Abstract
Background
Vascular endothelial growth factor tyrosine-kinase inhibitors (VEGFR-TKIs) have emerged as an effective targeted therapy in the treatment of cancer patients, the overall incidence and risk of proteinuria associated these drugs is unclear. We performed a systematic review and meta-analysis of published clinical trials to quantify the incidence and risk of proteinuria associated with VEGFR-TKIs.
Methodology
Databases from PubMed, Web of Science and abstracts presented at ASCO meeting up to May 31, 2013 were searched to identify relevant studies. Eligible studies included prospective phase II and III trials evaluating VEGFR-TKIs in cancer patients with adequate data on proteinuria. Statistical analyses were conducted to calculate the summary incidence, Odds ratio (OR) and 95% confidence intervals (CIs) by using either random effects or fixed effect models according to the heterogeneity of included studies.
Principal Findings
A total of 6,882 patients with a variety of solid tumors from 33 clinical trials were included in our analysis. The incidence of all-grade and high-grade (grade 3 or higher) proteinuria was 18.7% (95% CI, 13.3%–25.6%) and 2.4% (95% CI, 1.6%–3.7%), respectively. Patients treated with VEGFR-TKIs had a significantly increased risk of all-grade (OR 2.92, 95%CI: 1.09–7.82, p = 0.033) and high-grade proteinuria (OR 1.97, 95%CI: 1.01–3.84, p = 0.046) when compared to patients treated with control medication. No evidence of publication bias was observed.
Conclusions
The use of VEGFR-TKIs is associated with a significant increased risk of developing proteinuria. Physicians should be aware of this adverse effect and should monitor cancer patients receiving VEGFR-TKIs.
Introduction
Angiogenesis plays an important role in the growth, invasion, and metastasis of malignancies [1]–[5], and this process is mainly driven by vascular epithelial growth factor (VEGF). During the past decades, angiogenesis inhibitors targeting VEGF signaling pathway are the furthest along in clinical development [6]–[8]. Indeed, therapies that inhibit the VEGF pathway, including VEGF monoclonal antibody bevacizumab and vascular epithelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs) such as sorafenib, sunitinib, vandetanib, pazopanib axitinib, and regorafenib, have shown clinical efficacy in the treatment of several malignancies and have been approved for use in cancer treatments by regulatory agencies [9]–[17].
However, as with many therapeutic agents, significant side effects are associated with VEGF-targeted agents, including thrombosis, bleeding, hypertension, gastrointestinal perforation and renal toxicity [18]–[35]. Proteinuria is the predominant renal toxicities. Two previous meta-analyses have demonstrated that the use of bevacizumab is associated with a significantly increased risk of developing all-grade (RR, 1.4 with low-dose bevacizumab; 95% confidence interval [CI], 1.1 to 1.7; RR, 2.2 with high dose; 95% CI, 1.6 to 2.9) and high-grade proteinuria (RR, 4.79; 95% CI 2.71 to 8.46) in comparison with controls [19], [36]. Additionally, there is evidence that proteinuria is most probably related to the pharmacological action of VEGF-targeted drugs: the inhibition of the VEGF pathway [37]. Thus proteinuria may also occur with VEGFR-TKIs, which also target the VEGF signal pathway. Indeed, proteinuria associated with VEGFR-TKIs has been reported with a substantial variation in the incidences, ranging from 1.9 to 57.8% in clinical trials [38], [39]. Moreover, a recent abstract presented at 2013 American Society of Clinical Oncology (ASCO) conference shows that the use of axitinib is associated with a significantly increased risk of developing high-grade proteinuria [40]. However, the overall incidence and risk of proteinuria with other VEGFR-TKIs has not yet to be systematically defined. Therefore, we conducted a systematic review of the literature to identify prospective clinical trials of VEGFR-TKIs and performed a meta-analysis of the published results to estimate the incidence and risk of developing proteinuria.
Methods
Data sources
Study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [41], [42] (see Checklist S1). We searched the Pubmed (data from 1990 to May 2013) for relevant trials. Key words were sorafenib, nexavar, BAY43-9006, sunitinib, sutent, SU11248, pazopanib, votrient, GW786034, vandetanib, caprelsa, ZD6474, axitinib, AG-013736, cediranib, AZD2171, tivozanib, regorafenib, Linifanib, ABT-869, clinical trials and cancer. The search was limited to prospective clinical trials published in English. The search strategy also used text terms such as angiogenesis inhibitors and vascular endothelial growth factor receptor-tyrosine kinase inhibitors to identify relevant information. We also performed independent searches using Web of Science databases between January 1, 1990, and May 31, 2013, to ensure that no clinical trials were overlooked. Additionally, we searched the clinical trial registration website (http://www.ClinicalTrials.gov) to obtain information on the registered prospective trials. We also searched abstracts and virtual meeting presentations from the American Society of Clinical Oncology (http://www.asco.org/ASCO) conferences that took place between Jan 2004 and Jan 2013. Each publication was reviewed and in cases of duplicate publication only the most complete, recent, and updated report of the clinical trial was included in the meta-analysis
Study Selection
The primary goal of our study was to determine the overall incidence of proteinuria associated with VEGFR-TKIs and establish the association between treatments with VEGFR-TKIs and the risk of developing proteinuria. Thus, only prospective phase II and III trials evaluating VEGFR-TKIs in cancer patients with adequate data on proteinuria were incorporated in the analysis. Phase I trials were omitted due to multiple dose level and limited sample sizes. Clinical trials that met the following criteria were included: (1) prospective phase 2 or 3 trials involving cancer patients; (2) participants assigned to treatment with VEGFR-TKIs (alone or in combination at any dosage or frequency); and (3) available data regarding events or incidence of proteinuria and sample size.
Data Extraction and Clinical End Point
Data abstraction was conducted independently by two investigators, and any discrepancy between the reviewers was resolved by consensus. For each study, the following information was extracted: first author's name, year of publication, trial phase, number of enrolled subjects, treatment arms, number of patients in treatment and controlled groups, underlying malignancy, median age, median treatment duration, median progression-free survival, adverse outcomes of interest (proteinuria), name and dosage of the VEGFR-TKIs agents. Proteinuria in these studies were assessed and recorded according to the National Cancer Institute's common terminology criteria for adverse events (version2 or 3), which had been widely used in cancer clinical trials [43]. Major differences between the two versions included a particular category for proteinuria in version 3, which included grade 1–5 (table 1). For this study, we simply separated proteinuria into all grades and high-grade (grade 3–5) for our analysis.
Table 1. National Cancer Institute' toxicity grading criteria version 2 and 3 for proteinuria.
| Grade | Version 2 or 3 |
| 1 | Dipstick 1+ or 0.15 to 1.00 g/24 h |
| 2 | Dipstick 2+ to 3+ or 1.0 to 3.5 g/24 h |
| 3 | Dipstick 4+ or >3.5 g/24 h |
| 4 | Nephritic syndrome |
| 5 | Version 2 none; version 3 death |
Statistical Analysis
For the calculation of incidence, trials assigning patients to the treatment with VEGFR-TKIs as a single agent were used to define the incidence of proteinuria related to VEGFR-TKIs alone. The proportion of patients with proteinuria and 95% confidence interval (CI) were derived for each study. To calculate odds ratio (OR), patients assigned to VEGFR-TKIs were compared only with those assigned to control treatment in the same trial. We used the Peto method to calculate odds ratio (ORs) and 95%CI confidence intervals (CIs) of high-grade proteinuria because this method provides the best confidence interval coverage and is more powerful and relatively less biased than the fixed or random-effects analysis when dealing with low event rates [44]. Between-study heterogeneity was estimated using the χ2-based Q statistic [45]. Heterogeneity was considered statistically significant when P heterogeneity<0.1. If heterogeneity existed, data was analyzed using a random effects model. In the absence of heterogeneity, a fixed effects model was used. A statistical test with a p-value less than 0.05 was considered significant. For comparing the incidence difference among difference tumor types and VEGFR-TKIs, we calculate the relative risk (RR) of proteinuria with RCC and other VEGFR-TKIs by using incidence of proteinuria with non-RCC or sorafenib as controls. The quantitative 5-point Jadad scale was used to assess the quality of included trials based on the reporting of the studies' methods and results [46]. We then performed sub-group analysis based on the quality of included trials: low quality (≤3) versus high quality (>3). The presence of publication bias was evaluated by using the Begg and Egger tests [47], [48]. All statistical analyses were performed by using Version 2 of the Comprehensive MetaAnalysis program (Biostat, Englewood, NJ) and Open Meta-Analyst software version 4.16.12 (Tufts University).
Results
Study selection and characteristics
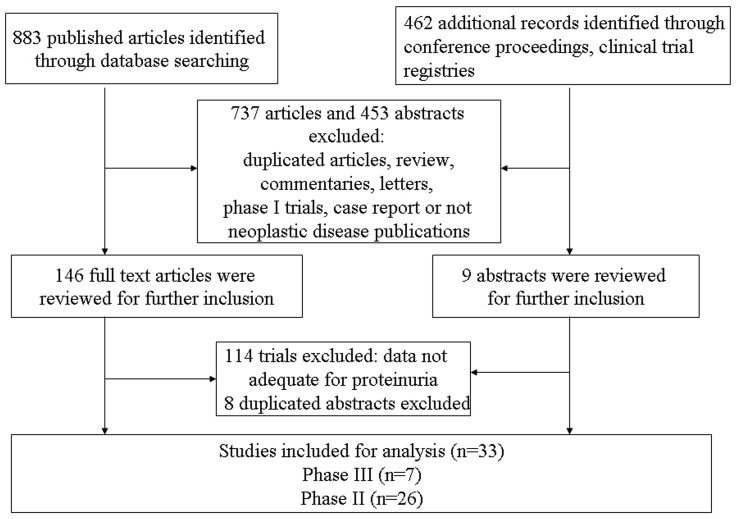
A total of 883 potentially relevant studies were retrieved electronically, 851 of which were excluded for the reasons shown in figure 1. The remaining 32 trials were included in the review. One additional conference abstract was located as a result of hand searching. Finally, a total of 33 publications were therefore included in the review. The baseline characteristics of each trial are presented in Table 2. A total of 6882 patients were available for the meta-analysis. According to the inclusion criteria of each trial, patients were required to have an adequate renal, hepatic and hematologic function. Underlying malignancies included breast cancer [49], renal cell carcinoma [39], [50]–[59], thyroid cancer [60]–[62], pancreatic cancer [63], [64], soft tissue sarcoma [65], [66], glioblastoma [67], [68], hepatocellular carcinoma [38], [69], small-cell lung cancer [70], ovarian cancer [71], nasopharyngeal carcinoma [72], non-small-cell lung cancer [73], malignant mesothelioma [74], alveolar soft part sarcoma [75] and colorectal cancer [76]–[79]. The median Jadad score of the fourteen randomized controlled trials was 3: five of them had Jadad scores of 5 which mentioned the concealment of allocation clearly in the randomization process, and provided the number of patients who withdrew from the trials. One trial did not mention the method for randomization process, thus had Jadad scores of 4. And six trials, did not mention the method for randomization and blinding of allocation clearly in the randomization process, thus had Jadad scores of 3. Another two trials had Jadad scores of 2.
Figure 1. Selection process for clinical trials included in the meta-analysis.
Table 2. Baseline characteristics of the 33 trials included in the meta-analysis (n = 6882).
| Authors/year/phase | Histology | Patients enrolled | Treatment Arm | Median age (years) | Median treatment duration (months) | Median PFS/TTP (months) | Median OS (months) | No. for analysis | No. of high-grade proteinuria | Jadad score |
| Rixe O. et al/2007/II | RCC | 52 | Axitinib 5 mg bid po | 59 | 9.4 | 15.7 | 29.9 | 52 | 0 | N/A |
| Cohn E.W. et al./2008/II | Thyroid cancer | 60 | Axitinib 5 mg bid po | 59 | 4.8 | 18.1 | NR | 60 | 3 | N/A |
| Spano J.P. et al./2009/II | Pancreatic cancer | 103 | Axitinib 5 mg bid po+GEM | 65.0 | 3.8 | 4.2 | 6.9 | 68 | 0 | 3 |
| GEM | 61.0 | NR | 3.7 | 5.6 | 31 | 0 | ||||
| Sleijfer S. et al/2009/II | STS | 142 | Pazopanib 800 mg qd po. | NR | NR | NR | NR | 142 | 0 | N/A |
| Batchelor T.T. et al/2010/II | Glioblastoma | 31 | Cediranib 45 mg/d | 53 | NR | 3.9 | 7.6 | 31 | 1 | N/A |
| Bible K.C. et al/2010/II | Thyroid cancer | 39 | Pazopanib 800 mg | 63 | 11.2 | NR | NR | 39 | 0 | N/A |
| Hsu C.H, et al./2010/II | Hepatocellular carcinoma | 53 | Sorafenib 400 mg bid po | 57 | 3.7 | 3.7 | 7.4 | 53 | 0 | N/A |
| Iwamoto F.M. et al/2010/II | Glioblastoma | 35 | Pazopanib 800 mg | 53 | NR | NR | NR | 35 | NR | N/A |
| Jonasch E. et al/2010/II | RCC | 80 | Sorafenib 400 mg bid | 62.4 | NR | 7.39 | NR | 40 | 1 | 2 |
| Sorafenib 400 mg+IFN | 60.7 | NR | 7.56 | 27.04 | 40 | 2 | ||||
| Mayer E.L. et al/2010/II | Breast cancer | 46 | PTX+Bev+Sunitinib | 58 | 2.6 | NR | NR | 23 | 0 | 2 |
| PTX+Bev | 52 | 3.3 | NR | NR | 23 | 0 | ||||
| Ramalingam S.S. et al./2010/II | SCLC | 25 | Cediranib 45 mg or 30 mg po | 61 | NR | 2 | 6 | 25 | 3 | N/A |
| Robinson E.S. et al./2010/II | Ovarian cancer | 31 | Cediranib 45 mg po | 57 | 2.8 | NR | NR | 31 | 0 | N/A |
| Lim W.T. et al/2011/II | Nasopharyngeal carcinoma | 33 | Pazopanib 800 mg | 50 | NR | 4.4 | 10.8 | 33 | 1 | N/A |
| Tan E.H. et al./2011/II | NSCLC | 139 | Linifanib 0.1 mg/kg or 0.25 mg/kg po | 62 | NR | 3.0 | 9.0 | 139 | 11 | N/A |
| Tannir N.M. et al./2011/II | RCC | 53 | Linifanib 0.25 mg/kg po | 61 | NR | 5.4 | 14.5 | 53 | 3 | N/A |
| Tomita Y. et al/2011/II | RCC | 64 | Axitinib 5 mg bid po | 63 | 10.9 | 11.0 | NR | 64 | 6 | N/A |
| Campbell N.P. et al./2011/II | Malignant mesothelioma | 51 | Cediranib 45 mg or 30 mg qd po | NR | NR | 1.8 | 4.4 | 51 | NR | N/A |
| Kindler H.L. et al/2012/II | Pancreatic cancer | 17 | Sorafenib 400 mg bid po+GEM | 62 | NR | 3.2 | 4.0 | 17 | 0 | N/A |
| Mulders P. et al/2011/II | RCC | 71 | Cediranib 45 mg | 60 | NR | 12.1 | NR | 53 | 0 | 5 |
| Placebo | 61 | NR | 2.8 | NR | 18 | 0 | ||||
| Nosov D.A. et al/2012/II | RCC | 272 | Tivozanib 1.5 mg qd po. | 56 | NR | NR | NR | 272 | 5 | N/A |
| Rini B. et al/2012/II | RCC | 152 | Sorafenib+AMG-386 10 mg/kg | 60 | NR | 9.0 | NR | 50 | 2 | 4 |
| Sorafenib+AMG-386 3 mg/kg | 58 | NR | 8.5 | NR | 51 | 0 | ||||
| Sorafenib+placebo | 59 | NR | 9.0 | NR | 50 | 0 | ||||
| Schmoll H.J. et al/2012/III | CRC | 1422 | Cediranib 20 mg+mFOLFOX6 | 59 | 6.7 | 9.9 | 22.8 | 705 | 7 | 3 |
| Bevacizumab+mFOLFOX6 | 60 | 7.0 | 10.3 | 21.3 | 704 | 6 | ||||
| Wells S.A. et al/2012/III | Thyroid cancer | 331 | Vandetanib 300 mg | 50.7 | 21.0 | NR | NR | 231 | 0 | 5 |
| Placebo | 53.4 | 9.3 | 19.3 | NR | 99 | 0 | ||||
| Motzer R. et al/2012/III | RCC | 517 | Tivozanib 1.5 mg/d | 59 | NR | 11.9 | NR | 259 | 3 | 3 |
| Sorafenib 400 mg | 59 | NR | 9.1 | NR | 257 | 2 | ||||
| Van der Graaf W.T. et al./2012/III | STS | 372 | Pazopanib 800 mg | 51.9 | 3.8 | 4.6 | 11.9 | 239 | 1 | 5 |
| Placebo | 56.7 | 1.9 | 1.6 | 10.4 | 123 | 0 | ||||
| Cunningham D. et al/2013/II | CRC | 210 | Cediranib 30 mg+FOLFOX | NR | 5.0 | 5.8 | 14.3 | 70 | 0 | 3 |
| Cediranib 20 mg+FOLFOX | NR | 5.4 | 7.2 | 16.8 | 73 | 6 | ||||
| Bev+FOLFOX | NR | 6.3 | 7.8 | 19.6 | 66 | 2 | ||||
| Grothey A. et al/2013/III | CRC | 1052 | Regorafenib 160 mg | 61 | 2.8 | NR | 6.4 | 500 | 7 | 5 |
| Placebo | 61 | 1.8 | NR | 5.0 | 253 | 1 | ||||
| Hainsworth J.D. et al/2013/II | RCC | 55 | Pazopanib 800 mg qd po | 60 | 6 | 7.5 | NR | 55 | 7 | N/A |
| Infante J.R. et al./2013/II | CRC | 126 | Axitinib 5 mg bid+FOLFOX | 61 | NR | 11.0 | 18.1 | 42 | 1 | 3 |
| Bevacizumab+FOLFOX | 64 | NR | 15.9 | 21.6 | 43 | 0 | ||||
| Axitinib+bevacizumab+FOLFOX | 59 | NR | 12.5 | 19.7 | 41 | 2 | ||||
| Kummar S. et al/2013/II | Alveolar soft part sarcoma | 46 | Cediranib 30 mg | 27 | NR | NR | NR | 46 | 1 | N/A |
| Motzer R. et al/2013/III | RCC | 723 | Axitinib 5 mg bid po | 61 | 6.4 | 8.3 | 20.1 | 359 | 11 | 3 |
| Sorafenib 400 mg bid po | 61 | 5.0 | 5.7 | 19.2 | 355 | 4 | ||||
| Sternberg C.N. et al/2013/III | RCC | 435 | Pazopanib 800 mg po qd | 59 | 7.4 | 9.2 | 22.9 | 290 | 7 | 5 |
| Placebo | 60 | 3.8 | 4.2 | 20.5 | 145 | 0 | ||||
| Toh H.C. et al/2013/II | Hepatocellular carcinoma | 44 | Linifanib 0.25 mg/kg po | 62.5 | NR | 3.7 | 9.7 | 44 | 0 | N/A |
Abbreviations: PFS, progression-free survival; OS, overall survival; RCC, renal cell cancer; NSCLC, non-small-cell lung carcinoma; SCLC, small-cell lung cancer; CRC, Colorectal cancer; STS, soft tissue sarcoma; Bev, bevacizumab; GEM, gemcitabine; PTX, paclitaxel; NR, not reported; N/A, not applicable.
Incidence of all-grade proteinuria events
A total of 3,701 patients receiving VEGFR-TKIs single agents in 23 trials were available for analysis. In two phase III trials, patients in both groups received VEGFR-TKIs single agent, thus both arms were included in this analysis [53], [58]. There were 604 total proteinuria events among these patients. The highest incidence (57.8%; 95% CI, 45.2%–69.2%) as observed in a phase II trial of renal cell cancer patients treated with axitinib [39], and the lowest incidence was observed in a phase III trials of soft tissue sarcoma patients treated with pazopanib in which two proteinuria event occurred [66]. Using a random-effects model (χ2-based Q statistic test: Q = 400.96; P<0.001; I 2 = 94%), the summary incidence of all-grade proteinuria events in patients receiving VEGFR-TKIs was 18.7% (95% CI, 13.3%–25.6%, table 3).
Table 3. Incidence for proteinuria with VEGFR-TKIs according to drugs and tumor types.
| Grade | Categories | No. of studies | Proteinuria events | Sample size | Incidence (%;95%CI) | Relative risk (95%CI) | P values |
| All-grade | Overall | 23 | 604 | 3701 | 18.7% (13.3–25.6%) | NA | NA |
| Non-RCC | 14 | 261 | 1635 | 18.5% (10.7–29.9%) | 1 | NA | |
| RCC | 9 | 343 | 2066 | 18.4% (11.5–28.3%) | 1.05(0.88–1.25) | 0.60 | |
| Sorafenib | 4 | 108 | 715 | 11.6% (4.3–27.6%) | 1 | NA | |
| Axitinib | 4 | 97 | 535 | 20.2% (6.9–46.7%) | 1.24 (0.92–1.68) | 0.15 | |
| Pazopanib | 5 | 131 | 761 | 13.5% (3.9–37.6%) | 1.17 (0.88–1.54) | 0.27 | |
| Vandetanib | 1 | 23 | 231 | 10.0% (6.7–14.5%) | 0.62 (0.39–1.00) | 0.05 | |
| Regorafenib | 1 | 35 | 500 | 7.0% (5.1–9.6%) | 0.42 (0.28–0.63) | 0.001 | |
| Cediranib | 5 | 73 | 192 | 37.8% (27.5–49.3%) | 3.45 (2.41–4.92) | 0.001 | |
| Tivozanib | 2 | 76 | 531 | 9.6% (0.9–54.3%) | 0.94 (0.68–1.29) | 0.70 | |
| Linifanib | 3 | 61 | 236 | 27.3% (18.6–38.1%) | 1.96 (1.37–2.80) | 0.002 | |
| High-grade | Overall | 25 | 76 | 3812 | 2.4% (1.6–3.7%) | NA | NA |
| Non-RCC | 14 | 28 | 1613 | 2.3% (1.2–4.4%) | 1 | NA | |
| RCC | 11 | 48 | 2199 | 2.5% (1.4–4.4%) | 1.26 (0.79–2.02) | 0.33 | |
| Sorafenib | 5 | 6 | 795 | 0.9% (0.4–1.9%) | 1 | NA | |
| Axitinib | 4 | 20 | 535 | 4.6% (2.2–9.2%) | 5.11 (2.04–12.8) | 0.0005 | |
| Pazopanib | 6 | 16 | 798 | 2.2% (0.6–6.9%) | 2.69 (1.05–6.91) | 0.04 | |
| Vandetanib | 1 | 0 | 231 | 0% | 0.26 (0.01–4.67) | 0.36 | |
| Regorafenib | 1 | 7 | 500 | 1.4% (0.7–2.9%) | 1.87 (0.62–5.59) | 0.26 | |
| Cediranib | 5 | 5 | 186 | 3.9% (1.4–10.3%) | 3.63 (1.10–12.03) | 0.03 | |
| Tivozanib | 2 | 8 | 531 | 1.5% (0.8–3.1%) | 2.01(0.69–5.83) | 0.20 | |
| Linifanib | 3 | 14 | 236 | 6.8% (3.9–11.4%) | 8.29 (3.15–21.83) | 0.0001 |
Incidence of high-grade proteinuria events
A total of 3,812 patients from 25 trials were available for analysis. There were 76 high-grade proteinuria events among these patients. The highest incidence (12.7%; 95% CI, 6.2%–24.4%) as observed in a phase II trials of renal cell cancer patients treated with pazopanib [57] and no cases of high-grade proteinuria was observed in two trials treated with sorafenib [38], [56], two trials treated with cediranib [54], [71], two trials treated with pazoapnib [60], [65], one trial treated with axitinib [50], one trial treated with vandetanib [62], and one trial treated with linifanib [69], respectively. Using a random-effects model (heterogeneity test: Q = 72.46; P<0.001; I 2 = 64%), the summary incidence of high-grade proteinuria events in patients receiving VEGFR-TKIs was 2.4% (95% CI, 1.6%–3.7%, table 3).
Incidence of proteinuria in patients with RCC vs. non-RCC malignancy
In order to explore the relationship between VEGFR-TKIs associated proteinuria and tumor types, we further analyzed the incidence of proteinuria in patients with RCC and non-RCC cancers. Among patients with RCC, the summary incidences of all grade and high grade proteinuria was 18.4% (95%CI: 11.5–28.3%) and 2.5% (95%CI:1.4–4.4%) using a random effects model; while for those patients with non-RCC malignancies, the summary incidences of all grade and high grade proteinuria were 18.5% (95%CI: 10.7–29.9%) and 2.3% (95%CI: 1.2–4.4%) using a random effects model. In addition, there was no significant difference detected between RCC and non-RCC cancer in terms of the incidence of VEGFR-TKIs-associated all grade proteinuria (RR 1.05, 95% CI 0.88, 1.25, P = 0.60) and high grade proteinuria (RR 1.26, 95% CI 0.79, 2.02, P = 0.33) (table 3).
Differences in proteinuria incidence among various VEGFR-TKIs
When stratified by each VEGFR-TKIs, the incidence of all-grade proteinuria was 11.6% (95%CI: 4.3–27.6%) for sorafenib, 20.2% (6.9–46.7%) for axitinib, 13.5% (95%CI: 3.9–37.6%) for pazopanib, 10.0% (95%CI:6.7–14.5%)vandetanib, 7.0% (95%CI: 5.1–9.6%) for regorafenib. 37.8% (95%CI: 27.5–49.3%) for cediranib, 9.6% (95%CI: 0.9–54.3%) for tivozanib, and 27.3% (95%CI, 18.6–38.1%) for linifanib, respectively. As for high-grade proteinuria, the incidence was 0.9% (95%CI: 0.4–1.9%) for sorafenib, 4.6% (2.2–9.2%) for axitinib, 2.2% (95%CI: 0.6–6.9%) for pazopanib, 0.0% for vandetanib, 1.4% (95%CI:0.7–2.9%) for regorafenib. 3.9% (95%CI: 1.4–10.3%) for cediranib, 1.5% (95%CI: 0.8–3.1%) for tivozanib, and 6.8% (95%CI, 3.9–11.4%) for linifanib, respectively (table 3). The risk of developing proteinuria significantly varied among VEGFR-TKIs. Compared with sorafenib, cediranib (RR 3.45, 95%CI: 2.41–4.92, p = 0.001) and linifanib (RR 1.96, 95%CI: 1.37–2.80, p = 0.002) significantly increased the risk of developing proteinuria, while vandetanib (RR 0.62, 95%CI: 0.39–1.00, p = 0.05) and regorafenib (RR 0.42, 95%CI: 0.28–0.63, p = 0.001) significantly decreased the risk of developing proteinuria. As for high-grade proteinuria, axitinib (RR 5.11, 95%CI: 2.04–12.8, p = 0.0005), pazopanib (RR 2.69, 95%CI: 1.05–6.91, p = 0.04), cediranib (RR 3.63, 95%CI: 1.10–12.03, p = 0.03) and linifanib (RR 8.29, 3.15–21.83, p = 0.001) significantly increased the risk of developing proteinuria when compared to sorafenib (table 3).
Odds Ratio of proteinuria events
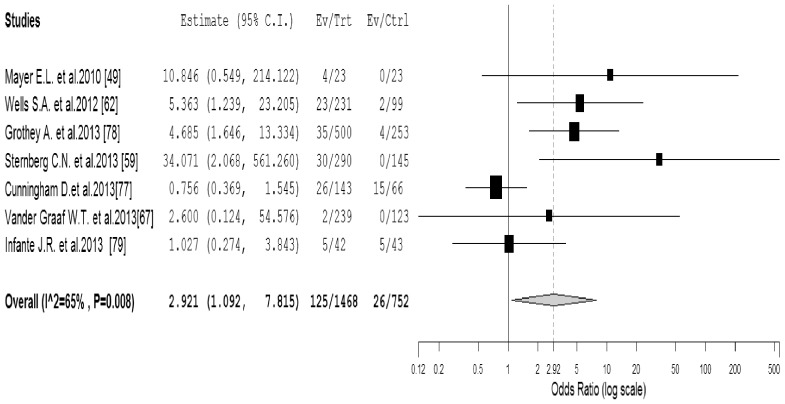
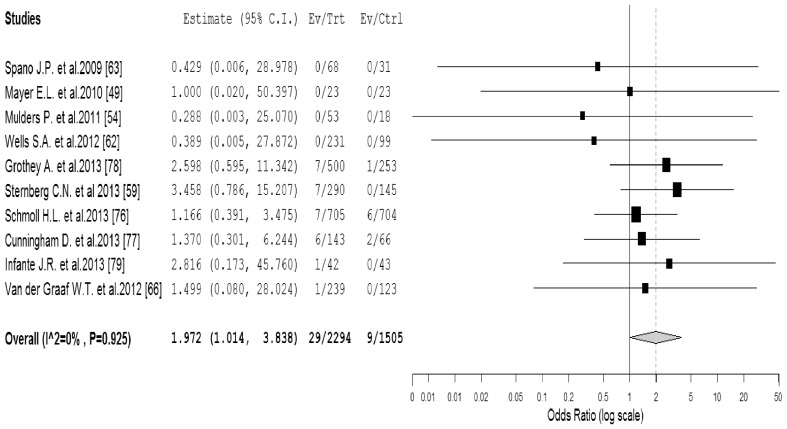
To investigate the specific contribution of VEGFR-TKIs to the development of proteinuria events and exclude the influence of confounding factors such as underlying malignancy, and other therapeutic interventions, we therefore determined the odds ratio (OR) of VEGFR-TKIs associated proteinuria events. Two phase III trials were excluded for OR analysis as both group received VEGFR-TKIs agents [53], [58]. A total of 2,220 patients in the 7 RCTs were included for calculating the OR of all-grade proteinuria events, the combined results demonstrated that the use of VEGFR-TKIs was associated with a significantly increased risk of developing all-grade proteinuria events with an OR of 2.92 (95%CI: 1.09–7.82, p = 0.033, figure 2 ) using a random-effects model (I 2 = 65%, p = 0.008). Due to significant heterogeneity among the included trials, we then performed sub-group analysis according to the quality of included trials. Our results showed that the use of VEGFR-TKIs significantly increased the risk of proteinuria in high-quality trials (OR 5.48, 95%CI: 2.49–12.03, p<0.001), but not for low-quality trials (OR 1.05, 95%CI: 0.42–2.61, p = 0.92). As for high-grade proteinuria events, a total of 3,799 patients in the 10 RCTs were included for analysis. The combined OR showed that the use of VEGFR-TKIs significantly increased the risk of high-grade proteinuria events among cancer patients (OR 1.97, 95%CI: 1.01–3.84, p = 0.046, figure 3 ) using a fixed effects model (I 2 = 0%, p = 0.93). We also performed sub-group analysis based on quality of included trials to investigate the potential risk difference. Again, the use of VEGFR-TKIs significantly increased the risk of high-grade proteinuria in high-quality trials (OR 3.44, 95%CI: 1.21–9.78, p = 0.02), but not for low-quality trials (OR 1.35, 95%CI: 0.57–3.19, p = 0.50).
Figure 2. Odds ratio of all-grade proteinuria associated with VEGFR-TKIs vs control.
Figure 3. Odds ratio of high-grade proteinuria associated with VEGFR-TKIs vs control.
Publication bias
No evidence of publication bias was detected for the OR of all-grade and high-grade proteinuria events in this study by the funnel plot (figure 4), Egger's test and Begg' test (OR of all-grade proteinuria: Egger's test p = 0.09, Begg's test p = 0.76; OR of high-grade proteinuria: Egger's test p = 0.17, Begg's test p = 0.45).
Figure 4. Funnel plot of standard error by log-odds ratio for all-grade and high-grade proteinuria.
Discussion
Although low grade proteinuria (grade 1–2) is typically asymptomatic and decreases after anti-VEGF treatment ends, serious proteinuria (grade 3–5) including nephrotic syndrome may cause significant morbidity with a possible consequence of renal failure and fatality during anti-VEGF therapy; concerns have arisen regarding the risk of proteinuria with the use of these drugs. Two previous meta-analyses have demonstrated that VEGF monoclonal antibody bevacizumab is associated with a significantly increased risk of developing proteinuria [19], [36]. In addition, the authors identify a relationship between bevacizumab dosage and proteinuria (all-grade: RR 1.4 for low dosage versus 2.2 for high dose; high-grade: RR 2.62 for low dosage versus 8.56 for high dosage) [36]. And that report also demonstrates that patients with renal cell carcinoma (RCC) have significantly increased risk for developing proteinuria when compared to non RCC patients [36]. However, no published article explores the association between proteinuria and VEGFR-TKIs, which also target VEGF signaling pathways. As a result, we conduct this study to investigate the overall incidence and risk of proteinuria in cancer patients treated with VEGFR-TKIs.
Our meta-analysis, included 6,882 patients from 33 clinical trials, demonstrates that the pooled incidence of all-grade and high-grade proteinuria is 18.7% (95% CI, 13.3%–25.6%) and 2.4% (95% CI, 1.6%–3.7%), which is higher than that of bevacizumab reported by Wu S. et al. (all-grade: 13.3%; high-grade: 2.2%) [36]. We also find that the use of VEGFR-TKIs is associated with a significantly increased risk of developing all-grade (OR 2.92, 95%CI: 1.09–7.82, p = 0.033) and high-grade proteinuria (OR 1.97, 95%CI: 1.01–3.84, p = 0.046). As VEGFR-TKIs are increasingly used in the routine treatment of cancer patients and in the setting of clinical trials in combination with other agents, it is important that oncologists, internists, and nephrologists monitor and manage proteinuria appropriately to ensure that patients receive maximum benefit from VEGFR-TKIs therapy.
The pathogeneses of VEGF inhibitor-induced proteinuria are not thoroughly understood. Vitro studies have found that VEGF is constitutively produced by podocytes with a function of activating VEGF receptor 2 on glomerular capillary endothelial cells, and its inhibition may cause a loss of endothelial fenestrations and podocytes and reduced proliferation of endothelial cells [80], [81]. Human and animal data suggests that proper VEGF expression is important to maintain the structure and function of the glomerulus. Overexpression or underexpression of VEGF may cause glomerulopathy. In Vuorela P et al's study [82], elevated levels of soluble VEGFR-1 protein, an endogenous antagonist of the VEGF pathway, are observed in the amniotic fluid of preeclamptic women. In animal studies, underexpression of VEGF results in glomerulopathy characterized by nephrotic-range proteinuria, endotheliosis, and hyaline deposits that resemble the pathological lesions seen in renal biopsy specimens from patients with preeclampsia [83]. And overexpression of VEGF also leads to proteinuria from a collapsing focal segmental glomerulosclerosis, a lesion also seen with human immunodeficiency virus associated nephropathy [80]. Additionally, VEGFR-TKIs-associated proteinuria may be a consequence in part of increased intraglomerular pressure resulting from hypertension. However, hypertension may not play a major role in the development of proteinuria, because the glomerular injury from reduced VEGF expression of podocytes preceded hypertension in a murine conditional knockout model [84].
Adequate and aggressive management of severe proteinuria could be essential for many patients, because severe proteinuria is an independent risk factor for renal disease. However, there are no evidence-based guidelines for the management of VEGFR-TKIs-associated proteinuria. According to the manufacturer package insert for pazopanib and axitinib [85], [86], baseline and periodic urinalysis during treatment is recommended with follow up measurement of 24-hour urine protein as clinically indicated. Interrupt VEGFR-TKIs and dose reduce for 24-hour urine protein ≥3 grams; discontinue VEGFR-TKIs for repeat episodes despite dose reductions. Additionally, blockade of the renin-angiotension system may have specific benefit in those hypertensive patients with proteinuria, thus it is reasonable to initiate angiotension converting enzyme inhibitors (ACEI) or angiotension receptor blockers (ARB) as first-line therapy for anti-VEGF-targeted patients with hypertension and proteinuria [87], although this remains to be validated in randomized, controlled studies.
Meta-analysis is considered as a useful tool for analyzing rare and unintended effects of a treatment because it could allow synthesis of data and achieve more stable estimates of effects. However, there are several limitations needed to be considered. First, our findings are influenced by the limitation of individual trials included in the analysis, such as the use of dipstick assessment for proteinuria, no specification of nephrotic syndrome for National Cancer Institute's Common Terminology Criteria grading, and completeness of follow-up; baseline proteinuria is also not mentioned in these trials. Secondly, this is a meta-analysis at study level; therefore we do not have access to individual patient data. Thus we could not establish risk factors associated with the development of proteinuria. Nevertheless, it is important to point out that meta-analysis from individual patients data can also carry bias, as data may only be available to limited numbers of research groups. Third, although proteinuria events are prospectively collected for each individual study, this analysis is retrospective, and there are potentially important differences among the studies, including differing tumor types, dosage and administration schedule of VEGFR-TKIs, periods of study conduct and study investigators. All of these would increase the clinical heterogeneity among included trials, which also make the interpretation of a meta-analysis more problematic. Finally, all these studies exclude patients with poor renal, hematological, and hepatic functions, and are performed mostly at major academic centers and research institutions; the analysis of these studies may not apply to patients with organ dysfunctions and in the community, and the overall incidences of proteinuria from this study may be overestimated.
Conclusions
In summary, the current meta-analysis suggests that the use of VEGFR-TKIs significantly increase the risk of developing proteinuria in cancer patients. As this class of drugs is used increasingly in patients with metastatic cancers, physicians should be aware of this adverse effect and should monitor cancer patients receiving VEGFR-TKIs. Further studies are recommended to focus on uncovering the mechanisms of VEGFR-TKIs-induced proteinuria, as well as investigating risk differences among different VEGFR-TKIs and tumor types.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
The authors have no funding or support to report.
References
- 1. Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 2. Sherwood LM, Parris EE, Folkman J (1971) Tumor angiogenesis: therapeutic implications. New England Journal of Medicine 285: 1182–1186. [DOI] [PubMed] [Google Scholar]
- 3. Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 5. Zetter BR (1998) Angiogenesis and tumor metastasis. Annual review of medicine 49: 407–424. [DOI] [PubMed] [Google Scholar]
- 6. Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249–257. [DOI] [PubMed] [Google Scholar]
- 7. Ferrara N, Hillan KJ, Gerber HP, Novotny W (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 8. Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69 Suppl 3: 4–10. [DOI] [PubMed] [Google Scholar]
- 9. Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, et al. (2012) OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30: 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, et al. (2013) AVEREL: A Randomized Phase III Trial Evaluating Bevacizumab in Combination With Docetaxel and Trastuzumab as First-Line Therapy for HER2-Positive Locally Recurrent/Metastatic Breast Cancer. J Clin Oncol 31: 1719–1725. [DOI] [PubMed] [Google Scholar]
- 11. Qi WX, Tang LN, He AN, Shen Z, Yao Y (2011) The role of vandetanib in the second-line treatment for advanced non-small-cell-lung cancer: a meta-analysis of four randomized controlled trials. Lung 189: 437–443. [DOI] [PubMed] [Google Scholar]
- 12. Wells SA, Gosnell JE, Gagel RF, Moley J, Pfister D, et al. (2010) Vandetanib for the Treatment of Patients With Locally Advanced or Metastatic Hereditary Medullary Thyroid Cancer. JOURNAL OF CLINICAL ONCOLOGY 28: 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, et al. (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 14. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, et al. (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356: 125–134. [DOI] [PubMed] [Google Scholar]
- 15. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 16. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, et al. (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356: 115–124. [DOI] [PubMed] [Google Scholar]
- 17. Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, et al. (2011) Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 364: 501–513. [DOI] [PubMed] [Google Scholar]
- 18. Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, et al. (2007) Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99: 1232–1239. [DOI] [PubMed] [Google Scholar]
- 19. Zhu X, Wu S, Dahut WL, Parikh CR (2007) Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 49: 186–193. [DOI] [PubMed] [Google Scholar]
- 20. Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S (2008) Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 300: 2277–2285. [DOI] [PubMed] [Google Scholar]
- 21. Hapani S, Chu D, Wu S (2009) Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta-analysis. Lancet Oncol 10: 559–568. [DOI] [PubMed] [Google Scholar]
- 22. Je Y, Schutz FA, Choueiri TK (2009) Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol 10: 967–974. [DOI] [PubMed] [Google Scholar]
- 23. Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J (2010) Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 28: 2280–2285. [DOI] [PubMed] [Google Scholar]
- 24. Qi WX, Min DL, Shen Z, Sun YJ, Lin F, et al. (2012) Risk of venous thromboembolic events associated with VEGFR-TKIs: A systematic review and meta-analysis. Int J Cancer 132: 2967–2974. [DOI] [PubMed] [Google Scholar]
- 25. Qi WX, Lin F, Sun YJ, Tang LN, He AN, et al. (2013) Incidence and risk of hypertension with pazopanib in patients with cancer: a meta-analysis. Cancer Chemother Pharmacol 71: 431–439. [DOI] [PubMed] [Google Scholar]
- 26. Qi WX, He AN, Shen Z, Yao Y (2013) Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol 76: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi WX, Shen Z, Lin F, Sun YJ, Min DL, et al. (2013) Incidence and Risk of Hypertension with Vandetanib in Cancer Patients: A Systematic review and Meta-analysis of clinical trials. Br J Clin Pharmacol 75: 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ibrahim EM, Kazkaz GA, Abouelkhair KM, Bayer AM, Elmasri OA (2013) Sunitinib adverse events in metastatic renal cell carcinoma: a meta-analysis. Int J Clin Oncol 18: 1060–1069. [DOI] [PubMed] [Google Scholar]
- 29. Cortes J, Calvo V, Ramirez-Merino N, O'Shaughnessy J, Brufsky A, et al. (2012) Adverse events risk associated with bevacizumab addition to breast cancer chemotherapy: a meta-analysis. Ann Oncol 23: 1130–1137. [DOI] [PubMed] [Google Scholar]
- 30. Qi WX, Sun YJ, Tang LN, Shen Z, Yao Y (2014) Risk of gastrointestinal perforation in cancer patients treated with vascular endothelial growth factor receptor tyrosine kinase inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol 89: 394–403. [DOI] [PubMed] [Google Scholar]
- 31. Qi WX, Tang LN, Sun YJ, He AN, Lin F, et al. (2013) Incidence and risk of hemorrhagic events with vascular endothelial growth factor receptor tyrosine-kinase inhibitors: an up-to-date meta-analysis of 27 randomized controlled trials. Ann Oncol 24: 2943–2952. [DOI] [PubMed] [Google Scholar]
- 32. Wu S, Chen JJ, Kudelka A, Lu J, Zhu X (2008) Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol 9: 117–123. [DOI] [PubMed] [Google Scholar]
- 33. Funakoshi T, Latif A, Galsky MD (2013) Risk of hypertension in cancer patients treated with sorafenib: an updated systematic review and meta-analysis. J Hum Hypertens 27: 601–611. [DOI] [PubMed] [Google Scholar]
- 34. Hurwitz HI, Saltz LB, Van Cutsem E, Cassidy J, Wiedemann J, et al. (2011) Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol 29: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 35. Sonpavde G, Je Y, Schutz F, Galsky MD, Paluri R, et al. (2013) Venous thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: A systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 87: 80–89. [DOI] [PubMed] [Google Scholar]
- 36. Wu S, Kim C, Baer L, Zhu X (2010) Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol 21: 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Launay-Vacher V, Deray G (2009) Hypertension and proteinuria: a class-effect of antiangiogenic therapies. Anticancer Drugs 20: 81–82. [DOI] [PubMed] [Google Scholar]
- 38. Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, et al. (2010) Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol 53: 126–131. [DOI] [PubMed] [Google Scholar]
- 39. Tomita Y, Uemura H, Fujimoto H, Kanayama HO, Shinohara N, et al. (2011) Key predictive factors of axitinib (AG-013736)-induced proteinuria and efficacy: a phase II study in Japanese patients with cytokine-refractory metastatic renal cell Carcinoma. Eur J Cancer 47: 2592–2602. [DOI] [PubMed] [Google Scholar]
- 40. Kuei A, Wu S (2013) The risk of proteinuria with the angiogenesis inhibitor axitinib in patients with cancer. J Clin Oncol 31: abstr 439. [Google Scholar]
- 41. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NCI, Cancer Therapy Evaluation Program. CTC v 2.0 and common terminology criteria for adverse events criteria V3.0 (CTCAE). Available: http://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm. Assessed 2013 January 27.
- 44. Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23: 1351–1375. [DOI] [PubMed] [Google Scholar]
- 45. Zintzaras E, Ioannidis JP (2005) Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 28: 123–137. [DOI] [PubMed] [Google Scholar]
- 46. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, et al. (1998) Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 352: 609–613. [DOI] [PubMed] [Google Scholar]
- 47. Yusuf S, Peto R, Lewis J, Collins R, Sleight P (1985) Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27: 335–371. [DOI] [PubMed] [Google Scholar]
- 48. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 49. Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, et al. (2010) SABRE-B: an evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Ann Oncol 21: 2370–2376. [DOI] [PubMed] [Google Scholar]
- 50. Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, et al. (2007) Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol 8: 975–984. [DOI] [PubMed] [Google Scholar]
- 51. Jonasch E, Corn P, Pagliaro LC, Warneke CL, Johnson MM, et al. (2010) Upfront, randomized, phase 2 trial of sorafenib versus sorafenib and low-dose interferon alfa in patients with advanced renal cell carcinoma: clinical and biomarker analysis. Cancer 116: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tannir NM, Wong YN, Kollmannsberger CK, Ernstoff MS, Perry DJ, et al. (2011) Phase 2 trial of linifanib (ABT-869) in patients with advanced renal cell cancer after sunitinib failure. Eur J Cancer 47: 2706–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Motzer R, Nosov D, Eisen T, Bondarenko I, Lesovoy V, et al. (2013) Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a Phase III randomized, open-label, multicenter trial. J Clin Oncol 31: 3791–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mulders P, Hawkins R, Nathan P, de Jong I, Osanto S, et al. (2012) Cediranib monotherapy in patients with advanced renal cell carcinoma: results of a randomised phase II study. Eur J Cancer 48: 527–537. [DOI] [PubMed] [Google Scholar]
- 55. Nosov DA, Esteves B, Lipatov ON, Lyulko AA, Anischenko AA, et al. (2012) Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol 30: 1678–1685. [DOI] [PubMed] [Google Scholar]
- 56. Rini B, Szczylik C, Tannir NM, Koralewski P, Tomczak P, et al. (2012) AMG 386 in combination with sorafenib in patients with metastatic clear cell carcinoma of the kidney : A randomized, double-blind, placebo-controlled, phase 2 study. Cancer 118: 6152–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hainsworth JD, Rubin MS, Arrowsmith ER, Khatcheressian J, Crane EJ, et al. (2013) Pazopanib as Second-Line Treatment After Sunitinib or Bevacizumab in Patients With Advanced Renal Cell Carcinoma: A Sarah Cannon Oncology Research Consortium Phase II Trial. Clin Genitourin Cancer 11: 270–275. [DOI] [PubMed] [Google Scholar]
- 58. Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, et al. (2013) Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14: 552–562. [DOI] [PubMed] [Google Scholar]
- 59. Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, et al. (2013) A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 49: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 60. Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, et al. (2010) Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 11: 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, et al. (2008) Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26: 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, et al. (2012) Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30: 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, et al. (2008) Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet 371: 2101–2108. [DOI] [PubMed] [Google Scholar]
- 64. Kindler HL, Wroblewski K, Wallace JA, Hall MJ, Locker G, et al. (2012) Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs 30: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, et al. (2009) Pazopanib, a Multikinase Angiogenesis Inhibitor, in Patients With Relapsed or Refractory Advanced Soft Tissue Sarcoma: A Phase II Study From the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC Study 62043). JOURNAL OF CLINICAL ONCOLOGY 27: 3126–3132. [DOI] [PubMed] [Google Scholar]
- 66. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, et al. (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379: 1879–1886. [DOI] [PubMed] [Google Scholar]
- 67. Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, et al. (2010) Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol 28: 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Iwamoto FM, Lamborn KR, Robins HI, Mehta MP, Chang SM, et al. (2010) Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol 12: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Toh HC, Chen PJ, Carr BI, Knox JJ, Gill S, et al. (2013) Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 119: 380–387. [DOI] [PubMed] [Google Scholar]
- 70. Ramalingam SS, Belani CP, Mack PC, Vokes EE, Longmate J, et al. (2010) Phase II study of Cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute #7097). J Thorac Oncol 5: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Robinson ES, Matulonis UA, Ivy P, Berlin ST, Tyburski K, et al. (2010) Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol 5: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lim WT, Ng QS, Ivy P, Leong SS, Singh O, et al. (2011) A Phase II Study of Pazopanib in Asian Patients with Recurrent/Metastatic Nasopharyngeal Carcinoma. Clinical cancer research 17: 5481–5489. [DOI] [PubMed] [Google Scholar]
- 73. Tan EH, Goss GD, Salgia R, Besse B, Gandara DR, et al. (2011) Phase 2 trial of Linifanib (ABT-869) in patients with advanced non-small cell lung cancer. J Thorac Oncol 6: 1418–1425. [DOI] [PubMed] [Google Scholar]
- 74. Campbell NP, Kunnavakkam R, Leighl N, Vincent MD, Gandara DR, et al. (2012) Cediranib in patients with malignant mesothelioma: a phase II trial of the University of Chicago Phase II Consortium. Lung Cancer 78: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kummar S, Allen D, Monks A, Polley EC, Hose CD, et al. (2013) Cediranib for Metastatic Alveolar Soft Part Sarcoma. J Clin Oncol 2013 31: 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, et al. (2012) Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol 30: 3588–3595. [DOI] [PubMed] [Google Scholar]
- 77. Cunningham D, Wong RP, D'Haens G, Douillard JY, Robertson J, et al. (2013) Cediranib with mFOLFOX6 vs bevacizumab with mFOLFOX6 in previously treated metastatic colorectal cancer. Br J Cancer 108: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, et al. (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 79. Infante JR, Reid TR, Cohn AL, Edenfield WJ, Cescon TP, et al. (2013) Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: A randomized phase 2 study. Cancer 119: 2555–2563. [DOI] [PubMed] [Google Scholar]
- 80. Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, et al. (2003) Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest 111: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schrijvers BF, Flyvbjerg A, De Vriese AS (2004) The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int 65: 2003–2017. [DOI] [PubMed] [Google Scholar]
- 82. Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, et al. (2000) Amniotic fluid–soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol 95: 353–357. [DOI] [PubMed] [Google Scholar]
- 83. Kincaid-Smith P (1991) The renal lesion of preeclampsia revisited. Am J Kidney Dis 17: 144–148. [DOI] [PubMed] [Google Scholar]
- 84. Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, et al. (2008) VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.U.S. Food and Drug Administration. FDA Briefing Document, Oncologic Drugs Advisory Committee Meeting, NDA 20324 Axitinib (Inlyta). http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202324lbl.pdf assessed Jun 11, 2013
- 86.US Food and Drug Administration. Highlights of Prescribing Information. Votrient (pazopanib) tablets. Available: http://wwwaccessdatafdagov/drugsatfda_docs/label/2009/022465lblpdf Accessed 2013 June 11.
- 87. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43: S1–290. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)