Abstract
Regeneration of injured tubular cells occurs after acute tubular necrosis primarily from intrinsic renal cells. This may occur from a pre-existing intratubular stem/progenitor cell population or from any surviving proximal tubular cell. In this study, we characterize a CD24-, CD133-, and vimentin-positive subpopulation of cells scattered throughout the proximal tubule in normal human kidney. Compared to adjacent ‘normal’ proximal tubular cells, these CD24-positive cells contained less cytoplasm, fewer mitochondria, and no brush border. In addition, 49 marker proteins are described that are expressed within the proximal tubules in a similar scattered pattern. For eight of these markers, we confirmed co-localization with CD24. In human biopsies of patients with acute tubular necrosis (ATN), the number of CD24-positive tubular cells was increased. In both normal human kidneys and the ATN biopsies, around 85% of proliferating cells were CD24-positive – indicating that this cell population participates in tubular regeneration. In healthy rat kidneys, the novel cell subpopulation was absent. However, upon unilateral ureteral obstruction (UUO), the novel cell population was detected in significant amounts in the injured kidney. In summary, in human renal biopsies, the CD24-positive cells represent tubular cells with a deviant phenotype, characterized by a distinct morphology and marker expression. After acute tubular injury, these cells become more numerous. In healthy rat kidneys, these cells are not detectable, whereas after UUO, they appeared de novo – arguing against the notion that these cells represent a pre-existing progenitor cell population. Our data indicate rather that these cells represent transiently dedifferentiated tubular cells involved in regeneration.
Keywords: proximal tubules, progenitor cell, regeneration, acute tubular necrosis
Introduction
The proximal tubules and the thick ascending limbs of the loop of Henle are susceptible to ischaemic or toxic injury. Hypoxic episodes or exposure to nephrotoxins often leads to acute loss of these tubular cells via necrosis or apoptosis. Such injuries are transient since renal tubular cells can regenerate after such insults. The origin of the tubular cells that replenish the epithelial population after such damage is controversial. Several studies, in part using genetic fate mapping techniques, showed that tubular cell regeneration after ischaemia–reperfusion injury is predominantly, if not completely, accomplished by intrinsic, surviving tubular cells, without the contribution of extrarenal and extratubular cells [1–4]. These studies could not distinguish whether regeneration relies on an intratubular progenitor- or stem-cell subpopulation or whether any surviving tubular cell has the potential to divide. An experimental study in murine ischaemia–reperfusion injury using timed metabolic labelling suggested that the proliferation of proximal tubule cells after injury cannot be attributed to a specified pre-existing tubular progenitor cell population. Co-staining for tubular cell injury and differentiation markers suggested that any surviving proximal tubular epithelial cells may dedifferentiate and proliferate after injury [5].
In contrast, some studies have characterized and isolated a distinct cell population from the proximal tubules [6–8]. These cells were characterized by expression of CD24 and/or a glycosylated isoform of CD133. CD24 is a glycosyl phosphatidylinositolanchored protein [9] expressed on immature cells [10] and usually absent from cells that have reached their final differentiation stage. CD133 is a pentaspanning transmembrane glycoprotein localizing to cellular protrusions. CD133 is widely expressed in immature cells but also in differentiated epithelial cells. In contrast, specific human glycosylated epitopes (CD133/1 and CD133/2) have been reported to be expressed only in immature cells, such as haematopoietic stem/progenitor cells, tissue-specific progenitor cells, and developing intestinal and neuronal cells [11–14]. Since CD24 and CD133 are characteristically associated with immature cells, it was proposed that the CD24- and CD133-positive cells in the proximal tubules represented a progenitor population. In addition, Lindgren et al showed sphere formation in vitro, which has also been observed with stem cells in culture [7], and Angelotti et al showed that these cells displayed resistance to apoptotic stimuli and when injected in models of tubular injury, exerted regenerative potential [8]. However, Kim et al found that only a small number of the CD133-positive tubular cells expressed the proliferation marker PCNA [6]. Therefore, the significance of these cells in tubular regeneration is still unclear. The aim of this study was to perform a detailed analysis of the previously described CD24- and CD133-positive proximal tubular cells. Using human biopsies, we examined the role of this population in tubular regeneration. In addition, we studied the origin of the scattered cells in rat kidneys.
Materials and methods
Patient material (see the Supporting information for details)
Macroscopically normal kidney tissue was obtained from the nephrectomized kidneys of five patients with renal cell carcinoma and snap-frozen in liquid nitrogen. We also studied six frozen biopsies of patients with reperfusion injury after kidney transplantation and two frozen nephrectomy specimens of the transplant kidneys of two patients with recurrent primary focal segmental glomerulosclerosis (FSGS). In addition, we studied the kidney biopsy specimens of four patients who had anti-neutrophil cytoplasmic autoantibody (ANCA)-positive crescentic glomerulonephritis. The experiments were approved by the Local Ethical Committee.
Electron microscopy
Small fragments of the kidney biopsies were immersion-fixed in 2.5% glutaraldehyde dissolved in 0.1 m sodium cacodylate buffer, pH 7.4, overnight at 4°C and washed in the same buffer. The tissue fragments were post-fixed in Palade buffered 1% OsO4 for 1 h, dehydrated, and embedded in Epon812 (Merck, Darmstadt, Germany). Ultrathin sections were used and contrasted with 4% uranyl acetate for 45 min and subsequently with lead citrate for 4 min at room temperature. Sections were examined in a JEOL 1200 EX2 electron microscope (JEOL, Tokyo, Japan).
Immunoelectron microscopy
Tubular CD24 expression was examined by indirect immunoelectron microscopy (IEM), using immunoperoxidase labelling on 20 µm frozen sections. One-millimetre-thick kidney slices were immersion-fixed in a mixture of 10 mm periodate, 75 mm lysine, and 2% paraformaldehyde, pH 6.2 (PLP), for 3 h. The slices were washed in PBS for 30 min and cryoprotected by immersion in 2.3 m sucrose solution for 1 h. Finally, tissues were snap-frozen in liquid nitrogen. Cryosections (20 µm) were rinsed in PBS for 1 h and then incubated with the anti-CD24 mAb diluted in PBS containing 1% bovine serum albumin (BSA) for 18 h at 4°C, followed, after three washes with PBS, by incubation with a peroxidase-labelled rabbit anti-mouse IgG (Dako, Glostrup, Denmark) diluted in PBS containing 1% BSA. After three washes in PBS, the sections were incubated in PBS, pH 7.4, containing diaminobenzidine (DAB) medium for 10min, followed by DAB with the addition of 0.003% H2O2 for 7 min. The sections were washed in distilled water, post-fixed in Palade buffer containing 1% OsO4 for 30min at 4°C, dehydrated, and embedded in Epon812 (Merck). Ultrathin sections were examined in a JEOL 1200 EX2 electron microscope (JEOL).
Immunofluorescence
For immunofluorescence (IF), 2 µm acetone-fixed cryostat sections and 4 µm paraffin sections were cut from the snap-frozen human kidney specimens and methyl Carnoy’s solution-fixed rat kidneys, respectively. The sections were incubated with the primary antibodies listed in Table 1 diluted in PBS containing 1% BSA for 45 min. To detect the mouse and rabbit primary antibodies, the sections were incubated with goat anti-mouse Alexa-488 or -546 and goat anti-rabbit Alexa-488 or -546 (Molecular Probes, Leiden, The Netherlands), respectively. The secondary antibodies were diluted in PBS containing 1% BSA and incubated for 30 min. The goat anti-CXCR4 and goat anti-KIM-1 primary antibodies were detected with a donkey anti-goat Dylight549 (Dianova, GmbH, Hamburg, Germany). For the double immunofluorescence staining of CD24 (SN3) and CD133 (clone 293C3), the following subclass-specific secondary antibodies were used: goat anti-mouse IgG1 Alexa-488 and goat anti-mouse IgG2b-Alexa-546, respectively. CD133 and annexin 3 were also stained on 4 µm formalin-fixed paraffin sections. To this end, the paraffin sections were deparaffinized and antigen retrieval was performed by heat induced epitope retrieval (HIER) in Tris–EDTA buffer, pH 9. CD133 was detected by the indirect immunoperoxidase procedure as described previously [15].
Table 1.
Primary antibodies
| Antibody/clone | Host species | Reactivity | Dilution | Supplier/reference |
|---|---|---|---|---|
| Gravin/AKAP12 | Rabbit pAb | m, r | 1 : 100 | Irwin H Gelman Laboratory |
| Annexin A2/C-10 | Mouse | m, r, h | 1 : 100 | Santa Cruz Biotechnology, CA, USA |
| Annexin A3 | Rabbit | Human | 1 : 100 | Sigma-Aldrich, St Louis, MO, USA |
| Aquaporin 1 | Rabbit pAb | m, r,h | 1 : 50 | Alpha Diagn Int, San Antonio, TX, USA |
| Aquaporin 2 | Rabbit pAb | r, h | 1 : 100 | Abcam, Cambridge, UK |
| Calbindin | Rabbit pAb | m, h | 1 : 50 | Abcam, Cambridge, UK |
| CD133 (AC133) | Mouse mAb | Human | 1 : 10 | Miltenyi Biotec GmbH |
| CD133/2 (293C3) | Mouse mAb | Human | 1 : 10 | Miltenyi Biotec GmbH |
| CD24 | Mouse mAb | Human | 1 : 20 | Santa Cruz Biotechnology, CA, USA |
| CD44/(156-3C11) | Mouse mAb | Human | 1 : 100 | Abcam, Cambridge, UK |
| CD44 (OX-50) | Mouse mAb | Rat | 1 : 100 | AbDSerotec, Düsseldorf, Germany |
| Claudin-1 | Rabbit pAb | m, r, h | 1 : 50 | Abcam, Cambridge, UK |
| Claudin-3 | Rabbit pAb | m, r, h | 1 : 50 | Thermo scientific, Kalamazoo, MI, USA |
| CXCR4 | Goat pAb | m, r, h | 1 : 10 | Santa Cruz Biotechnology, CA, USA |
| Integrin αV | Rabbit pAb | Human | 1 : 50 | Sigma-Aldrich, St Louis, MO, USA |
| KIM-1 | Goat pAb | Human | 1 : 100 | R&D Systems, Inc, Minneapolis, MN, USA |
| KIM-1 | Goat pAb | Rat | 1 : 200 | R&D Systems, Inc, Minneapolis, MN, USA |
| LTA-FITC | Lectin | m, r, h | 1 : 100 | Vector Laboratories, Burlingame, CA, USA |
| Megalin | Rabbit pAb | Human | 1 : 50 | Sigma-Aldrich, St Louis, MO, USA |
| Ki-67/Mib-1 | Mouse mAb | Human | 1 : 100 | Dako, Glostrup, Denmark |
| S100A6 | Rabbit pAb | Human | 1 : 50 | Sigma-Aldrich, St Louis, MO, USA |
| SSeCKS | Rabbit pAb | m, r | 1 : 100 | Irwin H Gelman Laboratory |
| Tamm–Horsfall protein | Rabbit pAb | m, r, h | 1 : 50 | Santa Cruz Biotechnology, CA, USA |
| Vimentin | Rabbit pAb | Human | 1 : 50 | Sigma-Aldrich, St Louis, MO, USA |
| Vimentin/V9 | Mouse mAb | r, h | 1 : 100 | Dako, Glostrup, Denmark |
m = mouse; r = rat; h= human; mAb = monoclonal antibody; pAb = polyclonal antibody.
Quantification of CD24/LTA/Ki-67-positive cells
To quantify the relative number of proliferating cells expressing CD24, triple immunostaining for CD24, the proliferation marker Ki-67, and the brush border with FITC-labelled LTA lectin was performed. The numbers of Ki-67-positive cells in proven proximal tubuli (showing segmental positivity for the LTA-FITC lectin) that were either positive or negative for CD24 were counted.
Experimental model and experimental design
The animal experiments were approved by the local review boards. Wistar rats (Jackson Laboratory, Bar Harbor, ME, USA) were housed under constant temperature and humidity, 12 h/12 h daylight cycle, with ad libitum access to drinking water and food. Young 7-week-old male Wistar rats (n = 10) underwent unilateral ureteral obstruction (UUO), inducing tubular cell injury and tubulointerstitial fibrosis. Five days after UUO, the rats were sacrificed and the obstructed kidney that underwent UUO and the healthy contralateral kidneys were sampled from each rat. In addition, the kidneys from five 34-week-old rats (four female and one male) were sampled. The kidneys were fixed in methyl Carnoy’s solution and embedded in paraffin.
Vimentin immunostaining and quantification
To assess the number of proximal tubular cells that express vimentin in the control and UUO kidneys, immunostaining for vimentin was performed. The indirect immunoperoxidase procedure was performed as described previously [15]. The 4-µm-thick paraffin sections were incubated with the anti-vimentin (V9) antibody (Dako). Evaluation of the vimentin immunostaining was performed on blinded sections. Fifteen microscopic images, with a 20× objective, were taken in the cortical region of each kidney. Within these images, the number of vimentin-positive cells was counted. Only those vimentin-positive cells that were present in proximal tubules were counted. The values are expressed as means ± SD. For multiple comparisons, ANOVA was used and post-hoc analysis was carried out with Tukey’s test. A value of p < 0.05 was considered statistically significant.
Results
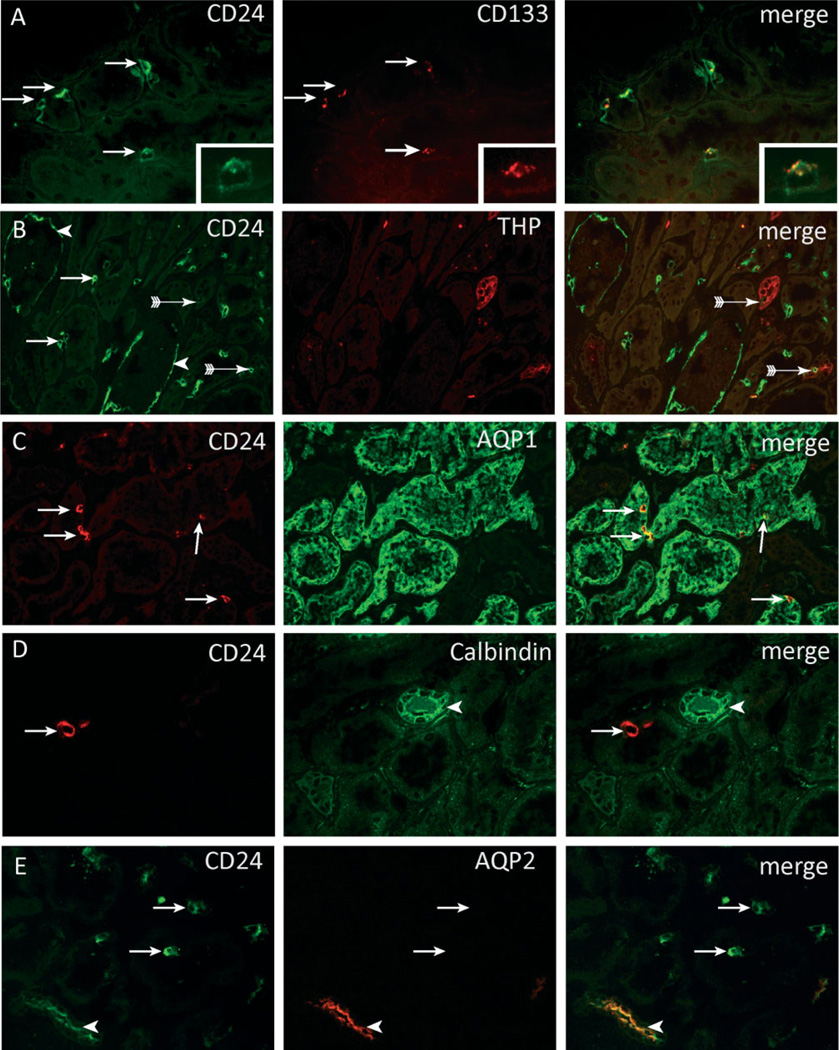
Using immunofluorescence staining in normal human kidneys, we could detect tubular expression of CD24 and CD133. Co-staining of CD24 and CD133 showed a complete overlay, although CD133 expression was weaker and sometimes more difficult to detect (Figure 1A). In addition to tubular staining, we also detected expression of both cell surface markers on the parietal epithelial cells of Bowman’s capsule as reported previously (Figure 1B, arrowhead) [16]. CD24-positive cells were scattered as single cells or in small groups of 2–4 cells throughout the kidney cortex (Figures 1A–1E, arrows). We used double immunofluorescence staining for CD24 and markers specific for different segments of the nephron to localize these cells within the different nephron segments. These cells were predominantly localized within the proximal tubules, as demonstrated by co-localization of CD24 and aquaporin-1, a marker expressed by the proximal tubules and the thick descending limb of the loop of Henle (Figure 1C, arrows). Only very few cells were seen in the thick ascending limb, as defined by positive staining for the Tamm–Horsfall glycoprotein (THP) (Figure 1B, arrows with tails). We did not observe co-localization in the distal convoluted or connecting tubules, which stain for calbindin (Figure 1D). The collecting ducts, expressing aquaporin-2, showed significant co-localization with CD24 (Figure 1E). This was not in a scattered pattern, but all the aquaporin-2-positive collecting duct cells showed apical CD24 staining.
Figure 1.
Localization of CD24- and CD133-positive cells within the kidney. (A – E) Within normal human kidney, CD24-positive cells were scattered as single cells or in small groups of 2 – 4 cells throughout the kidney cortex (arrows). The CD24-positive cells in proximal tubuli co-expressed the cell surface marker CD133 (A, arrows). The insets show a higher magnification of a CD24- and CD133-positive cell. CD24 is expressed on both the basal and the apical membrane. CD133 is expressed on the apical membrane. CD24 expression was also observed in parietal cells (PECs) on Bowman’s capsule (B, arrowheads). Co-staining with CD24 and THP showed rare cells that expressed both markers (B, arrows with tail). The majority of the CD24-positive cells were present in aquaporin-1-positive proximal tubular structures (C, arrows). No co-localization was observed between calbindin (D, arrowhead) and CD24. Aquaporin-2-positive collecting ducts cells showed apical staining for CD24 (E, arrowhead).
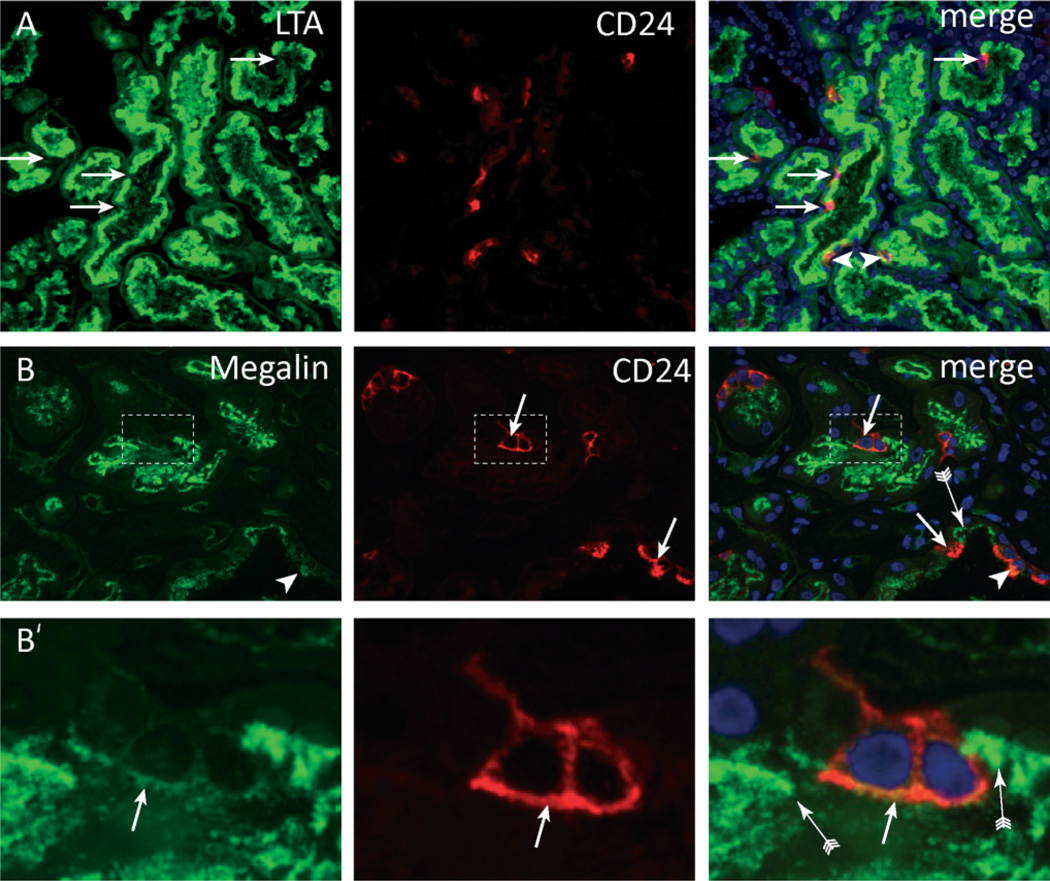
To determine whether these are distinct and functionally different cells, the presence of markers characteristic for a differentiated functional proximal tubular cell was assessed. The brush border was visualized using the lectin Lotus tetragonolobus agglutinin (LTA) and the protein receptor megalin. The vast majority (approximately 70%) of CD24-positive cells did not stain with LTA (Figure 2A) and showed no or only weak expression of megalin (Figures 2B and 2B’). Only a few CD24-positive cells were positive for LTA and showed expression of megalin (Figure 2, arrow-heads). In comparison, strong staining of both markers was detected on the neighbouring CD24-negative proximal tubular cells.
Figure 2.
CD24-positive cells lack a brush border. Double staining for (A) Lotus tetragonolobus lectin (LTA) and (B) megalin showed that most CD24-positive cells lacked expression of these brush border molecules (arrows). (B’) Magnifications of panel B showing that the CD24-positive cells show no or weak staining for megalin. Neighbouring CD24-negative cells were megalin-positive (B, B’, arrow with tail). A small number of CD24-positive cells showed a normal LTA or megalin-positive brush border (A, B, arrowheads).
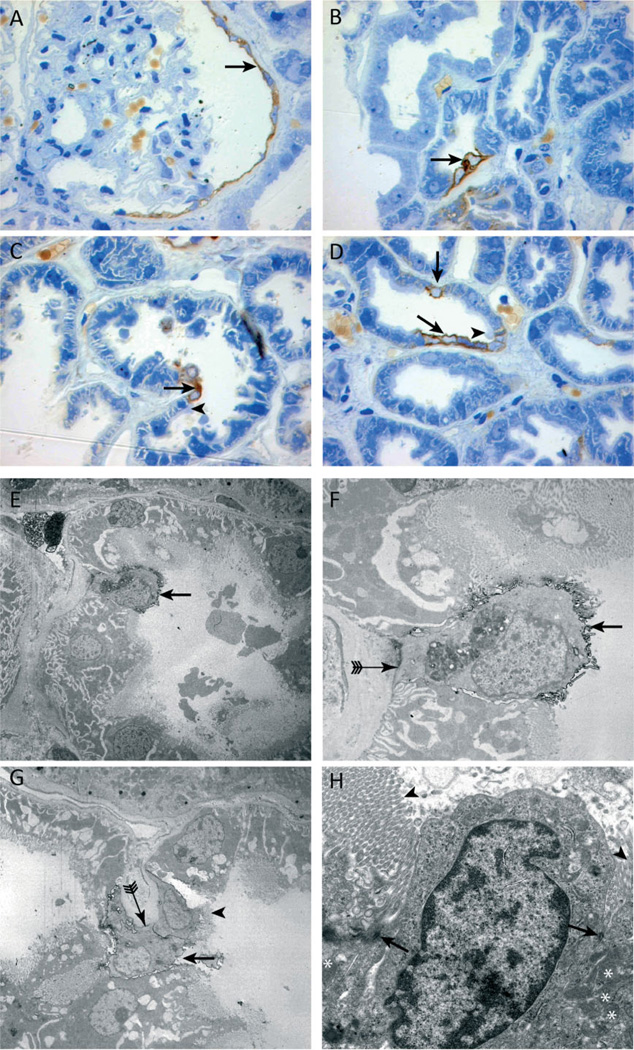
For a detailed ultrastructural morphological analysis of these cells, immunostaining of semi-thin kidney sections (Figures 3A – 3D) in combination with (immuno)-electron microscopy was performed (Figures 3E–3H). Compared with the neighbouring CD24-negative proximal tubular cells, CD24-positive cells were less differentiated, showing less cytoplasm, fewer mitochondria, lack of basolateral invaginations (Figures 3F and 3G, arrow with tails), and lack of a mature brush-border (Figures 3E–3G, arrows), which showed an abrupt stop at the adjoining CD24-negative cells (Figure 3H, arrowhead). CD24-positive cells were preferentially localized at the tips of intraluminal protrusions or within the inner aspect of narrow bends of tubules where the cells may be exposed to increased mechanical stress. Importantly, desmosomes with neighbouring differentiated tubular cells were still preserved (Figure 3H, arrows).
Figure 3.
Ultrastructural analysis of CD24 immunolabelled cells in semi-thin and ultrathin sections. (A – D) CD24 (brown staining) is expressed by the parietal epithelial cells of Bowman’s capsule (A, arrow) and a few proximal tubular cells (B – D, arrows). An abrupt termination of the brush border was visible on cells adjacent to the CD24-positive cells (C, D, arrowheads). Immunoelectron (E – G) and transmission electron microscopy (H). The morphology of CD24-positive cells (black outlining, E – G, arrows) differed from that of normal proximal tubular cells. CD24-positive cells contained less cytoplasm, fewer mitochondria, and the brush border was mostly rudimentary or absent compared with the brush border of adjacent CD24-negative cells (G, H, arrowheads). Of note, some CD24-positive cells contained a brush border. Panel G shows two CD24-positive cells adjacent to each other, one of which lacks a brush border (G, arrow) and the other does not (G, arrowhead). In addition, the basolateral membrane lacked the typical invaginations (F, G, arrow with tail). Electron microscopy revealed desmosomes between the two morphologically different proximal tubular cells (H, arrowheads).
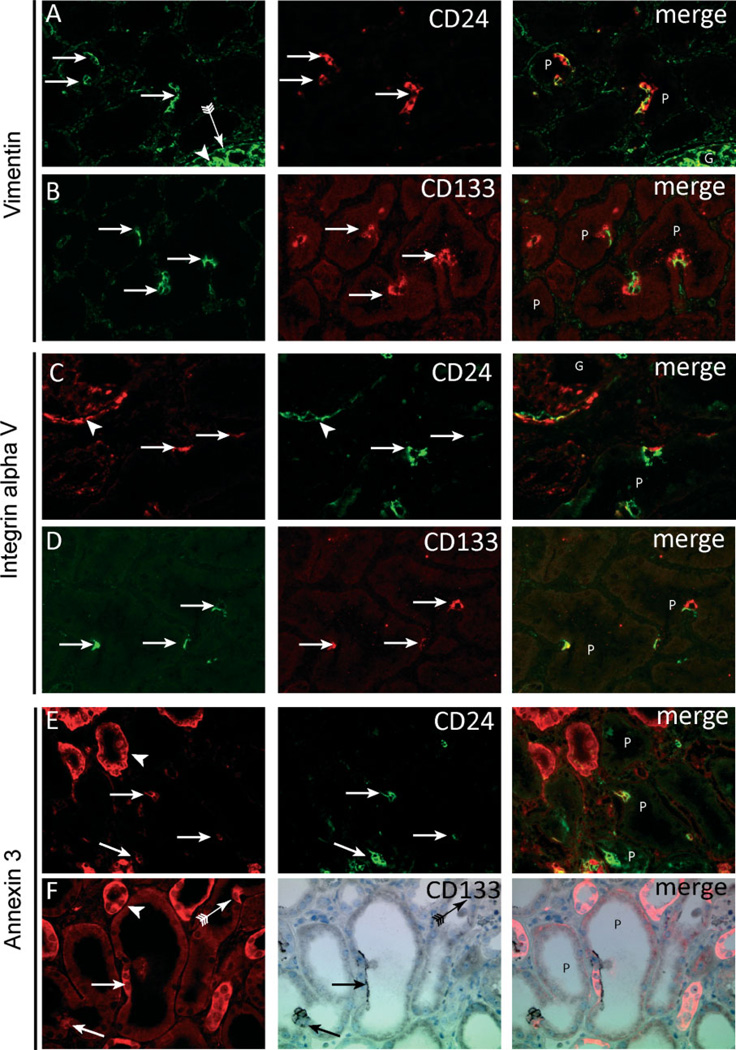
Next, markers previously reported to be expressed by dedifferentiated proximal tubular cells were tested. CD24- (Figure 4A) and CD133 (Figure 4B)-positive proximal tubular cells showed complete co-localization with vimentin, supporting the notion that these cells are the same as those described by Lindgren et al [7]. In addition, the cells expressed other markers associated with tubular cell injury/regeneration such as integrin αV (Figures 4C and 4D) and annexin 3 (Figures 4E and 4F). A review of the literature and the protein expression database The Human Protein Atlas (http://www.proteinatlas.org) [17] resulted in a list of an additional 46 proteins (Table 2) that are expressed in a scattered pattern throughout the proximal tubules of normal human kidneys (Supplementary Figure 1). Costaining of CD24 for a subset of these proteins (Table 2) revealed co-expression with CD24, ie annexin 2 (not shown), claudin-7 (not shown), S100A6, claudin-3, and CXCR4 (Supplementary Figures 2A–2C). In addition, AKAP12 co-localized with vimentin (Supplementary Figure 2D).
Figure 4.
CD24- and CD133-positive cells exhibit distinct marker expression. In contrast to neighbouring cells, CD24- and CD133-positive proximal tubular cells (A – F, arrows) show expression of vimentin (A, B), integrin αV (C, D), and annexin 3 (E, F). Vimentin (A, B) was strongly expressed by podocytes (A, arrowhead) and parietal epithelial cells (A, arrow with tail) but also showed co-localization in the CD24- and CD133-positive proximal tubular cells (A, B, arrows, respectively). Integrin αV (C, D) was expressed by the parietal cells (C, arrowhead) and the CD24- and CD133-positive proximal tubular cells (C, D, arrows, respectively). Annexin 3 (E, F) was expressed in distal tubuli (arrowhead) and in the CD24- and CD133-positive proximal tubular cells (arrows). The arrow with tail in panel F shows an annexin 3 cell negative for CD133. G = glomerulus; P = proximal tubules.
Table 2.
Markers expressed by scattered proximal tubular cells
| Gene | Full name or synonym | Co-staining with CD24 or CD133/reference (s) |
|---|---|---|
| 14-3-3 theta | ||
| AKAP5 | A-kinase anchor protein 5 | |
| AKAP12 | A-kinase anchor protein 12 | |
| Annexin A2 | Yes | |
| Annexin A3 | Yes | |
| Annexin A5 | ||
| Annexin A11 | ||
| Annexin A13 | ||
| Bcl2 | B-cell lymphoma 2 | [7] |
| CD146 | Melanoma cell adhesion molecule | |
| CD24 | Heat stable antigen CD24 | |
| c-FOS | ||
| CD44 | ||
| Claudin 1 | ||
| Claudin 3 | Yes | |
| Claudin 4 | ||
| Claudin 7 | Yes | |
| CSF-3 | Colony stimulating factor 3 | |
| CXCR4 | Fusin | |
| Cyclin D1 | ||
| Cyclin E1 | ||
| E-cadherin | Epithelial cadherin | |
| EGF receptor pathway substrate 8 | ||
| hspB1 | Heat shock protein 27 | |
| hspB5 | Crystallin, alpha B | |
| hspB6 | Heat shock protein 20 | |
| IKBKA | l-kappa-B-kinase alpha | |
| IKBKA | l-kappa-B-kinase beta | |
| Integrin alpha V | CD51 | Yes |
| Keratin 7 | Cytokeratin 7 | [7] |
| Keratin 18 | Cytokeratin 18 | |
| Keratin 19 | Cytokeratin 19 | [7] |
| NEDD4 | Neuronal precursor cell-expressed developmentally downregulated 4 | |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | |
| NFKB2 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2 | |
| NFKBIE | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, epsilon | |
| Osteopontin | Secreted phosphoprotein 1 | |
| OSM | Oncostatin M | |
| OSMR | Oncostatin M receptor | |
| Pax-2 | Paired box gene 2 | |
| RGS4 | Regulator of G-protein signalling 4 | |
| RELA | Transcription factor p65 | |
| S100A6 | S100 calcium binding protein A6 | Yes |
| Scinderin-1 | ||
| Sox9 | SRY-box containing gene 9 | |
| STAT1 | Signal transducer and activator of transcription 1 | |
| Delta-9-desaturase | Stearoyl-CoA desaturase | |
| VCAM-1 | Vascular cell adhesion molecule 1 | Yes |
| Vimentin | Yes/[7] | |
| Zonab | ZO-1-associated nucleic acid binding protein |
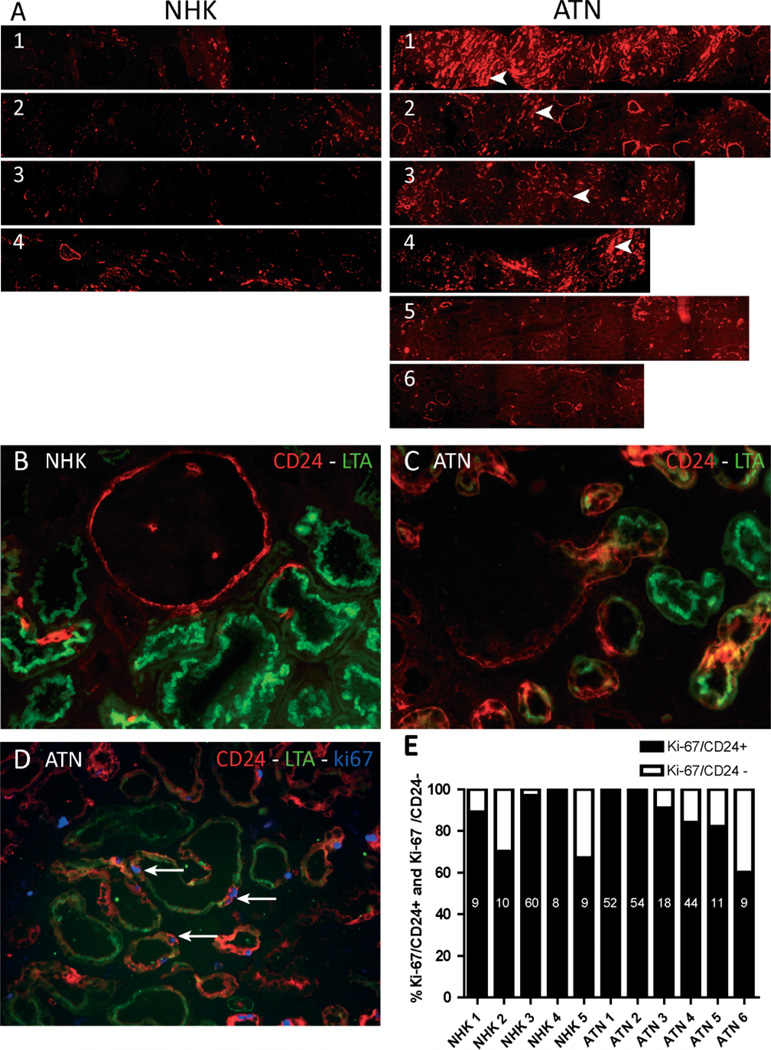
To investigate whether the CD24-positive cells are involved in cellular regeneration after acute injury, the expression of CD24 was studied in six biopsies of patients with acute kidney injury due to ischaemia–reperfusion injury after transplantation (designated ATN 1–6). By light microscopy, four of the biopsies showed marked injury of the tubular cells in periodic acid Schiff (PAS) staining (Supplementary Figure 2A, ATN 1–4), whereas the morphology was relatively preserved in biopsies 5 and 6 (Supplementary Figure 3A, ATN 5 and 6). The difference in the extent of tubular injury between ATN 1–4 and ATN 5 and 6 was confirmed by the difference in the expression of kidney injury molecule-1 (KIM-1), a marker for proximal tubular injury [18,19], as shown for ATN1 and ATN6 (Supplementary Figure 3B), and by the serum creatinine levels and urinary protein to creatinine ratios (Supplementary Table 1). The CD24 staining pattern in the ATN biopsies was compared with normal human kidneys (designated NHK 1–5). The ATN biopsies showed focal areas with a marked increase in the number of CD24-positive tubular cells (Figure 5A, ATN 1–4, arrowheads). Quantitative assessment of the increased number of CD24-positive cells in the ATN biopsies proved to be difficult due to this focal aspect. To illustrate the quantitative differences, with non-biased images, the entire biopsy is shown in one scan. The ATN biopsies contained large foci or even complete tubular cross-sections positive for CD24 (Figures 5B and 5C). CD24 staining was markedly increased in ATN patients with severe morphological tubular damage. In contrast, CD24 staining in the patients with limited injury was comparable to the staining pattern in the NHK sections. Importantly, increased expression of CD24 and CD133 was also observed in the tubules of patients with proteinuric glomerular diseases [ANCA-associated glomerulonephritis (not shown)] and recurrent primary FSGS (Supplementary Figure 4).
Figure 5.
Increased number of CD24-positive cells in acute tubular necrosis. (A) Comparison of microscopic scans of whole biopsies of normal human kidneys (NHK) with biopsies of acute tubular necrosis (ATN) showed that the number of CD24-positive tubular structures was increased in most (1 – 4) of the ATN biopsies. The low CD24 expression in 5 – 6 correlated with the relative mild necrosis visible in the PAS staining of these biopsies and the relatively low serum creatinine levels. The increased number of CD24-positive cells in ATN (C) compared with NHK (B) correlated with a high number of proliferating cells in the ATN biopsies (D, Ki-67-positive cell). (D) Triple staining of CD24, LTA, and Ki-67 showed that Ki-67 was expressed predominantly in CD24-positive proximal tubular cells (arrows). (E) Quantitative Ki-67, CD24, and LTA triple staining. The bars show the relative percentage of Ki-67-positive cells that are either CD24-positive (black bar) or CD24-negative (grey bar). The vast majority (~70 – 100%) of proliferating cells in both NHK and ATN biopsies were CD24-positive. Data labels: number of Ki-67 cells analysed in each biopsy.
To evaluate the role of the CD24-positive proximal tubular cells in regeneration, we tested whether the proliferating cells in acute kidney injury and control biopsies were CD24-positive using triple staining for the proliferation marker Ki-67 (MIB-1), CD24, and the proximal tubular marker LTA. In fact, the vast majority of proliferating cells were CD24-positive (Figure 5D, arrows). Quantitative assessment of the triple staining revealed that on average, 84 ± 15% of the proliferating cells in normal human kidney and 86 ± 15% in the ATN biopsies were CD24-positive (Figure 5E).
The cells characterized in this study can be a preexisting population that is always present or, alternatively, these cells could be derived from mature differentiated proximal tubular cells. Unfortunately, ‘normal’ renal tissue from tumour nephrectomies of patients with normal renal function (tissue samples were taken away from the neoplastic lesion) still showed foci of acute and chronic tubular injuries by light microscopy. These areas were characterized by dilated and/or atrophic tubuli with or without mild interstitial fibrosis. Nevertheless, the increased presence of proliferating CD24-positive cells in ATN biopsies is evident.
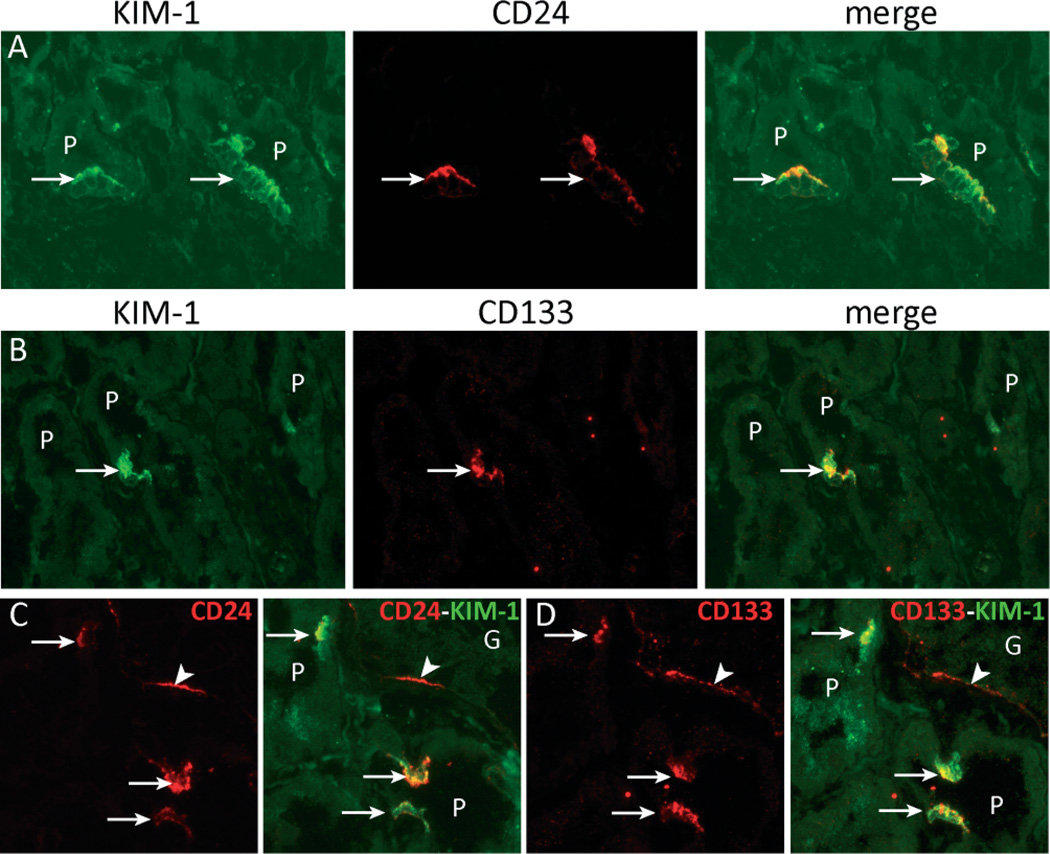
To determine whether the CD24- and CD133-positive cells in the ‘normal’ human kidney might be injured/regenerating proximal tubular cells, we performed double immunostaining for CD24 and CD133 with KIM-1. Both CD24 and CD133 co-localized with KIM-1, arguing for tubular cell injury (Figure 6).
Figure 6.
CD24- and CD133-positive proximal tubular cells express kidney injury molecule-1 (KIM-1). (A – D) Double immunofluorescence staining showing co-localization between CD24 or CD133 and KIM-1 in the CD24- and CD133-positive proximal tubule cell population (arrows). Panels C and D are serial sections stained for CD24/KIM-1 and CD133/KIM-1, respectively. CD24 and CD133 are expressed by the cells of Bowman’s capsule (arrowhead) and by the same KIM-1-positive proximal tubule cells (arrows).
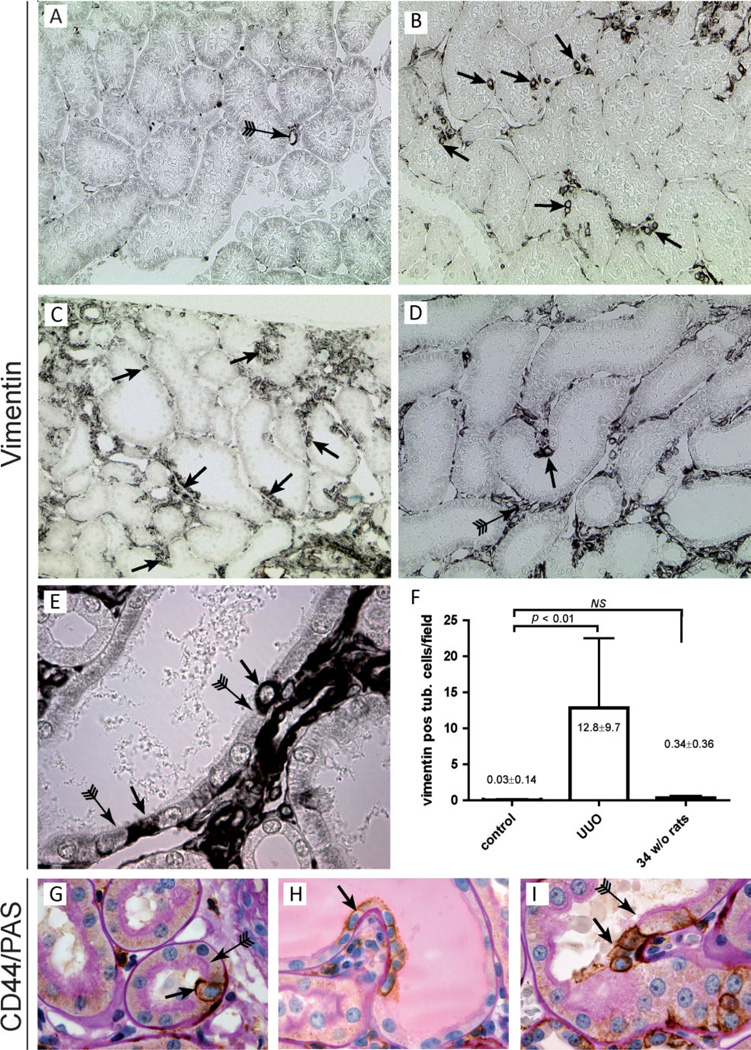
To further assess whether these cells are pre-existing or derived from normal tubular cells after tubular injury, healthy rat kidneys were first analysed. Since specific staining of CD24 and the glycosylated CD133 epitope was not possible in rodent tissues, immunohistochemistry for vimentin was performed, since this marker reliably labelled the CD24/CD133-positive cell population in tubuli of human kidneys. Unilateral ureteral obstruction (UUO) was performed in 7-week-old rats. First, we examined the contralateral control kidneys. Vimentin expression was present in podocytes, parietal cells, around peritubular capillaries, and in interstitial cells. However, the number of vimentin-positive proximal tubular cells was negligible (Figure 7A). Next, we assessed vimentin expression in the obstructed UUO kidneys. The morphology of the UUO kidneys was characterized by extensive tubular damage and regeneration with associated fibrosis. In the UUO kidneys, vimentin-positive cells were located in groups or as single cells throughout the tubular structures, frequently at the tips of intraluminal protrusions of the tubuli as already observed in human kidney (see above) (Figures 7B–7D, arrows). In areas with pronounced tubular injury, long stretches of vimentin-positive cells were observed. Similar to the CD24-positive cells in humans, the scattered vimentin-positive cells showed no or only a rudimentary brush border (Figure 7E, arrows). We confirmed these observations by quantification of the number of vimentin-positive proximal tubular cells in the obstructed and the uninjured contralateral kidneys of young rats (Figure 7F). The number of vimentin-positive tubular cells was also assessed in aged (34-week-old) rat kidneys. The morphology of these aged kidneys was still preserved and we did not detect a significant increase in the number of vimentin-positive tubular cells (Figure 7F).
Figure 7.
Detection of scattered vimentin- and CD44-positive tubular cells in the unilateral ureteral obstruction model (UUO). (A) Vimentin expression in the contralateral control kidney. Vimentin is exclusively expressed in the peritubular capillaries (10× magnification). (B – D) In kidneys that underwent UUO, de novo expression of vimentin was observed in proximal tubular cells. The cells were frequently scattered single cells or small groups of cells (arrows). Also in the interstitium, the expression of vimentin was increased (D, arrow with tail). (B) 20×, (C) 10×, and (D) 20× magnification. (E) Higher magnification (60×) of a proximal tubule showing scattered vimentin-positive cells with an undeveloped brush border (arrows) juxtaposed to differentiated proximal tubular cells with a normal brush border (arrows with tail). (F) Quantitative analysis of the number of vimentin-positive tubular cells per microscopic field (20× magnification) in the contralateral and UUO kidneys of 7-week-old rats and in the kidneys of aged 34-week-old rats. (G – I) CD44/PAS co-staining shows single or small groups of CD44-positive tubular cells with an undeveloped brush border (arrows) juxtaposed to differentiated proximal tubular cells with a normal brush border (arrows with tail) (60× magnification).
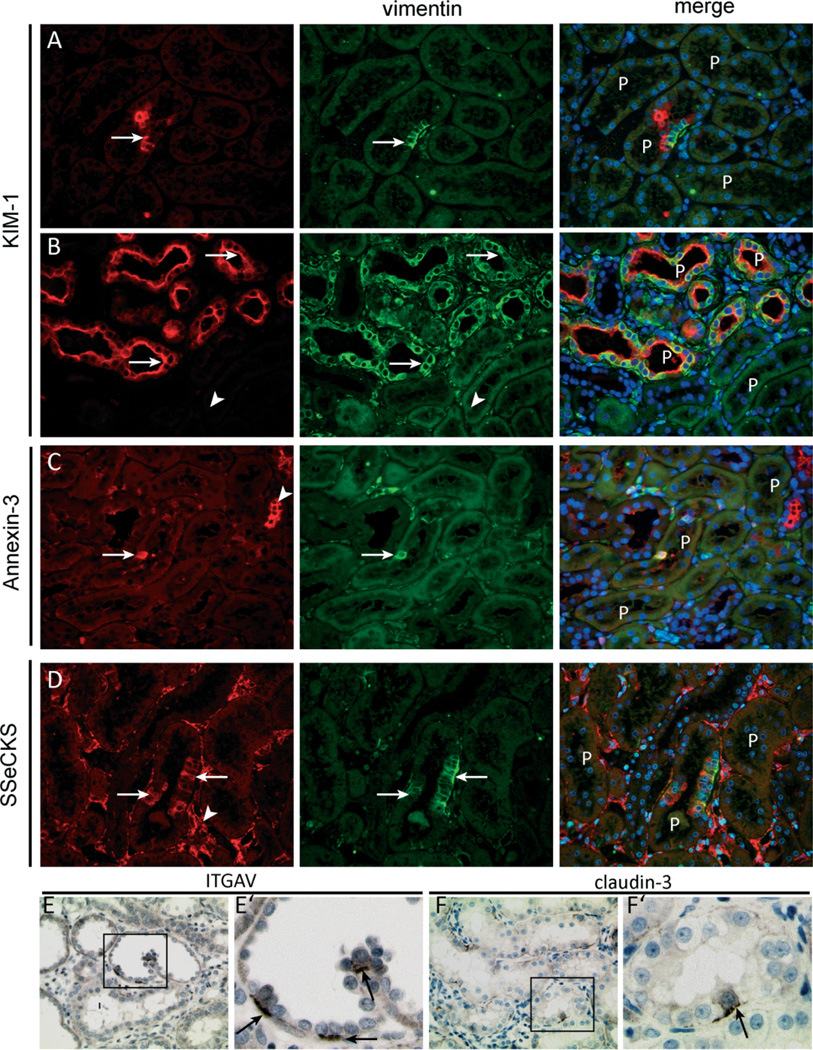
A similar staining pattern was observed in the tubuli of rat kidneys stained for CD44. Normal rat kidneys showed no tubular staining for CD44. In contrast, in affected tubuli, single cells or small groups of cells expressed de novo CD44. Like the vimentin-positive cells, these cells mostly lacked a brush border and were again frequently found at the tips of intraluminal protrusions of the tubuli (Figures 7G–7I). To assess whether the vimentin-positive cells observed in the injured rat kidneys were similar to the CD24-, CD133-, and vimentin-positive cells found in the human kidneys, we performed co-immunostaining for vimentin with markers found also on the cells in human kidneys, ie KIM-1, annexin 3, and SSeCKS, the rodent orthologue of human AKAP12. Like the CD24-, CD133-, and vimentin-positive cells in human kidneys, we observed co-staining for vimentin with KIM-1, annexin 3, and SSeCKS (Figures 8A–8D). In addition, integrin-αV and claudin-3 were, as observed in the human kidney, expressed basolaterally by proximal tubular cells that were interspersed between integrin-αV- and claudin-3-negative cells (Figures 8E and 8F).
Figure 8.
Vimentin-positive proximal tubular cells exhibit distinct marker expression. (A, B) The vimentin-positive cells in the injured rat kidneys co-expressed KIM-1. Vimentin and Kim-1 co-expression was seen in single cells or small groups of cells (A, arrow) but also in complete proximal tubular structures in segments of the kidney with more advanced tubular injury (B, arrows). Adjacent normal-appearing tubuli were mostly negative for both markers (B, arrowhead). (C) Within proximal tubuli, vimentin co-localized with annexin 3 (arrow). Annexin 3 was also expressed in other tubular structures (arrowhead). (D) Vimentin-positive proximal tubular cells co-expressed SSeCKS (arrows), which was also expressed by endothelial cells (arrowhead) and parietal cells (not shown). (E, F) Within the affected rat kidneys, both integrin αV and claudin-3 were expressed by a few proximal tubuli in a scattered pattern. E’ and F’ show a zoomed picture of the insets in E and F, respectively, and show mostly basolateral staining of both markers in a subset of the proximal tubular cells.
Discussion
Our data describe four major findings. First, CD24 expression within normal human kidney revealed a scattered population of proximal tubular cells with a distinct phenotype at light-microscopic and ultrastructural levels. Based on the localization within the kidney and co-expression of CD24, CD133, and vimentin, we propose that this proximal tubular cell population is identical to that described previously by Moll et al [20] and Kim et al [6] and which was recently isolated by Lindgren et al [7] and Angelotti et al [8].
Second, we showed that the CD24-positive cells within proximal tubules are the major proliferating cell population and thereby involved in the regeneration of injured proximal tubular cells. Only a small fraction of the CD24-positive cells express the proliferation marker Ki-67. This finding is similar to that described by Kim et al [6], who showed that the number of tubular cells co-expressing CD133 and Ki-67 was low. Nonetheless, when analysing only the proliferating cells, the vast majority of the proliferating cells co-expressed CD24.
Third, we showed that the different morphological appearance is associated with a different expression pattern of several proteins. We present 49 markers that are expressed within the proximal tubules exclusively by the scattered cells (Table 2 and Supplementary Figure 1). Co-staining of CD24 with a subset of these markers (ie vimentin, integrin αV, annexin 2, annexin 3, claudin-3, claudin-7, CXCR4, S100A6, and AKAP12) confirmed that they recognize one and the same cell population. The identification of these new markers in these cells is of great importance and could facilitate further studies on the cellular and molecular processes involved in tubular regeneration.
Fourth, based on the similarity in morphology, localization, and marker expression between the CD24/CD133-positive human cells and the vimentin-positive rat cells, we conclude that they resemble the same distinct proximal tubular cell population. In healthy rats, this population is virtually absent in healthy kidneys – independent of age. Instead, they only appeared after tubular injury induced by UUO. This finding strongly argues against the notion that the scattered cell population is a pre-existing (progenitor) cell subpopulation and is in line with the findings of Humphreys et al [5] and Vogetseder et al [2]. Both studies showed that the proliferation capacity of the renal proximal tubule involves the bulk of proximal tubular epithelial cells [2,5]. In addition, Humphreys et al showed that after injury, new epithelial cells arise from replication of the surviving cells which were often injured and dedifferentiated, indicated by de novo KIM-1 and reduced basolateral Na/K/ATPase staining, respectively [5]. Vogetseder et al showed that many proximal tubular cells in the S3 segment were in the G1 phase (cyclin D1-positive) and represented a reserve that stays ready to undergo division if proliferation is urgently required [2]. This would implicate a tight control of the G1→S cell cycle progression. In this regard, the expression of SSeCKS/AKAP12 in the scattered proximal tubular cells in both human and rat kidneys is of interest. SSeCKS/AKAP12 regulates G1→S progression by controlling the expression and cellular compartmentalization of cyclin D [21,22].
Are there additional arguments that the scattered cell population is simply derived from proximal tubular cells that dedifferentiated in order to replicate? In this study, we showed that the scattered cells have a simplified morphology similar to those cells described in many morphological studies on proximal tubular injury and regeneration [23–26]. Based on these studies, this phenotype is a result of the loss of the brush border due to shedding and flattening and simplification of the cell. In addition, desmosomes typical for proximal tubular cells were still preserved. Not only the morphology, but also many of the markers expressed by the scattered cells have been associated with tubular regeneration in injured kidneys rather than with stem or progenitor cells. Markers such as CD44, osteopostin [27], oncostatin M and receptor [28], S100A6, annexin A2 [29], integrin αV, Pax2 [30], vimentin [26], Bcl2, and nFATc1 [31] have been shown to be up-regulated in humans as well in experimental models with tubular injury and regeneration.
We showed here that the expression of CD24 and CD133 was up-regulated in tubules of patients with acute kidney injury, but also in patients with proteinuria, such as idiopathic FSGS (Supplementary Figure 2) or crescentic glomerulonephritis. This expression pattern is similar to that of the above-mentioned markers of tubular injury and regeneration. This raises the question of whether the presence of markers such as CD24 and CD133 in proximal tubuli should be interpreted differently from the neo-expression of markers previously reported. In other words, both cell surface markers might be associated with de- or re-differentiation after tubular injury, rather than a progenitor cell function. Expression of CD24 and the glycosylated epitope of CD133 is not restricted to stem or progenitor cells, as we were able to show for CD24, which was expressed by all cells of the collecting duct and has been reported by others [10,32–36]. These markers, similar to other molecules such as intermediate filaments or certain transcription factors expressed in progenitor cells, are also expressed in other, often less-differentiated cell types after injury during regeneration. For instance, CD24 is expressed de novo in regenerating muscle fibres [33], and studies have proposed that CD133 can act as a stress marker [35,37]; for example, CD133 expression in human glioma cells was not necessarily related to the stem cell phenotype but rather a stress response. Similarly, stem cell factor (SCF) normally associated with progenitor properties was shown to be an important survival factor for injured proximal tubular epithelial cells [38].
Lindgren et al have isolated the CD24/CD133- positive cell population from the human kidneys and have demonstrated the characteristics of stem/progenitor cells in vitro, ie clonal expansion and sphere formation [7]. In addition, CD24/CD133 proximal tubular cells injected into SCID mice affected by acute tubular injury were able to engraft within the kidney, generate novel tubular cells, and improve renal function [8]. These properties were not shared by other differentiated tubular cells of the adult kidney. Since our study indicated that CD24 and CD133 proximal tubular cells are dedifferentiated cells, it is of interest whether cells that have been dedifferentiated towards a more mesenchymal phenotype have different characteristics in such assays and may be mistaken for stem/progenitor cells.
Why do we find this scattered population in normal human kidneys and not in healthy rats? The most likely explanation is that the normal kidneys from tumour nephrectomies were from older patients with a certain degree of benign nephrosclerosis and a mild tubular injury associated with the nephrectomy procedure. Previous studies have also shown that mild chronic and acute tubular injury can be present in normal human kidney [39,40]. In comparison, the analysed rat kidneys were from perfectly healthy specific pathogen-free animals. We found that the scattered cell population was even in aged rats, a very rare finding.
In summary, normal human kidney proximal tubuli contain scattered, less specialized cells which can be detected using the cell surface markers CD24 and CD133, but also other markers of tubular injury and regeneration such as vimentin. These cells participate in regeneration of the tubular cells, particularly after acute kidney injury. In the UUO rat model, these scattered cells were formed de novo, arguing against a pre-existing progenitor population.
Supplementary Material
Acknowledgments
This work was supported by TP17 SFB/Transregio 57 of the German Research Foundation (DFG; to JF and MJM), the German Ministry for Science and Education (BMBF, 01 GN 0804) and the eRARE Consortium ‘Rare-G’ (01 GM 1208A; to MJM), the Genzyme Renal Innovation Program (GRIP; to BS and MJM), the Dutch Kidney Foundation (C07.2245; to BS), and by a financial research grant from Deutsche Forschungsgemeinschaft (BO 3755/1-1; to PB and BS). JF and MJM are members of the SFB/Transregio 57 DFG Consortium ‘Mechanisms of Organ Fibrosis’.
Footnotes
No conflicts of interest were declared.
Author contribution statement
BS, PB, and KB conceived and carried out experiments. HD, PJ, and JB carried out the (immuno)- electron microscopic studies. SS and FM carried out experiments. IG provided the AKAP12 and SSeCKS antibodies. BS, JF, JV, JW, and MM were involved in the supervision of the study and writing the manuscript. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1.Vogetseder A, Palan T, Bacic D, et al. Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007;292:C807–C813. doi: 10.1152/ajpcell.00301.2006. [DOI] [PubMed] [Google Scholar]
- 2.Vogetseder A, Picard N, Gaspert A, et al. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol. 2008;294:C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys D, Valerius TM, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Song J, Czerniak S, Wang T, et al. Characterization and fate of telomerase-expressing epithelia during kidney repair. J Am Soc Nephrol. 2011;22:2256–2265. doi: 10.1681/ASN.2011050447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphreys BD, Czerniak S, DiRocco DP, et al. Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci U S A. 2011;108:9226–9231. doi: 10.1073/pnas.1100629108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim K, Park BH, Ihm H, et al. Expression of stem cell marker CD133 in fetal and adult human kidneys and pauci-immune crescentic glomerulonephritis. Histol Histopathol. 2011;26:223–232. doi: 10.14670/HH-26.223. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren D, Bostrom AK, Nilsson K, et al. Isolation and characterization of progenitor-like cells from human renal proximal tubules. Am J Pathol. 2011;178:828–837. doi: 10.1016/j.ajpath.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelotti ML, Ronconi E, Ballerini L, et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 9.Pierres M, Naquet P, Barbet J, et al. Evidence that murine hematopoietic cell subset marker J11d is attached to a glycosylphosphatidylinositol membrane anchor. Eur J Immunol. 1987;17:1781–1785. doi: 10.1002/eji.1830171216. [DOI] [PubMed] [Google Scholar]
- 10.Shirasawa T, Akashi T, Sakamoto K, et al. Gene expression of CD24 core peptide molecule in developing brain and developing non-neural tissues. Dev Dyn. 1993;198:1–13. doi: 10.1002/aja.1001980102. [DOI] [PubMed] [Google Scholar]
- 11.Corbeil D, Roper K, Hellwig A, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275:5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Hamazaki TS, Ohnuma K, et al. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 13.Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 14.Kemper K, Sprick MR, de Bree M, et al. The AC133 epitope, but not the CD133 protein, is lost upon cancer stem cell differentiation. Cancer Res. 2010;70:719–729. doi: 10.1158/0008-5472.CAN-09-1820. [DOI] [PubMed] [Google Scholar]
- 15.Floege J, Hackmann B, Kliem V, et al. Age-related glomerulosclerosis and interstitial fibrosis in Milan normotensive rats: a podocyte disease. Kidney Int. 1997;51:230–243. doi: 10.1038/ki.1997.28. [DOI] [PubMed] [Google Scholar]
- 16.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 17.Uhlen M, Oksvold P, Fagerberg L, et al. Towards a knowledge-based Human Protein Atlas. Nature Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 18.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 19.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 20.Moll R, Hage C, Thoenes W. Expression of intermediate filament proteins in fetal and adult human kidney: modulations of intermediate filament patterns during development and in damaged tissue. Lab Invest. 1991;65:74–86. [PubMed] [Google Scholar]
- 21.Burnworth B, Pippin J, Karna P, et al. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. Lab Invest. 2012;92:499–510. doi: 10.1038/labinvest.2011.199. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Nelson P, Gelman IH. SSeCKS, a major protein kinase C substrate with tumor suppressor activity, regulates G(1)→S progression by controlling the expression and cellular compartmentalization of cyclin D. Mol Cell Biol. 2000;20:7259–7272. doi: 10.1128/mcb.20.19.7259-7272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grone HJ, Weber K, Grone E, et al. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987;129:1–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Houghton DC, Hartnett M, Campbell-Boswell M, et al. A light and electron microscopic analysis of gentamicin nephrotoxicity in rats. Am J Pathol. 1976;82:589–612. [PMC free article] [PubMed] [Google Scholar]
- 25.Venkatachalam MA, Bernard DB, Donohoe JF, et al. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978;14:31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 26.Wallin A, Zhang G, Jones TW, et al. Mechanism of the nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest. 1992;66:474–484. [PubMed] [Google Scholar]
- 27.Lewington AJ, Padanilam BJ, Martin DR, et al. Expression of CD44 in kidney after acute ischemic injury in rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R247–R254. doi: 10.1152/ajpregu.2000.278.1.R247. [DOI] [PubMed] [Google Scholar]
- 28.Pollack V, Sarkozi R, Banki Z, et al. Oncostatin M-induced effects on EMT in human proximal tubular cells: differential role of ERK signaling. Am J Physiol Renal Physiol. 2007;293:F1714–F1726. doi: 10.1152/ajprenal.00130.2007. [DOI] [PubMed] [Google Scholar]
- 29.Cheng CW, Rifai A, Ka SM, et al. Calcium-binding proteins annexin A2 and S100A6 are sensors of tubular injury and recovery in acute renal failure. Kidney Int. 2005;68:2694–2703. doi: 10.1111/j.1523-1755.2005.00740.x. [DOI] [PubMed] [Google Scholar]
- 30.Imgrund M, Grone E, Grone HJ, et al. Re-expression of the developmental gene Pax-2 during experimental acute tubular necrosis in mice 1. Kidney Int. 1999;56:1423–1431. doi: 10.1046/j.1523-1755.1999.00663.x. [DOI] [PubMed] [Google Scholar]
- 31.Langworthy M, Zhou B, de Caestecker M, et al. NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol. 2009;20:311–321. doi: 10.1681/ASN.2008010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akashi T, Shirasawa T, Hirokawa K. Gene expression of CD24 core polypeptide molecule in normal rat tissues and human tumor cell lines. Virchows Arch. 1994;425:399–406. doi: 10.1007/BF00189578. [DOI] [PubMed] [Google Scholar]
- 33.Figarella-Branger D, Moreau H, Pellissier JF, et al. CD24, a signaltransducing molecule expressed on human B lymphocytes, is a marker for human regenerating muscle. Acta Neuropathol. 1993;86:275–284. doi: 10.1007/BF00304142. [DOI] [PubMed] [Google Scholar]
- 34.Florek M, Haase M, Marzesco AM, et al. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005;319:15–26. doi: 10.1007/s00441-004-1018-z. [DOI] [PubMed] [Google Scholar]
- 35.Griguer CE, Oliva CR, Gobin E, et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS One. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shmelkov SV, Butler JM, Hooper AT, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen HL, Pistollato F, Hoeppner DJ, et al. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- 38.Stokman G, Stroo I, Claessen N, et al. Stem cell factor expression after renal ischemia promotes tubular epithelial survival. PLoS One. 2010;5:e14386. doi: 10.1371/journal.pone.0014386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan C, Pasternack B, Shah H, et al. Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol. 1975;80:227–234. [PMC free article] [PubMed] [Google Scholar]
- 40.Goyal VK. Changes with age in the human kidney. Exp Gerontol. 1982;17:321–331. doi: 10.1016/0531-5565(82)90032-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.