Abstract
Objectives
We sought to review the dysphagia-related outcomes and quality of life in a series of patients with upper esophageal sphincter (UES) dysfunction treated with cricopharyngeal (CP) botulinum toxin (BTX) injection, and to identify patient characteristics or CP muscle histologic features that predict efficacy of BTX injection.
Methods
A retrospective chart review was performed on patients with UES dysfunction who underwent CP BTX injection. Dysphagia-related quality-of-life questionnaires based on the Eating Assessment Tool (EAT-10) were mailed to patients.
Results
Forty-nine patients (30 female, 19 male; average age, 59 ± 16 years) with UES dysfunction have been treated at our institution with CP BTX injection since 2000. Seventeen of these patients also underwent CP myotomy. Injections of BTX were occasionally repeated after the treatment effect subsided, and the BTX dose varied widely (average, 39 ± 19 units). Improvement in symptoms was noted by 65% of patients. The overall complication rate was minimal, although many patients complained of transient worsening of dysphagia after CP BTX injection. Biopsy specimens of the CP muscle were evaluated in the subset of patients with CP BTX injection who proceeded to myotomy, with results of neuropathic, myopathic, and mixed histologic subtypes. The EAT-10 scores demonstrated a general trend toward improved swallowing outcomes after CP BTX injection.
Conclusions
This study reviewed findings from the largest published series of BTX treatment of UES dysfunction and evaluated the efficacy, patient satisfaction, and complications of this procedure. Dysphagia-related quality-of-life outcomes appear to be improved after CP BTX injection.
Keywords: botulinum toxin, cricopharyngeal muscle, dysphagia, upper esophageal sphincter
Dysphagia associated with cricopharyngeal (CP) muscle dysfunction has a significant impact on overall patient quality of life. The oropharyngeal swallowing mechanism is a complex orchestration of multiple coordinated movements, eventually leading to the active relaxation of the CP muscle to allow bolus propagation through the upper esophageal sphincter (UES) and into the cervical esophagus. The CP muscle is therefore often a target of surgical interventions for dysphagia, including bougie or balloon dilation, myotomy, and chemodenervation by treatment with botulinum toxin (BTX). Dilation of the UES is often effective and of low risk, but also has a short clinical effect. Transcervical myotomy of the CP muscle is effective in treating CP dysphagia, but has significant risks of infection, salivary fistula formation, and recurrent laryngeal nerve injury.1,2 Endoscopic laser CP myotomy was described by several authors in the early 1990s, with evidence of successful treatment in multiple patient series.3 Cricopharyngeal myotomy improves UES opening, but will not alter pharyngeal muscle contractile forces, and therefore may not benefit every patient with CP dysphagia.4 Injection of BTX into the CP muscle to treat dysphagia was first described in 1994 by Schneider et al,5 in a series of 7 patients, as an alternative treatment to the more-invasive myotomy procedures. Botulinum toxin inhibits the tonic and active contraction of muscles by inhibiting the release of acetylcholine across the neuromuscular junction; therefore, it will primarily benefit patients with hypertonicity of the UES and a retained ability to complete pharyngeal bolus formation.6 Cricopharyngeal injection of BTX has distinct appeal in patients who are not ideal candidates for longer general anesthesia or in whom the temporary nature of BTX injection is warranted. It may be advantageous to pursue CP injection of BTX in patients in whom multilevel dysphagia is suspected and in whom the clinician suspects that there may be some detriment to treatment directed at the UES. Additionally, CP injection of BTX is a diagnostic tool used by clinicians to identify patients who may potentially benefit from CP myotomy.7
Because the CP muscle is often the target of interest for these treatments, there has also been some investigation of this muscle for further characterization. The CP muscle is a truly unique muscle that is composed of striated muscle fibers that are predominantly slow-twitch (type I), as opposed to fast-twitch (type II). Innervation for this muscle is generally believed to be from the pharyngeal plexus, which has contributions from the vagus nerve (both the recurrent and superior laryngeal nerves), the glossopharyngeal nerve, and sympathetic nerve fibers from the cervical ganglia.8 Few studies have evaluated the muscle fiber subtype histology of the CP muscle, but it appears to have a unique adenosine triphosphatase staining pattern indicating the highly oxidative nature of the tissue. This feature is more characteristic of fetal musculature than of adult striated muscle9 and allows maintenance of basal tonicity, with occlusion of the esophageal lumen at rest and relaxation during swallowing to allow passage of the food bolus.8 Dysphagia can result from failed relaxation or hypertrophy of the CP muscle and/or altered coordination between pharyngeal peristalsis and opening of the UES.4 Alterations of the muscle histology could potentially impact CP muscle function; in addition, BTX injection may potentially have an impact on the muscle itself. Since the initial introduction of BTX injection for dysphagia, several authors have published their experience with BTX for the treatment of CP dysfunction.
A review of the literature identified 20 studies that focused on the use of CP injection of BTX, dating back to 1994 (Table 1).5-7,10-26 Only 2 series were of more than 20 patients; the majority of articles presented either case reports or limited series of 10 or fewer patients. The largest study included 34 patients. The causes of CP dysfunction in these published series encompassed numerous diagnoses, including neurologic diseases such as Parkinson's disease, multiple sclerosis, diabetic neuropathy, external-beam radiation treatment, cerebrovascular accident, and others. The dosages and administration techniques of BTX were also quite variable. There were also different types of BTX type A administered: Dysport (Ipsen, Paris, France) and Botox (Allergan, Irvine California). The Dysport doses delivered to the CP muscle ranged from 60 to 180 units (U), and the Botox doses ranged from 4 to 120 U. The techniques for administration of BTX to the CP muscle included endoscopic injection under general anesthesia or mask ventilation, percutaneous injection with or without electromyographic guidance, and injection via flexible endoscopy. In general, the majority of patients reported improved swallowing function: approximately 75% in combined analysis. Complications reported in the literature were infrequent and included transient vocal fold paresis, temporary worsening of dysphagia, neck cellulitis, and aspiration pneumonia. There were no reported deaths in the literature that were directly related to CP injection of BTX. On the basis of this review, CP BTX injection appears to be effective in patients with UES dysfunction.
Table 1. Literature summary.
| Authors | Year | No. of Pts | Botox Dose (Units) | Dysport Dose (Units) | Improvement | Method of Delivery | Causes | Complications |
|---|---|---|---|---|---|---|---|---|
| Schneider et al5 | 1994 | 7 | 80-120 | 5/7 (71%) | General anesthesia, direct esophagoscopy | Stroke, CN palsies, supraglottic or oropharyngeal cancer, reflux disease | None | |
| Atkinson and Rees10 | 1997 | 5 | 5-20 | 4/5 (80%) | CT-guided injection | Stroke, CN palsies, bulbar palsy | Left vocal fold paresis, aspiration pneumonia when injection wore off | |
| Blitzer and Brin7 | 1997 | 6 | 10 | 6/6 (100%) | Percutaneous injection | CVA, partial pharyngectomy, small Zenker's diverticulum | None | |
| Brant et al11 | 1999 | 1 | 100 | 1/1 (100%) | Flexible endoscopy | CVA | None | |
| Alberty et al12 | 2000 | 10 | 30 | 10/10 (100%) | Endoscopy under general anesthesia | CVA, idiopathic polymyositis | None | |
| Shaw and Searl13 | 2001 | 12 | 25-50 | 10/12 (83%) | Endoscopy under general anesthesia (9), flexible endoscopy (2), open technique (2) | Progressive neuropathy, oculopharyngeal dysphagia, skull base tumor resection, total laryngectomy, CVA, partial pharyngectomy, CN X neuropathy | Pharyngeal tear, worsening dysphagia | |
| Haapaniemi et al14 | 2001 | 4 | 14-50 | 3/4 (75%) | Endoscopy under general anesthesia | Brain stem stroke, inclusion body myositis, peripheral motor neuropathy, CVA | None | |
| Moerman et al15 | 2002 | 4 | 100 | 4/4 (100%) | General anesthesia | Head and neck cancer resection including total laryngectomy, radiation | None | |
| Parameswaran and Soliman16 | 2002 | 12 | 10-30 | 11/12 (92%) | Endoscopic injection with mask ventilation and apneic technique | Idiopathic, radiation, CVA, total laryngectomy, ALS, Parkinson's disease | Neck cellulitis (concurrent thyroglossal duct excision) | |
| Zaninotto et al17 | 2004 | 21 | 4-10 | 9/21 (43%) | Percutaneous with EMG | CNS disease, peripheral neuropathies, idiopathic | Death of aspiration (attributed to underlying disease) | |
| Liu et al18 | 2004 | 2 | 100 | 2/2 (100%) | Flexible EGD under conscious sedation | Inclusion body myositis | None | |
| Chiu et al19 | 2004 | 1 | 120 | 1/1 (100%) | General anesthesia and direct laryngoscopy | Brain stem stroke | None | |
| Murry et al6 | 2005 | 13 | 100 | 11/13 (85%), other 2 had improvement after second injection | EMG-guided transcutaneous approach | Stroke, extirpative head and neck surgery, cranial neuropathies, MVC, chemical inhalation, radiation therapy or lymphoma | None | |
| Kim et al20 | 2006 | 8 | 100 | 5/8 (62.5%) | Flexible endoscopy | CVA | None | |
| Masiero et al21 | 2006 | 2 | 25, 30 | 2/2 (100%) | Percutaneous injection | CVA | None | |
| Restivo et al22 | 2006 | 12 | 60 | 12/12 (100%) | EMG-guided transcutaneous approach | Diabetic neuropathy | None | |
| Suzukia et al23 | 2007 | 1 | 5 | 1/1 (100%) | Percutaneous injection | Spinal muscular atrophy type 2 | Transient worsening of dysphagia | |
| Krause et al24 | 2008 | 1 | 180, 150 | 1/1 (100%), 0/1 (0%) | Endoscopic injection with propofol sedation | Spasticity secondary to SAH | None | |
| Alfonsi et al25 | 2010 | 34 | 15 | 17/34 (50%) | EMG-guided transcutaneous approach | MS, multiple system atrophy (cerebellar variant and Parkinson variant), Parkinson's disease, progressive supranuclear palsy, ataxia-telangiectasia | None | |
| Restivo et al26 | 2011 | 14 | 20 | 14/14 (100%) | Percutaneous injection with EMG guidance | MS | None |
CN — cranial nerve; CT — computed tomography; CVA — cerebrovascular accident or stroke; ALS — amyotrophic lateral sclerosis; EMG — electromyography; CNS — central nervous system; EGD — esophagogastroduodenoscopy; MVC — motor vehicle collision; SAH — subarach-noid hemorrhage; MS — multiple sclerosis.
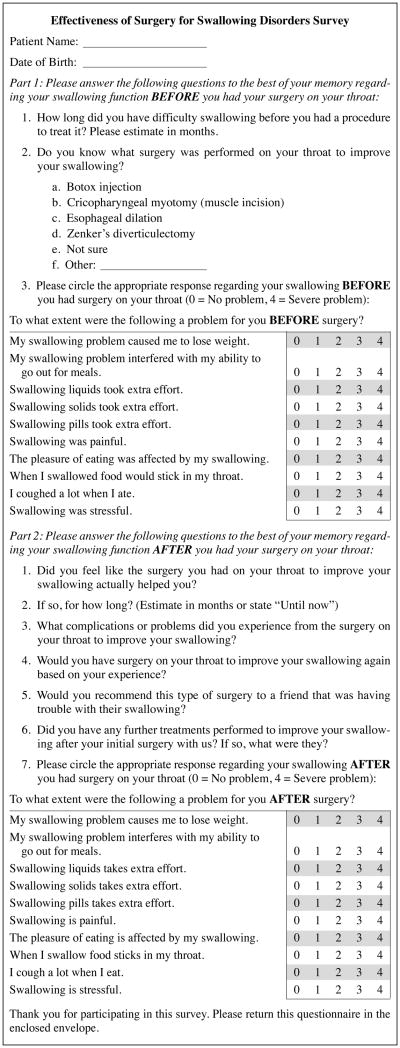
We hypothesized that CP BTX injection was a useful treatment for dysphagia in patients with UES dysfunction and that the identification of patient-related clinical factors and CP muscle histology of patients who respond to CP BTX injection may be used in the decision to perform this intervention. We report a series of 49 patients who received injections of Botox into the CP muscle — the largest patient series in the literature to date. We used a customized version of the Eating Assessment Tool (EAT-10) dysphagia-related quality of life instrument to retrospectively assess patient satisfaction with CP injection of BTX at a single institution (Fig 1). We demonstrated that CP injection of BTX is a relatively safe, well-tolerated treatment for dysphagia related to CP dysfunction, with good efficacy in the majority of patients, and that further work is needed to define the population of patients who might have a poor response to BTX.
Fig 1.
Patient questionnaire.
Methods
Patient Selection and Chart Review
The Institutional Review Board of the Medical College of Wisconsin approved this study. Institutional surgical databases were searched to identify CPT codes of eligible patients. All patients who had undergone injection of BTX type A (Botox) into the CP muscle to treat dysphagia since January 1, 2000, were identified. The inclusion criteria for this study were age greater than 18 years, ability to speak English, available telephone number or postal address, and a documented history of CP dysphagia based on clinical history with supporting radiographic or manometric evidence of UES dysfunction. Patients were excluded from the study if they were noted to have extrinsic compression of the esophagus and/or pharynx, intraluminal disease such as tumor or esophageal web, Zenker's diverticulum, or a history of total laryngectomy. A subgroup of 17 patients who underwent both BTX injection and CP myotomy was also included in the study.
A retrospective chart review was performed on the patients who met the inclusion criteria. Electronic medical records were reviewed to collect demo-graphic information, clinical history, preprocedure diagnostic study results, procedure details, postprocedure diagnostic study results, and outcomes.
Dysphagia Outcomes Survey
Identified patients were mailed a retrospective questionnaire based on a validated dysphagia-related quality-of-life instrument, the EAT-10,27 and asked to comment on the dysphagia symptoms they had before and after the intervention (Fig 1). If no response was received to the mailed survey, the patients were contacted by telephone and the questionnaire was completed by interview.
Cricopharyngeal Muscle Histopathology
The patients who underwent CP myotomy in addition to the BTX injection routinely had a biopsy of the CP muscle performed at the time of the myotomy. The CP muscle specimens were processed and stained in the Medical College of Wisconsin Nerve and Muscle Laboratory by previously described methods.28 The specimens were interpreted by a neurologist with fellowship training in neuromuscular pathology (S.S.J.) and were classified as showing predominantly neurogenic, myopathic, or mixed neurogenic and myopathic changes in accordance with previously detailed criteria.9,28
Statistical Analysis
Patient subgroup analysis was performed with Student's t-test, Fisher's exact test, or χ2 analysis, with the level of significance (p) set at 0.05. Data are presented as average values with standard deviation or data range where appropriate.
Results
Patient Demographics and Characteristics
Forty-nine patients underwent CP BTX injections to treat dysphagia at our institution over the 12-year period between 2000 and 2012 (Table 2), including 19 men and 30 women. Thirty-two (65%) of these patients were treated exclusively with BTX injection. Of the subgroup of 17 patients (35%) who underwent CP myotomy at some point during their treatment, 11 (64%) proceeded to CP myotomy because they had beneficial effects from the BTX injection and desired a more permanent treatment. Two patients underwent CP myotomy initially and after a return of symptoms proceeded to BTX injection for both diagnostic and therapeutic purposes. One patient had the BTX injection and CP myotomy performed at that same time in an attempt to enhance the effect of the myotomy. Three patients had minimal to no improvement with the BTX injection and proceeded to CP myotomy as a different treatment option.
Table 2. Patient demographics.
| Total (n = 49) | BTX Only (n = 32) | BTX and Myotomy (n = 17) | |
|---|---|---|---|
| Male | 19 (39%) | 9 (28%) | 10 (58%) |
| Female | 30 (61%) | 23 (71%) | 7 (41%) |
| Number deceased | 8 (16%) | 6 (19%) | 2 (12%) |
| Mean age and range (y) | 59 (27-91) | 61 (34-91) | 54 (27-83) |
| Feeding tube | 7 (14%) | 3 (9%) | 4 (23%) |
| Aspiration pneumonia | 5 (10%) | 2 (6%) | 3 (18%) |
| Prior head and neck irradiation | 4 (8%) | 3 (9%) | 1 (6%) |
| Prior head and neck surgery | 15 (30%) | 7 (22%) | 8 (47%) |
| Prior CVA | 2 (4%) | 0 | 2 (12%) |
| History of neurodegenerative disease | 24 (49%) | 17 (53%) | 7 (41%) |
| Esophageal dysfunction or dysmotility | 12 (24%) | 9 (28%) | 3 (18%) |
BTX — botulinum toxin.
The mean age at the time of the first injection was 59 years (range, 27 to 91 years). Many patients had severe levels of swallowing dysfunction, including 7 patients with feeding tubes in place and 5 patients with a history of aspiration pneumonia before intervention. Esophageal dysmotility was documented on swallow study or esophageal manometry in 12 of the 49 patients (24%) before injection. Twenty of the 49 patients (40%) had esophageal manometry performed before injection. Of these studies, 16 (80%) demonstrated impaired or shortened dilation of the UES and/or disrupted coordination of the swallow and UES relaxation, 2 demonstrated a hypertensive UES, 1 was normal, and 1 was referenced as “abnormalities of UES.” Forty-five of the 49 patients (92%) had preintervention videofluoroscopic swallow studies, all of which revealed some element of UES dysfunction. Eight study reports made direct reference to some component of pharyngeal dysphagia in addition to UES dysfunction. There were 15 patients with prior head and neck surgical procedures for issues other than dysphagia, and 4 patients with a history of head and neck irradiation. The potential causes of the CP dysphagia varied widely in this group, including cranial nerve neuropathy, prior spinal surgery, cerebrovascular accident, multiple sclerosis, prior chemotherapy and cervical radiation treatment, prior tracheostomy, traumatic brain injury, and idiopathic CP hypertrophy. Nearly half of the patients (24 of 49; 49%) had some history of neurologic dysfunction before the first injection, although only 2 patients had a history of stroke. Eight of these 49 patients (16%) are now deceased.
Clinical Utilization and Response to Intervention
A total of 62 BTX injections were administered during the studied time period to our identified group of 49 patients, and all patients received BTX type A (Botox). Five patients received a total of 2 BTX injections, and 4 patients received a total of 3 BTX injections. The BTX was generally diluted into 0.4 to 0.5 mL total volume of sterile saline solution (final concentration, 20 to 25 U per 0.1 mL). The average dose of BTX was 39 U, but the range of dosages was wide (15 to 100 U). The reasoning for the use of certain dosages was not entirely specified. The BTX was injected either by a transcutaneous technique in the clinic with electromyographic guidance or by direct suspension laryngoscopy to identify the horizontal component of the posterior belly of the CP muscle under direct visualization. The complication rate was low; only 16 patients (33%) reported mild side effects or minor complications, and there were no severe complications or deaths. The patients with mild complications included 2 who had episodes of nonspecific postoperative chest pain or tightness, with a negative workup for further cardiac or acute cause after injection, and 1 who reported worsened upper airway congestion. The remainder of the reported complications were temporary mild worsening of dysphagia, belching, increased mucus production, and worsening of reflux. These symptoms typically resolved after several weeks. One patient had worsening of dysphagia and required hospitalization for intravenous administration of fluids.
Overall, 32 of 49 patients (65%) indicated some improvement in swallowing function during their follow-up clinic visit. This improvement was noted from clinical documentation and/or posttreatment videofluoroscopic swallow study. Post hoc subgroup analysis was performed in an attempt to associate response to CP injection of BTX with multiple identified patient history parameters, including age, history of reflux, history of previous irradiation, history of head and neck surgery, history of documented esophageal motility disorders, history of previous documented neurologic or neurodegenerative condition, and history of stroke. None of these parameters reached statistical significance on further analysis (Fisher's exact test).
Cricopharyngeal Muscle Histopathology
Cricopharyngeal myotomy was performed in 17 patients: 13 via a transcervical approach and 4 via endoscopic transoral carbon dioxide laser–assisted CP myotomy. Fourteen patients underwent the CP myotomy after BTX injection, 2 patients had CP myotomy before the injections, and 1 patient had the CP myotomy and injection concurrently. Cricopharyngeal muscle specimens were submitted for histopathologic analysis from 14 (82%) of these patients (both transcervical and endoscopic procedures). Three patients demonstrated patterns of generalized neuropathy (fiber type grouping), 7 demonstrated generalized myopathic findings, and 4 had mixed histologic findings. The 2 patients who had the BTX injection after the CP myotomy had myopathic and mixed histologic features noted on biopsy specimens. Representative examples of CP muscle histopathologic specimens are displayed in Fig 2, demonstrating myopathic (parts A and B) and neuropathic (parts C and D) patterns. There were 2 samples that were suboptimal, and no further diagnostic features were identified. Six of 11 patients with myopathic or mixed histologic typing on CP biopsy analysis reported subjective improvement of symptoms after BTX injection, whereas all 3 of the patients with neuropathic changes had symptom improvement. No statistically significant association between specific CP muscle histology patterns and benefit from CP BTX could be established because of the overall small number of biopsy results (Fisher's exact test, p > 0.05).
Fig 2.
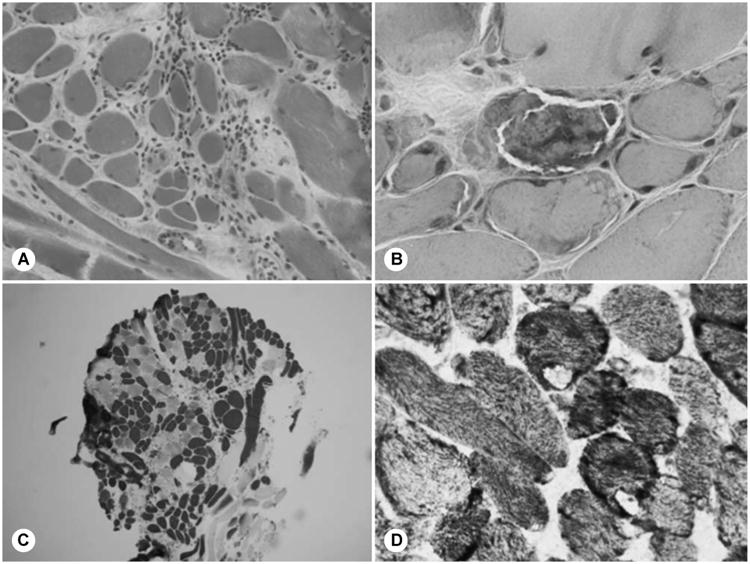
Cricopharyngeal muscle histopathologic specimens. Cryostat sections of cricopharyngeal muscle show myopathic (A,B) and neurogenic (C,D) changes. A) Inflammatory myopathy with mononuclear inflammatory infiltrate, 2 necrotic fibers, and increased fiber size variability (hematoxylin and eosin; original ×40). B) Ragged red fibers (modified Gomori trichrome; original ×100). C) Mild fiber type grouping (toluidine blue adenosine triphosphatase; original ×4). D) Two target fibers (nicotinamide adenine dinucleotide tetrazolium reductase; original ×100).
Dysphagia-Related Quality-of-Life Survey
Patients' answers on the dysphagia-related quality-of-life questionnaires were analyzed, and the results are displayed in Fig 3. A total of 28 patients responded to the mailing or follow-up telephone call and completed the questionnaire (57%). Eighteen patients who had BTX injection only completed the survey, and 5 patients who had BTX injection and CP myotomy completed the survey. The average EAT-10 score before any of these interventions was 28 ± 8 (Fig 3A) — a level that is equal to the levels of dysphagia seen in head and neck cancer populations.27 All interventions (CP BTX injection and both CP myotomy and CP BTX injection) trended toward similar improvements in EAT-10 scores, although because of the extreme variation in patient responses, no statistical significance was attained (Student's t-test, p > 0.05). Eleven of the 18 patients (61%) who underwent treatment with BTX reported some subjective improvement in swallowing function on our survey analysis — close to the 65% with improvement noted on the clinical records (Fig 3B). Four of the 5 patients (80%) who had treatment with BTX and later underwent CP myotomy indicated eventual improvement in their dysphagia. The majority of respondents indicated that they would repeat BTX treatment (55%) or CP BTX injection and myotomy (80%).
Fig 3.
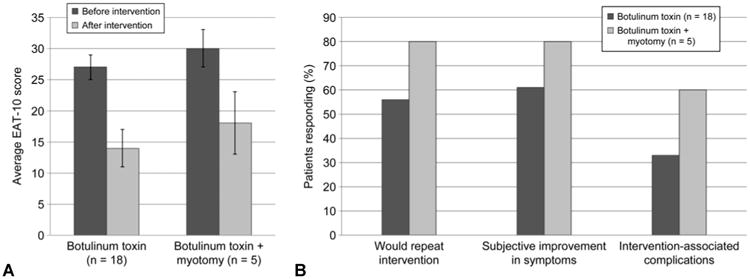
A) Eating Assessment Tool (EAT-10) questionnaire results presented as averaged response scores for patients with botulinum toxin (BTX) injection and those with BTX injection and myotomy. Error bars — standard error of mean. B) Subjective patient impressions of various interventions used to treat upper esophageal sphincter dysfunction, including injection of cricopharyngeal muscle with BTX or combination of BTX injection and myotomy, usually in succession. Data are percentages of patients who responded affirmatively to each question.
Discussion
Dysfunction of the UES can arise from a wide variety of causes, and the care of this disorder is also very diverse. The current operative management of UES dysfunction is quite variable, and there are little data to support the relative superiority in outcomes of one intervention over another. Injection of the CP muscle with BTX has been performed by many clinicians since its initial description in 1994.5 In addition, there is no significant risk of mediastinitis or infection after CP BTX injection, as evidenced by our patient series and review of the literature. Unfortunately, there are no standardized techniques or protocols for administration of CP BTX. In this report, we present findings from 49 patients in an attempt to clarify the role and efficacy of CP BTX injection in management of UES dysfunction. No clear trends were identified to predict who would benefit from CP injection of BTX, but the procedure appears to be well tolerated, with minimal side effects.
This report is the largest series to date of patients who received CP injection of BTX for treatment of UES dysfunction. Of the 49 patients included in the study, 65% had some improvement after treatment with BTX. This overall rate of improvement seems to correlate to the 61% improvement rate noted in our post hoc survey analysis of this same patient population, and to the 75% rate of success in the literature. It should be noted that the degree of improvement in swallowing function varied significantly among our patient population, and this variability is also seen in the literature. It remains difficult to discern those who may not benefit from treatment with BTX on the basis of our data analysis and review of the literature. The dosage of BTX varies in our single tertiary care center, as it does among the various case reports in the literature. The decision-making process for each specific dose for patients is not entirely described in clinical records, but overall trends in our practice demonstrate an increasing dose profile over time.
Patient survey questionnaires provided additional subjective data regarding the efficacy of CP injection of BTX in our population. In the initial validation of the EAT-10 instrument by Belafsky et al,27 patients with oropharyngeal dysphagia had an average EAT-10 score of 23 ± 12 and those with a history of head and neck cancer had an average score of 22 ± 14. Our series of patients with CP dysphagia had a similar recalled preoperative average baseline EAT-10 score of 28 ± 8, demonstrating the power and utility of this dysphagia-related quality-of-life instrument in dysphagia outcomes research. All treatment subgroups in our study had significant improvements in modified EAT-10 scores after their treatment, whether BTX injection or both BTX and CP myotomy. The retrospective nature of our survey certainly induced bias in the measurement of surgical treatment outcomes, but it is encouraging to note that all of these treatments for surgical dysphagia have perceived patient benefit. Patients also generally benefited from myotomy if they noted improved swallowing with BTX injection. Additionally, we have now integrated the EAT-10 questionnaire into our clinical practice and have each dysphagia patient complete this questionnaire at clinic visits.
Several prior studies have attempted to investigate specific patient characteristics that predict successful treatment of CP dysphagia with BTX. Multiple variables have been considered, including technique of injection, underlying causative diagnosis of the dysphagia, CP muscle histopathology, dysfunction of oral and pharyngeal swallowing mechanisms, and esophageal dysmotility and disease. Injection of BTX directly into the CP muscle and avoidance of injection into the pharyngeal constrictors is a crucial component of this surgical technique, and localization of the CP muscle with electromyographic guidance or direct endoscopic visualization may improve outcomes.29 Accuracy of the injection is vital in using this technique as a diagnostic tool before CP myotomy. Several patients in our series received multiple injections due to a failed initial response after clinic injection, and demonstrated improvement after a second intervention in the operating room. Injection technique failure may also explain improvement in patients' symptoms after CP myotomy despite disappointing results after CP BTX injection.10,14,22,26 Consideration for a second injection under direct visualization should be entertained in those patients who initially fail to demonstrate symptom improvement after the first injection. Patients who continue to have dysphagia despite intervention may have a prolonged transition between the oral and pharyngeal stages of swallowing, further suggesting a role for electrophysiological evaluation.25 This evaluation may identify the component of altered coordination and patients who would not benefit from CP BTX injection.
Although there has not been any identified correlation between the cause of the dysphagia and the efficacy of CP BTX injection, further investigation of CP muscle histopathologic features may provide information on the relationship between muscle function and subsequent treatment response. The histopathologic nature of the CP muscle is the subject of some debate in the published literature. In a previous study,28 we compared CP muscle specimens of patients who had undergone prior BTX injection to control specimens (patients with no prior history of BTX injection who were undergoing CP myotomy) and noted an increased incidence of neuropathic or mixed changes in patients with prior BTX injection, although this was not statistically significant. Zaninotto et al17 noted that CP muscle specimens after BTX injection demonstrated a more fibrotic phenotype. Although analysis of muscle fiber composition in small studies has not demonstrated clear trends, findings from our group suggested that relative myogenic and neurogenic histopathologic patterns in CP muscle specimens may predict response to BTX injection.30 In the current patient series, the group of patients who underwent treatment with BTX before myotomy did not demonstrate a clear predominance of any specific pathologic subtype on CP muscle analysis. The sample sizes from both studies are unfortunately too small for us to draw generalized conclusions regarding this trend, and further study is required to clarify this relationship. Understanding the neuromuscular and histopathologic patterns underlying CP muscle dysfunction may provide better clinical ability to predict response to specific treatments in the future.
There were minimal complications in our patient series, and no life-threatening issues or deaths were associated with BTX treatment. The most common complaint in our series was temporary worsening of dysphagia, often associated with increased belching or reflux sensation. The cause is likely induced muscle weakening of the UES after injection, and may perhaps suggest proper localization of the injection. Patients seem to recover from this symptom after several weeks and often experience beneficial effects of BTX at that time. One of our patients required hospitalization for intravenous fluid hydration due to this worsening dysphagia. Another patient with severe multifactorial dysphagia eventually underwent gastrostomy tube placement due to ongoing lack of response to both CP BTX injection and endoscopic CP myotomy. There were no episodes of vocal fold paresis in our series, but it has been documented previously.10 These results are reassuring that BTX injections are in fact not excessively invasive and that they have few side effects and no major complications.
In 65% of this patient series, CP BTX injection was effective in the treatment of UES dysfunction. Our ability to predict which patients will respond to CP BTX is still evolving because of the multifactorial causes of CP dysfunction and dysphagia. Because it is difficult to identify patients who may not respond well to the intervention, a trial of CP BTX injection is currently the only way to determine which patients will benefit. Our data suggest that patients who do respond will have improved quality of life and nutritional status with minimal associated risks from this procedure. Cricopharyngeal electromyography was not routinely performed in our patient series; however, it may provide additional information on pharyngeal and UES function before intervention. This information and additional histopathologic information may lead to identifying ideal candidates for CP BTX injection. Further research is needed to further define the utility of preoperative CP electromyography and postoperative CP muscle histopathologic analysis in the management of patients with CP dysfunction.
Acknowledgments
The authors thank Dr Michael Collins of the Medical College of Wisconsin Department of Neurology for his assistance in reviewing the histopathologic data and images, and Dr Robert Toohill for his critical review of the manuscript.
Footnotes
Presented at the meeting of the American Broncho-Esophagological Association, San Diego, California, April 18-19, 2012.
References
- 1.Kelly JH. External approach to cricopharyngeus muscle (CP) myotomy. Oper Techn Otolaryngol. 1997;8:193–8. [Google Scholar]
- 2.McKenna JA, Dedo HH. Cricopharyngeal myotomy: indications and technique. Ann Otol Rhinol Laryngol. 1992;101:216–21. doi: 10.1177/000348949210100304. [DOI] [PubMed] [Google Scholar]
- 3.Dauer E, Salassa J, Iuga L, Kasperbauer J. Endoscopic laser vs open approach for cricopharyngeal myotomy. Otolaryngol Head Neck Surg. 2006;134:830–5. doi: 10.1016/j.otohns.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Allen J, White CJ, Leonard R, Belafsky PC. Effect of cricopharyngeus muscle surgery on the pharynx. Laryngoscope. 2010;120:1498–503. doi: 10.1002/lary.21002. [DOI] [PubMed] [Google Scholar]
- 5.Schneider I, Thumfart WF, Pototschnig C, Eckel HE. Treatment of dysfunction of the cricopharyngeal muscle with botulinum A toxin: introduction of a new, noninvasive method. Ann Otol Rhinol Laryngol. 1994;103:31–5. doi: 10.1177/000348949410300105. [DOI] [PubMed] [Google Scholar]
- 6.Murry T, Wasserman T, Carrau RL, Castillo B. Injection of botulinum toxin A for the treatment of dysfunction of the upper esophageal sphincter. Am J Otolaryngol. 2005;26:157–62. doi: 10.1016/j.amjoto.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Blitzer A, Brin MF. Use of botulinum toxin for diagnosis and management of cricopharyngeal achalasia. Otolaryngol Head Neck Surg. 1997;116:328–30. doi: 10.1016/S0194-59989770267-5. [DOI] [PubMed] [Google Scholar]
- 8.Sivarao DV, Goyal RK. Functional anatomy and physiology of the upper esophageal sphincter. Am J Med. 2000;108(suppl 4a):27S–37S. doi: 10.1016/s0002-9343(99)00337-x. [DOI] [PubMed] [Google Scholar]
- 9.Schulze SL, Rhee JS, Kulpa JI, Danielson SK, Toohill RJ, Jaradeh SS. Morphology of the cricopharyngeal muscle in Zenker and control specimens. Ann Otol Rhinol Laryngol. 2002;111:573–8. doi: 10.1177/000348940211100702. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson SI, Rees J. Botulinum toxin for cricopharyngeal dysphagia: case reports of CT-guided injection. J Otolaryngol. 1997;26:273–6. [PubMed] [Google Scholar]
- 11.Brant CQ, Siqueira ES, Ferrari AP., Jr Botulinum toxin for oropharyngeal dysphagia: case report of flexible endoscopeguided injection. Dis Esophagus. 1999;12:68–73. doi: 10.1046/j.1442-2050.1999.00015.x. [DOI] [PubMed] [Google Scholar]
- 12.Alberty J, Oelerich M, Ludwig K, Hartmann S, Stoll W. Efficacy of botulinum toxin A for treatment of upper esophageal sphincter dysfunction. Laryngoscope. 2000;110:1151–6. doi: 10.1097/00005537-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Shaw GY, Searl JP. Botulinum toxin treatment for cricopharyngeal dysfunction. Dysphagia. 2001;16:161–7. doi: 10.1007/s00455-001-0074-8. [DOI] [PubMed] [Google Scholar]
- 14.Haapaniemi JJ, Laurikainen EA, Pulkkinen J, Marttila RJ. Botulinum toxin in the treatment of cricopharyngeal dysphagia. Dysphagia. 2001;16:171–5. doi: 10.1007/s00455-001-0059-7. [DOI] [PubMed] [Google Scholar]
- 15.Moerman M, Callier Y, Dick C, Vermeersch H. Botulinum toxin for dysphagia due to cricopharyngeal dysfunction. Eur Arch Otorhinolaryngol. 2002;259:1–3. doi: 10.1007/pl00007520. [DOI] [PubMed] [Google Scholar]
- 16.Parameswaran MS, Soliman AMS. Endoscopic botulinum toxin injection for cricopharyngeal dysphagia. Ann Otol Rhinol Laryngol. 2002;111:871–4. doi: 10.1177/000348940211101002. [DOI] [PubMed] [Google Scholar]
- 17.Zaninotto G, Marchese Ragona R, Briani C, et al. The role of botulinum toxin injection and upper esophageal sphincter myotomy in treating oropharyngeal dysphagia. J Gastrointest Surg. 2004;8:997–1006. doi: 10.1016/j.gassur.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Liu LWC, Tarnopolsky M, Armstrong D. Injection of botulinum toxin A to the upper esophageal sphincter for oropharyngeal dysphagia in two patients with inclusion body myositis. Can J Gastroenterol. 2004;18:397–9. doi: 10.1155/2004/360537. [DOI] [PubMed] [Google Scholar]
- 19.Chiu MJ, Chang YC, Hsiao TY. Prolonged effect of botulinum toxin injection in the treatment of cricopharyngeal dysphagia: case report and literature review. Dysphagia. 2004;19:52–7. doi: 10.1007/s00455-003-0029-3. [DOI] [PubMed] [Google Scholar]
- 20.Kim DY, Park CI, Ohn SH, Moon JY, Chang WH, Park SW. Botulinum toxin type A for poststroke cricopharyngeal muscle dysfunction. Arch Phys Med Rehabil. 2006;87:1346–51. doi: 10.1016/j.apmr.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Masiero S, Briani C, Marchese-Ragona R, Giacometti P, Constantini M, Zaninotto G. Successful treatment of longstanding post-stroke dysphagia with botulinum toxin and rehabilitation. J Rehabil Med. 2006;38:201–3. doi: 10.1080/16501970500515840. [DOI] [PubMed] [Google Scholar]
- 22.Restivo DA, Marchese-Ragona R, Lauria G, Squatrito S, Gullo D, Vigneri R. Botulinum toxin treatment for oropharyngeal dysphagia associated with diabetic neuropathy. Diabetes Care. 2006;29:2650–3. doi: 10.2337/dc05-2486. [DOI] [PubMed] [Google Scholar]
- 23.Suzukia Y, Sano N, Shinonaga C, Fukuda M, Hyodo M, Morimoto T. Successful botulinum toxin treatment of dysphagia in a spinal muscular atrophy type 2 patient. Brain Dev. 2007;29:662–5. doi: 10.1016/j.braindev.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Krause E, Schirra J, Gürkov R. Botulinum toxin A treatment of cricopharyngeal dysphagia after subarachnoid hemorrhage. Dysphagia. 2008;23:406–10. doi: 10.1007/s00455-007-9132-1. [DOI] [PubMed] [Google Scholar]
- 25.Alfonsi E, Merlo IM, Ponzio M, et al. An electrophysiological approach to the diagnosis of neurogenic dysphagia: implications for botulinum toxin treatment. J Neurol Neurosurg Psychiatry. 2010;81:54–60. doi: 10.1136/jnnp.2009.174698. [DOI] [PubMed] [Google Scholar]
- 26.Restivo DA, Marchese-Ragona R, Patti F, et al. Botulinum toxin improves dysphagia associated with multiple sclerosis. Eur J Neurol. 2011;18:486–90. doi: 10.1111/j.1468-1331.2010.03189.x. [DOI] [PubMed] [Google Scholar]
- 27.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10) Ann Otol Rhinol Laryngol. 2008;117:919–24. doi: 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 28.Merati AL, Tseng J, Blumin JH, Toohill RJ, Jaradeh S. Comparative neuromuscular histopathology of cricopharyngeal achalasia patients with and without previous botulinum toxin treatment. Ann Otol Rhinol Laryngol. 2007;116:375–80. doi: 10.1177/000348940711600510. [DOI] [PubMed] [Google Scholar]
- 29.Moerman MBJ. Cricopharyngeal Botox injection: indications and technique. Curr Opin Otolaryngol Head Neck Surg. 2006;14:431–6. doi: 10.1097/MOO.0b013e328010b85b. [DOI] [PubMed] [Google Scholar]
- 30.Davis MV, Merati AL, Jaradeh SS, Blumin JH. Myosin heavy chain composition and fiber size of the cricopharyngeus muscle in patients with achalasia and normal subjects. Ann Otol Rhinol Laryngol. 2007;116:643–6. doi: 10.1177/000348940711600903. [DOI] [PubMed] [Google Scholar]