Abstract
Objectives/Hypothesis
The purposes of this preclinical study were to investigate histologic and rheologic outcomes of Microendoscopy of Reinke’s space (MERS)-guided minithyrotomy and to assess its instrumentation.
Study Design
Human cadaveric and in vivo animal study.
Methods
Three human cadaveric larynges were treated with MERS-guided placement of Radiesse VoiceGel and immediately evaluated histologically for biomaterial location. In the second part of this investigation, two scarred porcine larynges were treated with MERS-guided placement of HyStem-VF and rheologically evaluated 6 weeks later. Student t tests determined differences in viscoelastic properties of treated/untreated vocal folds. Sialendoscopes and microendoscopes were subjectively compared for their visualization capacity.
Results
MERS imaged the subepithelial area and vocal ligament, guiding both tissue dissection and biomaterial positioning. Sialendoscopes provided adequate visualization and feature incorporated working channels. Enhanced image clarity was created in a gas-filled rather than saline-filled environment, per rater judgment. Histological analysis revealed desirable biomaterial positioning with MERS. Per rheological analysis, viscoelastic properties of the MERS-treated porcine vocal folds compared to uninjured vocal folds 6 weeks following treatment did not statistically differ.
Conclusions
MERS-guided laryngoplasty using sialendoscopes yielded satisfactory biomaterial positioning in the short-term and normalized rheologic tissue properties in the long-term, contributing to proof of concept for MERS in the treatment of scarring. Strengths of MERS include direct, real-time visualization of Reinke’s space and an ability to manipulate surgical instruments parallel to the vocal fold edge while maintaining an intact epithelium. Future work will explore the clinical utility of MERS for addressing scarring, sulcus vocalis, and other intracordal processes.
Keywords: Laryngoscopy, Reinke’s space, sialendoscopy, vocal fold scarring, minithyrotomy
INTRODUCTION
Shortcomings of contemporary surgical approaches to Reinke’s space have recently been acknowledged.1 Despite the need for surgical precision in treating disorders of the lamina propria, conventional methods for accessing and visualizing Reinke’s space have been indirect. The vocal fold lamina propria has traditionally been accessed transorally through an incision in the epithelium, and guided by endoscopic visualization from above the larynx. Over 10 years ago, a direct approach, “Gray’s minithyrotomy,” was introduced that permits surgical manipulation of the vocal fold lamina propria in a subepithelial plane through a window in the thyroid cartilage.2,3 The surgeon is guided by intraoperative fiberoptic imaging from above the larynx to grossly detect the dissecting instrumentation through the translucent vocal fold epithelium. The clinical utility of Gray’s minthyrotomy is currently being established, and the most commonly reported complication is inadvertent mucosal perforation.4 In an effort to improve surgical precision through direct visualization of Reinke’s space, in 2008 a minimally invasive technique–Microendoscopy of Reinke’s space (MERS)5 was introduced.
Direct visualization of Reinke’s space using MERS could enhance the precision of microdissection, diminish the risk of mucosal perforation and biomaterial extrusion, and improve diagnostic accuracy.6 MERS may also have the potential to guide biomaterial placement to the inferomedial vocal fold edge, which has generated theoretical support for improving the biomechanics of mucosal wave propagation.2 In our initial investigation of a human cadaveric larynx model, two external laryngeal access points (thyroid cartilage, cricothyroid membrane) were identified that provided free navigation and imaging of Reinke’s space using traditional laryngoscopes.5 Further investigation into smaller caliber instruments with infusion ports, such as sialendoscopes, was warranted. Sialendoscopes are semi-rigid instruments that were originally introduced in the 1990s for intraductal salivary gland investigation. They offer a small diameter and channels for visualization, irrigation, and/or instrument usage.
In an effort to develop MERS as a clinically feasible approach, we evaluated multiple endoscopes (microendoscopes, sialendoscopes) for their capacity to access and image Reinke’s space. Following MERS-guided injection of a biomaterial, outcomes were assessed by histologically locating the injected biomaterial in an uninjured human cadaver model and by rheologically evaluating the complex shear modulus in a porcine model of mature scar.
MATERIALS AND METHODS
Experiment 1: Localization of Injected Biomaterial in Human Cadaver Model
Tissue
Three frozen, unpreserved human larynges were obtained according to established protocols from the University of Iowa Deeded Body Program. Consultation with the University Institutional Review Board identified that federal guidelines exempt cadaver studies from specific protocol review.
Surgical procedures
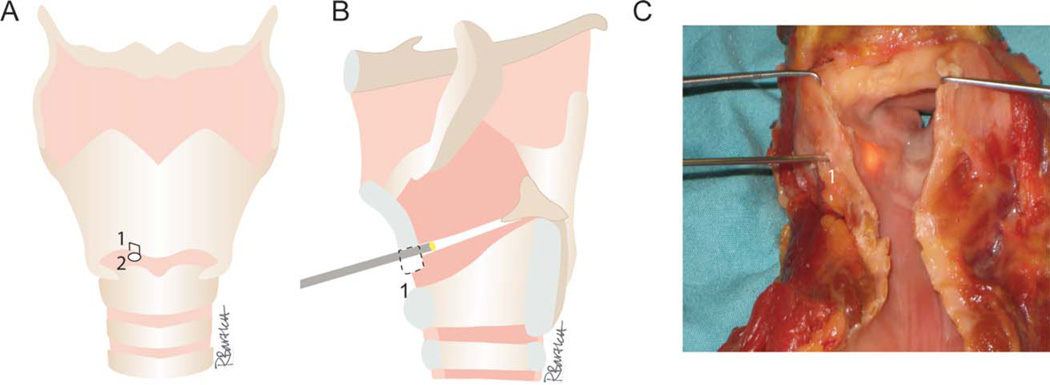
A laryngofissure was performed to improve visualization of the procedure. Access to Reinke’s space was provided through a 5-mm fenestration located 6 mm lateral to the midline, 6 mm above the lower border of the thyroid cartilage, and a puncture in the cricothyroid membrane, as previously described5 (see Fig. 1). Separation of the undersurface of the epithelium from the vocal ligament was accomplished via hydrodissection (saline instillation) and blunt/sharp dissection (including the endoscope tip and CO2 laser).
Fig. 1.
Microendoscopy of Reinke’s space. (A) MERS access points include a 5-mm fenestration located 6-mm lateral to the midline and 6 mm above the lower border of the thyroid cartilage (1) and a puncture of the cricothyroid membrane (2). (B) A microendoscope is advanced through the thyroid cartilage fenestration to Reinke’s space. (C) Laryngofissure permits view of the transilluminated tip of a Marchal sialendoscope in Reinke’s space.
Evaluation of Reinke’s space was performed with one microendoscope and two sialendoscopes from Karl Storz: a Hopkins Telescope 0° (#7218 AA) with a Xenon 300 light source, All-In-One Erlangen Miniature Straight Forward Telescope 0° (#11574A), and All-In-One Marchal Miniature Straight Forward Telescope 0° (#11575A) (Table I). Real-time images of Reinke’s space were obtained with a high-definition digital capture system.
TABLE I.
Instrument Comparison for MERS.
| Tool | Catalog # | Dimensions | Stiffness | Ports | Compatible instrumentation |
|---|---|---|---|---|---|
| Hopkins Telescope 0° | Storz, #7218 AA | diameter = 2.7 mm length = 18 cm |
rigid | none | none |
| All-In-One, Erlangen Miniature Straight Forward Telescope 0° | Storz, #11574A | diameter = 1.6 mm length = 10 cm |
semi-flexible | working port: 0.85 mm; irrigation port: 0.25 mm | stone extractor, guide wire, laser probe, balloon catheter, foreign body forceps, microdrill |
| All-In-One, Marchal Miniature Straight Forward Telescope 0° | Storz, #11575A | diameter = 1.3 mm length = 12 cm, distal angle = 5° |
semi-rigid | working port: 0.65 mm; irrigation port: 0.25 mm | stone extractor, guide wire, laser probe, microdrill |
| OnPoint 1.2 mm Scope | Biomet Microfixation, #24–3000 | diameter = 1.2 mm | semi-rigid | 1.9 mm outer sheath with luer port for water, air insufflation | cannula, cannula plug, trocar, obturator |
| FSC200 Micro-endoscope 0–80° | Schoelly Imaging, #96.0272a | diameter = 2.7 mm length = 11 cm |
rigid | none | trocar |
To assess the functionality of using common surgical instruments concurrently with MERS, the Omniguide flexible fiberoptic CO2 laser fiber was considered. The CO2 laser (outer diameter 0.9 mm) was too large for the working channel of the sialendoscope and was therefore placed through a larger needle (18-gauge spinal needle)/blunt trochar. Visualization of the laser tip was obtained with the Marchal sialendoscope in line with the laser in the cricothyroid puncture, and with the Hopkins Telescope 0° through the unused access point. The laser was used to make incisions in Reinke’s space, on the vocal ligament, and on the undersurface of the epithelium with helium gas (30–60 PSI). CO2 laser settings were set to provide pulses (200 msec) of 4 watts, resulting in an average power of 1.6 watts.
Radiesse Voice Gel (#7000M0, lots 1006464/1006876) from BioForm Medical, Inc. (San Mateo, CA) was infused through the Marchal sialendoscope port with a 27-gauge needle to separate the epithelium from the vocal ligament. Whereas Radiesse is designed for augmentation of a vocal fold, we have used it here as a “spacer,” as our intention was to evaluate biomaterial placement in this cadaveric tissue with the MERS technique. To prevent egress of the Radiesse, access sites were closed with suture and placement of adjacent muscle.
Histology
Larynges were fixed in formalin, decalcified, embedded in celloidin, cut into 30 µm to 35 µm coronal sections, stained with hematoxylin and eosin, and visualized with light microscopy (20–600 × magnification).
Experiment 2: Treatment Outcomes in a Porcine Model of Scarring
Tissue
Two 6-month-old female Yorkshire pigs were included. The care of the animals was in accordance with an approved Institutional Animal Care and Use Committee protocol at the University of Wisconsin-Madison and the NIH Guide for the Care and Use of Laboratory Animals.7
Surgical procedures
Pigs were positioned supine and the skin overlying the cricothyroid membrane was shaved/cleaned. General anesthesia was induced with Telazol (2–7 mg/kg) and xylazine (1–2.2 mg/kg) with atropine (0.05 mg/kg). The animals underwent endotracheal intubation and placed on isoflurane (2–3.5%). Buprehnorphine (0.1 mg/kg) was included during induction for pain and postoperative recovery. Transoral direct suspension laryngoscopy was used to visualize the larynx intraorally throughout the procedure using a 6-mm zero degree Storz endoscope (SDC PRO2, Stryker Endoscopy, Kalamazoo, MI) attached to a 3-chip digital camera and light source. Injury was created unilaterally in each pig and the contralateral vocal fold served as an unscarred control. In pig 1, a 4-mm cup biopsy forceps was used to create a left-sided longitudinal mucosalstripping injury of the lamina propria down to superficial thyroarytenoid muscle in the anterior-posterior direction, in an effort to mimic sulcus vocalis type II (sulcus vergeture). In pig 2, the 4-mm cup biopsy forceps was used to create a right-sided coronal injury perpendicular to the vocal fold edge to mimic a mid-vocal fold biopsy procedure. The OnPoint 1.2 Scope System (#24–3000, Biomet Microfixation, Jacksonville, FL) and the Marchal sialendoscope were used during the injury procedures (Table I). For pain management, Carprofen (2.2 mg/kg) was provided twice daily for 72 hours.
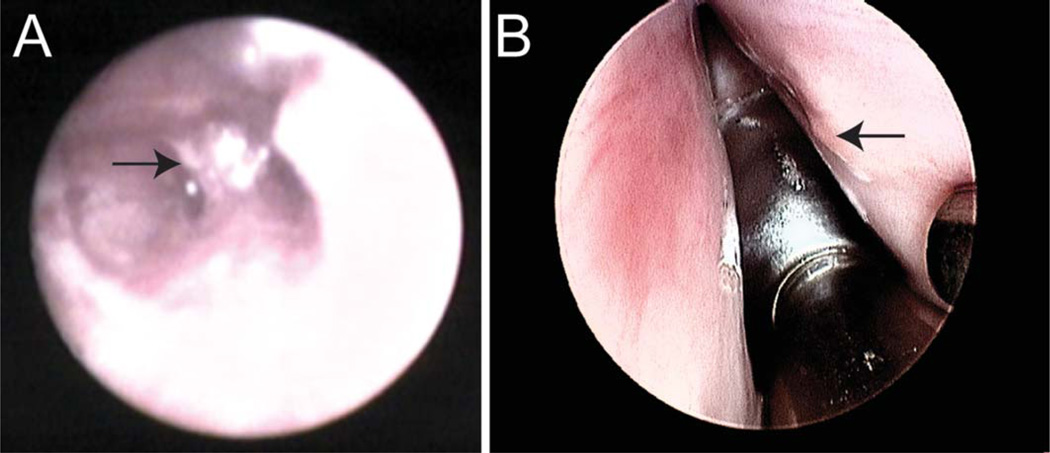
Six weeks later, the injured vocal folds were treated with MERS-guided injection of a hyaluronic acid-based hydrogel, HyStem-VF (Biotime, Inc, Alameda, CA). The pigs were anesthetized, intubated, and provided pain management as described above. The putative scar was identified with the FSC200 Microendoscope (#96.0272a, prototype provided by Schoelly Fiberoptic, Worcester, MA) in pig 1 (Fig. 2A) and with the 6-mm zero degree Storz transoral endoscope in pig 2 (Fig. 2B). Dissection was accomplished in pig 1 with a 27-gauge needle, followed by a stylet and concurrent imaging from the FSC200 Microendoscope (with air insufflation) and the transoral endoscope. Dissection in pig 2 was guided by imaging from the Erlangen sialendoscope.
Fig. 2.
Porcine scar. Putative scar (arrow) as observed within Reinke’s space in the left vocal fold of pig 1 in the 6 weeks following injury with the FSC200 Microendoscope 0/80° placed in the thyroid cartilage fenestration (A). Scar as observed on the right vocal fold of pig 2 in the 6 weeks following injury with a 6-mm zero degree Storz transoral endoscope (B).
An injection of HyStem-VF (0.8 cc in pig 2 and 0.7 cc in pig 1) was performed with suture closure of the anterior access site through the thyroid cartilage. HyStem-VF is a hyaluronic acid-based hydrogel and was selected for its viscoelastic properties that make it suitable for use in the vocal fold mucosa.8–10 Pigs were euthanized 4 weeks postinjection with Euthasol (1 ml/10 lb body weight) after full sedation with Telazol (2–7 mg/kg) and xylazine (1–2.2 mg/kg); larynges were excised, snap frozen in liquid nitrogen,11 and shipped to University of Iowa for rheological analysis.
Rheology
Larynges were stored at −80°C for 4 days prior to testing. Each larynx was submerged in phosphate buffered saline and thawed overnight at 4°C. See Klemuk et al. (2010)12 for a comprehensive description of sample processing. The specimens were placed between the stationary base and upper parallel plate of a Gemini stress-control rheometer (Malvern Instruments, UK). Wet/dry sandpaper (220-grit) was adhered to the upper and lower plates for all measurements. Samples were exposed to oscillatory shear stress at 32 frequencies across a 0.10 to 100 Hertz logarithmic scale. Rheology was performed by an individual blinded to the experimental condition. In order to prevent evaporation and heat loss, a thermal cover was placed over the unit. A water-jacketed control unit was coupled to the base plate to maintain a constant temperature (37°C ± 1.0°C). Samples were re-wetted with culture medium as needed.
Ordinary least squares method was used to estimate the slope and intercept, representing the relationship between the rheological values (G′, G″, or loss tangent) and frequency for each vocal fold. Paired t tests were used to evaluate the differences in the slopes and intercepts of treated and nontreated vocal folds. P values less than 0.001 were considered significant. To better characterize the relative variability in our data with respect to that found in a similar experimental sample, coefficients of variation (CV) were calculated. CV is defined as the standard deviation divided by the mean. All statistical analyses were performed using SAS v9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Surgical Technique (Experiments 1 and 2)
Access/surgical manipulation
Reinke’s space was entered through both access sites with all endoscopes and surgical instruments in the cadaveric and porcine larynges. Instruments (endoscope, needle, or laser) could be placed simultaneously in the two access sites to complement the exposure. During exploratory lasing with the Omniguide instrument, an inadvertent laser fenestration of the overlying epithelium occurred in one of the cadaveric larynges (Fig. 3B).
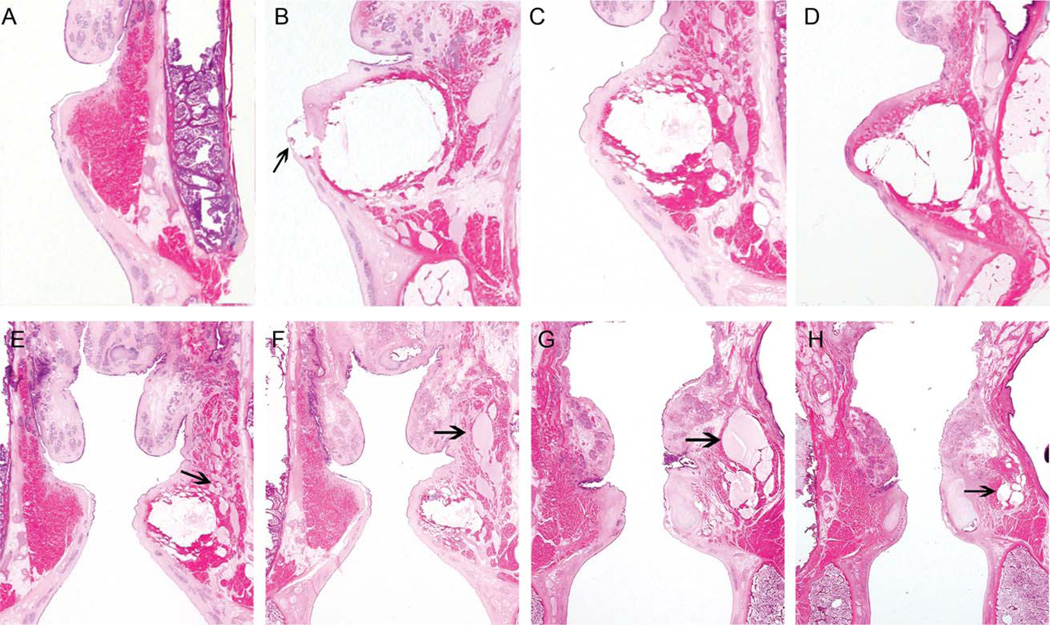
Fig. 3.
Placement of Radiesse Voice Gel in MERS-treated human cadaveric vocal folds. Coronal sections of a nontreated vocal fold (A), fenestrated right vocal fold (see arrow) of the first larynx (B), left vocal fold of the second larynx (C) and right vocal fold of the third larynx (D). The most desirable Radiesse positioning was in the third larynx (D), with infrafold placement projected to facilitate mucosal wave propagation.2 A, B, and D have been rotated 180° for ease of comparison. A sequence (E to H, anterior to posterior) identifying the permeation of Radiesse laterally through the paraglottic space to encroach on the pyriform sinus lateral to the vocal process (arrows) in the second larynx is shown. A small amount of lateral migration of the Radiesse was also found in the first larynx.
Imaging
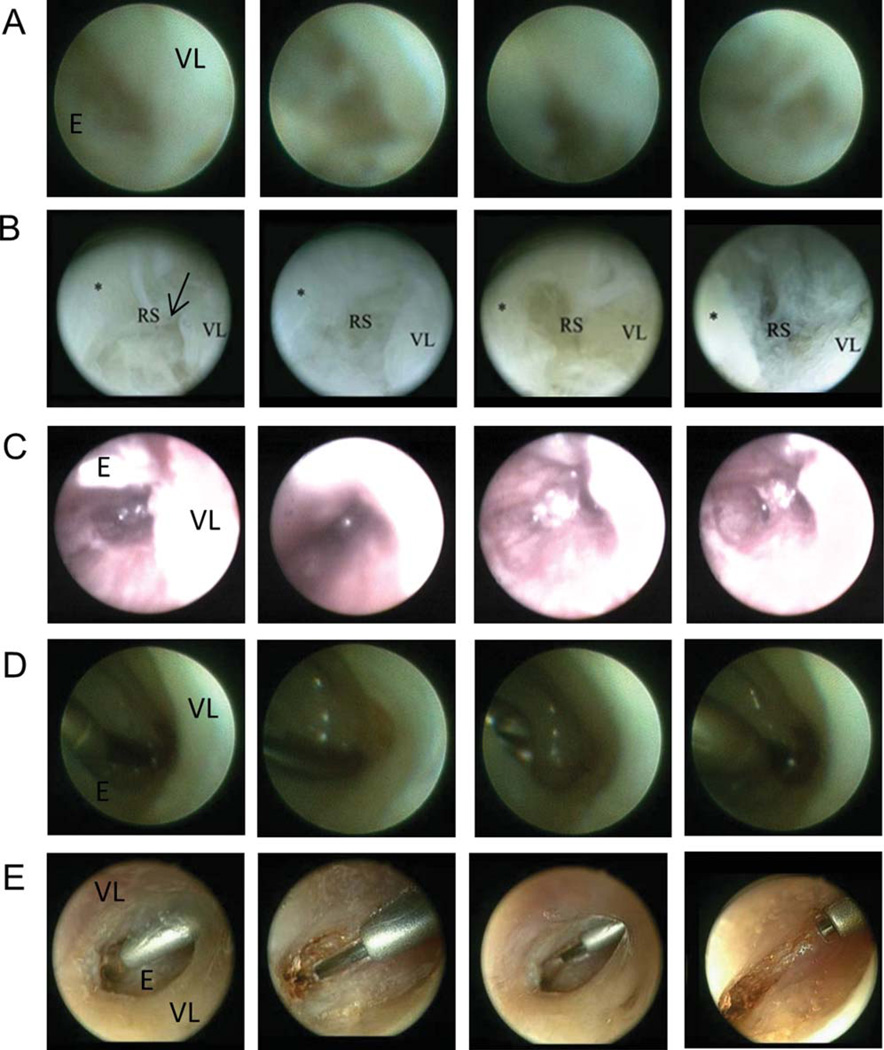
In the saline-infused environment, the resolution of the Marchal sialendoscope was not as clear as the larger Hopkins Telescope 0° instrument (Fig. 4A, 4B). Using the FSC200 Microendoscope, Reinke’s space was adequately visualized with the introducer and air insufflation (Fig. 4C). The best visual clarity was obtained when the Omniguide CO2 laser was in place, with the constant low flow of helium gas into Reinke’s space. The Hopkins Telescope 0° outperformed the Marchal sialendoscope in this environment (Fig. 4D, 4E).
Fig. 4.
Visualization of Reinke’s space. In order of ascending image clarity and the ability to identify Reinke’s space (RS), vocal ligament (VL), undersurface of the epithelium (E) and intervening collagen fibrils, instrumentation included: Marchal sialendoscope (saline) (A), Hopkins Telescope 0° (saline) (B), FSC200 Microendoscope (air) (C), Marchal sialendoscope (gas) (D), and Hopkins Telescope 0° (gas) (E). Images were taken through the access points denoted in Figure 1 (A–D), and through an alternate thyroid cartilage opening (E).
Histologic Assessment (Experiment 1)
MERS-guided minithyrotomy was successful in expanding Reinke’s space in all three cadaveric larynges (Fig. 3). The central portions of the expanded Reinke’s space demonstrated empty areas consistent with dropout of injectant in the course of specimen processing. Inferomedial biomaterial placement2 was best achieved in the third larynx (Fig. 3D), wherein MERS was performed through the cricothyroid membrane puncture. Additionally, observation of coarse, granular, pink-colored material lateral to the adjacent muscle in the first and second larynx and superior in the second larynx was found. Evaluation of serial sections of all specimens yielded the interpretation that these pockets represented diffused Radiesse mixed with serum and fixative.
Rheology (Experiment 2)
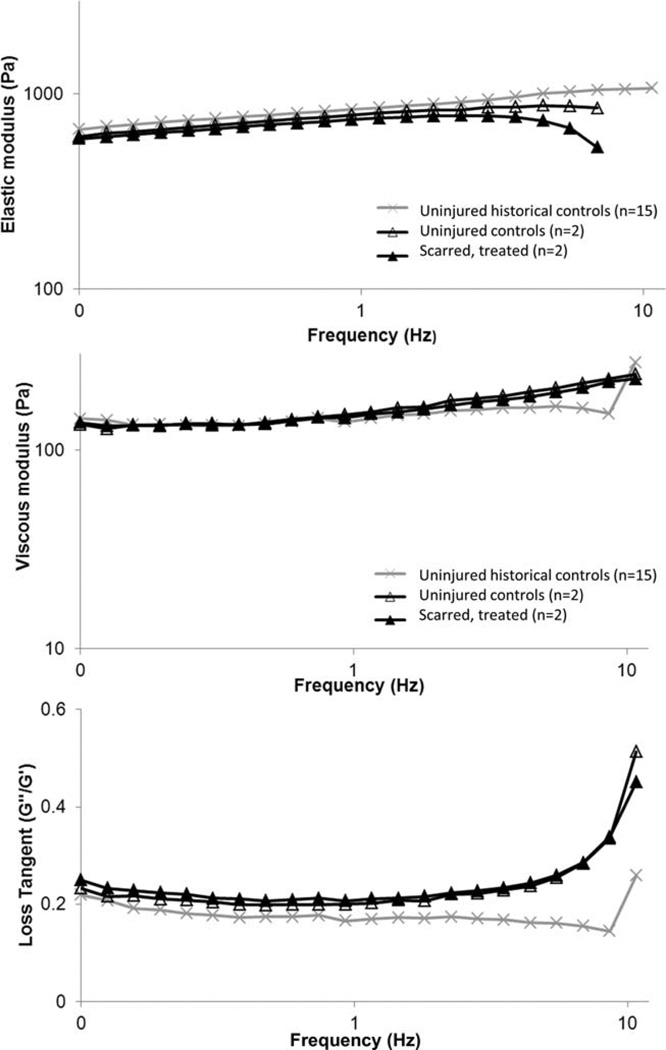
Valid data were recorded and analyzed up to 10 Hertz, after which inertial forces dominated the measurement. Estimated slopes and intercepts representing the relationship of rheologic values (G′, G″, or loss tangent) to frequency did not statistically differ between the treated and untreated vocal folds (see Fig. 5) (slopes: p >0.70; intercepts: p >0.39). This suggests that the MERS procedure coupled with injection laryngoplasty resulted in rheologic tissue properties within values of unscarred vocal folds. All vocal folds included in the study behaved as elastic solids with the elastic modulus (G′) dominating the viscoelastic response (G″/G′: 0.2–0.3). When mean values were calculated at each frequency (combining treated and untreated vocal folds together), the mean values and corresponding CVs were similar to previously recorded, unpublished measurements of unscarred porcine vocal fold mucosa (n = 15). The CV for G′ were 35% to 50% and for G″ were (23%–29%). At 1 Hz, the means of the four vocal folds for G′ and G″ were within 6% to 12% of the mean values from historical controls.
Fig. 5.
MERS-assisted laryngoplasty with HyStem-VF resulted in rheologic tissue properties within values of unscarred vocal folds. Elastic shear modulus (A), viscous modulus (B), and the loss tangent (C) in response to 0.1–10 Hertz of shear stress are reported for scarred MERS-treated vocal folds, uninjured controls, and uninjured historical controls (15 unaltered porcine vocal mucosa specimens). Estimated slopes and intercepts representing the relationship of rheologic values (G′, G″, or loss tangent) to frequency did not statistically differ between the treated and untreated vocal folds (slopes: p >0.70; intercepts: p >0.39).
DISCUSSION
Tissue architecture in the vocal fold lamina propria is known to vary widely with age, sex, and vocal hygiene status (e.g., smoking, phonotrauma).13–15 Disease processes affecting the larynx further modify the integrity of the superficial lamina propria in ways that, to date, have been assessed either indirectly or through incisions in the dorsal surface of the vocal fold. Direct visualization of the superficial lamina propria with microendoscopic instrumentation provides the researcher with the capacity to study this anatomic site in vivo. Translation of this technology to the clinician will enhance the management of patients with abnormalities of Reinke’s space by permitting direct access to the disease process with minimal disruption of adjacent tissue.
The two points of access identified in this investigation may not both be essential for all patients. The anterior thyroid cartilage fenestration could be made directly over the entry site into Reinke’s space to ease identification of the plane of dissection and to potentially better control biomaterial placement. This approach may be particularly useful in cases where scarring at the anterior vocal fold requires precise initiation of dissection immediately upon entry into Reinke’s space. In contrast, in a more conservative approach, the instrumentation could be placed in a “blinded” manner through the cricothyroid membrane (lateral to the area of interest) to decrease the risk of epithelial disruption.
We also found flexibility in the instrumentation used for dissecting the vocal mucosa from the ligament. Saline irrigation and blunt dissection with the tip of the endoscope were generally successful, however, in pig 2 infusion with saline early in the procedure did not gain entry into Reinke’s space and instead required dissection with needles. This may have been due to the presence of the fibrous scar tissue and is potentially more representative of the clinical population. Dissection with minimally traumatizing tools may be aided in the future with the development of instruments (e.g., microscissors) that fit through the working channels of the sialendoscopes. We found that insufflation of saline, helium (laser), and air were useful for maintaining the separation of the pocket in Reinke’s space. In future work, investigation into the use of other microdilators, such as the 0.7-mm balloon catheter (11588, Storz) that is designed for use in the Erlangen sialendoscope port, may also prove beneficial.
The initial description of MERS suggested that the ideal endoscope would be a small-caliber endoscope with an infusion port designed for instillation of saline under pressure into Reinke’s space and a second working channel sufficiently large to accommodate either a laser or cutting and grasping devices.5 In the present study, the functionality of sialendoscopes for these purposes was identified, along with a few shortcomings. Although the 0.65 (Marchal) and 0.85 mm (Erlangen) working channels were sufficiently large to permit instillation of biomaterials into Reinke’s space, they could not accommodate the Omniguide laser fiber (0.9 mm). Other laser fibers are sufficiently small to be used (e.g., Accuflex Holmium Laser Fiber, 0.15 mm). The sialendoscope optics were poorest in the saline environment, improved in the gas environment, but were still inferior to that provided by the larger Hopkins Telescope 0°. The potential for MERS to be used in the diagnosis of disorders involving the lamina propria may best be realized with the superior optics of a larger endoscope (such as the Hopkins Telescope 0°); however, patients with a known diagnosis may be best treated with the sialendoscopes that feature adequate optics and working channels. While the OnPoint 1.2-mm endoscope is small, easily transported, and features a disposable shaft, it had inferior optics (not shown) and lacked a working channel.
Analysis of the whole-organ sections identified the capacity of MERS to limit dissection and to position the biomaterial near the inferomedial portion of the vocal fold. Further, histology provided insight into the potential for migration of injected material in the larynx. It is possible that cadaveric tissue provided less resistance to diffusion; however, lateral migration toward the pyriform sinus has also been reported following Cymetra injection into the thyroarytenoid muscle in human subjects.16 It is unknown how the dynamic motion of the vocal folds in vivo influences diffusion of biomaterials of varying viscosities.
Continued advances in miniaturization, robotics and bioengineering will likely further enhance the capacity to restructure Reinke’s space with MERS in patients with scarring and other intracordal processes. Sophisticated biomaterials are being developed that will encourage extracellular matrix remodeling and prevent re-tethering.17 Rheologic data from our second experiment supports the concept that specific biomaterials placed in Reinke’s space can improve tissue biomechanics over a period of 6 weeks, suggestive of meaningful extracellular matrix repair. Specifically, we found that porcine vocal folds that underwent MERS-guided placement of HyStem-VF had elastic moduli (G′), viscous moduli (G″) and loss tangent values(G″/G′) across a frequency range (up to 10 Hz) that did not statistically differ from noninjured, untreated vocal folds. This relationship remained robust when the treated vocal folds (n = 2) were compared with a larger sample of noninjured vocal folds (historical controls; n = 15). Using MERS for injection of cell and biomaterial combinations is also of interest, as combination therapy likely improves wound healing in the vocal fold lamina propria more than cells or hydrogel treatment alone.18 To date, injectable materials used in the human vocal fold are classified as “monolithic,” with fixed chemistries that do not allow for cell encapsulation; HyStem-VF is a “living” hydrogel designed to crosslink in situ and therefore could be a vehicle for cell delivery.18,19
Limitations of the present study include the small sample size, lack of gene/protein level analysis, and lack of a control group that underwent minithyrotomy without MERS guidance. To date, our investigations have been designed to technically refine the instrumentation and procedure of MERS and to evaluate preliminary data. Future work will address the listed limitations and evaluate MERS-guided placement of cell/scaffold combinations.
CONCLUSION
MERS offers advances to existing procedures by providing direct visualization of the surgical plane, an ability to work parallel to the vocal fold edge (not perpendicular as is necessary with a microflap), and preservation of the epithelium. Our histologic and rheologic evaluation supports continued technical development and clinical study. Design of appropriately sized endoscopes with ideal optics and adequate working channels coupled with advances in injectable materials could translate this approach to clinical relevance.
ACKNOWLEDGEMENT
This work was funded by the Department of Otolaryngology at the University of Iowa and NIDCD R01 DC004336 (Thibeault). We also extend our gratitude to Glen E. Leverson, PhD (University of Wisconsin-Madison), for his assistance with statistical analysis, and to Douglas Van Daele, MD(University of Iowa Hospital and Clinics), for assistance with technical development of the MERS procedure.
Footnotes
Histological data was presented at the Middle Section of the Triological Society, Bonita Springs, Florida, January 10, 2009. The rheological data was presented at the 2013 Combined Sections Meeting in Scottsdale, Arizona, January 24, 2013.
Financial Disclosure: Karl Storz (Tuttlingen, Germany), Medtronic (Fridley, MN), Schoelly Fiberoptic, GmbH, Omniguide Laser (Cambridge, MA), and Biomet “OnPoint” (Jacksonville, FL) for provision of equipment. BioForm Medical (San Mateo, CA) for provision of Radiesse Voice Gel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Mallur PS, Gartner-Schmidt J, Rosen C. Voice outcomes following the Gray minithryotomy. Ann Otol Rhinol Laryngol. 2012;121:490–496. doi: 10.1177/000348941212100711. [DOI] [PubMed] [Google Scholar]
- 2.Gray SD, Bielamowicz S, Titze I, Dove H, Ludlow C. Experimental approaches to vocal fold alteration: introduction to the minithyrotomy. Ann Otol Rhinol Laryngol. 1999;108:1–9. doi: 10.1177/000348949910800101. [DOI] [PubMed] [Google Scholar]
- 3.Wexler D, Jiang J, Gray S, Titze I. Phonosurgical studies: fat-graft reconstruction of injured canine vocal cords. Ann Otol Rhinol Laryngol. 1989;98:668–673. doi: 10.1177/000348948909800902. [DOI] [PubMed] [Google Scholar]
- 4.Paniello RC, Sulica L, Khosla SM, Smith ME. Clinical experience with Gray’s minithyrotomy procedure. Ann Otol Rhinol Laryngol. 2008;117:437–442. doi: 10.1177/000348940811700606. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman HT, Bock JM, Karnell LH, Ahlrichs-Hanson J. Microendoscopy of Reinke’s Space. Ann Otol Rhinol Laryngol. 2008;117:510–514. doi: 10.1177/000348940811700707. [DOI] [PubMed] [Google Scholar]
- 6.Ford C. Commentary on “Microendoscopy of Reinke’s space”. Ann Otol Rhinol Laryngol. 2008;117:515–516. doi: 10.1177/000348940811700707. [DOI] [PubMed] [Google Scholar]
- 7.Guide for the care and use of laboratory animals. Washington DC: National Academies Press; 1996. Institute of Laboratory Animal Resources (US) [Google Scholar]
- 8.Caton T, Thibeault SL, Klemuk S, Smith ME. Viscoelasticity of hyaluronan and nonhyaluronan based vocal fold injectables: implications for mucosal versus muscle use. Laryngoscope. 2007;117:516–521. doi: 10.1097/MLG.0b013e31802e9291. [DOI] [PubMed] [Google Scholar]
- 9.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G. Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing. Tissue Eng. 2006;12:3201–3207. doi: 10.1089/ten.2006.12.3201. [DOI] [PubMed] [Google Scholar]
- 10.Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich GD. Vocal fold tissue repair in vivo using a synthetic extracellular matrix. Tissue Eng. 2006;12:2171–2180. doi: 10.1089/ten.2006.12.2171. [DOI] [PubMed] [Google Scholar]
- 11.Chan RW, Titze IR. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482–491. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- 12.Klemuk SA, Lu X, Hoffman HT, Titze I. Phonation threshold pressure predictions using viscoelastic properties up to 1,400 Hz of injectables intended for Reinke’s space. Laryngoscope. 2010;120:995–1001. doi: 10.1002/lary.20877. [DOI] [PubMed] [Google Scholar]
- 13.Hirano S, Minamiguchi S, Yamashita M, Ohno T, Kanemaru S, Kitamura M. Histologic characterization of human scarred vocal folds. J Voice. 2009;23:399–407. doi: 10.1016/j.jvoice.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Martins RH, Defaveri J, Custodio Domingues MA, de Albuquerque e Silva R, Fabro A. Vocal fold nodules: morphological and immunohistochemical investigations. J Voice. 2010;24:531–539. doi: 10.1016/j.jvoice.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Remacle M, Degols JC, Delos M. Exudative lesions of Reinke’s space. An anatomopathological correlation. Acta Otorhinolaryngol Belg. 1996;50:253–264. [PubMed] [Google Scholar]
- 16.Bock JM, Lee JH, Robinson RA, Hoffman HT. Migration of Cymetra after vocal fold injection for laryngeal paralysis. Laryngoscope. 2007;117:2251–2254. doi: 10.1097/MLG.0b013e3181462a16. [DOI] [PubMed] [Google Scholar]
- 17.Kutty JK, Webb K. Tissue engineering therapies for the vocal fold lamina propria. Tissue Eng Part B Rev. 2009;15:249–262. doi: 10.1089/ten.TEB.2008.0588. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BQ, Fox R, Chen X, Thibeault S. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. Laryngoscope. 2010;120:537–545. doi: 10.1002/lary.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett RS, Thibeault SL, Prestwich GD. Therapeutic potential of gel-based injectables for vocal fold regeneration. Biomed Mater. 2012;7:024103. doi: 10.1088/1748-6041/7/2/024103. [DOI] [PMC free article] [PubMed] [Google Scholar]