Summary
Chronic stress precipitates drug seeking behavior and alters the effects of drugs of abuse. Although it is known that chronic stress potentiates acute neurochemical and hyperthermic responses to the drug of abuse methamphetamine, no studies have investigated if and how chronic stress alters other physiological responses to methamphetamine. Therefore the objective of these studies was to determine if ten days of chronic unpredictable stress modulates corticosterone (CORT) responses to methamphetamine and furthermore how chronic stress may modulate methamphetamine-induced increases in hyperthermia and CORT. As chronic stress potentiates hyperthermic responses to serotonin 2 (5-HT2) stimulation and 5-HT2 receptors are important in mediating both hyperthermic and CORT responses, we also investigated if 5-HT2 antagonism would block hyperthermia and CORT secretion by the serial exposure to stress and methamphetamine (stress/methamphetamine). The results of these studies illustrate that stress potentiates methamphetamine-induced increases in body temperature and CORT secretion and that these increases are blocked by the 5-HT2 antagonist ketanserin. Furthermore, the combination of stress and methamphetamine depletes 5-HT content in the hippocampus seven days after methamphetamine administration which is blocked by the 5-HT2 antagonist ketanserin. Overall, these results indicate a pharmacological mechanism for the depletion of hippocampal 5-HT by the serial exposure to stress and methamphetamine and further illustrate the deleterious interactions between chronic stress and methamphetamine use.
Keywords: methamphetamine, serotonin receptors, corticosterone, stress, hyperthermia, neurotoxicity
Introduction
Chronic stress potentiates behavioral, neurochemical, and physiological responses to drug challenges and novel stressors. Specifically, chronic stress potentiates hyperthermic responses to serotonin 2 (5-HT2) receptor agonism (Matuszewich and Yamamoto, 2003). Chronic stress also potentiates the acute hyperthermic and CORT responses to a novel stressor (Bhatnagar et al., 2006) as well as the acute neurochemical and hyperthermic responses to the drug of abuse, methamphetamine (Tata et al., 2007). The acute neurochemical responses to methamphetamine are characterized by the release of dopamine and 5-HT (Kuczenski et al., 1995) and acute increases in 5-HT and downstream activation of 5-HT2 receptors have been implicated in the acute hyperthermic and CORT responses to the amphetamine derivative 3,4-methylenedioxymethamphetamine (Nash et al., 1988). Although the acute hyperthermic responses to methamphetamine are known to be increased following chronic stress, it is unknown how chronic stress modulates the CORT response to methamphetamine and the putative role of the 5-HT2 receptor in mediating hyperthermic and CORT responses to methamphetamine in chronically stressed rats. We hypothesize that chronic stress will potentiate CORT responses to methamphetamine and that potentiated hyperthermic and CORT responses will be blocked by the 5-HT2A/C antagonist, ketanserin
Since CORT and hyperthermia mediate the long-term toxicity of amphetamine derivatives to dopamine and 5-HT terminals in the brain (Johnson et al., 1989; Bowyer et al., 1994) and chronic stress potentiates toxicity to dopamine terminals in the striatum (Tata et al., 2007), we also wanted to investigate the effect of chronic stress and methamphetamine (stress/methamphetamine) on 5-HT content in the hippocampus given the link between 5-HT abnormalities and behavioral deficits in human methamphetamine abusers and the importance of the hippocampus in learning and memory (Thompson et al., 2004; Sekine et al., 2006). To this end, we hypothesized that the serial exposure to chronic stress and methamphetamine would deplete 5-HT in the hippocampus which would be blocked by the 5-HT2A/C antagonist, ketanserin.
Methods and Materials
Chronic Unpredictable Stress
Male Sprague-Dawley rats (175-200g) were purchased from Harlan Sprague-Dawley and housed 2-3 per cage in a temperature (21-23°C) and humidity controlled room with a 12 hour light/dark cycle (lights on at 0700h off at 1900h). All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, if available.
Rats were either handled daily or exposed to 10 days of chronic unpredictable stress: Day 1 1000h 50min cold room (4°C), 1300h 60min cage agitation; Day 2 1000h 60min restraint stress, 1800h lights on overnight (12h); Day 3 1000h 3h lights off, 1500h 50min cold room (4°C); Day 4 1100 50min cage agitation, 1800h food and water deprivation overnight (12h); Day 5 0900h 60min restraint stress, 1800h lights on overnight; Day 6 1500h 15min cold room individual housing, 1600h individual housing overnight; Day 7 1000h 60min restraint stress, 1800h food and water deprivation overnight; Day 8 1100h 30min cage agitation, 1500h individual housing overnight; Day 9 0900hr 15min cold room, 1800h lights on overnight; Day 10 1000h 3h lights off, 1300h 20min cage agitation.
Drug Administration
Methamphetamine hydrochloride (M8750) and ketanserin tartrate salt (S006) were purchased from Sigma-Aldrich and administered on Day 11, the day following the last stressor. In studies measuring plasma CORT levels, a single injection of methamphetmine (7.5 mg/kg) or saline (1 ml/kg) was administered at 0700h and rats killed one hour later. For temperature and tissue content studies, rats received 4 injections of saline (1 ml/kg) or methamphetamine (7.5 mg/kg) every 2h. In the ketanserin studies, rats were administered the 5-HT2 antagonist ketanserin (0.5, 1, and 3 mg/kg) 30min prior to each saline (1 ml/kg) or methamphetamine (7.5 mg/kg) injection.
Plasma Corticosterone
Rats were killed via rapid decapitation and trunk blood collected 1h after methamphetamine or saline administration. Samples were centrifuged at 800g for 15min at 4°C, plasma removed and re-centrifuged at 800g for 7min. The supernatant was analyzed for CORT using an EIA kit (Immunodiagnostic Systems).
Temperature Measurements
Rectal temperatures were measured 1h after each methamphetamine or saline injection via a Thermalert rectal probe thermometer. Baseline temperatures were taken prior to all injections.
5-HT Tissue Content
Rats were killed 7 days after methamphetamine or saline injections via rapid decapitation and whole hippocampi dissected as previously described (Tata et al., 2007). This time point was chosen based on the known time-course of high-dose methamphetamine (10mg/kg q 2hr × 4) to produce decreases in neurotransmitter content (Yamamoto and Bankson, 2005). Tissue was sonicated in 1200μL 0.25M perchloric acid and centrifuged at 14,000g for 20min at 4°C. The supernatant was analyzed for 5-HT using high-performance liquid chromatography with electrochemical detection as previously described (Breier et al., 2006). 5-HT was separated on a C18 column (100 × 2.0mm, 3μm particle size, Phenomenex, Torrance) and eluted with a mobile phase containing 32mM citric acid, 54.3 mM sodium acetate, 0.074mM EDTA, 0.215mM octyl sodium sulfate, and 3% methanol (pH 3.8). Compounds were detected with a LC-4C amperometric detector (BAS Bioanalytical) and data recorded using EZ Chrom Software (Scientific Software). The pellet was resuspended in 1200μL 1M NaOH and protein determined using the method of Bradford.
Statistical Analysis
CORT and 5-HT data were analyzed using a one-way ANOVA followed by Tukey's post hoc test. Temperature data were analyzed using a two-way repeated measures ANOVA with Tukey's post hoc test. Significance was set at p<0.05.
Results
Plasma CORT levels after methamphetamine or saline administration to stressed and control rats are illustrated in Figure 1A. Methamphetamine administered to rats not previously stressed (control/methamphetamine) resulted in a significant increase in plasma CORT (p<0.05 versus control/saline). In rats that were previously stressed, the CORT response to methamphetamine was potentiated (p<0.05 versus control/methamphetamine). The stress/methamphetamine increase in CORT was attenuated by the 5-HT2 antagonist ketanserin, administered 30 minutes prior to methamphetamine (Figure 1B). Although the lowest dose of ketanserin (0.5 mg/kg) did not alter stress/methamphetamine increases in CORT (p=0.250), the higher doses (1 and 3 mg/kg) attenuated stress/methamphetamine increases in CORT (p<0.05). Ketanserin alone was without effect as administration of ketanserin (1 mg/kg) prior to saline in stress pretreated rats (stress/saline/ketanserin) did not significantly change CORT levels (stress/saline 36.34±14.8ng/mL versus stress/saline/ketanserin 14.3±8.6ng/mL; p=0.117).
Figure 1.
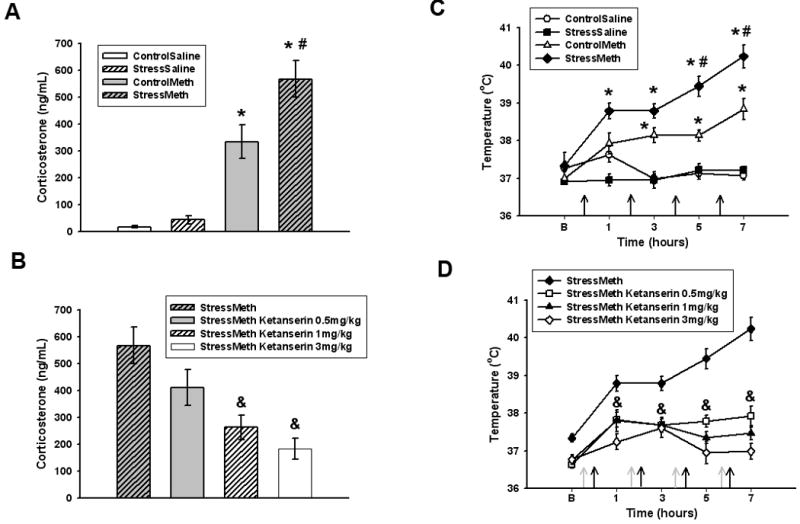
A. Effects of stress on methamphetamine-induced increases in CORT. *p<0.05 versus control/saline. #p<0.05 versus control/methamphetamine. B. Effects of ketanserin on stress/methamphetamine increases in CORT. &p<0.05 versus stress/methamphetamine. Data are expressed as mean ± SEM. Stress/methamphetamine is re-presented in panel B. n = 6 to 10 rats per group. C. Effects of stress on methamphetamine-induced increases in body temperature. Black arrows represent methamphetamine injections. *p<0.05 versus control/saline. #p<0.05 versus control/methamphetamine. D. Effects of ketanserin on stress/methamphetamine increases in body temperature. Grey arrows represent ketanserin injections. &p<0.05 versus stress/methamphetamine. Data are presented as mean ± SEM. Stress/methamphetamine is re-presented in panel D. B = baseline temperature. n = 6 to 8 rats per group. ControlSaline = control/saline, StressSaline = stress/saline, ControlMeth = control/methamphetamine, StressMeth = stress/methamphetamine
Figure 1C illustrates the effects of stress on methamphetamine (7.5 mg/kg × 4 q 2 hours) increases in body temperature. A significant time (F(3,207)=23.873, p<0.001), treatment (F(7,207)=6.136,p<0.001), and treatment by time interaction (F(21,207)=5.048, p<0.001) was observed. Methamphetamine alone (control/methamphetamine) increased body temperature (p<0.05 versus control/saline) and prior exposure to stress (stress/methamphetmine) potentiated this increase (p<0.05 versus control/methamphetamine). The stress/methamphetamine increase in temperature was attenuated by all doses of ketanserin administered 30min prior to each methamphetamine injection (Figure 1D, p<0.05 versus stress/methamphetamine). Ketanserin alone did not alter body temperature as there was no significant change in body temperature in stress/saline rats administered ketanserin (1 mg/kg) 30min prior to each saline injection (data not shown, p=0.632).
Hippocampal 5-HT concentrations are illustrated in Figure 2A. Methamphetamine (7.5mg/kg q 2hr × 4) administration to non-stressed rats (control/methamphetamine) did not alter hippocampal 5-HT 7 days after methamphetamine administration (p=0.947 versus control/saline); however, methamphetamine administration to rats that were previously stressed (stress/methamphetamine) resulted in a 40% depletion in 5-HT 7 days later (p<0.05 versus control/saline). The stress/methamphetamine decrease in 5-HT was reversed by all doses of ketanserin (Figure 2B, p<0.05 versus control/saline). Ketanserin alone was without effect as ketanserin (1 mg/kg) administered 30 minutes prior to saline injections in stress pretreated rats did not alter hippocampal 5-HT 7 days later (stress/saline 4.35±0.23pg/μg protein vs. stress/saline/ketanserin 4.44±0.25pg/μg protein, p=0.791).
Figure 2.
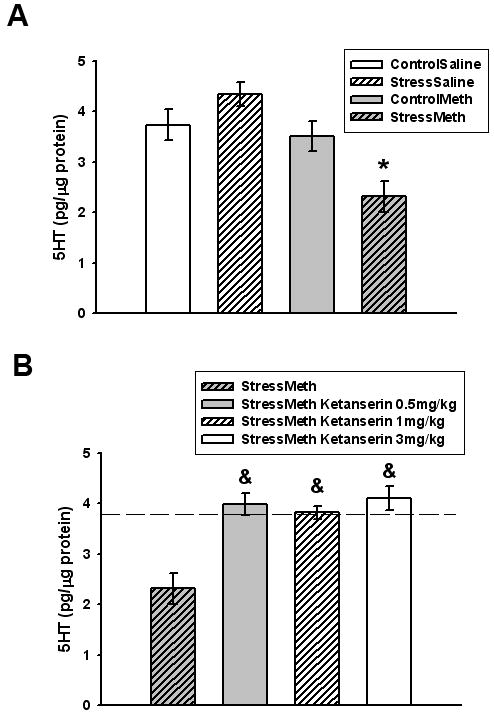
A. Effects of stress and methamphetamine on hippocampal 5-HT. *p<0.05 versus control/saline. B. Effects of ketanserin on stress/methamphetamine decreases in hippocampal 5-HT. &p<0.05 versus stress/methamphetamine. Data are presented as mean ± SEM. Stress/methamphetamine is re-presented in panel B. Dotted line = average control/saline values. n = 6 to 8 rats per group. ControlSaline = control/saline, StressSaline = stress/saline, ControlMeth = control/methamphetamine, StressMeth = stress/methamphetamine.
Discussion
This study examined the role of the 5-HT2 receptor in modulating acute hyperthermic and CORT responses and hippocampal 5-HT depletions in rats exposed to chronic stress followed by methamphetamine. The present findings demonstrate that stress potentiates methamphetamine-induced increases in CORT and hyperthermia. The combination of chronic stress and methamphetamine depleted hippocampal 5-HT, whereas methamphetamine alone had no effect. The increases in CORT produced by the serial exposure to chronic stress and methamphetamine were attenuated by the two highest doses of ketanserin, whereas the increases in hyperthermia and depletions of 5-HT were blocked by all doses of ketanserin.
The stress/methamphetamine potentiation of hyperthermia is in agreement with previously work (Tata et al., 2007), however, this study elucidates a pharmacological mechanism by which this occurs. Additionally, we report that stress potentiates methamphetamine-induced increases in plasma CORT which are blocked by the 5-HT2 receptor antagonist ketanserin. This potentiated CORT response is important in light of the fact that CORT mediates, in part, the rewarding and stimulating effects of psychostimulant drugs of abuse (Marinelli and Piazza, 2002). Therefore, a potentiated CORT response may enhance the rewarding effects of methamphetamine. Although CORT responses in chronically stressed rats were potentiated in response to methamphetamine, no significant increases in CORT were observed in stressed rats administered saline. This is in contrast to increases in basal CORT observed following numerous chronic stress paradigms and may be due to control/saline CORT levels being elevated after a saline challenge injection. Alternatively, CORT levels were also measured nearly 24hr after the last stressor at which point CORT may have returned to basal values. This phenomenon may also explain why stress alone had no effect on basal temperatures.
Although we demonstrate that stress/methamphetamine increases in CORT and hyperthermia are blocked by systemic administration of the 5-HT2 antagonist ketanserin, future studies will determine the subtype and distribution of 5-HT2 receptors that mediate this effect. As the hypothalamus is important in modulating HPA axis function and body temperature (Lin et al., 1998; Zhang et al., 2002), 5-HT2 receptors within that hypothalamus may be altered by stress and contribute to the potentiated CORT and hyperthermic responses to methamphetamine in chronically stressed rats. Future studies will test the effects of chronic unpredictable stress on hypothalamic 5-HT2 receptors and determine if local 5-HT2 antagonism attenuates the CORT and hyperthermic responses to methamphetamine.
The depletions in hippocampal 5-HT are similar to those observed in the striatum, where the combination of stress and methamphetamine but not methamphetamine alone depletes 5-HT (Tata et al., 2007). The lack of effect of methamphetamine alone is interesting in light of the fact that this dosing paradigm (7.5mg/kg q 2hr × 4) increases both hyperthermia and CORT. There may be a threshold level of hyperthermia and CORT that can be tolerated before depletions in neurotransmitter content occur. Chronic stress may serve to push physiological and neurochemical responses above this threshold level, which is in line with the potentiated glutamate responses that we have observed in the hippocampus after chronic stress (Raudensky and Yamamoto, 2007). Although we hypothesize that the stress-induced changes in 5-HT2 receptors may be responsible, future studies should also be designed to determine if similar effects are observed with methamphetamine alone at higher doses.
Overall, these studies illustrate that CORT and hyperthermic responses to methamphetamine are potentiated by chronic stress and that hippocampal 5-HT is depleted by the serial exposure to chronic stress and methamphetamine. Furthermore, these findings demonstrate that acute CORT and hyperthermic responses and hippocampal 5-HT depletions in stress/methamphetamine rats can be pharmacologically blocked by the 5-HT2 antagonist ketanserin. As 5-HT2 receptors are important mediators of the development and expression of methamphetamine-induced behavioral sensitization (Ago et al., 2007), 5-HT2 receptor antagonists may be beneficial in blocking physiological, neurochemical, and behavioral alterations produced by methamphetamine in chronically stressed rats. Therefore, 5-HT2 receptors may prove an important target in which to ameliorate a range of effects of methamphetamine that are either important in reward/addiction or play a role in depleting neurotransmitter content. The potential of 5-HT2 receptor antagonists to ameliorate the physiological and neurochemical effects of methamphetamine in chronically stressed rats is highly relevant in light of the high prevalence and comorbidity of drug abuse and stress (Marinelli and Piazza, 2002).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jamie Raudensky, McLean Hospital, Harvard Medical School, McLean Hospital MRC 114, 115 Mill St, Belmont, MA 02378.
Bryan K. Yamamoto, University of Toledo Health Sciences Center, Department of Neurosciences, Mail Stop 1007, 3000 Arlington Ave., Toledo, OH 43614
References
- Ago Y, Nakamura S, Kajita N, Uda M, Hashimoto H, Baba A, Matsuda T. Ritanserin reverses repeated methamphetamine-induced behavioral and neurochemical sensitization in mice. Synapse. 2007;61:757–763. doi: 10.1002/syn.20421. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. J Neurosci. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Stone DM, Bush LG, Hanson GR, Gibb JW. Glucocorticoids and 3,4-methylenedioxymethamphetamine (MDMA)-induced neurotoxicity. Eur J Pharmacol. 1989;161:181–188. doi: 10.1016/0014-2999(89)90841-8. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Tsay HJ, Su WH, Chueh FY. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol. 1998;274:R1260–1267. doi: 10.1152/ajpregu.1998.274.5.R1260. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia in male rats. Psychopharmacology (Berl) 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Res. 2007;1135:129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Tata DA, Raudensky J, Yamamoto BK. Augmentation of methamphetamine-induced toxicity in the rat striatum by unpredictable stress: contribution of enhanced hyperthermia. Eur J Neurosci. 2007;26:739–748. doi: 10.1111/j.1460-9568.2007.05688.x. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: cause and consequence of oxidative stress. Crit Rev Neurobiol. 2005;17:87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Damjanoska KJ, Carrasco GA, Dudas B, D'Souza DN, Tetzlaff J, Garcia F, Hanley NR, Scripathirathan K, Petersen BR, Gray TS, Battaglia G, Muma NA, Van de Kar LD. Evidence that 5-HT2A receptors in the hypothalamic paraventricular nucleus mediate neuroendocrine responses to (-)DOI. J Neurosci. 2002;22:9635–9642. doi: 10.1523/JNEUROSCI.22-21-09635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


