Abstract
This paper presents the first comprehensive investigation of PM2.5 and PM10 composition and sources in Saudi Arabia. We conducted a multi-week multiple sites sampling campaign in Jeddah between June and September, 2011, and analyzed samples by XRF. The overall mean mass concentration was 28.4 ± 25.4 μg/m3 for PM2.5 and 87.3 ± 47.3 μg/m3 for PM10, with significant temporal and spatial variability. The average ratio of PM2.5/PM10 was 0.33. Chemical composition data were modeled using factor analysis with varimax orthogonal rotation to determine five and four particle source categories contributing significant amount of for PM2.5 and PM10 mass, respectively. In both PM2.5 and PM10 sources were (1) heavy oil combustion characterized by high Ni and V; (2) resuspended soil characterized by high concentrations of Ca, Fe, Al, and Si; and (3) marine aerosol. The two other sources in PM2.5 were (4) Cu/Zn source; (5) traffic source identified by presence of Pb, Br, and Se; while in PM10 it was a mixed industrial source. To estimate the mass contributions of each individual source category, the CAPs mass concentration was regressed against the factor scores. Cumulatively, resuspended soil and oil combustion contributed 77 and 82% mass of PM2.5 and PM10, respectively.
Introduction
Atmospheric pollution in Saudi Arabia, as a serious consequence of the rapid economic and social growth associated with fuel over-consumption, has recently become of considerable interest. Also, just as they are worldwide, chronic illnesses such as cancer, cardiovascular and respiratory diseases constitute a serious public health problem in Saudi Arabia (NCR). In general, the studies of the effects of air pollution on chronic diseases are not conclusive due to the difficulty of assembling large cohorts, following subjects through a long enough period of time, and the difficulty in measuring personal exposures to ambient air pollution (Chen and Goldberg, 2009). However, most recent reviews agree that the human health effects should not be attributed simply to the total mass concentration, and call to establish the toxicity of particulate matter (PM) components (Fanning et al. 2007), specifically metallic elements (Lippmann and Chen, 2009), inorganic compounds (Schlesinger, 2007) and carbonaceous PM components (Mauderly and Chow, 2008). Identifying the sources of ambient air pollution will support further research of specific mechanistic pathways of pollution and diseases.
Jeddah is the second largest city and the most significant commercial center in the Kingdom of Saudi Arabia. The growth of the city over the last thirty years has been rapid and diverse, and continues to date. Unfortunately, due to lack of awareness and proper regulations, these development activities were accompanied by environmental degradation, and over the years the air quality progressively deteriorated. Most recent study by El-Assouli, et al. (2007) reported that PM10 in the city of Jeddah routinely exceeds the average hourly standard for PM10 (established by the Presidency of Meteorology and Environment, PME) of 80 μg/m3. Although most of that PM was contributed by sand storms, the PM10 extractable organic matter collected at 11 sites was found to be genotoxic. Similar to almost everywhere else in the world, Jeddah’s environment and its citizens’ health are affected by both stationary and mobile sources. Here, the stationary sources include an oil refinery, a desalinization plant, a power generation plant and several manufacturing industries (Figure 1). Many of the industrial facilities, such as the Jeddah oil refinery, were originally built in nonresidential areas, but with urban development that ensued, are now in the middle of highly populated areas, and some of Jeddah’s residential areas are particularly affected by several concrete factories. Additionally, somewhat unique PM source to this area are desert sand storms. Mobile sources of pollution (all forms of transportation) also became Jeddah’s most significant source of air pollution (Al-Jeelani, 1996) with 1.4 million cars currently registered in Jeddah. Although the early study by Nasralla (1983) concluded that concentrations of airborne particulates and other pollutants in Jeddah exceeded air quality standards, there is no research of sources of atmospheric PM in Jeddah. Several studies have been conducted to assess concentrations of total suspended particulates (TSP) and/or PM10, as well as their lead content (Nasrallah, 1984, Abulfaraj, et al., 1990, and Al-Jeelani, 1996). The PM2.5 studies are either limited to a specific component such lead in Saudi Arabia after leaded gasoline phase-out (Aburas, et al., 2011), or specific source, such as a study on roadside soil pollution (Kadi, 2009).
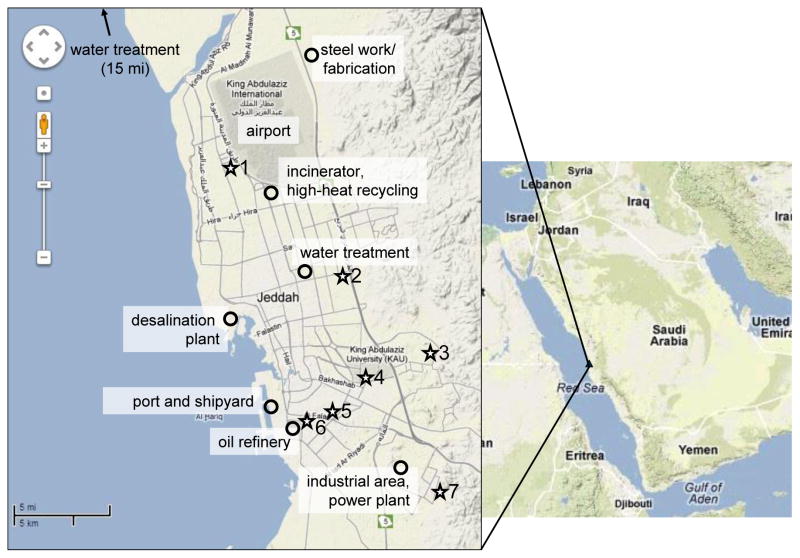
Figure 1.
Location of sampling sites (stars) and major particulate sources (circles) in Jeddah, Saudi Arabia.
This paper will be the first one ever to report the comprehensive elemental composition, concentrations, and sources of PM2.5 and PM10 in Jeddah, Saudi Arabia. The results of this study will establish the groundwork to evaluate the association between particulate air pollution and epigenetic modifications, and the onset of environmentally-related diseases.
METHODS
Particulate Sample Collection and Analysis
Jeddah, population 3.4 million, is located on the Red Sea coast in the western part of Saudi Arabia. The city is surrounded by mountains in the north-east, east and south-east. The city has a major airport in the northern part, and various industrial activities in southern Jeddah. Vehicle fuels used in Jeddah are mainly unleaded gasoline and diesel although some Pb is still permissible, as discussed further. The general climate of Jeddah is warm and moderate in winter; however, in summer it is characterized by high temperature and humidity. Rainfall is generally sparse. Meteorological data was obtained from PME. During the entire sampling campaign the prevailing winds were from west (32%) and north-west (40%). The average temperature was 33°C, and there was no precipitation.
The PM2.5 and PM10 samplers (Automated Cartridge Collector Unit (ACCU) Sampler) were installed during June – September, 2011, at seven sites throughout Jeddah. The sites and the location of the study area in relation to the Middle East are shown on Figure 1. Sampling sites in Jeddah were selected according to traffic densities and human activities – three in urban (Site 4 – University Campus, Site 5– Al-Nuzlah Al Yamaneyyah and Site 6 – Pitrumin), one in suburban (Site 3 – Al-Rughama), two in residential (Site 1 – Al-Muhammadiyah and Site 2 – Al-Rehab) and one in new residential (Site 7 – Al-Alfiyyah) areas. The urban and suburban areas in Jeddah are characterized by high traffic density and commercial activities. The residential areas are characterized by relatively high traffic density. In contrast, the new residential area is characterized by construction activities and low traffic density. Sampling inlets were installed at a height of 5 to 8 m above ground. Daily 24-hr samples were collected on Teflon filters (GelmanTeflo, 37 mm, 0.2 μm pore) from midnight to midnight every other day for two weeks at each site, but not concurrently at all sites, except for the University Campus where samples were collected for three months. The specific time periods at each site are shown in Table 1.
Table 1.
Sampling sites summary
| Site Name | Location type* | Sampling period in 2011 | Number of samples (PM2.5, PM10) |
|---|---|---|---|
| 1. Al-Muhammadiyah | residential | September 10–22 | 7, 7 |
| 2. Al-Rehab | residential | August 13–25 | 7, 7 |
| 3. Al-Rughama | suburban | July 30–August 11 | 7, 6 |
| 4. University Campus | urban | June 18–September 24 | 43, 40 |
| 5. Al-Nuzlah Al Yamaneyyah | urban | June 20–30 | 6, 6 |
| 6. Pitrumin | urban | July 2–14 | 7, 7 |
| 7. Al-Fiyyah | residential | July 16–28 | 7, 7 |
Urban= residential + commercial area
Suburban = urban area located at the city border
The description for gravimetric and elemental (via energy dispersive x-ray fluorescence, ED-XRF) analysis of all PM samples is described in Maciejczyk et al. (2005). Briefly, filter masses were measured on a microbalance (model MT5, Mettler-Toledo Inc., Highstown, NJ). Analyses for 33 elements (listed in Table 2 are elements above the detection limits) followed by nondestructive XRF (model EX-6600-AF, Jordan Valley) using five secondary fluorescers (Si, Ti, Fe, Ge, and Mo), and spectral software XRF2000 v 3.1 (U.S. EPA and ManTech Environmental Technology, Inc.).
Table 2.
Summary of PM concentrations (μg/m3) in Jeddah
| Site | PM2.5 | PM10 | PM2.5/PM10 | ||
|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||
| 1. Al-Muhammadiyah | 15.8 | 3.1 | 47.0 | 6.5 | 0.34 |
| 2. Al-Rehab | 18.0 | 4.0 | 68.9 | 20.5 | 0.26 |
| 3. Al-Rughama | 73.2 | 65.1 | 141.3 | 124.2 | 0.52 |
| 4. University Campus | 23.8 | 11.7 | 84.9 | 33.1 | 0.27 |
| 5. Al-Nuzlah | 29.1 | 14.1 | 73.5 | 10.1 | 0.40 |
| 6. Pitrumin | 31.1 | 5.8 | 107.1 | 28.7 | 0.29 |
| 7. Al-Alfiyyah | 24.5 | 11.8 | 99.4 | 43.9 | 0.25 |
| All sites | 28.4 | 25.4 | 87.3 | 47.3 | 0.33 |
Source Apportionment
To identify major particle source categories, Na, Mg, Al, Si, S, Cl, K, Ca, V, Mn, Fe, Ni, Cu, Zn, Se, Br, and Pb were used as independent variables in source apportionment. The PM2.5 source apportionment modeling also included Ti and Ba. The selection of species was based on existing knowledge of elemental tracers (e.g., Gordon 1988; Thurston et al. 2005) as well as XRF detectability. Several major constituents of PM, such as nitrates, elemental and organic carbon, were not measured. The SPlus Factor Analysis model with varimax rotations was applied to the PM2.5 or PM10 sample trace constituents collected at all sites. The number of factors in the final solution was determined by experiments with different number of factors, with the final choice based on the evaluation of the interpretability of the resulting source profiles. To obtain the daily mass contribution from each source, the “absolute” scores were regressed onto the gravimetric mass concentrations (Thurston and Spangler 1985, Maciejczyk and Chen 2005).
RESULTS AND DISCUSSION
PM mass and elemental concentrations
In total, 80 PM10 and 84 PM2.5 samples with 17 and 20 variables, respectively, were used for modeling purposes. The mean concentrations of elements with standard deviations, and total mass are shown in Tables 2 and 4. The overall mean mass concentration was 28.4 ± 25.4 μg/m3 for PM2.5, and 87.3 ± 47.3 μg/m3 for PM10. It is also apparent that PM concentrations also had significant spatial and temporal variability, with Site 3 driving the high average. All of these sites average concentrations exceeded both World Health Organization PM2.5 and PM210 annual averages of 10 and 20 μg/m3, respectively. The concentration of PM10 exceeded the European Union Air Quality Standard for daily average 50 ug/m3 in 87.5% of samples, and mean PM2.5 concentration exceeded the allowable EU annual PM2.5 average of 25 μg/m3. However, in comparison with U.S. 24-hr average standards, only 10% of PM10 samples exceeded the standard of 150 μg/m3, and 18% of PM2.5 samples exceeded the standard of 35 μg/m3. These results clearly indicated that the airborne particulate pollution has been high in Jeddah.
Table 4.
All sites summary of elemental concentrations (ng/m3) and enrichment factors.
| PM2.5 | PM10 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| mean | S.D. | % detected | EF | mean | S.D. | % detected | EF | |
| Na | 430 | 390 | 99 | 3 | 1600 | 830 | 100 | 3 |
| Mg | 300 | 510 | 79 | 2 | 1400 | 750 | 100 | 2 |
| Al | 800 | 1600 | 100 | 1 | 3300 | 2600 | 100 | 1 |
| Si | 2100 | 4800 | 100 | 1 | 11000 | 8300 | 100 | 1 |
| P | 80 | 43 | 79 | 18 | 160 | 62 | 96 | 5 |
| S | 3400 | 1300 | 100 | 3000 | 3500 | 1100 | 100 | 490 |
| Cl | 62 | 200 | 70 | 51 | 1600 | 1400 | 100 | 430 |
| K | 190 | 230 | 100 | 2 | 710 | 410 | 100 | 1 |
| Ca | 540 | 960 | 100 | 2 | 4400 | 2200 | 100 | 3 |
| Sc | 4.3 | 2.3 | 49 | 48 | - | - | ND | |
| Ti | 55 | 160 | 83 | 1 | 290 | 320 | 100 | 1 |
| V | 23 | 12 | 99 | 40 | 31 | 14 | 100 | 8 |
| Cr | 2.1 | 3.9 | 23 | 4 | 8.8 | 7.8 | 90 | 2 |
| Mn | 19 | 41 | 99 | 2 | 98 | 81 | 100 | 3 |
| Fe | 590 | 1400 | 100 | 1 | 3100 | 2800 | 100 | 1 |
| Co | 8.9 | 14 | 90 | 57 | 32 | 26 | 100 | 34 |
| Ni | 6.6 | 3.6 | 99 | 19 | 11 | 5.6 | 100 | 5 |
| Cu | 5.6 | 8.8 | 61 | 17 | 18 | 14 | 96 | 8 |
| Zn | 41 | 69 | 100 | 84 | 77 | 120 | 100 | 26 |
| As | 8.7 | 14 | 29 | 800 | 10 | 16 | 38 | 150 |
| Se | 2.9 | 1.9 | 49 | 14000 | 3.6 | 2.3 | 58 | 2400 |
| Br | 8.7 | 4.3 | 98 | 815 | 15 | 5.4 | 100 | 200 |
| Rb | 1.2 | 1.8 | 19 | 4 | 3.4 | 3 | 66 | 1 |
| Sr | 4 | 7.6 | 65 | 2 | 27 | 15 | 99 | 2 |
| Cd | 7.6 | 15 | 20 | 8800 | 98 | 85 | 88 | 15000 |
| Cs | 12 | 16 | 69 | 560 | 52 | 31 | 96 | 470 |
| Ba | 34 | 41 | 94 | 11 | 110 | 74 | 100 | 7 |
| Pb | 160 | 350 | 83 | 1500 | 200 | 420 | 90 | 300 |
Jeddah’s PM levels and ratio PM2.5/PM10 were compared with those in different locations across Europe and Asia (Table 3). The average ratio PM2.5/PM10 was 0.33 which was significantly lower than the most urban location. Only suburban Site 3 had a ratio of 0.52 comparable with the European urban ratios of 0.60 – 0.70. Other Jeddah’s sites clearly had a significant contribution from coarse PM resulting in an average PM2.5/PM10 ratio of 0.30. These were similar to the ones in Lebanon and India where PM10 concentrations was also high, most likely due to windblown dust, unpaved roads, and difference in urban landscaping.
Table 3.
Comparative concentrations of PM (μg/m3), and PM2.5/PM10 ratio
| Country | Site | PM2.5 | PM10 | PM2.5/PM10 | Reference |
|---|---|---|---|---|---|
| Saudia Arabia | Jeddah | 28.4 | 87.3 | 0.33 | This study |
| European Union | Central EU | 22–39 | 30–53 | ||
| Northern EU | 13–19 | 26–51 | 0.6–0.7 | Querol et al. (2004) | |
| Southern EU | 28–35 | 45–55 | |||
| Spain | Barcelona | 25, 27 | 39, 43 | 0.64, 0.62 | Pey et al. (2008), Querol et al. (2008) |
| Tarragona | 22.2 | 37.4 | 0.59 | Moreno et al. (2006) | |
| Eastern Spain | 33.9 | 50 | 0.69 | Rodríguez et al. (2004) | |
| Navarra | 17.4 | 28 | 0.63 | Aldabe et al. (2011) | |
| Greece | Athens | 40.2 | 76 | 0.53 | Chaloulakou et al. (2003) |
| Finokalia | 18.2 | 31 | 0.63 | Gerasopoulos et al. (2007) | |
| Italy | Milan | 45 | 63 | 0.71 | Marcazzan et al. (2003) |
| Turkey | Izmir (urban) | 64 | 80 | 0.80 | Yatkin and Bayram (2008) |
| Egypt | Cairo | 86 | 184 | 0.47 | Abu-Allaban et al. (2007) |
| Lebanon | Beirut | 28, 39 | 87, 104 | 0.32, 0.37 | Saliba et al. (2010) |
| China | Hong Kong | 42 | 80 | 0.53 | Ho et al. (2003) |
| Korea | Seoul | 49 | 51 | 0.98 | Kim et al. (2003) |
| India | Hyderabad City | 50 | 135 | 0.37 | Gummeneni et al. (2011) |
As shown in Table 4, the largest elemental contributors to the PM2.5 mass were S and Si, followed by Al, Fe, and Ca; while the largest contributors to the PM10 mass were Si and Ca, followed by S, Al, and Fe. The sum of the elements that were measured accounted for 36.5% of PM2.5 mass and 36.4% of PM10 mass. Reconstructed mass was computed from the elemental data for comparison with the measured mass concentrations for the same days. This was accomplished by using the following equations that account for the sum of typical PM components observed in the ambient atmosphere: ammonium sulfate, soil, sea salt (Malm et al. 1994; IMPROVE 2000):
As would be expected, given the lack of nitrate and carbon data analysis of the filters, and the possibility of unaccounted sources not represented by the equations, reconstructed mass concentrations are lower than those that were measured – 24.5 μg/m3 for PM2.5 (accounting for 87.0% of measured mass), and 63.4 μg/m3 for PM10 (or 72.2% of measured mass). Despite the missing speciation data, this calculation of reconstructed mass verifies that we had sufficient trace elemental data to be used in source apportionment. The difference between the actual measured PM2.5 and the reconstructed mass would therefore include ammonium nitrates, carbon particulates and any other PM component that had not been accounted for.
In order to investigate the sources of elements in Jeddah aerosol, the crustal enrichment factors (EFs) for each element, both in PM2.5 and PM10, were calculated to obtain preliminary information about elements originating both from human activities and natural processes. The enrichment factor for a generic element X with respect to a reference crustal element Y is defined as EFX = (X/Y)air/(X/Y)crust, where the ratio (X/Y) is the concentration ratio of X and Y in either aerosol sample or Earth crust. In the present study, Al was used as reference element Y, and the Earth crust chemical composition was taken from Taylor (1964) and Taylor and McLennan (1985). The mean EF values of the elements measured in PM2.5 and PM10 are summarized in Table 4. The EF values less than 10 indicate that the element has a significant crustal source, while EF values higher than 10 are ascribed to elements of anthropogenic origin (Biegalski et al., 1998). In Jeddah samples, EF values lower than 10 were found for Na, Mg, Al, Si, K, Ca, Ti, Cr, Mn, Fe, Rb, Sr in PM2.5 and Na, Mg, Al, Si, P, K, Ca, Ti, V, Cr, Mn, Fe, Ni, Cu, Rb, Sr and Ba in PM10. The similarities between elements in these fractions suggests that both PM2.5 and PM10 main sources are of a crustal type (e.g., soil and re-suspended dust), while anthropogenic sources have a lesser contribution. In fact, we found significant correlation between PM2.5 and PM10 mass concentrations (r = 0.77, p<0.001), suggesting that PM2.5 and PM10 in Jeddah come from similar sources, particularly elements Al, Si, K, Ti, Mn, and Fe that consistently exhibited EF values of 1. Slight EF elevations for other traditional crustal elements (Na, Mg, Ca, Mn, and Sr) indicate additional contributions from anthropogenic and human activities such as construction, wind-blown road dust, cement factories and building material manufacturing facilities.
In Jeddah, P, S, Cl, Sc, V, Co, Ni, Cu, Zn, As, Se, Br, Cd, Cs, Ba and Pb in PM2.5 and S, Cl, Co, Zn, As, Se, Br, Cd, Cs and Pb in PM10 had EFs higher than 10. The fast majority of the enriched elements (e.g., S), were more concentrated in PM2.5 than in PM10, which was consistent with the established fact that in an urban environment anthropogenic emissions are of combustion origin such as mobile sources (vehicle-exhaust emissions) and industrial activities (Birmili et al. 2006, Loughet et al. 2005, Sternbeck et al. 2002). High EFs for Zn and Pb, suggests traffic emissions, tire wear, incineration, fossil fuel combustion and construction activities. Elevated Ni, V, S, As and Se are usually attributed to fuel combustion (Gordon, 1988), while Cd, Zn, and Cl are due to incinerator type emissions (Gordon 1988, US EPA Speciate v 3.2, 2006). The presence of Cd and Cr, Cu and Ni were probably related to anthropogenic activities such as non-ferrous metal industries and chromium plating. Our consideration of EFs and the pattern of spatial variability of elements from site to site (data not shown) had enhanced our understanding of regional and local sources of pollution in Jeddah.
Sources of Jeddah PM2.5 aerosol – factor analysis
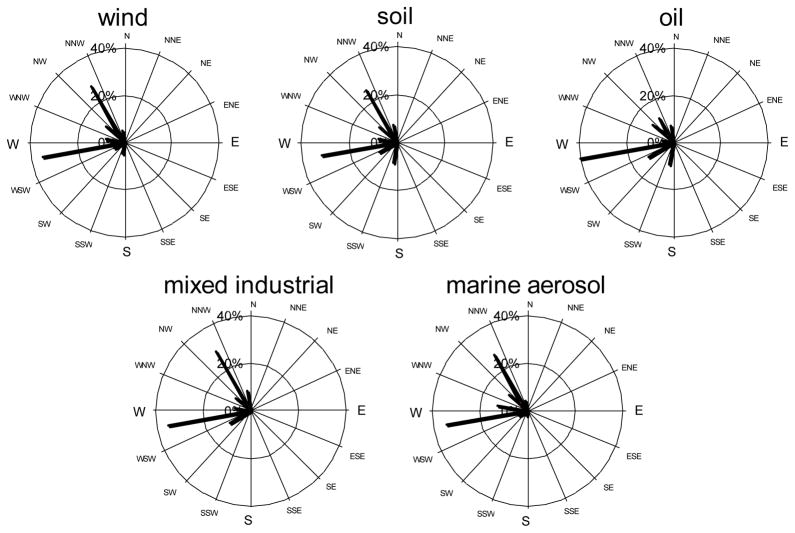
We used factor analysis with varimax rotation to predict five sources for PM2.5 and four sources for PM10, although between three and seven factors were modeled with each size fraction. The number of factors in the final analysis was based on the evaluation of the interpretability of the resulting source profiles. The factor loadings for each of the factor source category are shown in Figures 2 and 3. We let a pattern be limited to those variables with 10% or more of their variation involved in a pattern. Factors were interpreted by comparing the factor loadings for all factors and variables. In our modeling, we also considered the communalities for elements, which indicate the proportion of a variable’s total variation that is involved in the patterns, and percent total variance for each factor. The contributions of each source to overall mass were computed by each factor score regression onto daily mass, and the averages of daily mass contributions per source are shown in Table 5. In Figure 4, these daily source category contributions are paired with wind direction to form a pollution wind rose for Site 4, University Campus. Spatial analysis of data was possible by comparing data periods from Site 4 with other concurrent sampling sites, as also shown in Table 5.
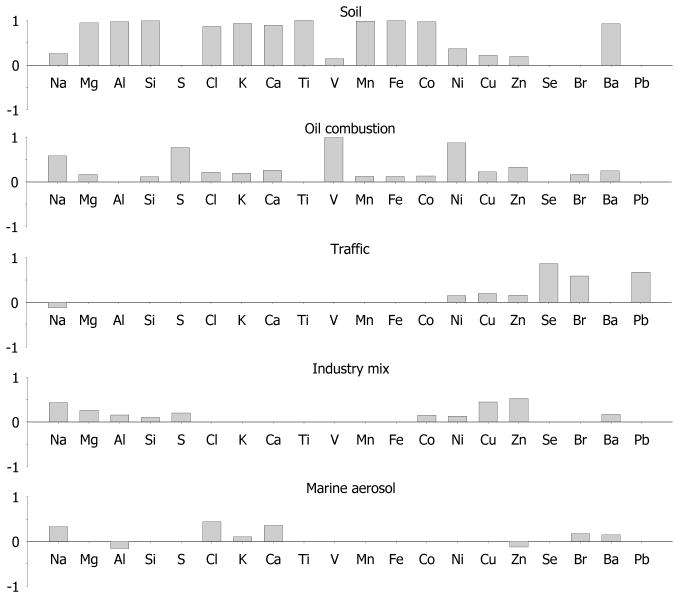
Figure 2.
Varimax rotated factor loadings for chemical species of PM2.5 combined from seven sites
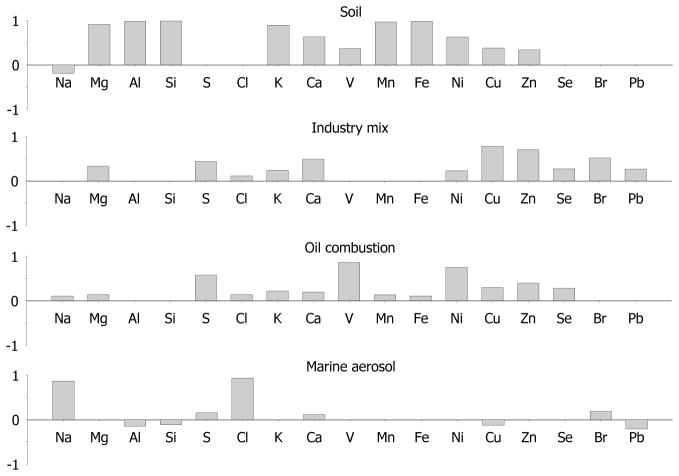
Figure 3.
Varimax rotated factor loadings for chemical species of PM10 combined from seven sites
Table 5.
Average mass source contribution (compared with concurrent data at University Campus Site 4) to PM2.5, in μg/m3
| Factor 1 soil | Factor 2 heavy oil combustion | Factor 3 traffic | Factor 4 Cu/Zn source | Factor 5 marine aerosol | |
|---|---|---|---|---|---|
| 1. Al-Muhammadiyah | 0.4 (0) | 12 (10) | 0.7 (1.1) | 0.3 (1.4) | 0.1 (0) |
| 2. Al-Rehab | 0.6 (0.2) | 11 (11) | 0.9 (0.9) | 0.9 (1.8) | 0 (0.1) |
| 3. Al-Rughama | 46 (11) | 18 (16) | 0.7 (0.2) | 3.8 (3.8) | 3.2 (0) |
| 4. University Campus | 2.7 | 14 | 0.8 | 2.6 | 0.1 |
| 5. Al-Nuzlah | 0 (0) | 28 (16) | 0.7 (0.8) | 2.0 (2.9) | 0 (0) |
| 6. Pitrumin | 0 (1.0) | 34 (15) | 0.7 (0.5) | 0.5 (2.3) | 0 (0.3) |
| 7. Al-Alfiyyah | 1.6 (4.2) | 17 (17) | 1.1 (1.4) | 2.3 (3.3) | 0 (0) |
| All sites | 5.4 | 17 | 0.8 | 2.1 | 0.3 |
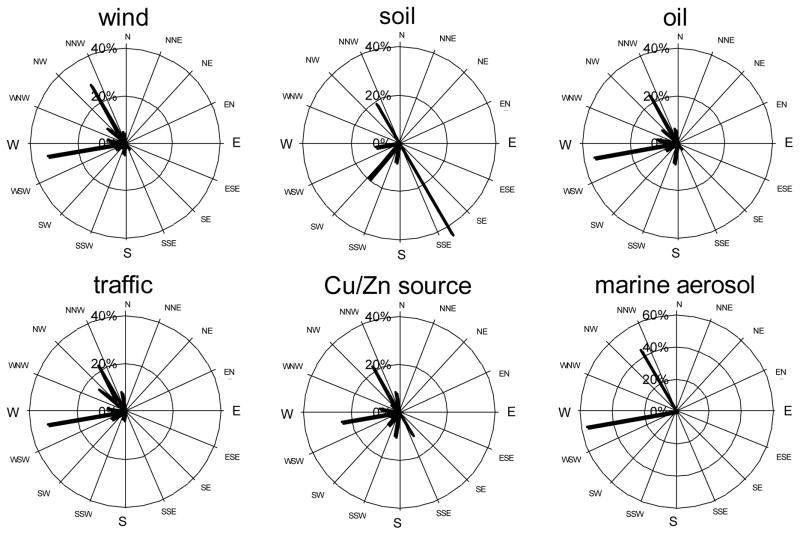
Figure 4.
Wind and pollution roses of each PM2.5 source category mass contribution to Site 4 (%).
Five factors were identified for PM2.5, and they explained 82.7% of total variance of the data set. The communalities for all species were high (>0.80), and the five factors identified were satisfactory. However, low communalities of Cu, Zn and Pb (0.3 – 0.5) indicated that these element concentrations cannot be entirely predicted from identified sources. The first factor that explained most of the total variance (51%) was heavily loaded with Al, Si, and Fe, classified as resuspended soil source category (e.g., Amato et al. 2009). Although the strongest in our factor analysis, this resuspended soil source had a mean mass contribution 5.4 μg/m3 (or 8.3% of total mass), and had almost uniformly low mass contribution of less than 3%. Sites 5 and 6, located in the western part of the city did not collect significant soil amounts. The wind rises at site 4 (Figure 4) confirms that even under prevailing westerly winds, soil contribution is rather small. The large soil contributions to samples at site 3 (46 μg/m3) were driven by several days of exceptionally high PM mass under south-east winds conditions on 7/30 and 8/3/2011 (115 and 150 μg/m3, respectively). Site 3 is located in the eastern suburb of the city, and under prevailing westerly winds is influenced the most by pollution from nearby Al-Haramain Road that has daily traffic density of 300 000 cars. However, on 7/30 and 8/3 the soil factor mass contribution was also high at the University Campus site (50 and 22 μg/m3, respectively), indicating that the whole city was experiencing an additional dust episode, that most likely was transported from elsewhere. Overall correlation between the University Campus and the concurrent sites for the resuspended soil source mass contribution was high (r = 0.97).
The second PM2.5 factor was rich in V, Ni, and S, and was assigned as emissions of oil combustion from the Jeddah oil refinery, electrical utilities and industrial and large commercial boilers, as well as residual oil combustion emitted from ocean-going ships docked in Jeddah’s port. We did not have sufficient data to attribute some of this source to transported emissions from oil producing Sudan and Egypt. Mass contribution from this source was 16.9 μg/m3 (or 69% of PM2.5 mass), and explained 15.7% of total variance. Overall, this source contribution had more spatial variation – while University Campus contributions were quite similar regardless of wind direction (10 – 17 μg/m3 or 61–69% contribution to PM2.5 mass, as shown in Table 5 and Fig. 4), Sites 5 and 6, located closest to the port and refinery had the highest Factor 2 contributions of 28 and 34 μg/m3, respectively. Other sites are not immediately downwind of the refinery or the seaport, and thus had lesser contribution. Even at a concurrent site 4 under exact westerly winds the mass contribution from oil was two-fold less. Thus, it seems that the average background concentration of oil combustion source is about 14 μg/m3, with local point sources having significant effect at immediate proximity. In fact, heavy oil combustion contributed overall 88 and almost 100% of total PM2.5 mass at sites 5 and 6. Site 3 located in the eastern suburbs, farthest from the port and refinery, had third highest contribution from that source category. Interestingly, the average Ni/V ratio was fairly consistent at all sites (0.24–0.28) except for Site 3 where it was 0.35, indicating possibly another source of V that was not satisfactorily resolved here. Poor correlation coefficient between Ni and V indicates that these elements were not always emitted from the same source(s) (Maciejczyk et al. 2010; Peltier and Lippmann, 2010). Correlation between the University Campus and concurrent sites was poor (r = 0.26), indicative of the local point source influences. Removing Site 3 improved that correlation to r = 0.57.
The third factor (8.4% of total variance) contained Pb, Br, and Se was assigned as traffic source. Atmospheric Pb and Br historically had been attributed to traffic emissions as these elements were bound to fuel additives. Although Saudi Arabia phased out the leaded gasoline consumption in January 2001, allowable Pb content in gasoline remains at 13 mg/L, which means that in high traffic density cities, EF for Pb still remained elevated. It was estimated that total Pb in consumed fuel in Jeddah is about 83.6 ton/year (Aburas et al. 2011). Additional vehicular Pb emissions are also caused by engine wear (Smichowskiet et al. 2008). Besides the motor vehicles, both Pb and Se are emitted by municipal incinerators and high heat recycling facilities (Emsley, 2011); one of each is located in northern part of Jeddah. Selenium, commonly attributed to the coal combustion in Eastern U.S., is also present in oil (U.S. EPA, 2003). However, here Se did not correlate with heavy oil combustion source but rather with traffic, and thus, here serves as tracer for fossil fuel combustion. Bromine is present in multitude of consumer products, and volatilizes easily when heated during combustion. Elements such as Br, As and Zn are considered as indicators of emission from fossil fuel combustion process, including vehicle exhaust (Gordon 1988; Pacyna and Pacyna 2001). Despite the world wide abatement measures for fuel additives, both Pb and Br are persistently present in both urban and rural environment in Europe and U.S. For example, Pb and Br in the daily TSP and PM10 samples from 1998–2000 collected in Germany were correlated, however not strongly with r(Pb, Br) = 0.56 (Lammel et al. 2002). Similarly, r = 0.61 was found in rural NY samples (Maciejczyk and Chen 2005; Maciejczyk et al. 2010). Here, we found r(Pb, Br) = 0.56 in PM2.5 and r(Pb, Br) = 0.51 in PM10. This factor mean contribution to PM2.5 mass concentration was 0.80 μg/m3 (or 3.7%). Although Pb has been banned in petrol for several years in Saudi Arabia, the levels of lead in the street dust of the urban areas can still reflect the significant degree of lead contamination from the past. We also noticed that the pollution rose for traffic source was virtually identical with overall wind rose, therefore, this can be considered as a regional source, further confirmed by the high inter-site correlation (r = 0.94) between University Campus and concurrent sites.
The fourth factor was characterized by high Cu and Zn representing the combined sources of industry and some additional mix of motor vehicle emission. This source category explained 4.5% of the total variance. Although Zn mostly originates from tire wear, it is also attributed to incinerator emissions and metal working (e.g., Morishita et al. 2011), with both facilities present in northern part of the city. The metallurgical processes could produce the largest emissions of Cu, Ni, and Zn (Pacyna, 1998), while exhaust emissions from road vehicles could also contain various amounts of Cu, Zn, and Ni (Pacyna, 1986; Lee et al. 1999). There is no typical metallurgical plant in Jeddah; however, there is a high-temperature metal recycling facility north of the city, and metal fabrication facility on both the north and the south parts of the city. Furthermore, welding, fine sanding, and other metal cutting activities occur in the Jeddah port and shipyard on the west side of the city. Spatial and temporal variation of this source was variable, with a mean mass contribution of 2.1 μg/m3 (or 8.2%), and poor concurrent site correlation (r = 0.31).
The last factor identified for PM2.5 was rich in Na and Cl, the main elements in sea salt, and thus was cautiously named marine aerosol. This was a weak source explaining 2.8% of total variance, with contribution to mass of 0.3 μg/m3 (or 0.4%), which was also, spatially, highly variable. Oddly enough, the highest contribution from that source was at the site furthest from the seashore. Thus, it is possible that it was some other source located in between Site 4 and 3, since during the same time period the contribution to Site 4 was null. As seen on Fig. 4, this source contributed to Site 4 exclusively under west and north-west winds. The true identity of this source remains unclear.
Sources of Jeddah PM10 aerosol – factor analysis
In PM10 factor analysis, four factors explained 75% of total variance. The loading for this PM fraction are shown in Fig. 3. The strongest Factor 1 explained 39.1% variance and was rich in Al, Si, and Fe, and, similarly to PM2.5, was identified as resuspended soil. Overall, this factor contributed to 64% of PM10 mass (or 55 μg/m3), although there were significant spatial and temporal variations (Table 6 and Figure 5). Sites 3 and 7 were affected the most by soil, but, we believe, for different reasons as confirmed by comparison to concurrent sampling at Site 4 (Table 6). Site 3 was down wind of a major highway and parcels of undeveloped land, and, therefore, had significantly higher soil factor contribution (128 μg/m3, or 87% of overall PM10 mass) than at Site 4 (37 μg/m3, or 49% mass). However, while at Site 7 soil contribution was 78 μg/m3, the concurrent Site 4 samples were also of comparable 82 μg/m3, thus indicating regional dust episode rather than influence of local source. There is no spike for soil under south-east winds at site 4 soil pollution rose because we did not collect the PM10 samples on 7/30 and 8/3 (the days for high soil mass in PM2.5 samples). We believe significant contribution from resuspended soil comes from traffic transporting goods from the seaport sugar plant, also located near the seaport, and refinery.
Table 6.
Average mass source contribution (compared with concurrent data at University Campus Site 4) to PM10, in μg/m3
| Factor 1 soil | Factor 2 mixed industrial | Factor 3 heavy oil combustion | Factor 4 marine aerosol | |
|---|---|---|---|---|
| 1. Al-Muhammadiyah | 21 (39) | 3.6 (6.9) | 13 (12) | 9.1 (5.9) |
| 2. Al-Rehab | 42 (46) | 7.3 (7.1) | 6.8 (8.3) | 6.8 (7.6) |
| 3. Al-Rughama | 128 (37) | 4.5 (5.7) | 8 (16) | 4.2 (3.2) |
| 4. University Campus | 51 | 9.0 | 13 | 6.7 |
| 5. Al-Nuzlah | 43 (58) | 5.8 (11) | 19 (9) | 8.8 (10) |
| 6. Pitrumin | 46 (47) | 7.4 (13) | 31 (12) | 5.5 (7.1) |
| 7. Al-Alfiyyah | 78 (82) | 10 (10) | 11 (24) | 5.6 (3.3) |
| All sites | 55 | 7.8 | 14 | 6.7 |
Figure 5.
Wind and pollution roses of each PM10 source category mass contribution to Site 4 (%).
Factor 2 (named mixed industrial) in PM10 showed high loadings of Cu, Zn, Br, S and Ca, and it explained about 13% of total variance. This was the only PM10 factor positively correlated with Pb. High contributions of Zn and Cu and was assigned to metallurgical industries and to diesel powered vehicles. Zn was present in tire wear dust as well as in tailpipe emissions due to its use in motor oil, and Cu was present in brake wear dust (López et al., 2011). This factor was likely a mixture of coarse emission of industrial and traffic sources similar to Factor 3 and 4 for PM2.5, however, we were not able to further split this source. Contribution of this source was somewhat variable, on average 7.8 μg/m3 (or 10% of PM10 mass), and at the Site 4 it followed the wind rose, and the concentrations there were almost higher than at any concurrent other sites.
The third factor had high loadings of V, Ni and S, and this clearly represented oil combustion similarly to PM2.5 Factor 2. The average mass contribution of this heavy oil combustion source was 14 μg/m3 (or 18% mass). Sites 5 and 6 in immediate proximity and downwind of Jeddah refinery and sea port had the largest contribution of 19 and 31 μg/m3 (or 26 and 29% PM10 mass). Site 1, which was also close to the seashore, was likely affected by ship exhaust. Temporal variation of oil source contributions to Site 4 confirms that this is like a point source, and thus highly affected by wind direction, as seen in Fig. 5. For example, during concurrent sampling at Sites 4 and 7, the overall contribution of oil source was 2-fold, quite possibly because Site 7 was not downwind of the refinery and port emissions. Notably, the northwest contribution is missing in site 4 PM10 pollution rose, compared to PM2.5. The fourth factor was loaded with Na and Cl, which we attributed to coarse sea salt of marine aerosol which contributed on average 6.7 μg/m3 of PM10 mass (or about 9% mass). As shown with pollution rose at Site 4, this source was reasonably expressed under favorable winds from the sea. Site 1 had larger overall contribution from the marine aerosol due to its proximity to the seashore.
Summary
This is the first study of elemental composition and sources of PM10 and PM2.5 in Saudi Arabia, and it reveals that airborne particulate matter in Jeddah is a serious problem. Using factor analysis, we determined that five and four source categories contributed to the mass concentration of PM2.5 and PM10, respectively, and estimated daily levels of source-apportioned PM mass concentrations for each of these categories. The PM2.5 mass in Jeddah was dominated by contribution from heavy oil combustion (69%), followed by resuspended soil (8.2%), Cu/Zn source (8.2%), traffic (3.7%), and marine aerosol (0.4%). While mass contributions from soil (64%), heavy oil combustion (18%), mixed industrial sources (18%) and marine aerosol (9.3%) were found in PM10. Our source apportionment modeling determined the contributions from individual sources within each size fraction and could be used to (1) better focus control strategies and (2) in a subsequent time-series analysis of health effects, in particular morbidity outcomes.
Acknowledgments
This work was funded by King Abdulaziz University (KAU), Jeddah, under grant number 4/00/00/252. The authors thank NYU and KAU for technical and financial support.
References
- Pacyna J. Sources inventories for atmospheric trace metals. In: Harrison RM, Van Grieke R, editors. Atmospheric Particles. IUPAC Series Anal Phys Chem Environ System. Vol. 5. Wiley; Chicester: 1998. pp. 385–423. [Google Scholar]
- Pacyna JM. In: Toxic Metals in the Atmosphere. Nriagu JO, Davidson CI, editors. Wiley; New York: 1986. [Google Scholar]
- Chen H, Goldberg M. The effects of outdoor air pollution on chronic illnesses. McGill J Med. 2009;12(1):58–64. [PMC free article] [PubMed] [Google Scholar]
- PME. Presidency of Meteorology and Environment. Jeddah; Saudi Arabia: 2001. www.pme.gov.sa. [Google Scholar]
- Abulfaraj A, Ahmed M, Mousli K, Erturk F. Measurement of ambient air lead concentrations in the city of Jeddah, Saudi Arabia. Environ Int. 1990;16(1):85. [Google Scholar]
- Aburas H, Zytoon M, Abdulsalam M. Atmospheric lead in PM2.5 after leaded gasoline phase-out in Jeddah city, Saudi Arabia. Clean-Soil, Air, Water. 2011;39(8):711–719. [Google Scholar]
- Al-Jahdali M, Bin Bisher A. Sulfur dioxide accumulation in soil and plant leaves around an oil refinery: A case study from Saudi Arabia. Amer J Environ Sciences. 2008;4(1):84–88. [Google Scholar]
- Al-Jeelani H. Ph D thesis. University of East Anglia; UK: 1996. Social and meteorological factors control air pollution in Jeddah city, Saudi Arabia. [Google Scholar]
- Amato F, Pandolfi M, Escrig A, Querol X, Alastuey A, Peya J, Perez N, Hopke PK. Quantifying road dust resuspension in urban environment by multilinear engine: a comparison with PMF2. Atmospheric Environment. 2009;43:2770–2780. [Google Scholar]
- Biegalski SR, Landsberger S, Hoff RM. Source-receptor modeling using trace metals in aerosols collected at three rural Canadian Great lakes Sampling Stations. J Air Waste Manage Assoc. 1998;48:227–37. doi: 10.1080/10473289.1998.10463680. [DOI] [PubMed] [Google Scholar]
- Birmili W, Allen AG, Bary F, Harrison RM. Trace metal concentrations and water solubility in size-fractionated atmospheric particles and influence of road traffic. Environmental Science and Technology. 2006;40 (4):1144–1153. doi: 10.1021/es0486925. [DOI] [PubMed] [Google Scholar]
- El-Assouli S, Al-Qahtani M, Milaat W. Genotoxicity of airborne particulates by comet and the salmonella mutagenicity test in Jeddah, Saudi Arabia. Int J Environ Res Public Health. 2007;4(3):216–223. doi: 10.3390/ijerph2007030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E, Froines J, Utell M, Lippmann M, Oberdorster G, Godleski J, Larson T. Accomplishments of the Particulate Matter (PM) Centers (1999–2005) and the role of interdisciplinary center-based. Report to EPA (online) 2007 doi: 10.1289/ehp.11543. http://www.epa.gov/ncer/science/pm/documents/11543.pdf. [DOI] [PMC free article] [PubMed]
- Freitas MC, Pacheco AMG, Verburg TG, Wolterbeek HT. Effect of particulate matter, atmospheric gases, temperature, and humidity on respiratory and circulatory diseases’ trends in Lisbon, Portugal. Environmental Monitoring and Assessment. 2010;162:113–121. doi: 10.1007/s10661-009-0780-5. [DOI] [PubMed] [Google Scholar]
- Fujiwara F, Jiménez Rebagliati R, Dawidowski L, Gómez D, Polla G, Pereyra V, Smichowski P. Spatial and chemical patterns of size fractionated road dust collected in a megacitiy. Atmospheric Environment. 2011;45:1497–1505. [Google Scholar]
- Gao Y, Anderson JR. Characterization of Chinese aerosols determined by individual-particle analysis. J Geophys Research. 2001;106(D16):18037–18045. [Google Scholar]
- Gordon GE. Receptor models. Environ Sci Technol. 1988;22:1132–1142. doi: 10.1021/es00175a002. [DOI] [PubMed] [Google Scholar]
- Guo H, Ding AJ, So KL, Ayoko G, Li YS, Hung WT. Receptor modeling of source apportionment of Hong Kong aerosols and the implication of urban and regional contribution. Atmos Environ. 2009;43:1159–69. [Google Scholar]
- Handler M, Puls C, Zbiral J, Marr I, Puxbaum H, Limbeck A. Size and composition of particulate emissions from motor vehicles in the Kaisermühlen-Tunnel, Vienna. Atmospheric Environment. 2008;42:2173–2186. [Google Scholar]
- IMPROVE. Spatial and Seasonal Patterns and Temporal Variability of Haze and its Constituents in the United States: Report III. 2000;Chapter 2 ( http://vista.cira.colostate.edu/improve/Publications/Reports/2000/2000.htm) [Google Scholar]
- Kadi M. Soil pollution hazardous to environment: A case study on the chemical composition and correlation to automobile traffic on the roadside soil of Jeddah city, Saudi Arabia. J Hazard Mater. 2009;168 (2–3):1280. doi: 10.1016/j.jhazmat.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Lammel G, Röhrl A, Schreiberm H. Atmospheric Lead and Bromine in Germany: Post-abatement Levels, Variabilities and Trends. Environ Sci & Pollut Res. 2002;9(6):397–404. doi: 10.1007/BF02987589. [DOI] [PubMed] [Google Scholar]
- Lippmann M. Targeting the components most responsible for airborne particulate matter health risks. J Expo Sci Environ Epidemiol. 2010 Mar;20(2):117–8. doi: 10.1038/jes.2010.1. [DOI] [PubMed] [Google Scholar]
- López ML, Ceppi S, Palancar G, Olcese Luis E, Tirao G, Toselli B. Elemental concentration and source identification of PM10 and PM2.5 by SR-XRF in Córdoba City, Argentina. Atmospheric Environment. 2011;45:5450–5457. [Google Scholar]
- Loughet GC, Schauer JJ, Park J-S, Shafer MM, De Minter JT, Weinstein JP. Emissions of metals associated with motor vehicle roadways. Environmental Science & Technology. 2005;39 (3):826–836. doi: 10.1021/es048715f. [DOI] [PubMed] [Google Scholar]
- Maciejczyk PB, Zhong M, Li Q, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice: II. The design of a concentrated ambient particulate matter exposure system for biometric telemetry monitoring. Inhalation Toxicology. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- Maciejczyk PB, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice: VIII. Source-related daily variations in in vitro responses to CAPs. Inhalation Toxicology. 2005;17:243–253. doi: 10.1080/08958370590912914. [DOI] [PubMed] [Google Scholar]
- Maciejczyk P, Zhong M, Lippmann M, Chen L. Oxidant generation capacity of source-apportioned PM2.5. Inhalation Toxicology. 2010 Dec;22(Suppl 2):29–36. doi: 10.3109/08958378.2010.509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm WC, Sisler JF, Huffman D, Eldred RA, Cahill TA. Spatial and seasonal trends in particle concentration and optical extinction in the United States. J Geo Res. 1994;99(D1):1347–1370. [Google Scholar]
- Marcazzan G, Vaccaro S, Valli G, Vecchi R. Characterization of PM10 and PM2.5 particulate matter in the ambient air of Milan (Italy) Atmospheric Environment (2001) 2001;35:4639–4650. [Google Scholar]
- Mauderly JL, Chow JC. Health effects of organic aerosols. Inhal Toxicol. 2008;20:257–288. doi: 10.1080/08958370701866008. [DOI] [PubMed] [Google Scholar]
- Mavroidis I, Chaloulakou A. Characteristics and expected health implications of annual PM10 concentrations in Athens, Greece. International Journal for Environment and Pollution. 2010;41:124–139. [Google Scholar]
- Mazzei F, D’Alessandro A, Lucarelli F, Nava S, Prati P, Valli G, et al. Characterization of particulate matter sources in an urban environment. Sci Total Environ. 2008;401:81–9. doi: 10.1016/j.scitotenv.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Momani K, Jiries A, Jaradat Q. Atmospheric Deposition of Pb, Zn, Cu, and Cd in Amman, Jordan. Turk J Chem. 2000;24:231–237. [Google Scholar]
- Monks P, Granier C, Fuzzi S, Stohl A, Willimas M, Akimoto H, Amann M, et al. Atmospheric composition change- Global and regional air quality. Atmos Environ. 2009;43(33):5268. [Google Scholar]
- Moreno T, Querol X, Alastuey A, Viana M, Salvador P, Sánchez de la Campa A, Artiñano B, de la Rosa J, Gibbons W. Variations in atmospheric PM race metal content in Spanish towns: illustrating the chemical complexity of the inorganic urban aerosol cocktail. Atmospheric Environment. 2006;40:6791–6803. [Google Scholar]
- Morishita M, Keeler GJ, Kamal AS, Wagner JG. Identification of ambientPM2.5 sources and analysis of pollution episodes in Detroit, Michigan using highly time-resolved measurements. Atmospheric Environment. 2011;45:1627–1637. [Google Scholar]
- Movassagh M, Vujic A, Foo R. Genome-wide DNA methylation in human heart failure. Epigenomics. 2011;3(1):103–109. doi: 10.2217/epi.10.70. [DOI] [PubMed] [Google Scholar]
- Nasralla M. Air pollution in the semitropical Saudi Urban area. Environment International. 1983;9(4):255–164. [Google Scholar]
- Nasrallah M. Lead in Jeddah urban dust. Environ Pollut B. 1984;8(2):133. [Google Scholar]
- National Cancer Registry. Cancer Incidence Report: 2005. Ministry of Health; Kingdom of Saudi Arabia: 2009. [Google Scholar]
- Pacyna J, Pacyna E. An assessment of global and regional emissions of tracemetals to the atmosphere from anthropogenic sources worldwide. Environmental Reviews. 2001;9:269–298. [Google Scholar]
- Peltier RE, Lippmann M. Residual oil combustion: 2. Distributions of airborne nickel and vanadium within New York City. J Expo Sci Environ Epidemiol. 2010;20(4):342–50. doi: 10.1038/jes.2009.28. [DOI] [PubMed] [Google Scholar]
- Pérez N, Pey J, Castillo S, Alastuey A, Viana M, Querol X. Interpretation of the variability of levels of regional background aerosols in the Western Mediterranean. Sci Total Environ. 2008;407:527–540. doi: 10.1016/j.scitotenv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Querol X, Alastuey A, Rosa J, Sanchez de la Campa A, Plana F, Ruiz C. Source apportionment analysis of atmospheric particulates in an industrialized urban site in southwestern Spain. Atmos Environ. 2002;36:3113–3125. [Google Scholar]
- Quiterio SL, Arbilla G, Silva CRS, Escaleira V. Metals in airborne particulate matter in the Industrial District of Santa Cruz, Rio de Janeiro, in an annual period. Atmos Environ. 2004;38:321–31. [Google Scholar]
- Samura A, Al-Agha O, Tuncel SG. Study of trace and heavy metals in rural and urban aerosols of Uludağ and Bursa (Turkey) Water, Air and Soil Pollution: Focus. 2003;3:111–129. [Google Scholar]
- Schlesinger RB. The Health Impact of Common Inorganic Components of Fine Particulate Matter (PM2.5) in Ambient Air: A Critical Review. Inhalation Toxicology. 2007;19:811–832. doi: 10.1080/08958370701402382. [DOI] [PubMed] [Google Scholar]
- Shridhar V, Khillare PS, Agarwal T, Ray S. Metallic species in ambient particulate matter at rural and urban location of Delhi. Journal of Hazardous Materials. 2010;175:600–607. doi: 10.1016/j.jhazmat.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Smichowski P, Gómez D, Frazzoli C, Caroli S. Traffic-related elements in airborne particulate matter. Applied Spectroscopy Reviews. 2008;43:23–49. [Google Scholar]
- Sternbeck J, Sjodin A, Andreasson K. Metal emissions from road traffic and the influence of resuspension - results from two tunnel studies. Atmospheric Environment. 2002;36:4735–4744. [Google Scholar]
- Taylor SR, McLennan SM. The continental crust: its composition and evolution. Oxford, England: Blackwells; 1985. p. 312. [Google Scholar]
- Taylor SR. Abundance of chemical elements in the continental crust: a new table. Geochimica et Cosmochimica Acta. 1964;28:1273–1285. [Google Scholar]
- Thurston GD, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Lall R, Larson TV, Liu H, Neas L, Pinto J, Stölzel M, Suh H, Hopke PK. Workshop on the Source Apportionment of Particulate Matter Health Effects: Inter-Comparison of Results and Implications. Environ Health Perspect. 2005 Dec;113(12):1768–74. doi: 10.1289/ehp.7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston GD, Spangler SD. A quantitative assessment of source contributions to inhalable particle matter pollution in metropolitan Boston. Atmos Environ. 1985;19:9–25. [Google Scholar]
- United Nations. U N Country Profile 2002: Saudi Arabia, United Nations. The World Summit on Sustainable Development; Johannesburg. 2002. [Google Scholar]
- Vallius M, Janssen NAH, Heirich J, Hoek G, Ruuskanen J, Cyrys J, Grieken RV, Hartog JJ, Kreyling WG, Pekkanen J. Sources and elemental composition of ambient PM2.5 in three European cities. Sci Total Environ. 2005;337:147–162. doi: 10.1016/j.scitotenv.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Vecchi R, Marcazzan G, Valli G, Ceriani A, Antoniazzi C. The role of atmospheric dispersion in the seasonal variation of PM1 and PM2.5 concentration and composition in the urban area of Milan (Italy) Atmos Environ. 2004;38:4437–46. [Google Scholar]
- Yongming H, Peixuan D, Junji C, Postmentier ES. Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Sci Total Environ. 2006;355:176–86. doi: 10.1016/j.scitotenv.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Zereini F, Alt F, Messerschmidt J, Wiseman C, Feldmann I, Von Bohlen A, Muller J, Liebl K, Puttmann W. Concentration and distribution of heavy metals in urban airborne particulate matter in Frankfurt am Main, Germany. Environmental Science and Technology. 2005;39:2983–2989. doi: 10.1021/es040040t. [DOI] [PubMed] [Google Scholar]