsal root fibers in the cat:. An experimental study with the Nauta Methods. Brain Res. 1969;13:338–359. doi: 10.1016/0006-8993(69)90292-3. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O, Tresch MC, Harris-Warrick RM. Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res Bull. 2000;53:649–659. doi: 10.1016/s0361-9230(00)00398-1. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Tresch MC. Gap junctions and motor behavior. Trends Neurosci. 2002;25:108–115. doi: 10.1016/s0166-2236(02)02038-6. [DOI] [PubMed] [Google Scholar]
- Korn H, Sotelo C, Crepel F. Electrotonic coupling between neurons in the lateral vestibular nucleus. Exp Brain Res. 1973;16:255–275. [PubMed] [Google Scholar]
- Labella LA, McCrea DA. Evidence for restricted central convergence of cutaneous afferents on an excitatory reflex pathway to medial gastrocnemius motoneurons. J Neurophysiol. 1990;64:403–412. doi: 10.1152/jn.1990.64.2.403. [DOI] [PubMed] [Google Scholar]
- Li X, Olson C, Lu S, Kamasawa N, Yasumura T, Rash JE, Nagy JI. Neuronal connexin36 association with zonula occludens-1 protein (ZO-1) in mouse brain and interaction with the first PDZ domain of ZO-1. Eur J Neurosci. 2004;19:2132–2146. doi: 10.1111/j.l460-9568.2004.03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan SD, Pickering AE, Gibson IC, Nolan MF, Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. J Physiol. 1996;495:491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Micevych PE. Gap junctions between lateral spinal motoneurons in the rat. Brain Res. 1989;495:362–366. doi: 10.1016/0006-8993(89)90229-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arnold AP, Zampighi G, Micevych PE. Androgenic regulation of gap junctions between motoneurons in the rat spinal cord. J Neurosci. 1988;8:4177–4138. doi: 10.1523/JNEUROSCI.08-11-04177.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Androgen regulates gap junction mRNA expression in androgen-sensitive motoneurons in the rat spinal cord. Neurosci Lett. 1991;131:159–162. doi: 10.1016/0304-3940(91)90603-q. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Arai Y, Urano A, Hyodo S. Effect of androgen on the expresssion of gap junction and beta-actin mRNAs in adult rat motoneurons. Neurosci Res. 1992;14:133–144. doi: 10.1016/0168-0102(92)90089-u. [DOI] [PubMed] [Google Scholar]
- Matthews MA, Willis WD, Williams V. Dendrite bundles in lamina IX of cat spinal cord: a possible source for electrical interaction between motoneurons? Anat Rec. 1971;171:313–328. doi: 10.1002/ar.1091710210. [DOI] [PubMed] [Google Scholar]
- Meier C, Dermietzel R. Electrical synapses--gap junctions in the brain. Results Probl Cell Differ. 2006;43:99–128. doi: 10.1007/400_013. [DOI] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Abelson L. Distribution of mRNAs coding for liver and heart gap junction proteins in the rat central nervous system. J Comp Neurol. 1991;305:96–118. doi: 10.1002/cne.903050110. [DOI] [PubMed] [Google Scholar]
- Motorina MV. Electrotonic synapses in the spinal cord of Mammals. Neurosci Behav Physiol. 1989;19:72–78. doi: 10.1007/BF01148414. [DOI] [PubMed] [Google Scholar]
- Motorina MV. Structural organization of the synaptic connections of the spinal cord motor neurons of mammals. Struc Behav Physiol. 1991;25:273–289. doi: 10.1007/BF02360038. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in in the mammalian central nervous system. Brain Res Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Nagy JI. Evidence for connexin36 localization at hippocampal mossy fiber terminals suggesting mixed chemical/electrical transmission by granule cells. Brain Res. 2012;1487:107–122. doi: 10.1016/j.brainres.2012.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Bautista W, Blakley B, Rash JE. Morphologically mixed chemical-electrical synapses in rodent vestibular nuclei as revealed by immunofluorescence detection of connexin36 and vesicular glutamate transporter-1. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.07.056. EPUB ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira ALR, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim T, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Personius KE, Balice-Gordon RJ. Activity-dependent editing of neuromuscular synaptic connections. Brain Res Bull. 2000;53:513–522. doi: 10.1016/s0361-9230(00)00384-1. [DOI] [PubMed] [Google Scholar]
- Personius KE, Balice-Gordon RJ. Loss of correlated motor neuron activity during synaptic competition at developing neuromuscular synapses. Neuron. 2001;31:395–408. doi: 10.1016/s0896-6273(01)00369-5. [DOI] [PubMed] [Google Scholar]
- Personius KE, Chang Q, Bittman K, Panzer J, Balice-Gordon RJ. Gap junctional communication among motor and other neurons shapes patterns of neural activity and synaptic connectivity during development. Cell Commun Adhes. 2001;8:329–333. doi: 10.3109/15419060109080748. [DOI] [PubMed] [Google Scholar]
- Personius KE, Chang Q, Mentis GZ, O'Donovan MJ, Balice-Gordon RJ. Reduced gap junctional coupling leads to uncorrelated motor neuron firing and precocious neuromuscular synapse elimination. Proc Natl Acad Sci. 2007;104:11808–11813. doi: 10.1073/pnas.0703357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Boulland J-L, Aspling M, Larsson M, Fremeau RT, Jr, Edwards RH, Storm-Mathisen J, Chaudhry FA, Broman J. Distribution of vesicular glutamate transporters 1 and 2 in the rat spinal cord, with a note on the spinocervical tract. J Comp Neurol. 2006;497:683–701. doi: 10.1002/cne.20987. [DOI] [PubMed] [Google Scholar]
- Rash JE, Dillman RK, Bilhartz BL, Duffy HS, Whalen LR, Yasumura T. Mixed synapses discovered and mapped throughout mammalian spinal cord. Proc Natl Acad Sci. 1996;93:4235–4239. doi: 10.1073/pnas.93.9.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freezefracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J Comp Neurol. 1997;388:265–292. doi: 10.1002/(sici)1096-9861(19971117)388:2<265::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE. Ultrastructure, histological, distribution, and freeze-fracture immunocytochemistry of gap junctions in rat brain and spinal cord. Cell Biol Int. 1998;22:731–749. doi: 10.1006/cbir.1998.0392. [DOI] [PubMed] [Google Scholar]
- Rash JE, Staines WA, Yasumura T, Pate D, Hudson CS, Stelmack GL, Nagy J. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin36 (Cx36) but not Cx32 or Cx43. Proc Natl Acad Sci. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins, and evidence for restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001a;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Davidson K, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes. 2001b;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Davidson KGV, Yasumura T, Kamasawa N, Nagy JI. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience. 2007a;147:938–956. doi: 10.1016/j.neuroscience.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Pouliot WA, Davidson KGV, Yasumura T, Furman CS, Royer S, Kamasawa N, Nagy JI, Dudek FE. Connexin36 vs connexin32, “miniature” neuronal gap junctions, and limited electrotonic coupling in rodent suprachiasmatic nucleus. Neuroscience. 2007b;149:350–371. doi: 10.1016/j.neuroscience.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethelyi M. The Golgi architecture of Clark’s column. Acta Morphologica Acad Sci Hung. 1968;16:311–330. [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Terminal patterns in cat spinal cord. III. Primary afferent collaterals. Brain Res. 1969;13:417–443. doi: 10.1016/0006-8993(69)90258-3. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Schneider TR, Foxe JJ, Engel K. Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci. 2008;31:401–409. doi: 10.1016/j.tins.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Shapovalov AI. Interneuronal synapses with electrical dual and chemical mode of transmission in vertebrates. Neuroscience. 1980;5:1113–1124. doi: 10.1016/0306-4522(80)90190-6. [DOI] [PubMed] [Google Scholar]
- Shrestha SS, Bannatyne BA, Jankowska E, Hammar I, Nilsson E, Maxwell DJ. Excitatory inputs to four types of spinocerebellar tract neurons in the cat and rat thoraco-lumbar spinal cord. J Physiol. 2012;590:1737–1755. doi: 10.1113/jphysiol.2011.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Smith CL. The development and postnatal organization of primary afferent projections to the rat thoracic spinal cord. J Comp Neurol. 1983;220:29–43. doi: 10.1002/cne.902200105. [DOI] [PubMed] [Google Scholar]
- Söhl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- Söhl G, Maxeiner S, Willecke K. Expression and functions of neuronal gap junctions. Nat Rev Neurosci. 2005;6:191–200. doi: 10.1038/nrn1627. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Palay SL. The fine structure of the lateral vestibular nucleus in the rat. II. Synaptic organization. Brain Res. 1970;18:93–115. doi: 10.1016/0006-8993(70)90459-2. [DOI] [PubMed] [Google Scholar]
- Stepien AE, Tripodi M, Arber S. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68:456–472. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Sypert GW, Fleshman JW, Munson JB. Comparison of monosynaptic actions of medial gastrocnemius group Ia and group II muscle spindle afferents on triceps surae motoneurons. J Neurophysiol. 1980;44:726–738. doi: 10.1152/jn.1980.44.4.726. [DOI] [PubMed] [Google Scholar]
- Szentagothai J. Pathways and subcortical relay mechanisms of visceral afferents. Acta Neuroveg. 1966;28:103–120. [Google Scholar]
- Tracey DJ, Walmsley B. Synaptic input from identified muscle afferents to neurones of the dorsal spinocerebellar tract in the cat. J Physiol. 1984;350:599–614. doi: 10.1113/jphysiol.1984.sp015220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nature. 2000;3:593–599. doi: 10.1038/75768. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Kiehn O. Synchronization of motor neurons during locomotion in the neonatal rat: predictors and mechanisms. J Neurosci. 2002;22:9997–10008. doi: 10.1523/JNEUROSCI.22-22-09997.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Want JJL, Gramsbergen A, Ijema-Paassen J, de Weerd H, Liem RSB. Dendrodendritic connections between motoneurons in the rat spinal cord: an electron microscopic investigation. Brain Res. 1998;779:342–345. doi: 10.1016/s0006-8993(97)01238-9. [DOI] [PubMed] [Google Scholar]
- Vivar C, Traub RD, Gutierrez R. Mixed electrical–chemical transmission between hippocampal mossy fibers and pyramidal cells. Eur J Neurosci. 2012;35:76–82. doi: 10.1111/j.1460-9568.2011.07930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton KD, Navarette R. Postnatal changes in motoneurone electronic coupling studied in the in vitro rat lumbar spinal cord. J Physiol. 1991;433:283–305. doi: 10.1113/jphysiol.1991.sp018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Paxinos G, Kayalioglu G. The spinal cord; A Christopher and Dana Reeve Foundation Text and Atlas. Amsterdam: Academic Press; 2009. [Google Scholar]
- Whittington MA, Traub RD. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Cowan AI, Brownstone RM. Heterogeneous electrotonic coupling and synchronization of rhythmic bursting activity in mouse Hb9 Interneurons. J Neurophysiol. 2007;98:2370–2381. doi: 10.1152/jn.00338.2007. [DOI] [PubMed] [Google Scholar]
- Wu S-X, Koshimizu Y, Feng Y-P, Okamoto K, Fujiyama F, Hioki H, Li Y-Q, Kaneko T, Mizuno N. Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res. 2004;1011:247–251. doi: 10.1016/j.brainres.2004.03.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Movie file of the image in Fig. 7E (z-stack of 9 µm), showing maintained association of Cx36-puncta (red) with vglut-1-positive nerve terminals (green) at all angles of rotation shown. Immunolabelled terminals are located on the initial dendrites and somata of a non-counterstained neurons (marked by asterisks) located in the medial area of lamina VII near the central canal.