Summary
Many intracellular bacterial pathogens undergo actin-based motility to promote cell-cell spread during infection [1]. For each pathogen, motility was assumed to be driven by a single actin polymerization pathway. Curiously, spotted-fever-group Rickettsia differ from other pathogens in possessing two actin polymerizing proteins. RickA, an activator of the host Arp2/3 complex, was initially proposed to drive motility [2, 3]. Sca2, a mimic of host formins [4, 5], was later shown to be required for motility [6]. Whether and how their activities are coordinated has remained unclear. Here, we show that each protein directs an independent mode of Rickettsia parkeri motility at different times during infection. Early after invasion, motility is slow and meandering, generating short, curved actin tails that are enriched with Arp2/3 complex and cofilin. Early motility requires RickA and Arp2/3 complex, and is correlated with transient RickA localization to the bacterial pole. Later in infection, motility is faster and directionally persistent, resulting in long, straight actin tails. Late motility is independent of Arp2/3 complex and RickA, and requires Sca2, which accumulates at the bacterial pole. Both motility pathways facilitate cell-to-cell spread. The ability to exploit two actin assembly pathways may allow Rickettsia to establish an intracellular niche and spread between diverse cells throughout a prolonged infection.
Results and Discussion
Rickettsia motility occurs in two phases with distinct movement parameters
Pathogens that undergo actin-based motility (ABM), including Listeria monocytogenes and Shigella flexneri, were assumed to deploy one protein that harnesses a single actin polymerization pathway, resulting in a mechanistically uniform mode of motility throughout infection [7]. However, spotted fever group (SFG) Rickettsia differ from L. monocytogenes and S. flexneri in having a slower doubling time (8-12 h versus 40-60 min) and longer persistence time in host cells (120 h or more versus 8-24 h) [8, 9]. Previous studies examined Rickettsia ABM primarily at 24-48 h post infection (hpi) [10-12], with one report of rare motility at 30 min post infection (mpi) [10]. These reports did not, however, quantify movement parameters or molecular requirements throughout infection. We sought to determine how ABM progressed during infection with Rickettsia parkeri, an emerging SFG pathogen that causes eschar-associated rickettsiosis in humans [13].
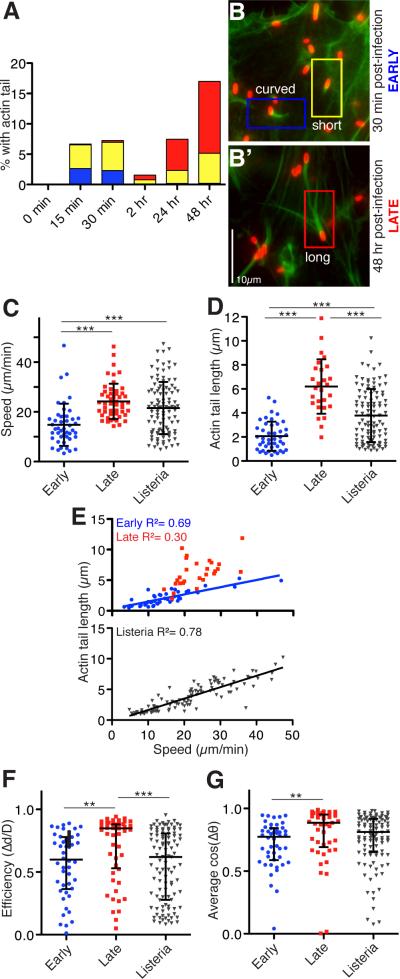
We investigated the appearance of actin tails at various times after infection of human microvascular endothelial (HMEC-1) cells. Early after invasion (15-30 mpi), actin tails were predominantly short or curved (Figure 1A, 1B). Most bacteria with actin tails were intracellular even at 15 mpi (Figure S1A), with a small extracellular fraction that may have been invading or escaping from host cells. At intermediate times post infection (2-12 hpi), few tails were observed (Figure 1A). At later times (24-48 hpi), tails were more frequent and were predominantly long (Figure 1A, 1B’). This suggests that R. parkeri transition through an early motile phase prior to bacterial replication, an intermediate phase with infrequent motility, and a late motile phase after replication commences.
Figure 1. R. parkeri motility occurs in early and late phases with distinct movement characteristics.
(A) Graph depicting the percentage of R. parkeri with actin tails that are curved (blue; > 90° bend in tail), short (yellow, < 2 bacteria lengths) or long (red, > 2 bacteria lengths) in HMEC-1 cells infected synchronously for the indicated times as described previously [14]. Results are the average from three independent experiments performed in duplicate. (B) R. parkeri infected cells were fixed at the indicated times, stained with anti-Rickettsia antibody (red) and for actin with Alexa Fluor 488-phalloidin (green). (C-G) Bacterial motility parameters in HMEC-1 cells expressing Lifeact-EGFP and infected with R. parkeri expressing mCherry [15] early (12-60 mpi) or late (48 hpi), or HMEC-1 cells expressing Lifeact-mCherry and infected with L. monocytogenes strain 10403S expressing GFP [16] at 8-12 hpi. (C) Speed of movement and (D) actin tail length for R. parkeri early (blue) and late (red), and for L. monocytogenes (grey). Mean ± SD, *** p<0.001 by ANOVA with Bonferroni's Multiple Comparison Test, from three separate experiments, with tracking and speed data in 60 s intervals for each bacterium. (E) Relationship between average speed and actin tail length for each bacterium, with best-fit linear regression. (F) Average efficiency of movement, calculated by dividing the total x-y displacement by the total distance moved over 60 s for each bacterium. (G) Path straightness for each bacterium, calculated by averaging cosines of the change in tangent angle between adjacent track segments (Δθ) over 60 s of movement. For (F, G), we analyzed variation around the medians due to the non-Gaussian distribution of the data. Data are median ± interquartile range, ** p<0.01, *** p<0.001 by Kruskal-Wallis test with Dunn's Multiple Comparison test. See also Figure S1, Movies S1-S3.
To discern whether the parameters of movement changed over time, we observed motility of individual R. parkeri early (15-60 mpi) and late (48 hpi) after infection of HMEC-1 cells (Movies S1-S2; Figure S1B, C), and compared these with the parameters of L. monocytogenes motility (8-12 hpi) (Movie S3; Figure S1D). Early R. parkeri motility was slower and produced shorter actin tails in comparison with late R. parkeri and L. monocytogenes motility (Figure 1C, D). Tail length and speed were well correlated for both early R. parkeri (R2 = 0.69) and L. monocytogenes motility (R2 = 0.78) (Figure 1E), similar to previous observations for L. monocytogenes [17]. However, there was little correlation for late R. parkeri motility (R2 = 0.30), as seen previously [18]. We also measured path curvature by calculating movement efficiency (displacement /distance traveled) and the average cos (Δθ) (Δθ = change in tangent angle between track segments) (Figure 1F, G) over 60 s. The median values for both curvature measures were close to 1 for late R. parkeri motility, indicating straighter trajectories. Both measurements were significantly different for early R. parkeri motility, reflecting more curved trajectories similar to those of L. monocytogenes. Thus, early and late R. parkeri motility differ in key parameters, suggesting that each phase is driven by a distinct actin polymerization mechanism.
Different host proteins are recruited and required for early and late motility
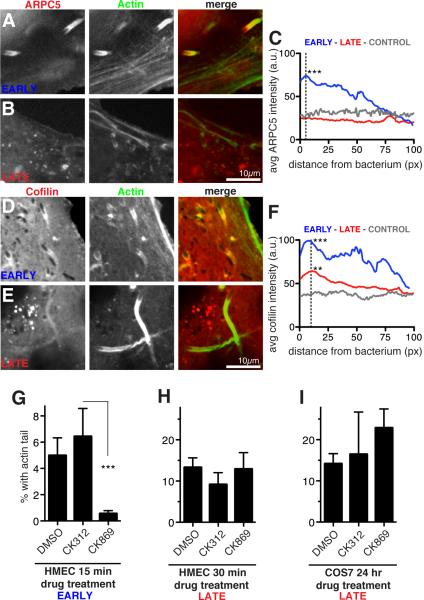
The observation that early R. parkeri motility resembles L. monocytogenes motility, which requires the host Arp2/3 complex [7], suggested that differences between early versus late R. parkeri motility result from differential utilization of proteins involved in Arp2/3-dependent actin network assembly and disassembly. The recruitment of the Arp2/3 complex to R. parkeri actin tails was examined in HMEC-1 cells expressing the mCherry-tagged ARPC5 subunit. mCherry-ARPC5 localized intensely to early R. parkeri actin tails in both live and fixed cells (Figure 2A, C), similar to Arp2/3 complex localization in L. monocytogenes tails [20, 21]. In fixed cells, the Arp3 subunit of native Arp2/3 complex was also observed in early tails (Figure S2A). In contrast, ARPC5 and Arp3 intensities were indistinguishable from controls in late R. parkeri actin tails (Figure 2B, C; Figure S2B), consistent with previous findings [12, 18, 22]. We also examined the localization of the actin severing and depolymerizing protein cofilin, which is enriched in cellular actin networks containing Arp2/3 [23]. In HMEC-1 cells expressing cofilin tagged with EGFP, EGFP-cofilin intensity was significantly higher in early R. parkeri actin tails compared with late tails, and intensity in both tail types was significantly higher than control intensity (Figure 2D-F; Figure S2C). Earlier qualitative observations also found cofilin to be more abundant in L. monocytogenes actin tails than late R. parkeri tails [12, 18]. The distinct composition of actin cytoskeletal proteins in early and late R. parkeri actin tails likely reflects differences in the mechanisms of actin assembly and dynamics.
Figure 2. Different host proteins are recruited and required for early and late motility.
(A-D) Confocal micrographs of live HMEC-1 cells stably expressing Lifeact-3xBFP (green) infected with R. parkeri for 30 mpi (early) or 48 hpi (late). Cells also expressed (A, B) mCherry-ARPC5 (red) or (D, E) EGFP-Cofilin (red) [19]. (C, F) Average intensities of line scans along the first 100 pixels (~6.5 μm) of actin tails proximal to bacteria, or control lines in various areas of transfected cells (a.u. = arbitrary units). Intensity measured for 26-36 tails in snapshots of multiple live cells after identical rolling circle background correction using ImageJ. Asterisks indicate significantly different average peak intensities (at 5 pixels for C and 10 pixels for F) compared with controls, with p-values of **p < 0.01 or ***p < 0.001, based on an ANOVA test with Bonferroni’s multiple comparison post test. (G) Percent of all bacteria with an actin tail in HMEC-1 cells infected with R. parkeri for 15 min to allow invasion, then treated with 1% DMSO or 100 μM inactive control (CK312) or Arp2/3 inhibitor (CK869) for 15 mpi, before fixation at 30 mpi. (H) HMEC-1 cells infected with R. parkeri for 48 h, then treated with 1% DMSO or 100 μM CK312 or CK869 for 30 min before fixation. (I) COS7 cells infected with R. parkeri for 24 h, then treated with 1% DMSO or 100 μM CK312 or CK869 for an additional 24 h (with fresh media plus inhibitors exchanged at 32 h, 40 h, and 48 h). For (G-I) cells were stained with Alexa Fluor 488-phalloidin and anti-Rickettsia antibody, and 5-10 random fields of view were imaged. Results represent the mean ± SD of two (G) or three (H, I) independent experiments performed in duplicate, *** p<0.001 indicated means vary significantly by ANOVA with Bonferroni's Multiple Comparison Test. See also Figure S2.
We tested for differences in the functional requirement for host Arp2/3 complex in early versus late R. parkeri ABM by treating infected HMEC-1 and Cos7 with the small-molecule Arp2/3 inhibitor CK869 [24], the inactive control compound CK312, or DMSO alone (Figure 2G-I; Figure S2). Early actin tail formation (30 mpi) was significantly reduced upon 15 min treatment with inhibitor relative to controls (Figure 2G, Figure S2D). In contrast, late motility (48 hpi) was unaffected by 30 min or 24 h treatment with Arp2/3 inhibitor (Figure 2H, I; Figure S2E). Thus, the molecular mechanisms of actin nucleation during early and late motility are distinct, with early motility exhibiting an Arp2/3-dependent mechanism similar to that used by L. monocytogenes [7].
Early motility requires RickA and late motility requires Sca2
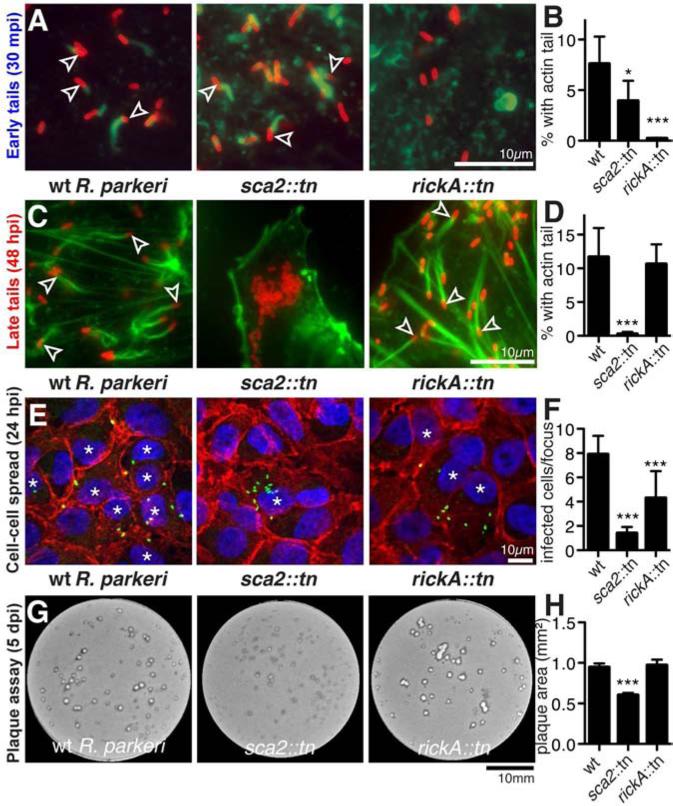
Differences in the requirement for host Arp2/3 complex in early versus late R. parkeri ABM suggested that the two bacterial actin-polymerizing proteins, RickA and Sca2, might also contribute differentially to each motility phase. Using the pMW1650/himar1 transposon system [25, 26] in R. parkeri, we isolated transposon insertion mutations in the rickA or sca2 genes (Figure S3A, B). The sca2::tn mutation causes the expression of a truncated 50 kDa protein, whereas the rickA::tn mutation eliminates detectable expression of RickA (Figure S3C). Neither mutation deleteriously affected the growth of R. parkeri (Figure S3D). Importantly, the rickA::tn mutant was completely defective in early tail formation (Figure 3A, B), but showed no defect in late tail formation (Figure 3C, D). Conversely, the sca2::tn mutant was completely deficient in late tail formation (Figure 3C, D) (similar to the sca2::tn mutant in R. rickettsii [6]), and exhibited a slight defect in early tail formation (Figure 3A, B), perhaps reflecting a small subset of early ABM driven by Sca2. Thus, the two phases of Rickettsia motility are mechanistically independent, with RickA and the Arp2/3 complex required for early motility, and Sca2 alone required for late motility.
Figure 3. Early actin-based motility requires RickA and late motility requires Sca2.
(A, C) HMEC-1 cells infected with wild type or mutant R. parkeri for (A) 30 min or (C) 48 h, stained with Alexa Fluor 488-phalloidin to label actin and anti-Rickettsia antibody. Arrowheads indicate bacteria with an actin tail. (B, D) Percentage of wild type or mutant R. parkeri with actin tails in HMEC-1 cells infected for (B) 30 min or (D) 48 h. Data are mean ± SD of 5 random fields of view in each of three independent experiments performed in duplicate. (E) Infectious foci formed by wild type or mutant R. parkeri in A549 cells, stained with DAPI (blue), anti-β-catenin antibody (red) and anti-Rickettsia antibody (green) (* infected cells). (F) Number of infected cells per focus for strains in (E). Data are mean ± SD for 10 foci. (G) Plaques formed by wild type or mutant R. parkeri in Vero cells 5 dpi. (H) Plaque area for strains in (G). Data are mean ± SEM of data from three independent experiments. *p<0.05, *** p<0.001 indicate significant variation of mean from wild-type values by ANOVA with Bonferroni's multiple comparison test. See also Figure S3.
We next investigated the function of RickA and Sca2 in R. parkeri cell-cell spread. The efficiency of spread at 24 hpi was determined by measuring the size of infectious foci in A549 cell monolayers, and at 5 d post infection (dpi) by measuring the size of plaques in Vero cell monolayers. At 24 hpi, both the rickA::tn and sca2::tn mutants exhibited significantly smaller foci than wild type, although the sca2::tn mutant phenotype was more severe (Figure 3E, F). At 5 dpi, however, only the sca2::tn mutant had significantly smaller plaques than wild type (Figure 3G, H), consistent with small plaques formed by a R. rickettsii sca2 mutant [6]. Thus, both RickA and Sca2 are important for efficient spread kinetics early in infection, whereas Sca2 is primarily important for spread later in infection.
A transition between phases is mediated by changes in RickA and Sca2 localization
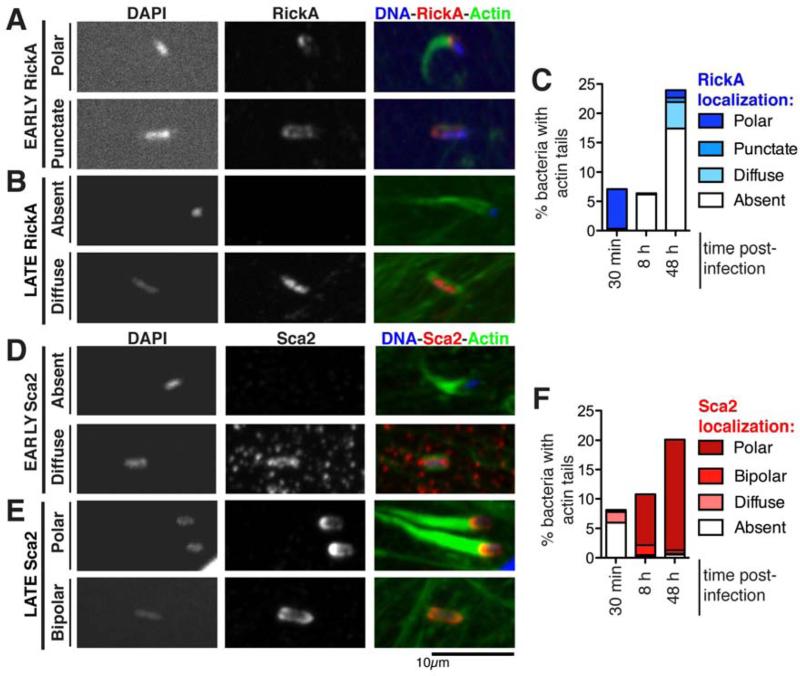
For L. monocytogenes and S. flexneri, polar localization of the bacterial actin polymerizing proteins ActA or IcsA is important for efficient ABM [27]. Previous studies reported diffuse localization of RickA [2, 15] and polar localization of Sca2 [4] late in infection. We hypothesized that changes in the localization or abundance of RickA and Sca2 might coordinate their activities in the two phases of motility. Surface-accessible protein localization was determined in a synchronized infection of HMEC-1 cells (Figure 4, Table S1). At 30 mpi, 97% of bacteria with actin tails exhibited robust RickA staining at the actin-polymerizing pole (Figure 4A, C). In bacteria expressing FLAG-tagged RickA [15], the tagged protein was also polar at 30 mpi (Figure S2A). In contrast, 96% of bacteria with early actin tails lacked Sca2 staining or exhibited weak and diffuse Sca2 (Figure 4D, F). At 8 or 48 hpi, 80-93% of bacteria with actin tails showed intense staining of Sca2 at the actin-polymerizing pole (Figure 4E, F). However, more than 90% of bacteria with late tails lacked RickA staining or had diffusely distributed or dispersed punctate RickA (Figure 4B, C). Thus, there was excellent correspondence of polar RickA localization with early tail formation, and polar Sca2 localization with late tail formation. We also quantified RickA and Sca2 protein levels by Western blotting relative to the control outer membrane protein OmpA, but did not observe statistically significant changes in their abundance (Figure S4A, B). Thus, if protein abundance does vary, it does so at the level of individual bacteria rather than the population as a whole. The correspondence between polar localization of each protein and formation of actin tails at each time point suggests that sequential polar localization of RickA followed by Sca2 regulates the transition between early and late motility.
Figure 4. A transition between early and late motility is accompanied by changes in the localization of RickA and Sca2.
(A, B) Localization of RickA using anti-RickA antibody [3] or (D, E) Sca2 using anti-Sca2 antibody [4] in HMEC-1 cells infected with wild type R. parkeri for 30 min (early) or 48 h (late). Cells were fixed and stained for immunofluorescence microscopy with DAPI to visualize DNA and Alexa Fluor 488-phalloidin to visualize actin. Time post infection and localization patterns are noted on the left. (C, F) The percentage of bacteria with the indicated RickA or Sca2 localization patterns was scored for 400-1200 bacteria over two independent experiments. Localization patterns correspond to those shown in (A, B, D, E), and only bacteria also having an actin tail are shown. See also Figure S4 and Table S1.
Models and functions for dual motility phases
Our results indicate that R. parkeri ABM proceeds in mechanistically independent phases. Early motility is driven by RickA and the host Arp2/3 complex, whereas late motility is driven by Sca2. The rickA and sca2 genes are highly similar in nearly all SFG Rickettsia, suggesting that biphasic motility is conserved among these species. However, the typhus group species R. typhi, which forms short actin tails [10], lacks rickA [28] and has a divergent sca2 [4], suggesting that it may undergo a single motility phase driven by a distinct mechanism. For SFG Rickettsia, a transition between phases is correlated with changes in RickA and Sca2 localization. Such changes could be attributed to temporal control over secretion, with RickA being secreted during or just following invasion, and Sca2 being secreted and localized during bacterial replication as seen for S. flexneri IcsA and other autotransporter proteins [29]. RickA and Sca2 localization and function might also be regulated by posttranslational modification. Changes in motility behavior and surface protein localization may define key stages of Rickettsia infection, and suggest that Rickettsia may undergo previously unappreciated developmental switches. It is also notable that early RickA-Arp2/3 and late Sca2-formin-like actin polymerization pathways result in fundamentally different movement parameters. This suggests that actin networks generated by different actin nucleators result in biophysically distinct forces and movements. Further study of Rickettsia motility may reveal how different actin nucleation pathways impact the movement of pathogens as well as host cell organelles and structures.
The execution of two ABM phases distinguishes SFG Rickettsia from other motile bacterial pathogens, which are thought to undergo a single mode of motility [1], although S. flexneri protrusion formation and spread are enhanced by the host formin Dia1 [30]. Why did SFG Rickettsia evolve to separately exploit both RickA-Arp2/3 and Sca2-formin-like pathways? Our results suggest that one function of both early and late ABM is to promote cell-cell spread. RickA-driven early motility might promote efficient spread kinetics from previously-infected cells before bacterial replication, as has been observed for vaccinia virus [31]. Sca2-mediated late motility appears to play a crucial role in spread once bacterial replication has commenced. The newly developed mouse model of R. parkeri infection [32] will ultimately be useful for illuminating how each motility mechanism enhances spread between the diverse cell types of the mammalian host. A second central function of actin polymerization is to modulate bacterial targeting by the host cell autophagy pathway. For L. monocytogenes, binding and recruitment of Arp2/3 complex is crucial for evading autophagy [33]. For S. flexneri, in contrast, association with actin appears to promote autophagy [34, 35]. For Rickettsia, however, the role of actin in autophagic targeting is poorly understood [34], and differential recruitment of Arp2/3 complex and actin early and late in Rickettsia infection may modulate autophagy to adapt Rickettsia to their long residence time in host cells. Thus, understanding why SFG Rickettsia exploit two actin polymerization pathways will reveal diverse evolutionary strategies by which pathogens exploit actin to establish an intracellular niche and avoid degradation.
Supplementary Material
Highlights.
Rickettsia undergo two phases of actin-based motility early and late in infection
Early motility is slower and curvier and requires bacterial RickA and host Arp2/3
Late motility is faster and straighter and requires bacterial Sca2
A phase transition involves changes in the localization of RickA and Sca2
Acknowledgements
We thank members of the Welch lab for helpful discussions, and Taro Ohkawa, John Nixon and Hedieh Attai for technical assistance. We thank Craig Roy for providing laboratory space for SR, Ted Hackstadt and Tina Clark for anti-Rickettsia antibodies, Chris Paddock for R. parkeri strains, and David Wood for the pMW1650 plasmid. This work was supported by NIH/NIAID grant AI074760 to MDW. RLL is supported by a Helen Hay Whitney Foundation postdoctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haglund CM, Welch MD. Pathogens and polymers: microbe-host interactions illuminate the cytoskeleton. J. Cell Biol. 2011;195:7–17. doi: 10.1083/jcb.201103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gouin E, Egile C, Dehoux P, Villiers V, Adams J, Gertler F, Li R, Cossart P. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nat. Cell Biol. 2004;427:457–461. doi: 10.1038/nature02318. [DOI] [PubMed] [Google Scholar]
- 3.Jeng RL, Goley ED, D'Alessio JA, Chaga OY, Svitkina TM, Borisy GG, Heinzen RA, Welch MD. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell. Microbiol. 2004;6:761–769. doi: 10.1111/j.1462-5822.2004.00402.x. [DOI] [PubMed] [Google Scholar]
- 4.Haglund CM, Choe JE, Skau CT, Kovar DR, Welch MD. Rickettsia Sca2 is a bacterial formin-like mediator of actin-based motility. Nat. Cell Biol. 2010;12:1057–1063. doi: 10.1038/ncb2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madasu Y, Suarez C, Kast DJ, Kovar DR, Dominguez R. Rickettsia Sca2 has evolved formin-like activity through a different molecular mechanism. Proc. Natl. Acad. Sci. USA. 2013;110:E2677–86. doi: 10.1073/pnas.1307235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleba B, Clark TR, Lutter EI, Ellison DW, Hackstadt T. Disruption of the Rickettsia rickettsii Sca2 autotransporter inhibits actin-based motility. Infect. Immun. 2010;78:2240–2247. doi: 10.1128/IAI.00100-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14:242–255. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portnoy DA, Auerbuch V, Glomski IJ. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J. Cell Biol. 2002;158:409–414. doi: 10.1083/jcb.200205009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welch MD, Reed SCO, Haglund CM. Establishing intracellular infection: Escape from the phagosome and intracellular colonization (Rickettsiaceae). In: Palmer GH, Azad AF, editors. Intracellular Pathogens II: Rickettsiales. ASM Press; Washington DC: 2012. pp. 154–174. [Google Scholar]
- 10.Heinzen RA, Hayes SF, Peacock MG, Hackstadt T. Directional actin polymerization associated with spotted fever group Rickettsia infection of Vero cells. Infect. Immun. 1993;61:1926–1935. doi: 10.1128/iai.61.5.1926-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinzen RA, Grieshaber SS, Van Kirk LS, Devin CJ. Dynamics of actin-based movement by Rickettsia rickettsii in vero cells. Infect. Immun. 1999;67:4201–4207. doi: 10.1128/iai.67.8.4201-4207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, Villiers V, Gounon P, Sansonetti PJ, Cossart P. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J. Cell Sci. 1999;112:1697–1708. doi: 10.1242/jcs.112.11.1697. [DOI] [PubMed] [Google Scholar]
- 13.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin. Infect. Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 14.Reed SCO, Serio AW, Welch MD. Rickettsia parkeri invasion of diverse host cells involves an Arp2/3 complex, WAVE complex and Rho-family GTPase-dependent pathway. Cell. Microbiol. 2012;14:529–545. doi: 10.1111/j.1462-5822.2011.01739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch MD, Reed SC, Lamason RL, Serio AW. Expression of an epitope-tagged virulence protein in Rickettsia parkeri using transposon insertion. PLoS ONE. 2012;7:e37310. doi: 10.1371/journal.pone.0037310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen A, Higgins DE. The 5′ untranslated region-mediated enhancement of intracellular listeriolysin O production is required for Listeria monocytogenes pathogenicity. Mol. Microbiol. 2005;57:1460–1473. doi: 10.1111/j.1365-2958.2005.04780.x. [DOI] [PubMed] [Google Scholar]
- 17.Theriot JA, Mitchison TJ, Tilney LG, Portnoy DA. The rate of actin- based motility of intracellular Listeria monocytogenes equals the rate of actin polymerization. Nature. 1992;357:257–260. doi: 10.1038/357257a0. [DOI] [PubMed] [Google Scholar]
- 18.Serio AW, Jeng RL, Haglund CM, Reed SC, Welch MD. Defining a core set of actin cytoskeletal proteins critical for actin-based motility of Rickettsia. Cell Host Microbe. 2010;7:388–398. doi: 10.1016/j.chom.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannherz HG, Gonsior SM, Gremm D, Wu X, Pope BJ, Weeds AG. Activated cofilin colocalises with Arp2/3 complex in apoptotic blebs during programmed cell death. Eur. J. Cell Biol. 2005;84:503–515. doi: 10.1016/j.ejcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Welch MD, Iwamatsu A, Mitchison TJ. Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes. Nature. 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]
- 21.Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J. Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlander RS, Way M, Ren Q, Howe D, Grieshaber SS, Heinzen RA. Effects of ectopically expressed neuronal Wiskott-Aldrich Syndrome protein domains on Rickettsia rickettsii actin-based motility. Infect. Immun. 2003;71:1551–1556. doi: 10.1128/IAI.71.3.1551-1556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koestler SA, Steffen A, Nemethova M, Winterhoff M, Luo N, Holleboom JM, Krupp J, Jacob S, Vinzenz M, Schur F, et al. Arp2/3 complex is essential for actin network treadmilling as well as for targeting of capping protein and cofilin. Mol. Biol. Cell. 2013;24:2861–2875. doi: 10.1091/mbc.E12-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolen BJ, Tomasevic N, Russell A, Pierce DW, Jia Z, McCormick CD, Hartman J, Sakowicz R, Pollard TD. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature. 2009;460:1031–1034. doi: 10.1038/nature08231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z-M, Tucker AM, Driskell LO, Wood DO. Mariner-based transposon mutagenesis of Rickettsia prowazekii. Appl. Environ. Microbiol. 2007;73:6644–6649. doi: 10.1128/AEM.01727-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark TR, Lackey AM, Kleba B, Driskell LO, Lutter EI, Martens C, Wood DO, Hackstadt T. Transformation frequency of a mariner-based transposon in Rickettsia rickettsii. J. Bacteriol. 2011;193:4993–4995. doi: 10.1128/JB.05279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg MB. Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 2001;65:595–626. doi: 10.1128/MMBR.65.4.595-626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLeod MP, Qin X, Karpathy SE, Gioia J, Highlander SK, Fox GE, McNeill TZ, Jiang H, Muzny D, Jacob LS, et al. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J. Bacteriol. 2004;186:5842–5855. doi: 10.1128/JB.186.17.5842-5855.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain S, van Ulsen P, Benz I, Schmidt MA, Fernandez R, Tommassen J, Goldberg MB. Polar localization of the autotransporter family of large bacterial virulence proteins. J. Bacteriol. 2006;188:4841–4850. doi: 10.1128/JB.00326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heindl JE, Saran I, Yi C-R, Lesser CF, Goldberg MB. Requirement for formin-induced actin polymerization during spread of Shigella flexneri. Infect. Immun. 2010;78:193–203. doi: 10.1128/IAI.00252-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doceul V, Hollinshead M, van der Linden L, Smith GL. Repulsion of superinfecting virions: a mechanism for rapid virus spread. Science. 2010;327:873–876. doi: 10.1126/science.1183173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grasperge BJ, Reif KE, Morgan TD, Sunyakumthorn P, Bynog J, Paddock CD, Macaluso KR. Susceptibility of inbred mice to Rickettsia parkeri. Infect. Immun. 2012;80:1846–1852. doi: 10.1128/IAI.00109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa Y, Ogawa M, Hain T, Yoshida M, Fukumatsu M, Kim M, Mimuro H, Nakagawa I, Yanagawa T, Ishii T, et al. Listeria monocytogenes ActA-mediated escape from autophagic recognition. Nat. Cell Biol. 2009;11:1233–1240. doi: 10.1038/ncb1967. [DOI] [PubMed] [Google Scholar]
- 34.Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 2010;8:433–444. doi: 10.1016/j.chom.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Mostowy S, Sancho-Shimizu V, Hamon MA, Simeone R, Brosch R, Johansen T, Cossart P. p62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 2011;286:26987–26995. doi: 10.1074/jbc.M111.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.