Abstract
Objectives
We present the second published case of laryngeal involvement in mitochondrial myopathy.
Methods
A patient with laryngeal involvement of mitochondrial myopathy is presented, together with a literature review.
Results
A 41-year-old man presented with progressive breathy dysphonia. His brother had mitochondrial myopathy. Biopsy of the biceps muscle demonstrated cytochrome C oxidase–negative ragged blue fibers confirming mitochondrial myopathy. Videostroboscopy showed marked vocal fold atrophy, but subsequent injection laryngoplasty did not significantly improve the patient’s voice, despite improved postoperative glottic closure.
Conclusions
Mitochondrial myopathy should be considered in the differential diagnosis of severe early-onset vocal fold atrophy.
Keywords: atrophy, dysphonia, injection laryngoplasty, mitochondrial myopathy, speech therapy, vocal cord, vocal fold
INTRODUCTION
Normal laryngeal neuromuscular function enables numerous physiological functions, such as protection of the airway from aspiration, voice production, and maintenance of adequate airflow for respiration. Neurologic deficits such as motor weakness, loss of laryngopharyngeal sensation, disrupted laryngeal muscle coordination, and abnormal protective laryngeal reflexes can impair all of these vital functions. Dysphonia can often be the presenting symptom of a progressive neurologic disorder because of altered laryngeal and pharyngeal closing force, altered palatal and oral mechanics, and inability to maintain adequate subglottic air pressure to sustain voicing. Altered neurologic function is often suspected when patients present with progressive dysphonia and/or dysphagia without associated mass lesions or antecedent neurologic injury such as stroke or surgical injury.1 Appropriate diagnosis of an underlying neurologic disorder is essential to providing proper treatment recommendations. A number of progressive neuromuscular disorders can cause dysphonia, dysphagia, and/or respiratory insufficiency, including Parkinson’s disease, amyotrophic lateral sclerosis, and progressive bulbar palsies. Pharyngeal symptoms have also been noted in systemic myopathies, including mitochondrial myopathy.2 However, only 1 prior case report has demonstrated findings of laryngeal involvement in mitochondrial myopathy.3 We present the second known case of laryngeal involvement of this rare disease, in a patient demonstrating severe early-onset vocal fold atrophy and consequent breathy dysphonia that was unresponsive to injection laryngoplasty and voice therapy.
CASE REPORT
A 41-year-old man presented to the otolaryngology clinic with a 1-year history of progressive dysphonia and dysphagia. He described his voice as primarily breathy and rough, and said he had occasional better voice quality in the early morning. He additionally complained of slowly worsening dysphagia with a regular sensation of solid foods sticking in his throat. He did not report any difficulty in breathing or stridor, but did complain of fatigue. He had previously seen a neurologist for symptoms of mild proximal muscle weakness, and had a medical history of type II diabetes mellitus and depression. He also reported a family history of neuromuscular disease. His older brother had complaints of proximal muscle weakness and ophthalmoplegia and had recently received a diagnosis of mitochondrial myopathy based on muscle biopsy. Another brother had a diagnosis of total ophthalmoplegia and was also being actively followed by a neurologist.
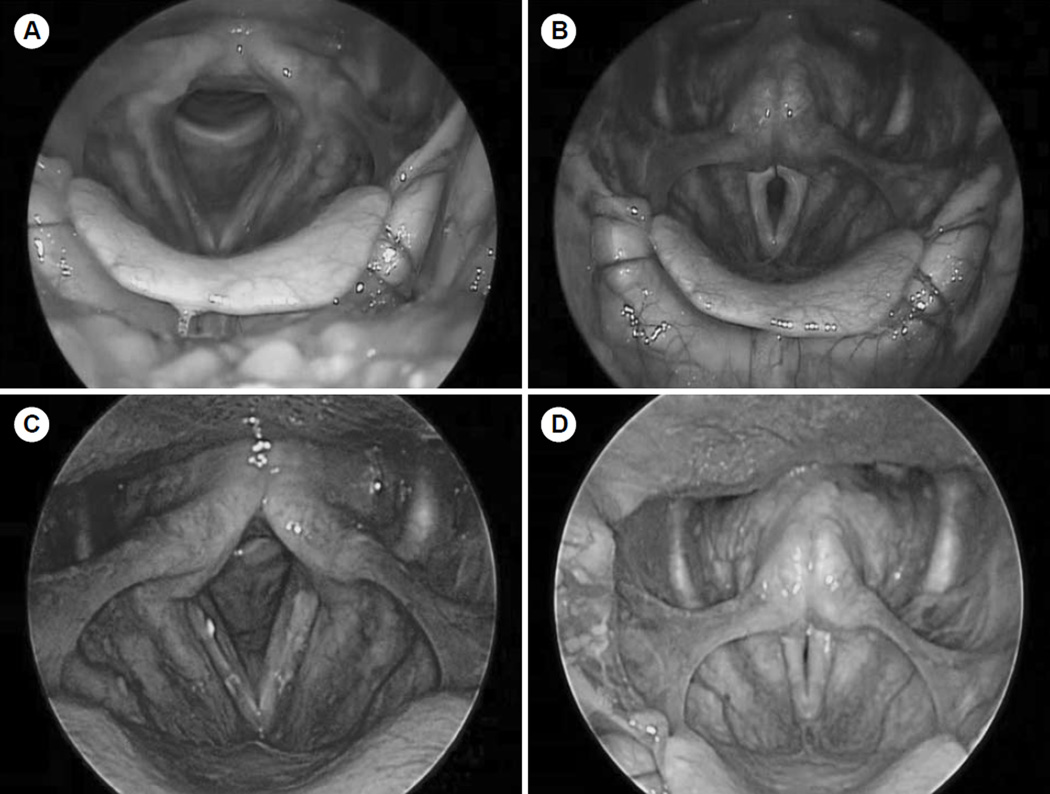
The initial clinical examination demonstrated a man who appeared much older than his stated age, with a somewhat haggard appearance. He had significant muscle wasting of his face and mild weakness of his palatal elevation and facial function. A voice evaluation revealed a severely breathy and strained voice without stridor. Rigid and flexible videostroboscopic laryngeal examinations demonstrated preserved true vocal fold movement with marked vocal fold atrophy and a large glottic gap maintained throughout the phonatory cycle with a large, flaccid mucosal wave (Fig 1A,B). Because of the patient’s marked glottic gap and severe breathy dysphonia, vocal fold augmentation was recommended to improve glottic closure. The patient was also referred for definitive neurologic evaluation before surgery because of his family history and unusual presentation.
Fig. 1.
Videostroboscopic laryngeal imaging. Preoperative abduction (A) and adduction (B) images on rigid videostroboscopy show marked vocal fold atrophy and large mucosal wave with persistent glottic gap. Postoperative abduction (C) and adduction (D) images show improved vocal fold bulk and improved glottic gap after injection laryngoplasty.
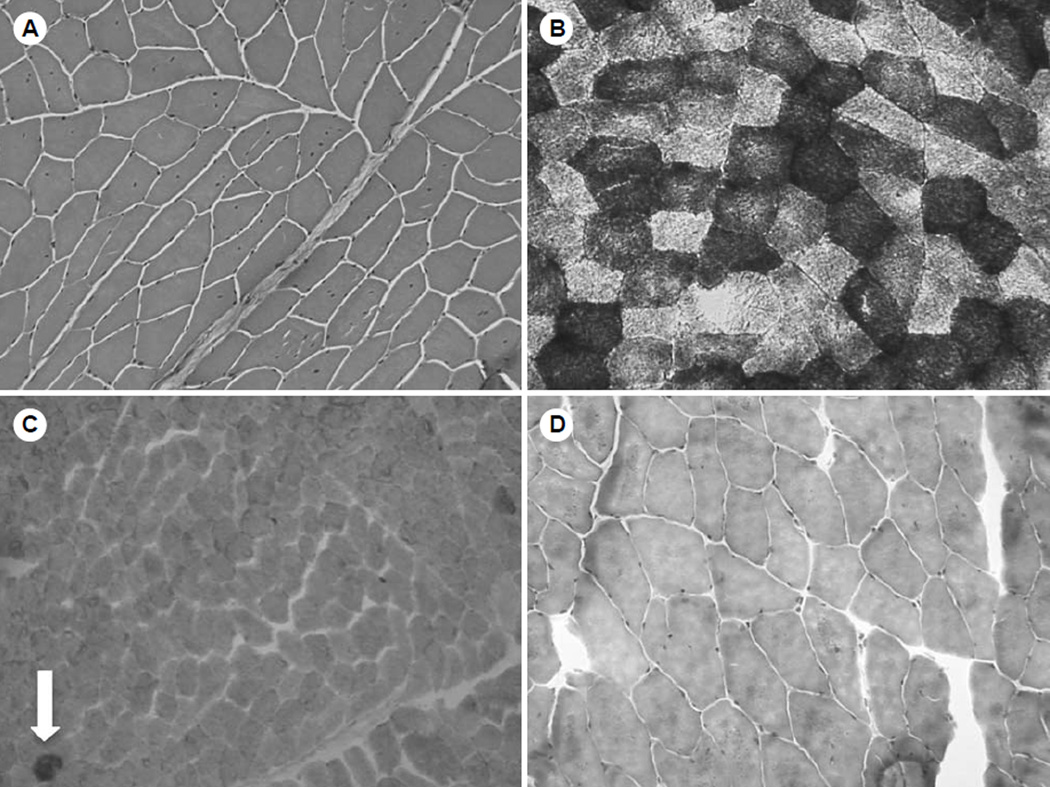
Electromyography demonstrated evidence of an inactive myopathy of the orbicularis oris muscle. Additionally, a biopsy of the left biceps muscle demonstrated findings indicative of mitochondrial myopathy (Fig 2). Hematoxylin and eosin staining showed multiple muscle fibers with central nuclei but no obvious increase in connective tissue between the muscle fibers or alterations in fiber size (Fig 2A). Congo red staining was negative for the presence of amyloid. The muscle biopsy specimen was analyzed with several different oxidative enzyme stains that often help in assessment of mitochondrial abnormalities. Nicotinamide adenine dinucleotide hydride tetrazolium reductase staining revealed many muscle fibers with a “moth-eaten” appearance in either the center or elsewhere in the muscle fiber, suggesting multiple areas of absent oxidative activity (Fig 2B). The presence of ragged fibers, which represent the subsarcolemmal accumulation of mitochondria often seen in mitochondrial myopathies, was analyzed by Gomori trichome and succinyl dehydrogenase (SDH) staining. There were no ragged red fibers on modified Gomori trichome staining (Fig 2D) or ragged blue fibers on SDH staining. However, cytochrome C oxidase (COX) staining showed 3 COX-negative ragged blue fibers under a lowpower field, indicative of mitochondrial myopathy (Fig 2C). Stains for proteins associated with muscular dystrophies, including dystrophin, merosin, and sarcoglycan (an associated glycoprotein), were also performed and showed normal findings. Further genetic testing was performed, including screens for limb girdle muscular dystrophy and oculopharyngeal muscular dystrophy; these were negative. Serum creatine kinase, lactic acid, and carnitine levels were measured to investigate potential categories of myopathy; these levels were all normal.
Fig. 2.
Immunohistochemical analysis of biceps muscle. A) Hematoxylin and eosin staining shows many muscle fibers with central nuclei. B) Staining with nicotinamide adenine dinucleotide hydride demonstrates that many muscle fibers have “motheaten” appearance. C) Staining for cytochrome C oxidase and succinyl dehydrogenase demonstrates 2 cytochrome C oxidase–negative ragged blue fibers (arrow) on low power. D) Modified Gomori trichrome stain demonstrates no obvious ragged red fibers.
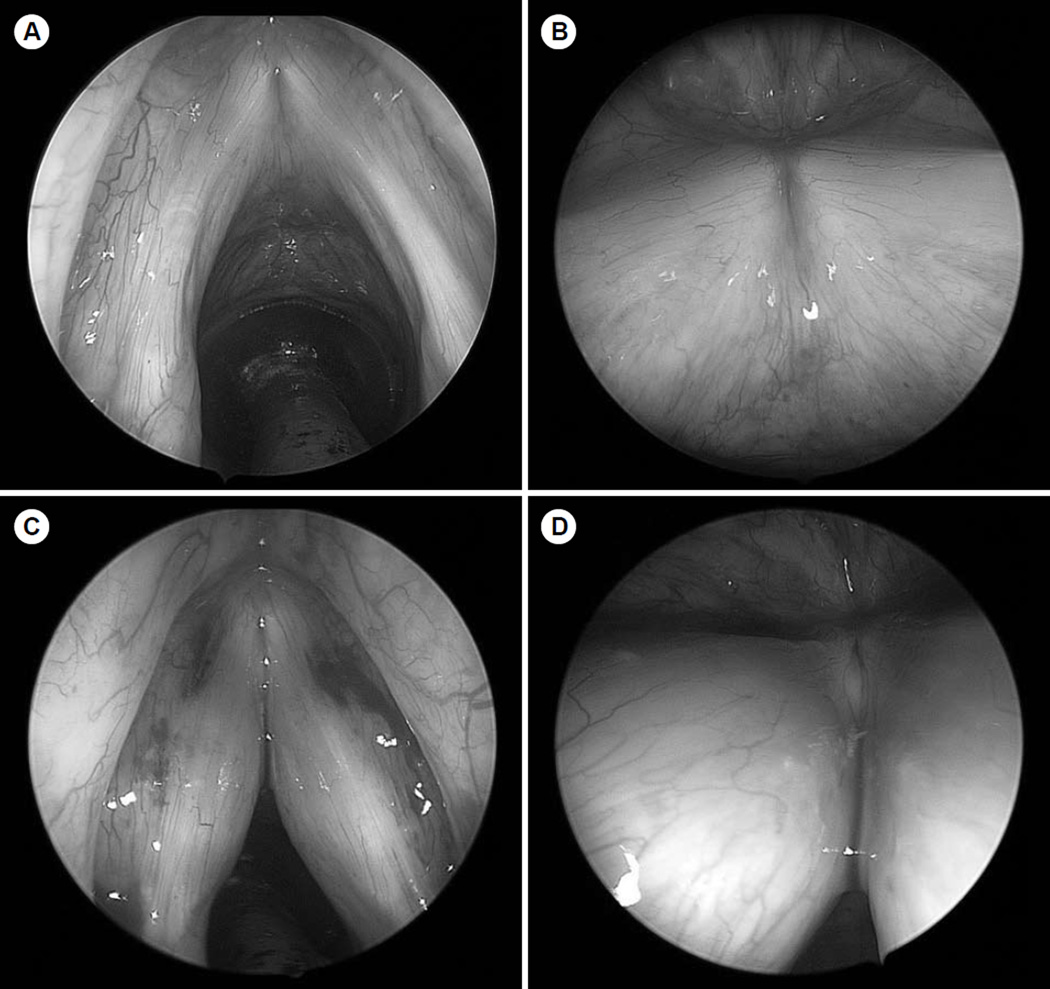
The patient was eventually taken to the operating room for suspension microlaryngoscopy with bilateral injection of micronized acellular human dermis into both vocal folds. Operative laryngoscopy demonstrated severe bilateral vocal fold atrophy without mucosal lesions (Fig 3A,B). The patient was given purposeful overinjection of the anterior and posterior parts of both vocal folds (Fig 3C,D). He tolerated the procedure well, and there were no complications. He returned for postoperative follow-up 3 weeks after injection, and his voice remained quite breathy and his speech was rather uncoordinated. On repeat stroboscopic examination (Fig 1C,D), his glottic closure was significantly improved, but he continued to have a large, flaccid mucosal wave with subsequent breathy dysphonia and a short maximum phonation time. The patient was referred for ongoing speech therapy to improve his vocal effort and breath support, but had minimal subsequent improvement in his voice. He was started on a regimen of co-enzyme Q (200 mg 3 times daily) to prevent progression of his mitochondrial myopathy, in addition to high-dose thiamine, riboflavin, and vitamin C supplementation. At the most recent clinical follow-up appointment, his breathy dysphonia re- mained severe but stable.
Fig. 3.
Intraoperative 0° and 70° telescopic images taken A,B) before and C,D) after injection laryngoplasty with micronized alloderm. Both folds were injected at anterior and posterior locations, and significant medialization effect was noted.
DISCUSSION
Mitochondrial myopathies are a diverse group of disorders that are maternally inherited and result in primary dysfunction of the mitochondrial oxidative respiratory chain. Because of varied genotype penetrance and disease severity, patients with this disorder have a wide spectrum of symptoms. They may present with slowly progressive peripheral muscle weakness, with multisystem organ failure, or solely with respiratory insufficiency requiring mechanical ventilation.4 Because of the heterogeneous nature of this group of disorders, many patients do not fit into one particular syndrome. Many forms of this disorder manifest in childhood or adolescence and have a very poor prognosis. However, some milder forms can present in adulthood, and they often include ptosis, ophthalmoplegia, and slowly progressive cranial nerve dysfunction. Dysphagia is a common theme of many mitochondrial myopathies, but only 1 previous case report describes a (single) patient with true vocal fold involvement.3 That patient presented in a fashion similar to that of our case, with slowly progressive weakness of the voice, poor cough, and poor glottic closure that was seen on stroboscopy and did not respond to speech therapy.
Diagnosis of mitochondrial myopathy can often be challenging. Electromyographic testing will often show evidence of active or inactive myopathy, as shown in our patient on the orbicularis oris muscle. Muscle biopsy and molecular genetic test- ing can also be performed to confirm the diagnosis.2,5 Several characteristic features can be noted on light microscopy, including detection of ragged red fibers on modified trichrome Gomori stain and ragged blue fibers on SDH stain. Absence of COX staining is also often seen in mitochondrial myopathy, as in our patient’s biopsy. The COX reaction has subunits in the nuclear and mitochondrial DNA that make the COX stain valuable in diagnosing mitochondrial myopathies.5 However, muscle immunohistochemical findings may appear normal in patients who have genetic study results consistent with mitochondrial dysfunction.5,6 Notably, the patient described in the literature had laryngeal involvement of mitochondrial myopathy and also had absent COX staining without evidence of ragged red fibers.3 Our case demonstrates the utility of a thorough neurologic evaluation in patients with unexplained early-onset vocal fold atrophy and dysphagia, and suggests that electromyographic testing and muscle biopsy may be appropriate in properly selected patients with severe early-onset vocal fold atrophy, especially if they have a family history of mitochondrial myopathy.
Laryngeal symptoms are rare in patients with mitochondrial myopathy, and therefore no standard laryngeal treatment regimen has been established for these patients. In the prior case of a patient with mitochondrial myopathy and laryngeal involvement, speech therapy alone was recommended.3 Typically, glottic insufficiency caused by various neurogenic disorders can be treated to at least some degree with injection laryngoplasty or medialization thyroplasty.7,8 Although these procedures may improve glottic closure and voice strength and quality, they do not necessarily improve the glottic closing force or tone necessary for phase symmetry. Medialization procedures alter the vocal fold in a static manner, but do not adjust for any dynamic alterations from the disease process that may be present.7 The vocal fold augmentation performed on our patient may have improved the bulk of the vocal folds, but it likely did not have an impact on their flaccidity, therefore resulting in limited improvement in his voice quality. In addition, vocal fold augmentation procedures do not improve overall respiratory support for voicing, and therefore may not be of benefit for all patients. This need for respiratory support appears to be part of the reason for the lack of improvement in our patient after vocal fold augmentation. It might have been appropriate to further evaluate the patient’s lung function with pulmonary function tests before surgical intervention to further determine the potential utility of this intervention. Speech therapy (including intensive therapies directed toward increasing breath support and volume, such as Lee Silverman Voice Therapy) may prove beneficial to this patient population.8 It appears that vocal fold augmentation alone may not improve glottic closing force sufficiently to improve voice in patients with mitochondrial myopathy.
The initial consideration of a neurodegenerative process as a diagnosis was essential for this patient’s management. Although the patient’s main complaint in our clinic was a weak voice, investigation into his other symptoms, including his palatal and facial weakness, was imperative. His loss of muscle tone and cranial nerve weakness were suggestive of a type of myopathy. These findings were supported by his family history and prompted a referral to a neurologist, as well as further workup with electromyography and muscle biopsy. During the initial assessment of a patient with dysphonia or dysphagia, evaluation of other clinical signs such as proximal muscle weakness, cranial neuropathies, muscle spasticity, and tremors is valuable, and these signs may represent manifestations of neurologic disease.9 Assessment of handwriting may provide additional information in identification of the cause of the patient’s dysphonia and potentially lead to more direct management and treatment options.10 These signs and symptoms should lead one to suspect other diagnoses, including neuromuscular disease, mitochondrial disorder, and autoimmune disease. Further workup including electromyography, biopsies, laboratory tests, and consultation by a neurologist is imperative in further management.
Conclusions
We describe the second known case of laryngeal involvement of mitochondrial myopathy. The lack of response to vocal fold augmentation in this patient suggests that mitochondrial myopathies with laryngeal involvement may not respond to vocal fold medialization because of underlying muscle dysfunction. Mitochondrial myopathy should be considered in the differential diagnosis of patients with severe early-onset vocal fold atrophy and breathy dysphonia.
REFERENCES
- 1.Woodson G. Management of neurologic disorders of the larynx. Ann Otol Rhinol Laryngol. 2008;117:317–326. doi: 10.1177/000348940811700501. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer G, Chinnery PF. Diagnosis and treatment of mitochondrial myopathies. Ann Med. 2011 Aug 25; doi: 10.3109/07853890.2011.605389. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartley C, Ascott F. Laryngeal involvement in mitochondrial myopathy. J Laryngol Otol. 1994;108:685–687. doi: 10.1017/s0022215100127835. [DOI] [PubMed] [Google Scholar]
- 4.Cros D, Palliyath S, DiMauro S, Ramirez C, Shamsnia M, Wizer B. Respiratory failure revealing mitochondrial myopathy in adults. Chest. 1992;101:824–828. doi: 10.1378/chest.101.3.824. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RW, Schaefer AM, Barron MJ, McFarland R, Turnbull DM. The diagnosis of mitochondrial muscle disease. Neuromuscul Disord. 2004;14:237–245. doi: 10.1016/j.nmd.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer AM, Blakely EL, Griffiths PG, Turnbull DM, Taylor RW. Ophthalmoplegia due to mitochondrial DNA disease: the need for genetic diagnosis. Muscle Nerve. 2005;32:104–107. doi: 10.1002/mus.20319. [DOI] [PubMed] [Google Scholar]
- 7.Damrose EJ, Berke GS. Advances in the management of glottic insufficiency. Curr Opin Otolaryngol Head Neck Surg. 2003;11:480–484. doi: 10.1097/00020840-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Sewall GK, Jiang J, Ford CN. Clinical evaluation of Parkinson’s-related dysphonia. Laryngoscope. 2006;116:1740–1744. doi: 10.1097/01.mlg.0000232537.58310.22. [DOI] [PubMed] [Google Scholar]
- 9.Rubin AD. Neurolaryngologic evaluation of the performer. Otolaryngol Clin North Am. 2007;40:971–989. doi: 10.1016/j.otc.2007.05.005. vi. [DOI] [PubMed] [Google Scholar]
- 10.Lange KW, Mecklinger L, Walitza S, et al. Brain dopamine and kinematics of graphomotor functions. Hum Mov Sci. 2006;25:492–509. doi: 10.1016/j.humov.2006.05.006. [DOI] [PubMed] [Google Scholar]