Abstract
The efficacy of reduced intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (HCT) for Ph+ acute lymphoblastic leukemia (ALL) is uncertain. We analyzed 197 adults with Ph+ ALL in first complete remission; 67 patients receiving RIC were matched with 130 receiving myeloablative conditioning (MAC) for age, donor type, and HCT year. Over 75% received pre-HCT tyrosine kinase inhibitors (TKI), mostly imatinib; 39% (RIC) and 49% (MAC) were MRDneg pre-HCT. At a median 4.5 years follow-up, 1-year transplant-related mortality (TRM) was lower in RIC (13%) than MAC (36%;p=0.001) while the 3-year relapse rate was 49% in RIC and 28% in MAC (p=0.058). Overall survival was similar (RIC 39% [95% CI:27–52] vs. 35% [95% CI:270–44];p=0.62). Patients MRDpos pre-HCT had higher risk of relapse with RIC versus MAC (HR 1.97;p=0.026). However, patients receiving pre-HCT TKI in combination with MRD negativity pre-RIC HCT had superior OS (55%) compared to a similar MRDneg population after MAC (33%; p=0.0042). In multivariate analysis, RIC lowered TRM (HR 0.6; p=0.057), but absence of pre-HCT TKI (HR 1.88;p=0.018), RIC (HR 1.891;p=0.054) and pre-HCT MRDpos (HR 1.6; p=0.070) increased relapse risk. RIC is a valid alternative strategy for Ph+ ALL patients ineligible for MAC and MRDneg status is preferred pre-HCT.
Keywords: Acute lymphoblastic leukemia, Philadelphia chromosome, reduced intensity conditioning, allograft, minimal residual disease, tyrosine-kinase inhibitor
INTRODUCTION
Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia (ALL) is the largest genetically defined subset, affecting about 25% of adults with ALL; particularly those older than 40 years.1 The poor survival of Ph+ ALL patients treated with chemotherapy alone (10%) has been substantially improved through the use of allogeneic hematopoietic cell transplantation (HCT) in first complete remission (CR1) and more recently, by combining tyrosine kinase inhibitors (TKI) with induction and post-remission chemotherapy.2–5 The anti-leukemia effect of HCT is through chemotherapy and/or radiation used in the preparative regimen and through an immune-mediated graft-versus-leukemia (GVL) effect.5–8 Although widespread use of TKIs has changed the landscape of Ph+ ALL management, myeloablative conditioning (MAC) followed by the allogeneic HCT remains the only established curative therapy. Incorporating TKIs into induction chemotherapy has not increased toxicity, but has substantially improved remission rates and facilitated more allotransplants in CR1.9, 10 Furthermore, several prospective clinical trials testing an imatinib-containing strategy consolidated with a MAC alloHCT showed overall survival (OS) ranging from 40–65%, which is markedly better than historical pre-imatinib controls (OS 20–40%).2–4, 11–13 However, many patients are not eligible for a conventional myeloablative conditioning regimen due to their age and comorbidities. High transplant-related mortality (TRM) remains a serious problem in older adults which negates the survival benefit gained through protection from relapse by full intensity conditioning and GVL.14 For these reasons, RIC HCT was developed to allow engraftment and harness the GVL effect while potentially limiting TRM in patients unfit for full intensity conditioning regimens
To date, there are no large-scale data on the efficacy of RIC HCT for Ph+ ALL. Most single institution studies lack detail on ALL subset-specific outcomes.15–19 The utility of RIC HCT for ALL was recently demonstrated in a CIBMTR study for Ph negative ALL, in which similar rates of TRM, relapse, and survival (43% vs 38%) between RIC and MAC were observed.20 A European Bone Marrow Transplant (EBMT) Registry study, which included 41 Ph+ patients in a RIC cohort, showed comparable OS between RIC and MAC groups.21 However, the limited details on minimal residual disease (MRD) and TKI use make the interpretation of these studies problematic. Indeed, the definition of remission in Ph+ ALL now routinely includes tools to assess the depth of remission by cytogenetic testing of interphase cells for t(9;22) (fluorescent in situ hybridization [FISH]) and PCR for detection of chimeric mRNA arising from BCR-ABL1 genomic recombination. FISH assay allows the sensitivity between 0.5–3%, while real-time PCR and nested PCR allow quantification of MRD to the 1:105-106 cell level.22 Both assays are widely used to monitor response and guide therapeutic choices.17,23–26 Several studies in adult Ph+ ALL have confirmed that patients with MRD persistence 6–10 weeks after initiating induction therapy have a higher risk of relapse, yet early myeloablative allogeneic donor HCT can sometimes overcome MRDpos and cure a subset of patients.25, 27 The sensitivity of Ph+ ALL to non-ablative chemotherapy/radiation and to GVL in the setting of RIC HCT is not well established. To address these issues, we performed a multicenter registry-based analysis investigating the outcomes of RIC allogeneic HCT for Ph+ ALL. Using a matched pair design, we examined a cohort of patients with Ph+ALL in CR1 and compared survival after RIC or MAC allogeneic transplantation, as well as the effect of TKI use and pre-HCT MRD status on transplant outcomes.
PATIENTS AND METHODS
Data source
The CIBMTR (Center for International Bone Marrow Transplant Research), a voluntary working group of more than 450 transplantation centers worldwide, collects data on consecutive allogeneic HCTs at a statistical center housed at both the Medical College of Wisconsin (Milwaukee, WI) and the National Marrow Donor Program (Minneapolis, MN). Patients are observed longitudinally with yearly follow-up. Computerized checks for errors and onsite audits of participating centers ensure data quality. The present study was conducted with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the Institutional Board and the Privacy Officer of the Medical College of Wisconsin.
Patient Selection
We included patients aged 18 and older with Ph+ALL in CR1 who had received HLA allele matched related or matched or mismatched unrelated donor (URD) HCT between 2000 and 2009. Well matched URD were either 8/8 or 6/6 matched at Class 1 as recommended by CIBMTR.26 Umbilical cord blood donors, mismatched related donor and ex vivo T cell-depleted grafts were excluded. Preparative regimens were classified either as RIC or MAC according to published consensus definitions.28 The CIBMTR definition of RIC included regimens containing melphalan ≤ 150mg/m2 (n=24), busulfan ≤ 9mg/kg orally (n=20), total body irradiation <5 Gy (TBI; n=11), fludarabine-TBI combinations (n=17), or fludarabine-based conditioning (n=5). The MAC preparative regimen consisted mostly of TBI (n=108) or busulfan combinations (n=22). RIC patients were matched with MAC patients on 3 factors: age (within 15 years), type of donor (related versus URD), and year of transplant (within 5 years). A supplemental data form was developed to collect: 1) presence of pre-HCT MRD in bone marrow immediately prior to conditioning tested by FISH and/or by PCR for the BCR-ABL (yes/no); and 2) use of TKIs (imatinib, nilotinib or dasatinib) delivered at any time prior to transplantation and the duration of TKI therapy. We also collected data on post-transplant TKI administration, defined as maintenance therapy (excluding treatment given for cytogenetic or morphologic relapse), start date and duration of maintenance. Retrospectively collecting the MRD data from many centers reflects the real world clinical practice where both BCR/ABL transcript levels and/or FISH analysis are often obtained in patients with morphologic CR. The stringency of MRD determination using each center’s testing sensitivity with these approaches could not be addressed in this multicenter analysis. Data on post-transplant MRD monitoring were not collected. The final study population excluded MAC patients not selected by the matching strategy (n=241) and those without supplemental data (n=32). The return rate on supplemental forms requested on 243 cases from 76 centers was 86.4%. Each participating center enrolled an average 2.5 cases (range 1–15 cases).
Definitions, Study Endpoints and Statistical Analysis
The primary outcomes were OS after HCT defined as the time from transplantation to death, disease-free survival (DFS), relapse incidence and TRM. Surviving patients were censored at the time of last contact. Secondary endpoints were grade II-IV acute graft-versus-host disease (aGVHD) and chronic GVHD (cGVHD). Probability of DFS and OS were calculated using the Kaplan-Meier estimator, with the variance estimated by Greenwood’s formula. Values for other endpoints were calculated using cumulative incidence curves to accommodate competing risks.29 We defined the MRD status as MRDpos (Ph+ by FISH positive and/or positive BCR-ABL by PCR) and pre-HCT MRDneg (Ph+ FISH negative and/or negative BCR-ABL by PCR). Use of TKI was defined as pre-HCT TKI (including 41 patients who received TKI both pre and post-HCT) or no TKI at any time-point. The risk factors considered in the stepwise model building procedures were conditioning regimen intensity (main effect), age, gender, pre-HCT MRD positivity, TKI use pre-HCT (yes/no), year of HCT and cGVHD as a time-dependent covariate. The potential interactions between the main effect (conditioning regimen) and MRD, TKI and other significant variables were examined.
RESULTS
Patients Characteristics
Data on 197 eligible patients from 14 different countries and 76 reporting centers were analyzed. Sixty-seven RIC patients were matched for analysis 1:2 (n=63) or 1:1 (n=4) to 130 MAC patients for age, donor type, and year of transplant (Table 1). Median age in the RIC and MAC groups was 54 and 50 years, respectively (p=0.02). WBC count at diagnosis and performance status at time of transplant were similar for both groups. Previous fungal infections were more common in the RIC group (12% vs 3%; p=0.006). RIC recipients had a longer median time from diagnosis to HCT (6 months (interquartile range 4.8–7.4 months) vs MAC: 5 months (interquartile range 4.2–6.8 months; p=0.03), but the time from diagnosis to CR1 was similar (median 42 days (interquartile range 34–82 days) and 52 days (interquartile range 31–111 days; p=0.76) in RIC and MAC groups. Over half of patients in both groups had co-existent morbidities or organ impairment (61% in RIC and 58% in MAC; p=0.12). Significantly more RIC patients had a pre-HCT comorbidity index of ≥1 as compared to MAC patients (19% vs 8%; p=0.03).30 Both RIC and MAC groups used peripheral blood grafts more often than bone marrow grafts and had similar use of related donors (39% and 38%) and matched URD (42% for both). GVHD prophylaxis was similar in both groups with cyclosporine or tacrolimus-containing-regimens used most often. RIC patients more often received anti-thymocyte globulin or campath (37% vs 17%; p=0.03). The remaining variables of donor/recipient sex, donor/recipient CMV status and year of transplant were balanced. Median follow-up of survivors was 49 months (range 3–108 months) for the RIC group and 61 months (range 3–119 months) for the MAC group.
Table 1.
Characteristics of adults with Ph+ ALL who received reduced intensity conditioning (RIC) allogeneic donor HCT in CR1 between 2000–2009 and a matched cohort receiving myeloablative (MAC) allogeneic HCT
| Variable | RIC | MAC | P-value |
|---|---|---|---|
| Number of patients | 67 | 130 | |
| Number of centers | 30 | 66 | |
| Age, median (range) | 54 (19–69) | 50 (19–66) | 0.02 |
| Sex | 0.94 | ||
| Male | 37 (55) | 71 (55) | |
| Female | 30 (45) | 59 (45) | |
| Karnofsky score | 0.36 | ||
| < 80% | 8 (12) | 8 (6) | |
| 80 – 100% | 54 (81) | 113 (87) | |
| Missing | 5 (7) | 9 (7) | |
| HCT Comorbidity Index | 0.03 | ||
| 0 | 54 (81) | 119 (92) | |
| ≥1 | 13 (19) | 11 (8) | |
| Fungal infection prior to HCT | 0.006 | ||
| No | 57 (85) | 126 (97) | |
| Yes | 8 (12) | 4 (3) | |
| Missing | 2 (3) | 0 | |
| WBC at diagnosis, ×10^9/L | 0.23 | ||
| < 30 | 41 (61) | 70 (54) | |
| 30 – 100 | 14 (21) | 23 (18) | |
| > 100 | 5 (7) | 24 (18) | |
| Missing | 7 (10) | 13 (10) | |
| Cytogenetic abnormalities | 0.34 | ||
| t(9:22) only | 32 (48) | 48 (37) | |
| t(9:22) and other | 25 (37) | 58 (45) | |
| Other | 4 (6) | 5 (4) | |
| Missing | 6 (9) | 19 (15) | |
| Time from diagnosis to 1st complete remission | 0.76 | ||
| Median (interquartile range) in days | 42 (34–82) | 52 (31–111) | |
| Missing | 1 (1) | 7 (5) | |
| Time from diagnosis to transplant | 0.02 | ||
| ≤ 5 months | 20 (30) | 64 (49) | |
| > 5 months | 46 (69) | 66 (51) | |
| Missing | 1 (1) | 0 | |
| Minimal residual disease pre-HCT | |||
| FISH tested | 59 (88) | 113 (87) | 0.75 |
| FISH neg/tested for MRD | 36/59 (54) | 63/113 (48) | |
| FISH pos/tested for MRD | 23/59 (34) | 50/113 (38) | |
| FISH not performed or unknown | 8 (12) | 17 (13) | |
| BCR/ABL PCR tested | 42 (63) | 82 (63) | 0.73 |
| PCR neg/tested for MRD | 21/42 (50) | 46/82 (35) | |
| PCR pos/tested for MRD | 21/42 (50) | 36/82 (28) | |
| PCR not performed or unknown | 24 (36) | 48 (37) | |
| MRDneg** | 26 (39) | 58 (49) | 0.79 |
| MRDpos* | 39 (58) | 62 (47) | |
| TKI use prior to HCT | 0.71 | ||
| No | 16 (24) | 28 (22) | |
| Yes | 51 (76) | 102 (78) | |
| Duration of TKI pre HCT, months | 0.41 | ||
| Median (range) | 7 (1–12) | 6 (1–54) | |
| <6 month | 28 (42) | 66 (51) | |
| ≥6 month | 15 (22) | 18 (14) | |
| TKI not given or unknown | 24 (36) | 48 (36) | |
| Conditioning regimen | N/A | ||
| MAC | |||
| TBI>500 cGy single dose or > 800cGy | 0 | 108 (83) | |
| Busulfan > 9 mg/kg + other | 0 | 22 (17) | |
| RIC (includes non-myeloablative) | |||
| Melphalan ≤ 150 mg/m2 | 24 (36) | 0 | |
| Busulfan ≤ 9 mg/kg | 20 (30) | 0 | |
| TBI ≤ 200 cGy dose# | 18 (26) | 0 | |
| Fludarabine + other | 5 (7) | 0 | |
| Total body irradiation | N/A | ||
| No | 45 (67) | 22 (17) | |
| Yes | 21 (31) | 108 (83) | |
| Missing | 1 (1) | 0 | |
| Type of donor | 0.10 | ||
| HLA - identical sibling | 26 (39) | 50 (38) | |
| Well - matched URD | 28 (42) | 54 (42) | |
| Partially – matched/ mismatched URD | 5 (7) | 21 (16) | |
| URD (matching unknown) | 8 (12) | 5 (4) | |
| Donor –Recipient sex match | 0.79 | ||
| Male-Male | 22 (33) | 47 (36) | |
| Male-Female | 18 (27) | 40 (31) | |
| Female-Male | 15 (22) | 24 (18) | |
| Female-Female | 12 (18) | 18 (14) | |
| Missing | 0 | 1 (<1) | |
| Donor-Recipient CMV match | 0.55 | ||
| Donor positive/Recipient positive | 27 (40) | 39 (30) | |
| Donor positive/Recipient negative | 9 (13) | 15 (12) | |
| Donor negative/Recipient positive | 14 (21) | 35 (27) | |
| Donor negative/Recipient negative | 15 (22) | 38 (29) | |
| Missing | 2 (3) | 3 (2) | |
| Graft type | 0.12 | ||
| Bone marrow | 15 (22) | 43 (33) | |
| Peripheral blood | 52 (78) | 87 (67) | |
| Year of transplant | 0.53 | ||
| 2001–2004 | 40 (59) | 70 (54) | |
| 2005–2009 | 27 (41) | 60 (46) | |
| GVHD prophylaxis | 0.36 | ||
| Tacrolimus ± other | 61 (46) | 72 (58) | |
| Cyclosporine ± other | 30 (45) | 47 (36) | |
| ATG/alemtuzumab | 25 (37) | 23 (17) | 0.032 |
| Other | 6 (8) | 9 (7) | |
| Median follow-up of survivors: months (range) | 49 (3–108) | 61 (3–119) | |
| Post-transplant therapy | |||
| Any TKI given as maintenance post-HCT | 0.02 | ||
| No | 46 (69) | 108 (83) | |
| Yes | 21 (31) | 22 (17) | |
| Duration of TKI given for maintenance post-HCT | 0.05 | ||
| < 3 months | 7 (10) | 4 (3) | |
| 3–12 months | 9 (13) | 9 (7) | |
| ≤ 12 months | 4 (6) | 9 (7) |
Includes both FISH and/or BCR/ABL PCR positive,
Includes FISH negative and BCR/ABL PCR negative or missing,
one patient received TBI <500 cGy single dose
Abbreviations: ALL acute lymphoblastic leukemia, RIC reduced intensity conditioning, MAC myeloablative conditioning, HCT hematopoietic cell transplantation, MRD minimal residual disease, TKI tyrosine kinase inhibitor, CMV cytomegalovirus, TBI total body irradiation, GVHD graft versus host disease,
MRD assessment and use of TKI inhibitors
All patients were in CR1 by morphologic criteria. Reflecting the clinical practice, more patients had pre-HCT bone marrow MRD evaluation by FISH (RIC: 89%; MAC: 88%) than PCR (RIC: 64%; MAC: 63%). 185 subjects (94% of all patients; RIC: 97%; MAC: 92%) were investigated by at least one MRD method prior to transplant at median time 25 days (interquartile range: 14–122 days; only 46% reported the MRD test date). Similar proportions of RIC and MAC recipients were FISHpos (34% vs 38%), PCR BCR/ABLpos (50% vs 35%) and positive by either or both methods (MRDpos 58% and 47%; p=0.79; Table 1).
Before 2005, 60% received TKI pre-transplant compared to nearly 90% after 2005 (p<0.01). All but 2 patients received imatinib pre-HCT (the pre-HCT TKI group); including who 2 received dasatinib pre-HCT. The median duration of TKI pre-HCT was 7 months (RIC) and 6 months (MAC). Among 153 patients given TKI pre-HCT, 41% became MRDneg pre-HCT. Among 44 patients not receiving TKI, 48% were MRDneg. The rate of MRD negativity was not better in those receiving prolonged pre-HCT TKI (>6 months 38% vs <6 months 42%; p=0.44).
Only 43 (<25%) patients (RIC n=21; MAC n=22) received TKI post-transplant for maintenance. Data on the drug, dose, and treatment duration were not available. The majority of patients (90% in RIC and 77% in MAC) who received TKI post-transplant were MRDpos pre-HCT. The median time to TKI administration after RIC HCT was 1.5 months (interquartile range: 0.9 −3.5 months), about 1 month earlier than MAC HCT (2.9 months [interquartile range 1.7–5.5 months]; p=0.095). Forty-one patients (20%) were not treated with TKI agents at any time.
DFS and OS
At 3 years, DFS and OS for the RIC group were 26% (95% CI 16–37%) and 39% (95% CI 27–52%), respectively and were similar to the MAC group (28% [95%CI 20–36%] and 35% [95%CI 27–44%]; Table 2). In univariate analysis, pre-HCT MRD status did not impact survival in either group (Figure 1B). Sex, peripheral WBC at diagnosis and year of HCT did not significantly affect OS in univariate analysis (male gender HR: 1.17 (95%CI 0.8–1.6); WBC>100×109/L: HR 0.6 [95%CI 0.32–1.1], 2005–2009 HR: 1.05 [95%CI 0.7–1.5]. Within the RIC group, both pre-HCT TKI and concomitant MRDneg status yielded the best 3-year OS (55% [95%CI 31–77%]), which compared favorably to the same patients (MRDneg with pre-HCT TKI) in the MAC group (33% ([95% CI 20–48%]); p= 0.0042).
Table 2.
Outcomes after HCT: Univariate analysis
| RIC | MAC | P-value | |||
|---|---|---|---|---|---|
| N Eval | Prob (95% CI) | N Eval | Prob (95% CI) | ||
| Relapse | 67 | 130 | |||
| 3-year | 49 (37–61)% | 28 (20–36)% | 0.058 | ||
| Treatment related mortality | 67 | 130 | |||
| 1-year | 13 (6–23)% | 36 (28–44)% | <0.001 | ||
| Disease free survival | 67 | 130 | |||
| 3-year | 26 (16–37)% | 28 (20–36)% | 0.75 | ||
| Overall survival | 67 | 130 | |||
| 3-year | 39 (27–52)% | 35 (27–44)% | 0.62 | ||
| Acute GVHD, grade II – IV | 66 | 130 | |||
| 100-day | 30 (20–42)% | 47 (39–56)% | 0.014 | ||
| Chronic GVHD at 3 years | 66 | 60 (47–72)% | 128 | 47 (38–56)% | 0.17 |
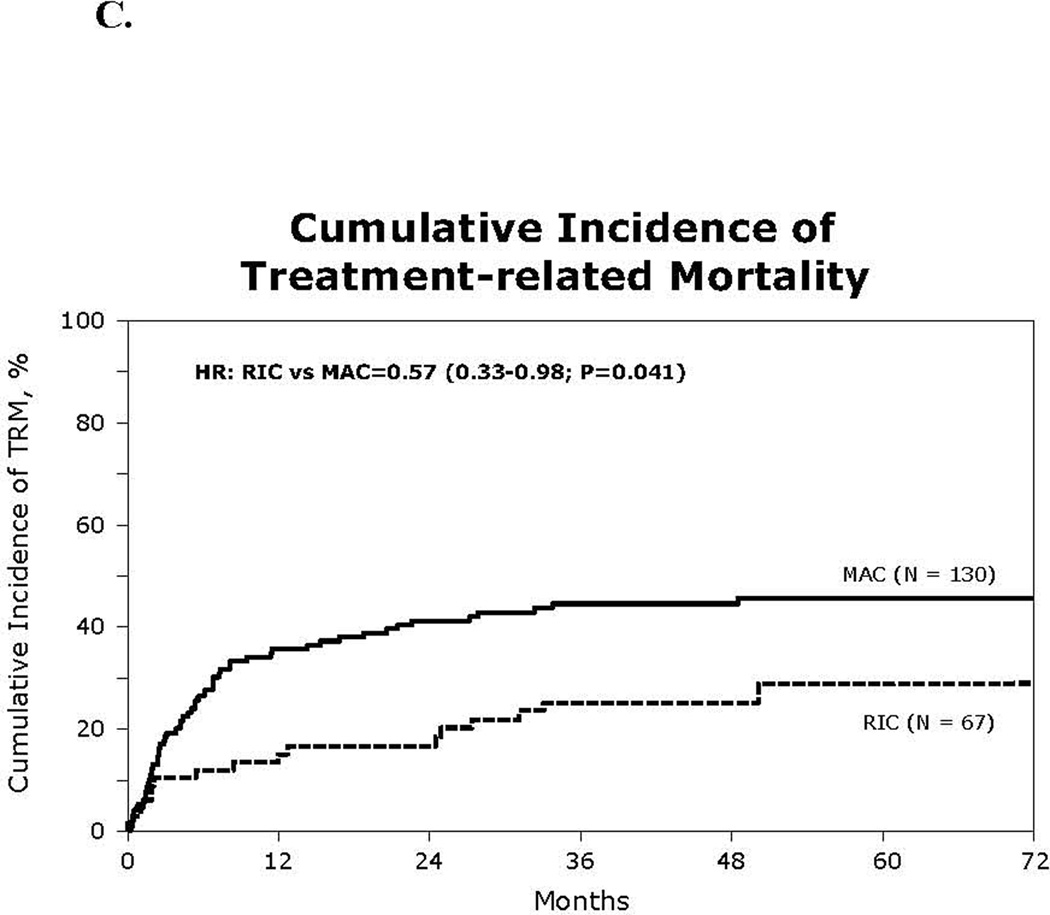
Figure 1.
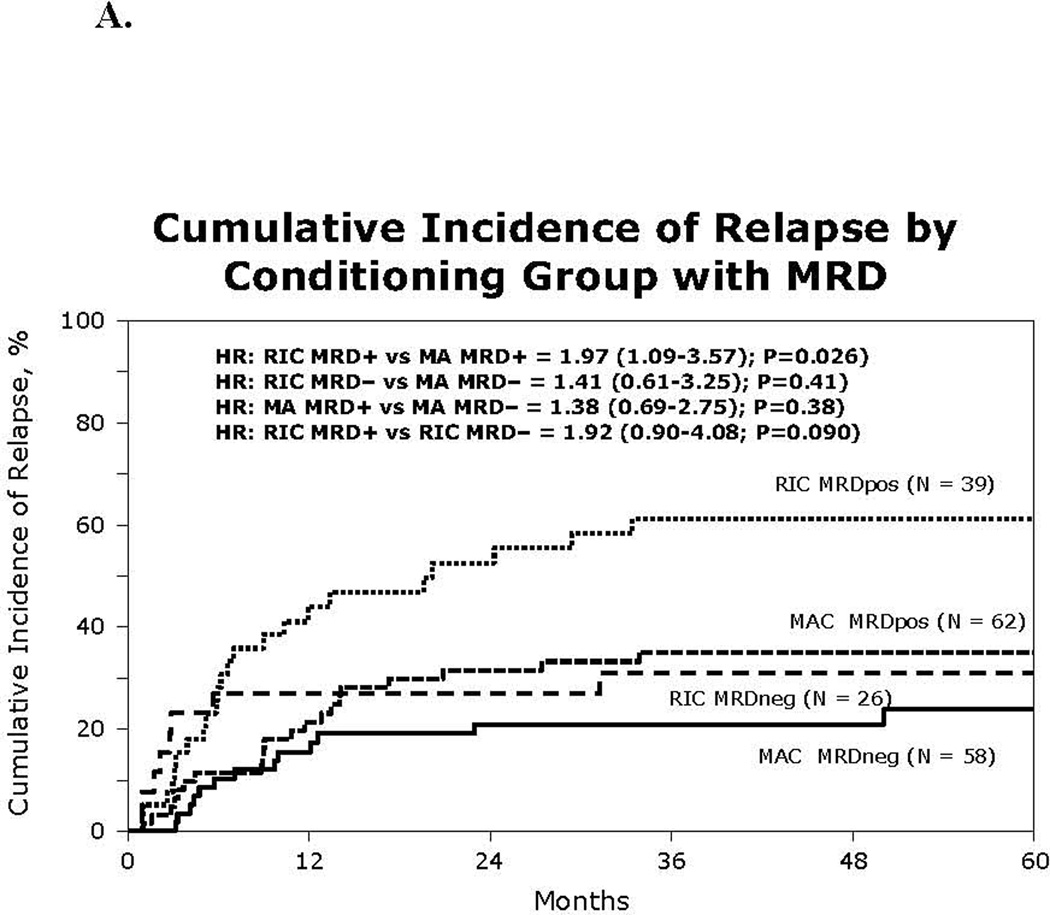
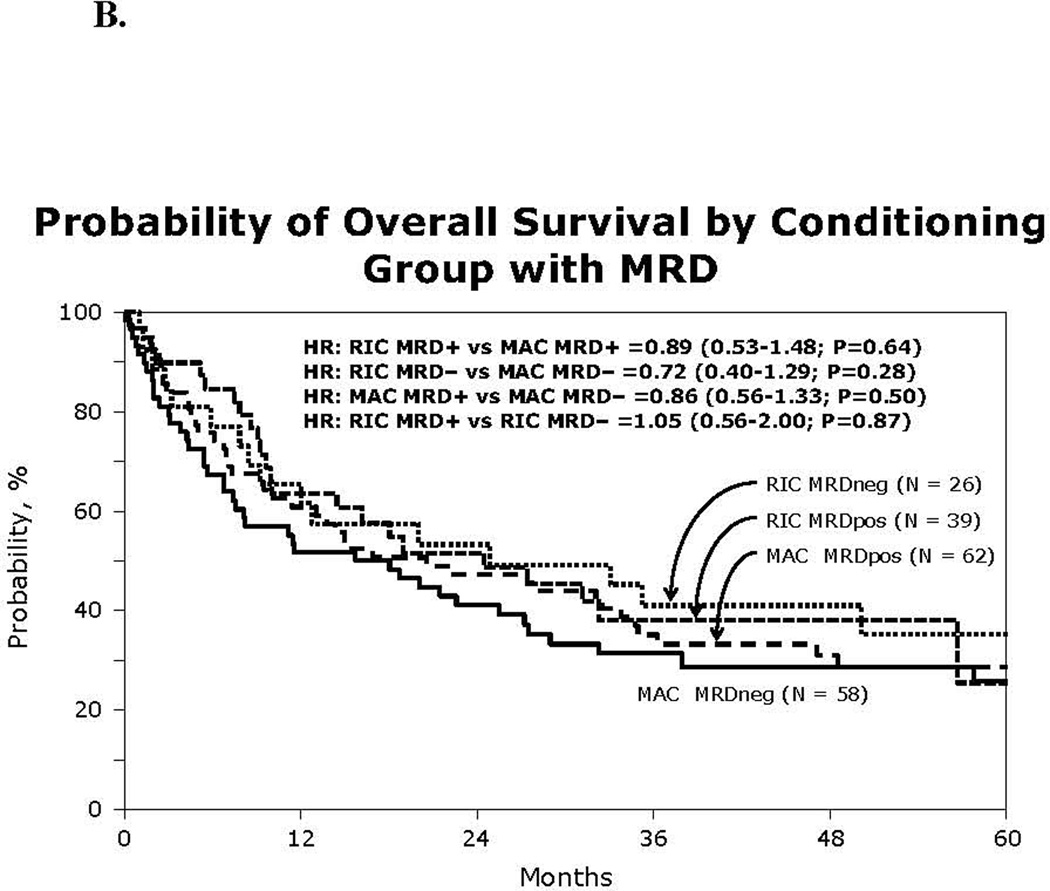
(A) Kaplan-Meier estimate of overall survival using reduced intensity or myeloablative conditioning HCT for Ph+ ALL by MRD status
(B) Cumulative incidence of relapse by pre HCT MRD and conditioning intensity
(C) Cumulative incidence of treatment related mortality
In multivariate analysis, RIC did not significantly influence OS (HR 0.87; p=0.48) or DFS (HR 1.1; p=0.58; Table 3). Age above 40 years was associated with significantly worse survival (HR 1.92), but pre-HCT MRD status, pre-HCT TKI use and the development of cGVHD did not significantly alter OS or DFS (Table 3).
Table 3.
Multivariate analysis of acute GVHD, chronic GVHD, treatment related mortality, relapse, disease free survival and overall survival
| Outcomes | N | HR (95% CI) | P-value |
|---|---|---|---|
| Acute GVHD: | |||
| Main effect: | |||
| Myeloablative conditioning | 130 | 1.00 | |
| Reduced intensity conditioning | 67 | 0.54 (0.33–0.88) | 0.014 |
| Chronic GVHD: | |||
| Main effect: | |||
| Myeloablative conditioning | 130 | 1.00 | |
| Reduced intensity conditioning | 67 | 1.23 (0.81–1.86) | 0.34 |
| Other factors: | |||
| Pre HCT TKI | |||
| Yes | 153 | 1.00 | |
| No | 44 | 0.88 (0.53–1.45) | 0.62 |
| Treatment related mortality: | |||
| Main effect: | |||
| Myeloablative conditioning | 130 | 1.00 | |
| Reduced intensity conditioning | 67 | 0.60 (0.35–1.02) | 0.059 |
| Other factors: | |||
| Age | |||
| ≤ 40 years | 42 | 1.00 | |
| > 40 | 155 | 3.24 (1.45–7.24) | 0.0042 |
| Minimal residual disease* | |||
| MRDneg | 84 | 1.00 | Poverall=0.33 |
| MRDpos | 101 | 0.72 (0.45–1.16) | 0.18 |
| MRD unknown | 12 | 0.65 (0.25–1.69) | 0.37 |
| Pre-HCT TKI | |||
| Yes | 153 | 1.00 | |
| No | 44 | 0.66 (0.38–1.15) | 0.14 |
| Chronic GVHD (time dependent) | |||
| No | 101 | 1.00 | |
| Yes | 96 | 1.74 (0.97–3.11) | 0.064 |
| Relapse: | |||
| Main effect: | |||
| Myeloablative conditioning | 130 | 1.00 | |
| Reduced intensity conditioning | 67 | 1.84 (1.15–2.94) | 0.011 |
| Other factors: | |||
| Age | |||
| ≤ 40 years | 42 | 1.00 | |
| > 40 | 155 | 1.02 (0.61–1.68) | 0.95 |
| Minimal residual disease* | |||
| MRDneg | 84 | 1.00 | Poverall=0.13 |
| MRDpos | 101 | 1.60 (0.96–2.67) | 0.070 |
| MRD unknown | 12 | 0.77 (0.22–2.78) | 0.69 |
| Pre-HCT TKI | |||
| Yes | 153 | 1.00 | |
| No | 44 | 1.88 (1.11–3.17) | 0.018 |
| Chronic GVHD (time dependent) | |||
| No | 101 | 1.00 | |
| Yes | 96 | 0.72 (0.40–1.31) | 0.28 |
| Disease free survival: | |||
| Main effect: | |||
| Myeloablative conditioning | 130 | 1.00 | |
| Reduced intensity conditioning | 67 | 1.08 (0.76–1.51) | 0.68 |
| Other factors: | |||
| Age | |||
| ≤ 40 years | 42 | 1.00 | |
| > 40 | 155 | 1.68 (1.11–2.56) | 0.015 |
| Minimal residual disease* | |||
| MRDneg | 84 | 1.00 | Poverall=0.56 |
| MRDpos | 101 | 1.06 (0.75–1.48) | 0.75 |
| MRD unknown | 12 | 0.67 (0.29–1.54) | 0.35 |
| Pre-HCT TKI | |||
| Yes | 153 | 1.00 | |
| No | 44 | 1.08 (0.75–1.54) | 0.69 |
| Chronic GVHD (time dependent) | |||
| No | 101 | 1.00 | |
| Yes | 96 | 1.02 (0.68–1.54) | 0.92 |
| Overall survival: | |||
| Main effect: | |||
| Myeloablative conditioning | 130 | 1.00 | |
| Reduced intensity conditioning | 67 | 0.85 (0.59–1.24) | 0.41 |
| Other factors: | |||
| Age | |||
| ≤ 40 years | 42 | 1.00 | |
| > 40 | 155 | 1.90 (1.19–3.04) | 0.0070 |
| Minimal residual disease* | |||
| MRDneg | 84 | 1.00 | Poverall=0.66 |
| MRDpos | 101 | 0.94 (0.65–1.34) | 0.71 |
| MRD unknown | 12 | 0.67 (0.28–1.62) | 0.37 |
| Pre-HCT TKI | |||
| Yes | 153 | 1.00 | |
| No | 44 | 1.01 (0.69–1.48) | 0.96 |
| Chronic GVHD (time dependent) | |||
| No | 101 | 1.00 | |
| Yes | 96 | 1.15 (0.77–1.74) | 0.49 |
TRM and Causes of Death
The cumulative incidence of TRM at day 100 was 10% in the RIC group (95% CI 4–19%) and 19% in the MAC group (95%CI 13–26%; p=0.11), while at 1 year it was almost 3-fold lower in the RIC than in the MAC group (13% vs 36%; p<0.001; Figure 1C). In adjusted multivariate analysis, RIC was associated with reduced TRM risk (HR 0.60; p=0.059). Age over 40 years increased the risk of TRM 3-fold and cGVHD increased TRM risk 1.7-fold (Table 3). The most common cause of death in the RIC group was relapse (n=13) followed by infection (n=10), organ failure (n=6), and GVHD (n=4). MAC patients died most often of GVHD (n=22), relapse (n=16), and infection (n=12). Death attributed to GVHD was more common after MAC compared to RIC HCT (p=0.1).
Relapse
The incidence of relapse at 3 years was higher in the RIC group (49%) than in the MAC group (28%) although not statistically significant (p=0.058; Table 2). Given that pre-HCT MRD and use of TKI could potentially modify relapse risk, we examined relapse risks in specified subgroups. The cumulative incidence of relapse at 3 years in pre-HCT MRDpos patients was significantly higher after RIC 61% (95%CI 45–76%]) than MAC HCT (35% [95% CI 24–48%]; HR 1.97 [1.09–3.57; p=0.026]; Figure 1A). However, pre-HCT MRDneg patients had similar relapse risks after RIC or MAC transplants (31% [95% 15–50%]) vs 21% [11–32%]); p=0.15). Low relapse rate was also observed in subset of patients who were pre-HCT BCR/ABLneg (RIC 16% [95%CI 7–28]; MAC 25% [9–46%]; p=0.36).
In the RIC group, pre-HCT TKI therapy was associated with 2-fold reduction in 3- year relapse incidence (38%[95% CI 25–52%]) as compared to no pre-HCT TKI (81% [95%CI 59–96%]; p=0.0039). In contrast, in the MAC group no protection from relapse by pre-HCT TKI was observed (26% [95%CI, 18%–35%] vs 33% [95%CI 17–52%]; p=0.51). Remarkably low rates of relapse were observed in patients who received pre-HCT TKI who were also pre-HCT MRDneg (RIC 17% [95%CI 4–37%] and MAC HCT 20% [95%CI, 10–33%]). For these patients, the conditioning regimen intensity did not influence relapse risk. The 3-yr relapse was not impacted by TKI post-transplant maintenance neither in the RIC cohort (TKI maintenance 59% vs. no TKI 45%; p=0.63) nor in the MAC cohort (TKI maintenance 27% vs no TKI 28%; p=0.26), although only 43 patients were treated with TKI post-transplant. The median time from HCT to relapse was similar at 11 months in the RIC group (range 1–103 months) and 9 months in the MAC group (range 1–119 months; p= 0.60).
In adjusted multivariate analysis, RIC was associated with increased risk of relapse (HR 1.84; p=0.011). While age (>40 years) and cGVHD did not influence relapse risk, no TKI use pre-HCT (HR 1.88; p=0.018) was independently associated with an increased risk of relapse. Pre-HCT MRDpos (HR 1.6, [95%CI 0.96–2.67]) was associated with increased relapse risk, but did not reach statistical significance (Table 3).
Donor lymphocyte infusions and second transplant
Fourteen patients received donor lymphocyte infusion (DLI) post-transplant (RIC 3; MAC 11). Six DLI infusions were administered for relapsed ALL and all were in the MAC cohort. None of the 6 patients survive. Three of 8 patients who received DLI for non-relapse indications are alive (two in RIC and one in MAC cohort). Seventeen other patients underwent second HCT for relapsed ALL (RIC cohort 6; MAC cohort 11) and three (17%) survive (2 in RIC and 1 in the initial MAC cohort) after their 2nd HCT.
GVHD
The cumulative incidence of Grade II-IV aGVHD at day 100 was lower in the RIC (30% [95%CI 20–42%]) than the MAC group (47% [95%CI 39–56%]; p=0.014). The incidence of cGVHD at 1 year was similar (RIC 46% [95% 34–58%] vs. MAC 41% [95%CI 32–50%]; p=0.38). Given the recent use of TKIs for treatment of cGVHD, we analyzed the relationship between GVHD and TKI administration after HCT. The median time from HCT to aGVHD diagnosis was 0.9 month (range 0.2–2.7 months), while the median time to start TKI therapy post-transplant was later: 2.3 months (range 0.5–36 months). More importantly, the time from HCT to cGVHD onset was similar in the subgroup treated with TKI (4.4 months) versus no TKI therapy (5.8 months). Additionally, TKI administration post HCT did not reduce the incidence of cGVHD as the proportion of patients with cGVHD was similar with or without post-HCT TKI (63% vs 45%; p= 0.87). In multivariate analysis, pre-HCT MRD and TKI use did not impact risks of acute or chronic GVHD. After adjusting for age, MRD and TKI use, the risk of aGVHD was significantly lower in the RIC group (HR 0.54; p=0.014; Table 3), while the risk of cGVHD was not altered by conditioning regimen intensity.
Discussion
To examine the role of conditioning regimen intensity, MRD and TKI influences on HCT for ALL, we conducted a multicenter retrospective matched-pair analysis of 197 Ph+ ALL patients undergoing RIC and MAC allogeneic HCT in CR1. The strength and clinical applicability of this study is enhanced by the incorporation of data on pre-HCT MRD status and administration of TKI pre-HCT-- two critical outcome-modifying variables. In addition, given the matched pair study design, the median age of 52 years closely reflects the HCT population with Ph+ ALL.
The main finding is that DFS and OS in Ph+ ALL were similar after RIC and MAC allogeneic HCT, confirming the curative potential of allogeneic HCT after a reduced intensity preparative regimen. The relatively mature follow-up of 4 years suggests that long-term survival can be achieved for about 40% of older patients with Ph+ ALL after RIC HCT. Hence, RIC extends the benefit of allotransplant to patients above age 50 and to those who are otherwise ineligible for conventional MAC HCT. While there is still considerable room for improvement, these results are encouraging given the disappointing long-term outcomes observed without HCT. In some studies, patients ineligible for HCT have been treated with TKI-based maintenance therapy.3, 4, 31, 32 Although early results with short follow-up were promising, late relapses still occurred after a median duration of remission of 20–25 months and this approach currently cannot be considered curative. Most relapses were associated with a highly resistant phenotype and BCR-ABL gene mutations including T315I.33, 34 As a result, the value of RIC HCT compared to chemotherapy plus TKI for older patients with Ph+ ALL remains a key issue for future prospective trials.
RIC HCT conferred the most significant benefit to the patients who were MRDneg prior to allograft and had received TKI pre-HCT. Indeed, the 2-fold increase in relapse in patients with pre-HCT MRDpos evident only in the RIC group suggests that a less intense regimen may not overcome the presence of residual detectable leukemia and that caution is needed when RIC is considered for MRDpos patients. Notably, higher use of ATG in RIC group may contribute to higher relapse risk.35 Nevertheless, the favorable outcomes in MRDneg patients suggest that achieving a MRD negative state prior to HCT is vitally important and highlight the need to use effective therapy or perhaps second/third generation TKI pre-HCT for those MRDpos patients in whom RIC is planned.38 In our study, both pre-HCT TKI and MRDneg status in RIC patients best protected patients from relapse (only 17% relapsed) and were associated with superior OS of 55%. Use of TKI pre-HCT could lead to deeper MRD negativity; however off-target immunomodulatory antileukemia effects of TKI have been also reported. Interestingly, recent clinical studies suggested development of BCR-ABL-specific cytotoxic T cells in the bone marrow of patients with Ph+ ALL during long-term imatinib treatment.36 In addition to TKI, other targeted therapeutic interventions such as blinatumomab and cellular infusions could also be of benefit if used prior to RIC HCT or peritransplant.36–40
Importantly, we showed that reducing the intensity of the preparative regimen can substantially lower risks of acute GVHD and TRM. This finding is particularly important because higher risks of TRM limit the utility of HCT, particularly for older individuals in whom Ph+ ALL is often diagnosed.14 The observed low TRM (13% at 1 year) is striking, even acknowledging the inherent selection bias associated with RIC patients, who are usually at a greater risk of transplant-related toxicity.
Because of the retrospective registry study design, we were not able to quantify the depth of MRD and its influence on survival. Both assays have limitations and while PCR is highly sensitive, false negative results can be seen due to exquisite susceptibility of RNA to degradation.22 Variations in transcript levels detectable by PCR complemented by assessment using FISH temper the implications of these data and highlight the need for prospective validation using standardized BCR/ABL testing. Nevertheless, Ph+ ALL remains an important ALL subset for which RIC HCT is being tested and until prospective trial results become available, our analysis provides real life, clinically relevant insights on allograft approaches for patients with Ph+ALL in morphologic remission. Current evidence suggests that MRDpos status after induction/consolidation chemotherapy predicts for an increased risk of relapse and worse survival. In a prospective study by Bassan et al. on 236 patients; 48% remained MRDpos after induction/consolidation and 36 patients (66%) underwent HCT rescuing a proportion of MRDpos by MAC HCT.41 Our data also suggest that MAC allotransplantation can reduce the adverse relapse risk conferred by a pre-HCT MRDpos status. Other studies reported evidence that MRDpos patients treated with allogeneic HCT can have successful outcomes,24–,26 although post-transplant persistence of MRDpos most often predicts imminent relapse and poor DFS. A recent GRALL report showed improved OS after imatinib-based chemotherapy followed by MAC HCT (50%) compared to no allo-HCT (33%). Although MRDpos predicted higher risk of relapse, it did not influence OS.4
The emerging future question of great importance is the value serial monitoring of BCR/ABL post-transplant and screening for BCR/ABL mutations. Impact of post-HCT TKI on relapse in this study has to be interpreted with caution because 70–80% of patients did not receive TKI post-HCT in maintenance. This might reflect the earlier era of study or poor tolerance of TKI post-transplant related to myelosuppression or other adverse effects as reported.11,42 A recent German prospective trial randomized Ph+ ALL patients after MAC HCT to receive either maintenance imatinib or pre-emptive therapy with imatinib for molecular relapse. They concluded that post-transplant imatinib was often delayed or interrupted, but no difference in survival was observed with either approach. The study reported excellent OS of 70% in both groups; however, only those patients who were alive at day 60 were included in the analysis.42
Other strategies of post-HCT manipulations such as DLI and second transplant are often available. Though rarely used in our cohort, these efforts did not significantly alter the outcome.
Our results provide directly applicable clinical data for clinicians and supports prospective application of RIC for Ph+ ALL patients who are ineligible for MAC such as the current prospective UKALL 14 clinical trial which offers RIC HCT to Ph+ ALL patients older than 40. Our results suggest that achieving MRDneg status may lead to low relapse and prolonged survival from either MAC or RIC HCT and that MRD status and fitness rather than a pre-defined age cut-off may better guide decisions about conditioning intensity prior to allogeneic HCT. The use of TKI post- transplant requires further study before it can be considered standard of care.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by Novartis providing the grant to CIBMTR for supplemental data collection. The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24 CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement U10 HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-12-1-0142 and N00014-13-1-0039 from the Office of Naval Research; and grants from Allos Therapeutics, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.;Gentium SpA; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
AUTHORSHIP AND CONFLICT OF INTEREST STATEMENT
No conflict of interest: Authors declare no competing financial interests.
V. B., D.W. - designed the study, assisted in supplemental data collection, interpreted data and wrote the manuscript
D.I.M. - assisted in data interpretation, analysis and writing the manuscript
H.W.; M.Z. - collected and analyzed data, performed statistical analysis
Other authors reviewed the analyses modified and approved the final manuscript.
REFERENCES
- 1.Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109(8):3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 2.Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25(1):41–47. doi: 10.1038/leu.2010.228. [DOI] [PubMed] [Google Scholar]
- 3.Ottmann OG, Wassmann B, Pfeifer H, Giagounidis A, Stelljes M, Duhrsen U, et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) Cancer. 2007;109(10):2068–2076. doi: 10.1002/cncr.22631. [DOI] [PubMed] [Google Scholar]
- 4.Tanguy-Schmidt A, Rousselot P, Chalandon Y, Cayuela JM, Hayette S, Vekemans MC, et al. Long-Term Follow-Up of the Imatinib GRAAPH-2003 Study in Newly Diagnosed Patients with De Novo Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A GRAALL Study. Biol Blood Marrow Transplant. 2013;19(1):150–155. doi: 10.1016/j.bbmt.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, Marks DI, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489–4496. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia--results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 7.Laport GG, Alvarnas JC, Palmer JM, Snyder DS, Slovak ML, Cherry AM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood. 2008;112(3):903–909. doi: 10.1182/blood-2008-03-143115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunault M, Harousseau JL, Delain M, Truchen-Graczyk M, Cahn JY, Witz F, et al. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high-dose therapy and autologous BMT: a GOELAMS trial. Blood. 2004;104(10):3028–3037. doi: 10.1182/blood-2003-10-3560. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DA, Faderl S, Cortes J, O’Brien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 10.de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–1413. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 11.Ribera JM, Oriol A, Gonzalez M, Vidriales B, Brunet S, Esteve J, et al. Concurrent intensive chemotherapy and Imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87–95. doi: 10.3324/haematol.2009.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanada M, Matsuo K, Suzuki T, Naoe T. Allogeneic hematopoietic stem cell transplantation as part of postremission therapy improves survival for adult patients with high-risk acute lymphoblastic leukemia: a metaanalysis. Cancer. 2006;106(12):2657–2663. doi: 10.1002/cncr.21932. [DOI] [PubMed] [Google Scholar]
- 13.Wassmann B, Pfeifer H, Stadler M, Bornhauser M, Bug G, Scheuring UJ, et al. Early molecular response to posttransplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2005;106(2):458–463. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- 14.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111(4):1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 15.Ram R, Storb R, Sandmaier BM, Maloney DG, Woolfrey A, Flowers ME, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96(8):1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medd PG, Peniket AJ, Littlewood TJ, Pearce R, Perry J, Kirkland KE, et al. Evidence for a GVL effect following reduced-intensity allo-SCT in ALL: a British Society of Blood and Marrow Transplantation study. Bone Marrow Transplant. 2013;48(7):982–987. doi: 10.1038/bmt.2012.261. [DOI] [PubMed] [Google Scholar]
- 17.Cho BS, Lee S, Kim YJ, Chung NG, Eom KS, Kim HJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia. 2009;23(10):1763–1770. doi: 10.1038/leu.2009.102. [DOI] [PubMed] [Google Scholar]
- 18.Stein AS, Palmer JM, O’Donnell MR, Kogut NM, Spielberger RT, Slovak ML, et al. Reduced-intensity conditioning followed by peripheral blood stem cell transplantation for adult patients with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2009;15(11):1407–1414. doi: 10.1016/j.bbmt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bachanova V, Verneris MR, DeFor T, Brunstein CG, Weisdorf DJ. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113(13):2902–2905. doi: 10.1182/blood-2008-10-184093. [DOI] [PubMed] [Google Scholar]
- 20.Marks DI, Wang T, Perez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 22.Radich JP, Kopecky KJ, Boldt DH, Head D, Slovak ML, Babu R, et al. Detection of BCR-ABL fusion genes in adult acute lymphoblastic leukemia by the polymerase chain reaction. Leukemia. 1994;8(10):1688–95. [PubMed] [Google Scholar]
- 23.Lee S, Kim DW, Cho B, Kim YJ, Kim YL, Hwang JY, et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse-transcription polymerase chain reaction. Br J Haematol. 2003;120:145–153. doi: 10.1046/j.1365-2141.2003.03988.x. [DOI] [PubMed] [Google Scholar]
- 24.Yanada M, Sugiura I, Takeuchi J, Akiyama H, Maruta A, Ueda Y, et al. Prospective monitoring of BCR-ABL1 transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia undergoing imatinib-combined chemotherapy. Br J Haematol. 2008;143(4):503–510. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
- 25.Pane F, Cimino G, Izzo B, Camera A, Vitale A, Quintarelli C, et al. Significant reduction of the hybrid BCR/ABL transcripts after induction and consolidation therapy is a powerful predictor of treatment response in adult Philadelphia-positive acute lymphoblastic leukemia. Leukemia. 2005;19(4):628–635. doi: 10.1038/sj.leu.2403683. [DOI] [PubMed] [Google Scholar]
- 26.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 28.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676–3678. doi: 10.1182/blood-2006-10-052746. [DOI] [PubMed] [Google Scholar]
- 32.Delannoy A, Delabesse E, Lheritier V, Castaigne S, Rigal-Huguet F, Raffoux E, et al. Imatinib and methylprednisolone alternated with chemotherapy improve the outcome of elderly patients with Philadelphia-positive acute lymphoblastic leukemia: results of the GRAALL AFR09 study. Leukemia. 2006;20(9):1526–1532. doi: 10.1038/sj.leu.2404320. [DOI] [PubMed] [Google Scholar]
- 33.Pfeifer H, Wassmann B, Pavlova A, Wunderle L, Oldenburg J, Binckebanck A, et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2007;110(2):727–734. doi: 10.1182/blood-2006-11-052373. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe K, Minami Y, Ozawa Y, Miyamura K, Naoe T. T315I mutation in Ph-positive acute lymphoblastic leukemia is associated with a highly aggressive disease phenotype: three case reports. Anticancer Res. 2012;32(5):1779–1783. [PubMed] [Google Scholar]
- 35.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riva G, Luppi M, Barozzi P, Quadrelli C, Basso S, Vallerini D, et al. Emergence of BCR-ABL-specific cytotoxic T cells in the bone marrow of patients with Ph+ acute lymphoblastic leukemia during long-term imatinib mesylate treatment. Blood. 2010;115(8):1512–1518. doi: 10.1182/blood-2009-06-230391. [DOI] [PubMed] [Google Scholar]
- 37.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 38.Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 39.Ottmann OG, Larson RA, Kantarjian HM, le Coutre PD, Baccarani M, Hochhaus A, et al. Phase II study of nilotinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;27(6):1411–3. doi: 10.1038/leu.2012.324. [DOI] [PubMed] [Google Scholar]
- 40.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric Antigen Receptor-Modified T Cells for Acute Lymphoid Leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113(18):4153–4162. doi: 10.1182/blood-2008-11-185132. [DOI] [PubMed] [Google Scholar]
- 42.Pfeifer H, Wassmann B, Bethge W, Dengler J, Bornhauser M, Stadler M, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1 positive acute lymphoblastic leukemia. Leukemia. 2012;27(6):1254–62. doi: 10.1038/leu.2012.352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


