Abstract
Glucocorticoids are steroid hormones regulated in a circadian and stres-associated manner to maintain various metabolic and homeostatic functions that are necessary for life. Synthetic glucocorticoids are widely prescribed drugs for many conditions including asthma, chronic obstructive pulmonary disease (COPD), and inflammatory disorders of the eye. Research in the last few years has begun to unravel the profound complexity of glucocorticoid signaling and has contributed remarkably to improved therapeutic strategies. Glucocorticoids signal through the glucocorticoid receptor, a member of the superfamily of nuclear receptors, in both genomic and non-genomic ways in almost every tissue in the human body. In this review, we will provide an update on glucocorticoid receptor signaling and highlight the role of GR signaling in physiological and pathophysiological conditions in the major organ systems in the human body.
Keywords: Stress hormones, inflammation, nuclear receptor, glucocorticoid response element
Glucocorticoids
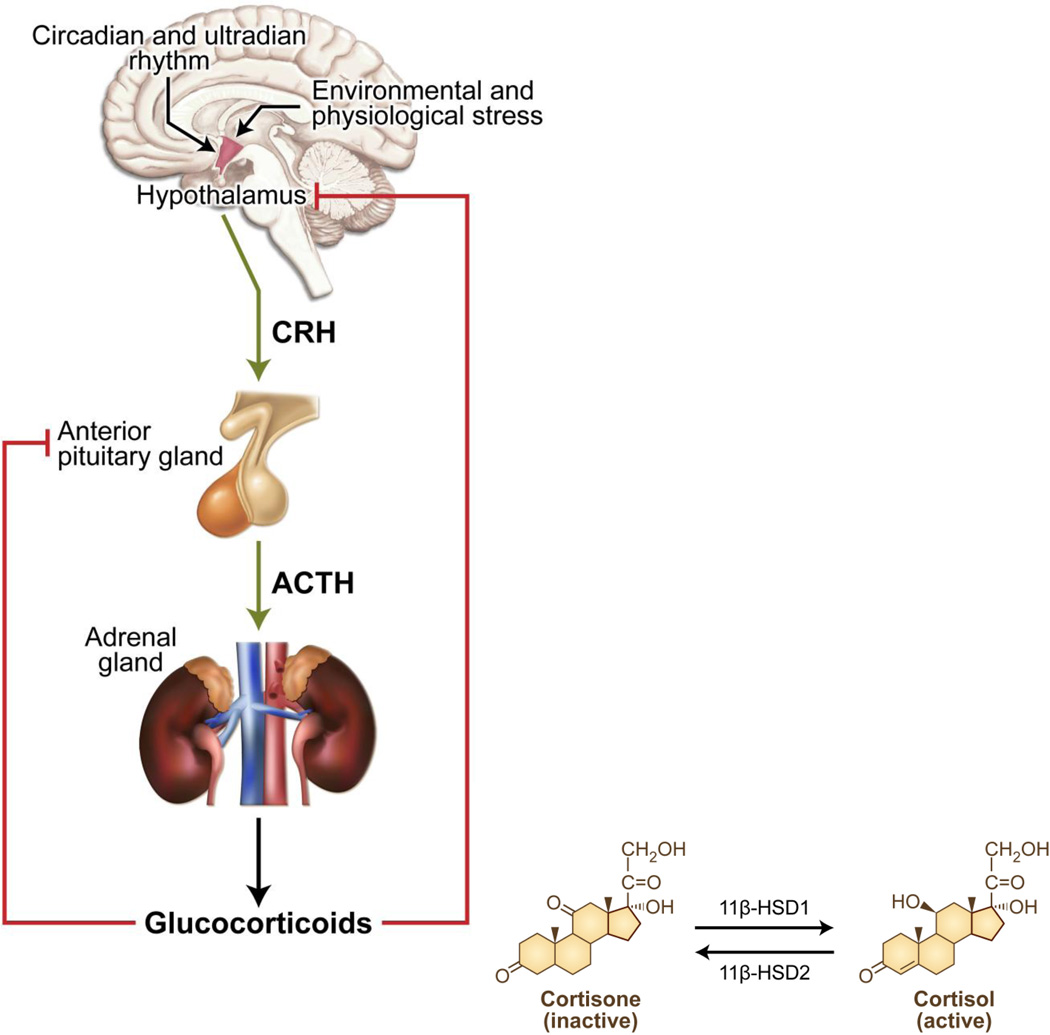
Natural glucocorticoids (cortisol in humans and corticosterone in rodents) are cholesterol-derived hormones secreted by the zona fasciculata of the adrenal glands. The synthesis and release of glucocorticoids is under dynamic circadian and ultradian regulation by the hypothalamic-pituitary-adrenal (HPA) axis (Figure 1) [1]. Furthermore, availability of natural glucocorticoids in tissues is regulated by corticosteroid binding globulin in serum and by locally expressed 11β-hydroxysteroid dehydrogenase enzymes (11β-HSD) [2] (Figure 1). Imbalance in glucocorticoid levels such as chronic elevation or deficiency can result in pathological conditions known as Cushing’s disease and Addison’s disease, respectively.
Figure 1.
Schematic representation of the regulation of glucocorticoid levels by the hypothalamic pituitary adrenal (HPA) axis. The synthesis and release of glucocorticoids is under the dynamic circadian and ultradian regulation by the periventricular nucleus of the hypothalamus. Corticotropin-releasing hormone (CRH) secreted by the hypothalamus stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland. In turn, ACTH induces the synthesis and secretion of cortisol from the cortex of the adrenal glands into the blood stream. In the blood, majority of the cortisol remains bound to corticosteroid-binding globulins in the blood. The biologically active form of the glucocorticoid is the unbound cortisol that can be converted to the inactive form, cortisone by type 2 11β-hydroxysteroid dehydrogenase. Type 1 11β-hydroxysteroid dehydrogenase converts the cortisone to cortisol. Homeostasis in glucocorticoid levels in maintained by the negative feedback loop suppressing ACTH levels in the anterior pituitary and CRH levels in the hypothalamus.
Synthetic glucocorticoids are drugs that resemble natural glucocorticoids. Prednisone/prednisolone, dexamethasone and budesonide are some of the commonly prescribed glucocorticoids. Synthetic glucocorticoids differ from natural glucocorticoids by their potency and metabolic clearance. Unlike natural glucocorticoids, dexamethasone is not susceptible to inactivation by 11β-HSD2, thereby increasing its local availability [3]. Furthermore, unlike natural glucocorticoids, synthetic glucocorticoids do not bind corticosteroid-binding globulin, thereby not being susceptible to their regulation of available levels.
The clinical use of glucocorticoids dates back to the late 1940s when Philip Hench successfully treated the symptoms of rheumatoid arthritis [4] with cortisone, for which he later received a Nobel Prize [5]. Since then, glucocorticoids have revolutionized the field of medicine; synthetic glucocorticoids are being prescribed for chronic inflammatory conditions including asthma, skin infections, and ocular infections, as well as for immunosuppression in patients undergoing organ transplant. In addition to their anti-inflammatory properties, corticosteroids have been exploited for their anti-proliferative and antiangiogenic actions for the treatment of cancers [6].
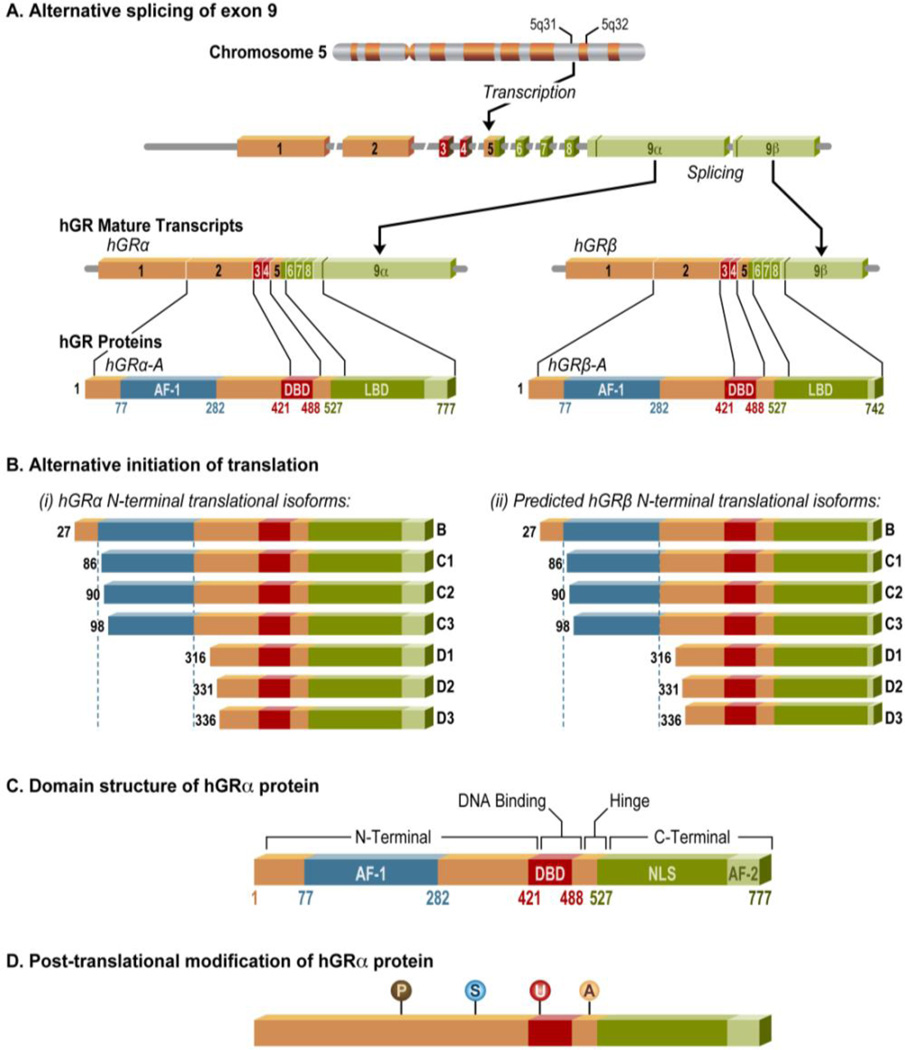
Both natural and synthetic glucocorticoids transduce their actions by binding to the glucocorticoid receptor (GR) (Figure 2). In the absence of glucocorticoids, the GR resides in the cytoplasm bound to chaperone proteins such as heat shock protein 90 (hsp90). Upon ligand binding, the GR undergoes a confirmational change that triggers its translocation to the nucleus, where it can exert its actions mainly through genomic (transactivation and transrepression) mechanisms. GR is the product of a single gene, NR3C1 located on chromosome 5q31–32 in humans, that undergoes alternative processing to yield multiple functionally distinct subtypes of GR. Diversity in GR signaling comes from the actions of different glucocorticoid-response elements (GREs) and multiple receptor isoforms generated by alternative splicing and alternative translation initiation [7]. Additionally, multiple posttranslational modifications including phosphorylation, acetylation, ubiquitination and SUMOylation (small ubiquitin related modifier) can alter the function of this transcription factor [8]. These mechanisms are summarized in Table 1.
Figure 2.
Genomic location and organization of the human glucocorticoid receptor. The human glucocorticoid receptor is located on chromosome 5q31–32 locus. (A) GR undergoes alternative processing to yield multiple functionally distinct subtypes of GR. GR contains 9 exons with the protein coding region formed by exons 2–9. Exon 1 forms the 5’-untranslated region. Alternative splicing of GR generates hGRα and hGRβ isoforms, which differ in their C-termini. (B) The GRα isoform undergoes alternative translation initiation in exon 2, generating eight additional isoforms of GR with truncated N-termini (GRα-A, GRα-B, GRα-C1, GRα-C2, GRα-C3, GRα-D1, GRα-D2, and GRα-D3). GR β is predicted to also generate eight β isoforms similar to hGRα. (C) GR is a modular protein containing an N-terminal transactivation domain (NTD), a central DNA-binding domain (DBD), a C-terminal ligand-binding domain (LBD), and a flexible “hinge region” separating the DBD and the LBD. The NTD has strong transcriptional activation function (AF1), which allows for the recruitment of coregulators and transcription machinery. Glucocorticoids bind the hydrophobic pocket of the LBD causing the second activation function (AF2), located in the LBD itself, to interact with coregulators. The DBD/hinge region junction and the LBD, each contain a nuclear localization signal that allows translocation to the nucleus. (D) GR undergoes multiple posttranslational modifications including phosphorylation (P), SUMOylation (S), ubiquitination (U) and acetylation (A).
Table 1.
Multiple mechanisms of glucocorticoid receptor-mediated regulation.
| GR Isoform |
|---|
| Alternative Splicing |
| GRα and GRβ |
| Alternative Translational Initiation |
| GRα-A, GRα-B, GRα-C1, GRα-C2, GRα-C3, GRα-D1, GRα-D2, and GRα-D3 |
| GRβ-A, GRβ-B, GRβ-C1, GRβ-C2, GRβ-C3, GRβ-D1, GRβ-D2, and GRβ-D3 |
|
Non-genomic effects: |
| Specific effects |
| Cytoplasmic GR |
| Membrane-bound GR |
| Non-specific effects |
| Not GR-mediated |
|
Genomic effects: |
| Direct effects |
| Simple GREs |
| Negative GREs |
| Composite GREs |
| Indirect effects |
| Tethered GREs |
|
Posttranslational Modifications: |
| Phosphorylation |
| Acetylation |
| Ubiquitination |
| SUMOylation |
Mechanism of glucocorticoid receptor signaling
Glucocorticoid Receptor
The GR is a modular protein containing an N-terminal transactivation domain (NTD), a central DNA-binding domain (DBD), a C-terminal ligand-binding domain (LBD), and a flexible “hinge region” separating the DBD and the LBD. The NTD has strong transcriptional activation function (AF1), which allows for the recruitment of coregulators and transcription machinery. Among the entire 48 members of the nuclear receptor superfamily, the DBD is the most conserved region. The two zinc finger motifs present in the DBD recognize and bind specific DNA sequences on target genes called glucocorticoid response elements (GREs). Upon ligand-binding, the second activation function (AF2), located in the LBD, interacts with coregulators. The DBD/hinge region and the LBD, each contain a nuclear localization signal that allows translocation to the nucleus via an importin-dependent mechanism [7].
GR isoforms
The human NR3C1 gene contains 9 exons with the protein coding region formed by exons 2–9. Exon 1 forms the 5’-untranslated region. Alternative splicing of GR generates hGRα and hGRβ isoforms, which are identical through amino acid 727, but differ in their C-termini [7]. The hGRα isoform binds to glucocorticoids, translocates to the nucleus, and recruits coregulators to exert transcriptional effects. However, the hGRβ isoform resides constitutively in the nucleus and acts as a natural dominant negative inhibitor of hGRα isoform. The hGRβ isoform can directly regulate genes that are not regulated by hGRα isoform. Although hGRβ has not been reported to bind glucocorticoid agonists, one antagonist RU486 (mifepristone) has been shown to bind to hGRβ and regulate its transcriptional activity [9]. These data show that hGRβ functions to negatively regulate the actions of the hGRα isoform as well as exert its own independent functions. GRβ isoforms also exist in mice and zebrafish, but are generated by an alternative splicing mechanism that is distinct from the GRβ in humans [10, 11]. GRγ, GR-A and GR-P are other less characterized GR isoforms, which have been associated with glucocorticoid insensitivity [7]. For example, GRγ expression was found to be lower in patients with acute lymphoblastic leukemia who responded well to glucocorticoid treatment than in patients who responded poorly to the treatment [12].
The GRα isoform also undergoes alternative translation initiation in exon 2, generating eight additional isoforms of GR with truncated N-termini (GRα-A, GRα-B, GRα-C1, GRα-C2, GRα-C3, GRα-D1, GRα-D2, and GRα-D3). GRβ may also generate eight β isoforms similar to the hGRα [13]. All of the GRα isoforms have similar glucocorticoid-binding affinities and interactions with GREs. Interestingly, the GRα-C isoforms are the most biologically active, while the GRα-D isoforms are the most deficient in glucocorticoid-mediated functions [14]. Intriguingly, the GRα-D isoform is constitutively present in the nucleus and bound to certain GRE-containing target genes [7]. Widespread tissue distribution of all transcriptional and translational isoforms of GR permit fine-tuning of GR signaling based on their relative availability in a given cell or a tissue type.
Genomic effects of GR
The classical effects of glucocorticoid signaling are the genomic actions, which depend on GR-mediated transcription and de novo protein synthesis.
Ligand-bound GR homodimerizes in the nucleus and exerts its transcriptional activation or repression by direct high-affinity binding to glucocorticoid response elements (GREs) found either in the promoters or the intragenic regions of glucocorticoid target genes (Figure 4). The gene encoding glucocorticoid-induced leucine zipper (GILZ) [15], serum/glucocorticoid regulated kinase 1 (SGK1) [16], Tristetraproline (TTP) [17], and Mitogen-activated protein kinase phosphatase-1 (MKP-1) [18] are examples of genes upregulated by activated GR. Examples of genes negatively regulated by GR are β-arrestin 2 [19], osteocalcin [18] and the GR gene, NR3C1 itself [20].
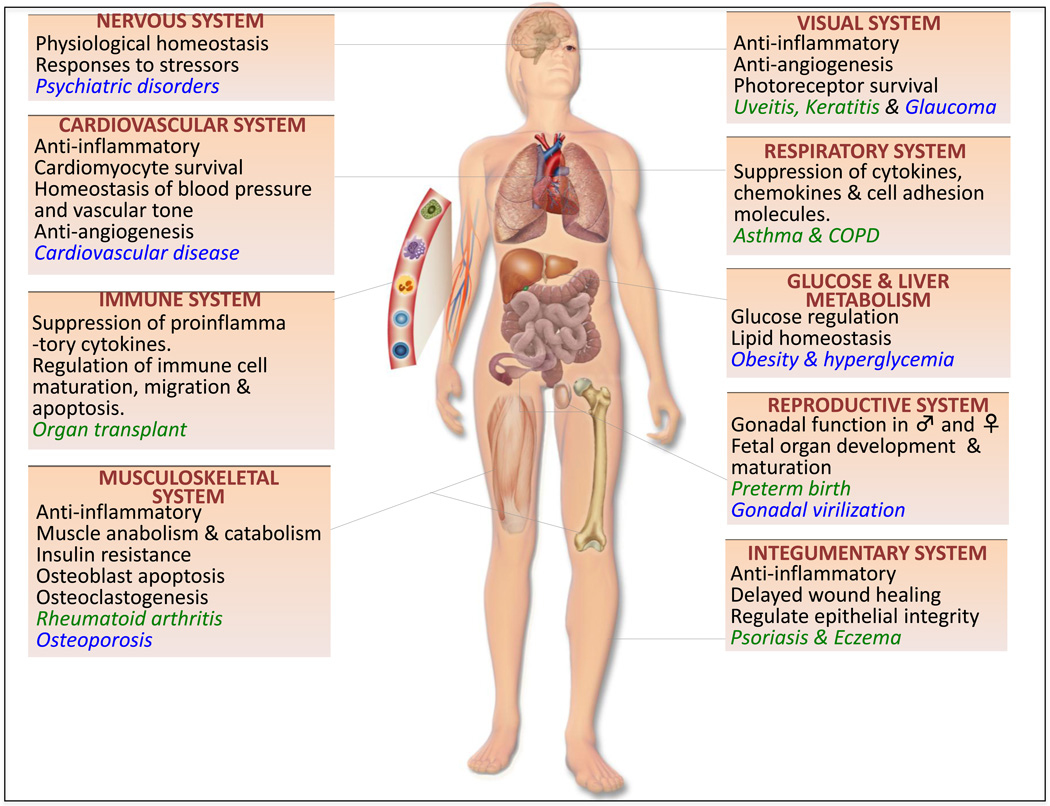
Figure 4.
Role of glucocorticoids in health and disease. This schematic represents the roles of glucocorticoids in major organ systems of the human body (black text), beneficial roles of glucocorticoids in the clinic (green text) and adverse outcomes in patients with elevated glucocorticoid levels (blue text).
A recent review by Beck et al., which comprehensively analyzed the characteristics of several artificial mutants of GR, sheds light on the complexity of GR function at the molecular and cellular level [21]. Typically, a “simple” GRE is classified as a DNA sequence of imperfect palindromic sequences containing two hexameric half sites separated by three base pairs. One-third of these bases are highly conserved, while two-thirds of these bases can be variable. It is this variability in DNA sequence within the GRE that dictates the outcome of GR-transcriptional activity [22]. Furthermore, glucocorticoid-bound GR binds to these “simple” GREs to mediate transactivation, rather than transrepression, by recruiting coactivators and chromatin-remodeling complexes [7, 23]. In addition, GR has been reported to bind a half-GRE site on tristetraprolin (TTP), an mRNA destabilizing gene, to decrease gene expression of pro-inflammatory cytokines [17]. GR also modulates gene expression by binding to “composite” GREs, wherein the target gene contains binding sites for GREs as well as other transcription factors. “Tethering” GREs are another way by which GR indirectly regulates gene expression. Despite the absence of DNA binding site, tethering GREs recruit other transcription factors that in turn are bound to GR [7]. For example, suppression of inflammation in diseases such as asthma and COPD occurs by GR tethering with pro-inflammatory transcription factors such as activator protein-1 (AP-1), nuclear factor-kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) [24]. Until recently, the mechanism of GR transrepression was thought to be primarily mediated by tethering GREs. However, a recent finding by Surjit et al. [25] has caused a paradigm shift in our understanding of the direct role of GR in transrepression. GR has been shown to bind to specific, widely prevalent, inverted palindromic sequences with a spacer ranging from 0–2 base pairs, called “simple” negative GREs (nGREs) on target genes. Upon GR binding, the nGRE sequence, recruits co-repressors (NCoR and SMRT), which further recruit histone deacetylases (HDACs) to exert gene repression. In addition, Hudson et al. have discovered through structural studies that binding of GR to nGREs prevents dimerization of GR, while the converse is true when GR binds to activating GREs [26]. Thus, DNA in the form of GREs modulates GR function by allowing selective-regulation of gene expression, whether it be for gene activation or repression. Similarly, the GREs can offer more than just a docking site for GR in the protein/DNA complex, since the DNA sequence in the GRE can serve as a ligand that can allosterically modulate the structure and the transcriptional activity of GR [22].
Non genomic effects of GR
Emerging evidence suggests that glucocorticoids can also exert their actions in a more rapid (within minutes), non-genomic signaling mechanism that does not require nuclear-GR mediated- transcription or translation (usually takes a few hours). These actions are thought to be mediated by the activation of signal transduction pathways, such as the mitogen activated protein kinase pathway, by the membrane-bound GR or the cytoplasmic GR [27, 28]. Additionally, rapid effects, not specific to GR, also occur as a result of physiochemical interactions of glucocorticoids with the cell membrane [29]. These rapid actions of the GR have been reported in various systems, including the cardiovascular, the immune and the neuroendocrine systems [27],[30], [31]. Exploiting these mechanisms to improve novel corticosteroids is currently underway.
Post-translational modifications
Post-translation modifications (PTM) to the GR also contribute to the diversity in transcriptional activity. Phosphorylation of GR has been reported to occur on several serine residues (S113, S134, S141, S143, S203, S211, S226, and S404) by a number of kinases including cyclin-dependent kinase, MAPK, glycogen synthase kinase-3 (GSK-3) and casein kinase II [8],[32]. Most phosphorylation sites have been shown to be modified only upon receptor activation by ligand binding. However, Galliher-Beckley et al. have recently shown that serine 134 residue can be phosphorylated by p38 MAPK in a hormone-independent manner [32]. Interestingly, studies in patients undergoing glucocorticoid treatment for asthma revealed a negative correlation between p38 MAPK activity and the patient’s response to GC therapy [33]. The use of inhibitors against p38 MAPK activity improved the anti-inflammatory effect of GC therapy in patients with asthma and chronic obstructive pulmonary disease (COPD) [34], providing an improved avenue for therapy.
There have been recent reports that the GR can be acetylated on Lysine 494 and 495 in response to glucocorticoids, and this modification correlates with impaired ability of GR to inhibit the actions of NF-κB [35]. Furthermore, knockdown of HDAC2 correlated with decreased sensitivity to corticosteroid treatment in primary alveolar macrophages, and overexpression of HDAC2 in alveolar macrophages from COPD patients exhibiting glucocorticoid resistance reestablished their sensitivity to glucocorticoid therapy [24]. Further supporting evidence comes from recent work published by Zhang et al., in which inhibition of HDAC using trichostatin A in rodents exacerbated the inflammatory response in an LPS-induced myocarditis model [36]. In addition, enhanced inflammatory response to LPS, and decreased HDAC activity were observed in rodents whose glucocorticoid pathway was attenuated either by RU486 or adrenalectomy, thereby suggesting that GR exerts anti-inflammatory actions by utilizing HDAC. An additional layer of GR regulation lies in ubiquitin-proteasome and SUMOylation processes. A lysine residue critical for GR ubiquitination and an E3 ubiquitin ligase were identified, and alterations to the lysine residue or the E3 ligase have been shown to modulate GR activity [37] [38]. SUMOylation regulates a variety of cellular functions such as protein subcellular localization, organization of chromatin structure, transcription and protein stability. GRα undergoes SUMOylation at specific lysine residues and this modification targets GR for degradation [7].
The role of glucocorticoids in health and disease
GR isoforms are expressed in nearly all tissue types and glucocorticoid signaling is almost ubiquitously prevalent in the various organ systems [7]. Due to their anti-inflammatory, anti-proliferative, pro-apoptotic and anti-angiogenic roles, glucocorticoids have been remarkably effective in treating various diseases and they have been at the forefront of basic science and pharmaceutical research for the past few decades. In this section, we summarize the recent progress that has been made in understanding the role of glucocorticoid/GR signaling in various organ systems (Figure 4).
Embryonic development
To elucidate the in vivo role of glucocorticoid signaling during development, Schutz and colleagues [39], [40] conducted genetic studies with GR knockout mice. GR−/− neonates die soon after birth due to respiratory failure arising from impaired lung development, indicating the important role of GR signaling in lung maturation [41, 42]. In addition, profound alterations in the regulation of liver, adrenal gland, brain and HPA axis were observed in GR−/− mice. Futhermore, thymocytes become resistant to apoptosis in the absence of GR [39]. Together, the presence of a functional GR during gestation is essential for postnatal survival as well as during development.
Nervous system
Signals caused by stress are mediated by glucocorticoid receptors that are expressed widely in the brain. Elevation in glucocorticoids has been implicated in psychiatric disorders such as schizophrenia, drug addiction, posttraumatic stress disorder (PTSD) and mood disorders [43]. Moreover, addiction to substances such as cocaine [44] and alcohol [45] has also been linked to aberrant GR signaling. Knockout and transgenic mice have aided in elucidation of the role of GR in the central nervous system [46, 47]. Several studies indicate that GR functions in the brain positively correlate with anxiety behavior [48]. For example, Tronche et al. showed that brain-specific deletion of GR resulted in mice with decreased anxiety and lower levels of despair-like behavior [40]. Consistently, mice overexpressing GR in the brain exhibit increased anxiety [48]. Since GR is either deleted or overexpressed in the entire brain in these studies, that too early in birth, it is impossible to attribute specific brain regions to the phenotypes observed. However, recent studies using mice lacking GR in specific regions of the brain- forebrain or the amygdala during adulthood have shed some light on the role of GR on the HPA axis and behavioral disorders [49]. GR in the forebrain has been shown to regulate HPA axis and behavior under stressed conditions, while GR in the amygdala has been shown to be playing an important role in memory acquisition and fear conditioning.
Visual System
Ophthalmologists have wisely utilized the anti-inflammatory and anti-angiogenic functions of glucocorticoids in treating various forms of ocular inflammation (e.g., conjunctivitis, keratitis, uveitis), macular edema, and macular degeneration [47]. In addition, glucocorticoids have been used to inhibit neovascularization in the eye that could lead to vision loss [50, 51]. Unfortunately, a subset of patients undergoing chronic systemic glucocorticoid therapy develop cataracts and are at a high risk for developing glaucoma arising from increased intraocular pressure [50, 52]. To minimize off-target effects of GR signaling within the eye, significant advances in drug delivery have introduced intraocular implants that release corticosteroids in a controlled manner [52]. Much of vision research involving steroids has focused on the effects of exogenous glucocorticoids, but not in understanding the role of glucocorticoid function in normal physiology. Response to glucocorticoid treatment in the eye implies a natural role for glucocorticoid signaling in the eye. Furthermore, by using rodent models, it has been shown that glucocorticoids confer protection in photoreceptors in the retina by preventing their apoptosis [53, 54].
Cardiovascular System
Corticosteroid levels should be in balance, because patients with excessive levels of corticosteroids (either endogenously or exogenously) are at a higher risk of developing cardiovascular disease, although it is unclear if these effects are direct or indirect actions of glucocorticoids on the heart [55]. Patients who developed Iatrogenic Cushing’s syndrome as a result of long-term glucocorticoid treatment were at a higher risk of developing cardiovascular disease compared to the people not receiving glucocorticoid treatment [56]. There is also evidence for the expression of GR and for the production of corticosteroids in the blood vasculature and the heart of humans; however, the molecular mechanism by which glucocorticoids-GR exert cardioprotection is poorly understood [57]. Moreover, the role of the GR in the heart is disputed because cardiomyocytes also express the mineralocorticoid receptor (MR), which can also bind glucocorticoids [58]. In the vasculature, glucocorticoids inhibit the production of vasodilators such as prostacyclin and nitric oxide, for maintaining blood pressure homeostasis [30]. In cardiomyocytes, GRs have been demonstrated to exert anti-apoptotic (e.g., attenuate cytokine induced pro-apoptotic signals) and anti-inflammatory actions (e.g., inhibition of NF-κB and upregulation of annexins) [59].
Interestingly, Ren et al. have recently reported that treatment of cardiomyocytes with synthetic glucocorticoid dexamethasone causes the cells to attain features characteristic of cardiac hypertrophy [60]. However, dexamethasone was also capable of reversing the apoptosis caused by starvation and exposure to TNFα, suggesting that glucocorticoids are cardio-protective under stressful conditions. Furthermore, in a recent in vivo study using LPS-induced myocarditis model, Zhang et al. [36] demonstrated that endogenous glucocorticoids are required for inhibiting myocardial inflammation. In addition to the above mentioned genomic effects, there exist reports on the non-genomic roles of GR in the cardiovascular system which are exerted through mitochondrial GR and serum and glucocorticoid-responsive kinase-1 (SGK-1) [30]. Collectively, glucocorticoid regulation of cell size, apoptosis, inflammatory state and vascular tone appear to be vital for proper cardiac function.
Immune System
Glucocorticoids are a gold standard for immune suppression in organ transplant patients. They exert their classic anti-inflammatory role by acting on nearly all cell types of the immune system [61]. For example, in dendritic cells (antigen presenting cells) glucocorticoids suppress dendritic cell maturation, thereby converting them into tolerogenic dendritic cells that possess weak T-cell energy. Dendritic cell migration and apoptosis are also controlled by glucocorticoids. Furthermore, it has been reported recently that glucocorticoids can induce differential effects in dendritic cells based on the GR isoform available [62], thereby providing a strategy for GR-isoform specific targeted therapy. Another key cell type in the immune system are the macrophages, which express pattern recognition receptors (such as Toll-like receptors) that sense infectious agents and harmful signals and activate the inflammasome complex which in turn controls pro-inflammatory cytokine release [63]. Interestingly, glucocorticoids have also been shown to positively regulate NLRP3, a component of the inflammasome complex in macrophages, to augment pro-inflammatory response [63]. Also, glucocorticoids cooperate with the pro-inflammatory molecule TNFα to induce toll like receptor 2 gene expression, thereby stimulating innate immunity [64]. In contrast, the GR has also been shown to enhance phagocytosis of neutrophils by macrophages, a desirable outcome by glucocorticoids to curb inflammation [65]. Neutrophils infiltrate the inflammatory site by following cues from cytokines and chemokines released from mast cells and endothelial cells. Glucocorticoids suppress migration of these neutrophils by repressing the expression of cell adhesion molecules [28]. However, glucocorticoids are also thought to suppress apoptosis of neutrophils by mechanisms including upregulation of anti-apoptotic proteins such as Mcl-1 an XIAP (reviewed in [66]). The effect of glucocorticoids on T cells is specific to the subtype of T cell. In proinflammatory T cells, glucocorticoids induce apoptosis, whereas glucocorticoids exert pro-survival effects in regulatory T cells [61] [65]. Consequently, mice lacking GR in T cells by gene targeted deletion displayed resistance to glucocorticoid-induced apoptosis. Glucocorticoids also act as immunomodulators to suppress B cell antibody production in various autoimmune disorders and in B- cell malignancies [67]. Glucocorticoids have been known to moderately influence the different phases of B cell activation, survival, proliferation and differentiation. Glucocorticoid treatment of B cells results in reduction in B cell numbers, proliferation of progenitor numbers and in IgG production in a dose-dependent manner and can also lower the level of the anti-apoptotic protein, Bcl-2, thereby making the B cells susceptible to glucocorticoid-induced apoptosis [61].
Respiratory system
Glucocorticoids, particularly the inhaled corticosteroids, are the most commonly prescribed drugs for the treatment of chronic inflammatory conditions of the respiratory tract. In asthma, multiple inflammatory genes in the airways are turned on by the activation of pro-inflammatory transcription factors, such as NF-kB and AP-1. Glucocorticoids, by inhibiting NF-kB and AP-1 activity, suppress the production and secretion of cytokines, chemokines and cell adhesion molecules by the airway epithelium. However, a vast majority of COPD patients and a minority of asthma patients fail to respond to glucocorticoid therapy even at high doses. Several studies have associated this corticosteroid resistance with changes in interferon-γ levels [68]. Furthermore, increased activity of MAPK, ERK and JNK pathways in the airways have been implicated in glucocorticoid insensitivity in respiratory disorders. Inhibitors to some of these pathways are currently in clinical trials, and based on the outcome of the trials [69], these compounds could be used in combination with corticosteroids to improve the treatment of respiratory conditions.
Glucose and liver metabolism
Metabolism and energy homeostasis is maintained by glucocorticoids. Under conditions of stress (such as starvation and exercise), glucocorticoid signaling regulates the liver to replenish glucose by glycogenolysis and gluconeogenesis. A physiological appreciation for GR signaling to regulate metabolic homeostasis can be found in cases with Cushing’s disease and Addison’s disease, where Cushing’s can result in central obesity, hyperglycemia, hypercholesterolemia, and fatty liver. In contrast, weight loss due to loss of appetite, skin discoloration, and hypoglycemia are seen in patients with Addison’s disease [70]. Moreover, numerous studies have reported metabolic abnormalities associated with dysfunctional signaling of the GR [70]. Studies using liver-specific knockout mice of growth hormone (GH), STAT5 and GR reveal that interplay between the GR and growth hormone pathways regulates the whole-body growth and metabolic functions [reviewed in [71]. For example, in the liver, GR has been shown to bind STAT5, a critical molecular player in growth hormone signaling. Moreover, liver-specific deletion of GR in mice resulted in decreased expression of genes downstream of STAT5 signaling, indicating that GR and GH pathways interact with each other. There is considerable evidence implicating GR signaling in maintaining glucose homeostasis. With diabetes and obesity on the high rise, further studies dissecting the complex actions of GR in the hepatic system would be beneficial.
Reproductive System
During development, gonads and adrenals share a common adrenogonadal primordium. Therefore, it is not surprising that glucocorticoids play a vital role in reproduction in both sexes. Physiologically, glucocorticoids regulate gonadal function at multiple levels along the hypothalamic-pituitary-gonadal axis [72]. Excessive glucocorticoids either as a result of synthetic glucocorticoid therapy or due to Cushings’ syndrome lead to various adverse effects resulting in impaired reproductive function [73].
Over the past decade, glucocorticoid therapy has drastically improved the outcome of preterm births by significantly decreasing neonatal mortality and morbidity by accelerating fetal lung maturation and survival in infants born <34 weeks of gestation [74]. However, antenatal glucocorticoid treatment can trigger premature signaling by the endogenous fetal glucocorticoid receptors, which in turn could predispose the infant to alterations in the hypothalamic-pituitary-adrenal axis (HPA), and metabolic and cardiovascular dysfunction. Studies conducted in animal models have shown that this developmental programming of antenatal glucocorticoids may have long lasting effects on the life of the offspring [75]. However, the extent of damage contributed by antenatal glucocorticoid therapy in preterm infants is controversial due to several factors. For example, intrauterine growth restricted (IUGR) babies are predisposed to be delivered prematurely. Preterm babies are most often subjected to acute therapy in the neonatal intensive care unit (NICU), which are stressors that could have a longterm affect on the health of these babies, making it difficult to tease apart the contribution of exogenous glucocorticoids from other factors such as IUGR, and assistance at NICU. Moreover, since it is unethical to not treat women predicted with preterm birth with glucocorticoids, it is difficult to identify the exogenous glucocorticoid-specific effects in the infants born prematurely. Interestingly, the rate of antenatal therapy is not high in Poland and a group of Polish researchers were able to perform whole-genome wide expression study comparing profiles of peripheral leukocytes from the blood of preterm born neonates either exposed to or not exposed to corticosteroids. The authors reported that the effects of antenatal glucocorticoids were mostly short-lived. Since the study was dealing with prematurely born infants, it had its own limitations in being unable to examine the steroid-specific effects on tissues besides the circulating leukocytes [76]. A major concern in the neonatal practice of antenatal glucocorticoid therapy is the association of glucocorticoid treatment to changes in development and regulation of the HPA axis in the offspring. However, studies involving preterm babies are confounded by the fact that there is attenuated HPA axis signaling due to prematurity. Although studies conducted in animal models suggest dysregulation of the HPA axis, studies conducted in humans have not clarified if antenatal or neonatal glucocorticoid therapy is known to cause any permanent neurodevelopmental consequences. Alexander and colleagues [77] have conducted an interesting study, where they examined cortisol activity in offspring born not prematurely, but at term, to hospitalized females who have undergone antenatal glucocorticoid treatment and compared them to controls born to hospitalized females not exposed to synthetic glucocorticoids. Results from this study suggest that antenatal synthetic glucocorticoid therapy may alter the developmental programming to have a long-lasting effect on the HPA axis of the offspring. Considering the benefits in preterm birth, antenatal glucocorticoids will however likely remain as an important treatment strategy in neonatal practice. Clearly, further longitudinal studies are needed to understand if the adverse outcomes seen in animal models are recapitulated in humans. Until then, the smallest dose of antenatal glucocorticoids required should be used to improve the survival of preterm babies.
Although glucocorticoids are beneficial in promoting fetal lung maturation, fetal exposure to glucocorticoids can have long-term repercussions affecting the fertility of the offspring. The importance of glucocorticoids in maintaining fertility is well-illustrated by the example of congenital adrenal hyperplasia (CAH). CAH is an autosomal recessive disorder caused by the decrease in 21-hydroxylase enzyme that is required for steroidogenesis of cortisol and aldosterone in the adrenal glands. Decrease in these steroid hormones signals the adrenals to produce more androgens, leading to virilization of genitalia in females [78] and subfertility in males [79]. CAH can be treated by substituting the loss of cortisol with synthetic glucocorticoids. Recently, other studies have looked into the beneficial effects of glucocorticoids in fertility. For example, women who had been treated with dexamethasone in addition to induced ovulation and intrauterine insemination (IUI) had about a four-fold increase in pregnancy rate compared to women that underwent ovulation induction and IUI [80].
Furthermore, there is an association between recurrent miscarriages and a polymorphism in NR3C1, suggesting the importance of an intact functioning GR for achieving a successful pregnancy [81]. Recent work has underscored the role of glucocorticoid receptor signaling in the uterine epithelium. In particular, the glucocorticoid signaling system communicates with the estrogen signaling pathway to tightly regulate the pro- and anti-inflammatory uterine milieu, perhaps as a mechanism for implantation [82]. Since most of the clinical studies are currently focused on the effects of antenatal steroid therapy, future studies addressing the physiological role of glucocorticoids in couples trying to achieve/maintain pregnancy would be beneficial.
Musculoskeletal system
Glucocorticoids are the first line of drugs for treating musculoskeletal disorders, such as rheumatoid arthritis (RA) where there is inflammation in the joints and the surrounding tissues. However, synthetic glucocorticoids used in RA therapy can have adverse side effects. Glucocorticoids can cause osteoporosis, and in fact, glucocorticoid-induced osteoporosis is one of the most unfavorable outcomes of long-term high dose glucocorticoid therapy, particularly in the elderly. Studies have shown that the GR is required for bone resorption and the mechanism is thought to be mediated by RANKL/OPG and Wnt signaling pathways [83, 84]. Furthermore, Jia et al. have reported that glucocorticoids can also act directly on osteoclasts to expand their life span [85]. GR-induced bone loss is a sum of apoptosis of osteoblasts (help make bone) and osteocytes (resorb bone) [5], increased osteoclastogenesis, and decreased vasculature, and thereby poor nutrient transport [86]. Recent work have focused on combination therapy to combat glucocorticoid-induced osteoporosis. For example, Ramli et al. have identified that Glycyrrhizic acid (isolated from Licorice root) was able to rescue rats from dexamethasone-induced bone loss [87]. Furthermore, parathyroid hormone (PTH) has been shown to attenuate the effects of prednisolone on bone loss in mice [88].
Upon injury, muscle regeneration utilizes the immune system and the inflammatory response to repair the injury to muscle. Glucocorticoids disrupt muscle energy homeostasis in multiple ways, by increase in muscle anabolism as well as catabolism, induction of proteolysis and by inhibiting regeneration of muscle [89]. For example, in conditions such as starvation or renal failure, glucocorticoids play an important role in regulating muscle mass. Glucocorticoids break down skeletal muscle by inhibiting their regeneration by attenuating myogenic cell proliferation and differentiation [89]. The IGF-1—PI3K—Akt pathway, the myostatin signaling pathway and the NF-κB pathway have been implicated as the primary pathways mediating glucocorticoid-mediated skeletal muscle catabolism. Increase in protein degradation is a consequence of chronic hypercortisolism or chronic administration of exogenous glucocorticoids. Studies have shown that glucocorticoids inhibit PI3K–Akt pathway to activate the ubiquitin-proteosome pathway, which in turn leads to the increased expression of machinery required for protein degradation [90] [91]. Besides protein catabolism, glucocorticoids inhibit muscle anabolism by inhibiting amino acid transport into muscles. mTOR, REDD1 and KLF15 are involved in glucocorticoid-mediated repression of protein synthesis [91]. Moreover, GR signaling activates transcription of genes associated with insulin resistance and muscular atrophy [92]. Clinically, glucocorticoid-induced skeletal muscle catabolism can present as glucocorticoid-induced myopathy or critical illness myopathy. Glucocorticoid-induced myopathy manifests as muscle weakness in the proximal muscles, often sparing the distal extremities, and is rather painless. This condition can be reversed by either discontinuation of steroids or lowering the administered dose. However, critical illness myopathy is more severe and is reported in patients receiving intensive care and are being treated with a combination of high-dose glucocorticoids and non-depolarizing neuromuscular blocking agents [91, 93]. This myopathy manifests as a symmetric diffused weakness of all extremities, reduced deep tendon reflexes and elevated serum creatinine kinase levels. Therefore, withdrawal of glucocorticoids remains to be the treatment of choice for glucocorticoid-induced muscle atrophy. In addition, activity of GR in the skeletal muscle has been shown to positively correlate with the metabolic syndrome [70].
Integumentary System
Topical corticosteroids are commonly used for treating cutaneous inflammatory conditions, such as eczema and psoriasis, due to their anti-proliferative and anti-inflammatory actions [94], [95]. However, the therapeutic benefits from the long-term use of glucocorticoids in patients are also accompanied by adverse effects such as skin atrophy and delayed wound healing. Whether the wound healing side effect is due to transactivation or transrepression by GR was poorly understood until recently when Perez and colleagues significantly moved the field forward, providing insight into the role of skin-specific GR [96, 97]. In particular, anti-proliferative effects of the GR in keratinocytes were shown to be regulated by transrepression. However, both GR transrepression and transactivation were found to negatively delay wound healing of keratinocytes [98]. In addition, GR was found to regulate eyelid development [97]. More recently, results from mice lacking GR in the skin (GREKO) demonstrated that the physiological role of GR in the skin is to regulate epithelial integrity and immune function [96]. Altogether, the knowledge gained from these studies could be applied to improving glucocorticoid therapy for cutaneous diseases, perhaps by using GR agonists that can preferentially activate transrepression by the GR and minimize transactivation.
Glucocorticoid resistance
Some patients being treated with glucocorticoids for inflammatory conditions, such as COPD and asthma, respond poorly to the treatment. This resistance to glucocorticoid therapy has become a major barrier in effectively treating patients with inflammatory diseases. Ongoing research indicates that multiple mechanisms contribute to glucocorticoid resistance in subpopulations of patients receiving glucocorticoid treatment. Increase in the inactive hGRβ isoform or a decrease in nuclear translocation of GR have been thought to contribute to this resistance. Repression of GR gene expression by glucocorticoid-induced GR binding to an nGRE on NR3C1 has been implicated to be a critical mechanism for glucocorticoid resistance [20]. Also, the ability of GRβ to modulate transcription, independent of GRα, could perhaps be one of the mechanisms by which GRβ antagonizes the actions of GRα [9]. Another mechanism is believed to be decreased GR signaling due to changes in GR phosphorylation [8]. Phosphorylation of GR by p38 MAPK has been shown to be a causal factor for glucocorticoid resistance in several patients. Mercerdo et al._[34] have shown that inhibition of p38 MAPK in peripheral blood monocytes isolated from patients exhibiting glucocorticoid resistance restores insensitivity by increasing GR nuclear translocation. In addition, the authors went on to show that p38 MAPK inhibition decreases phosphorylation of GR at S226 in a monocytic cell line, providing a mechanism by which phosphorylation of GR by p38 MAPK could contribute to glucocorticoid resistance. Future clinical studies will be required to prove that p38 MAPK inhibition will have beneficial effects on asthmatic patients with glucocorticoid resistance.
As discussed above, GR recruits HDACs to undergo deacetylation, which correlates with improved glucocorticoid sensitivity. By contrast, hyperacetylation of GR due to impaired HDAC2 function could result in glucocorticoid resistance. Genetic susceptibility, hyperactivity of pro-inflammatory transcription factors and or cytokines, and elevated expression of the multidrug resistance gene, MDR1, are some of the mechanisms believed to be contributing factors to glucocorticoid resistance [18]. Furthermore, several polymorphisms in the GR gene have been linked to changes in glucocorticoid sensitivity [99]. Thus, knowing if a patient has polymorphism(s) in their GR gene would potentially provide clues to predicting the state of a patient’s glucocorticoid sensitivity early on in therapy.
Glucocorticoid receptor polymorphism
Polymorphisms are found throughout the gene body (NR3C1) of the glucocorticoid receptor. NR3C1 gene with a polymorphism(s) results in a modified transcript that can have a varying degree of impact ranging from no affect to altered glucocorticoid sensitivity. The main polymorphisms include BclI, N363S, and ER22/23EK. The BclI restriction fragment length polymorphism is located in intron 2 of NR3C1 and comprises of a single nucleotide substitution of C>G. BclI has been associated with abdominal obesity, depression, memory, and hypersensitivity to glucocorticoid treatment [100–102]. N363S is a single nucleotide polymorphism (SNP) also located in exon 2 of the gene, which is determined by a single amino acid substitution from asparagine to serine. N363S is associated with improved glucocorticoid sensititvity [102]. ER22/23EK is a polymorphism located on exon 2 and comprises of two nucleotide substitutions, of which only the second polymorphism results in the substitution of an amino acid which has an impact on the tertiary structure of GR, thereby affecting activation of transcription. The presence of ER22/23EK polymorphism is reported to decrease glucocorticoid sensitivity [103], increase muscle mass [104], BMI, triglyceride levels and hs-CRP [103] and increased the risk of depression.
Polymorphisms in the GRβ gene have been associated with increased GR insensitivity. An A to G substitution in the 3’UTR at position 3669 of the GRβ gene has been shown to increase its mRNA stability, and is associated with rheumatoid arthritis [105]. In addition, results from a large study involving nearly 8000 human subjects suggest that homozygosity for this GR-9β polymorphism correlates with an increased risk of myocardial infarction and coronary heart disease [106]. GR polymorphisms are rare, therefore resulting in interpretations that may not be statistically significant. Hence, any conclusions drawn from studies characterizing polymorphisms should be considered with caution.
Optimizing glucocorticoid therapy
Every year, glucocorticoids (oral, inhaled and topical drugs) are saving lives or improving the quality of lives all around the world [107]. However, glucocorticoid therapy comes with several side effects. Endogenous corticosteroids can exert their effects through the GR and MR. Therefore, synthetic glucocorticoids are tailored towards exerting their effects primarily through GR to minimize effects arising from MR activity. Significant advancements have been made since the studies unraveling the complex mechanisms in the GR signaling have laid the foundation for new and improved drug design [2]. For example, fluticasone and budesonide are inhaled corticosteroids that can be used for long-term treatment of asthma with minimal systemic effects. The current areas of improvement in drug design are to i) develop selective GR agonists (SEGRAs) or modulators (SGRMs) that have an improved anti-inflammatory action and minimal sideeffects, ii) synthesize potent anti-inflammatory GR agonists that are less effective on MR (eg: prednisone), iii) target delivery of the drug to the site of inflammation (intra-vitreal injections, synovial injections in rheumatoid arthritis, use of liposomal glucocorticoids), iv) combination therapy, and v) to optimize the dose of glucocorticoids administered to the patients. The transcriptional activity of GR is dependent on the kind of the activating ligand glucocorticoid. For example, in a recent study, dexamethasone, prednisolone and a SEGRA (GW870086X) exhibited differential response in human trabecular meshwork cells, with SEGRA activity exhibiting potentially improved outcome in glaucoma [108]. Since all glucocorticoids are not created equal, conclusions drawn from one glucocorticoid may not be equivalent to that of another glucocorticoid. Therefore, in-depth studies of each synthetic glucocorticoid to evaluate their specific pharmacological properties are warranted. The differential gene-expression profile of different glucocorticoids can be advantageous in developing personalized medicine. Interestingly, recent work demonstrating that GR binds to GREs in β-arrestin 1 and 2 and modulates their gene expression to alter G-protein coupled receptor (GPCR) signaling may have beneficial implications in combination therapy using corticosteroids and GPCR-based drugs in the treatment of asthma and COPD [19]. An emerging area of approach is exploiting the rhythmic nature of glucocorticoid signaling with the goal of developing drugs that can be released in a manner that is in tune with the circadian pattern of GR signaling. Under normal physiological conditions, glucocorticoid levels are in synchrony with the circadian rhythm. Moreover, glucocorticoids can reset the circadian rhythm in the peripheral tissues without the involvement of the master clock residing in the suprachiasmatic nucleus of the hypothalamus [109]. Since the discovery of circadian regulation of glucocorticoid signaling, chrono(pharmaco)therapy has become the recent advancement in glucocorticoid therapy. Chrono(pharmaco)therapy integrates the regulation of circadian timing system and the delivery of drugs to optimize treatment options. Glucocorticoids being chronobiotic in nature (have the ability to regulate circadian rhythm) offer hope to patients suffering with symptoms from rheumatoid arthritic, asthma and COPD [4, 110].
Concluding Remarks
Glucocorticoids are a mainstay for their anti-inflammatory, immunosuppressive and inflammatory disease-modulating actions. Since the start of glucocorticoid therapy 60 years ago, the therapeutic implications of targeting GR in various organ systems have been well studied; however, less is known about the physiological role of the GR. From a mechanistic and tissue-specific perspective, numerous studies using transcriptomics have shed light on the magnitude of GR’s ability as a transcription factor. Being cognizant about recent knowledge on the mechanistic advances in transrepressing (anti-inflammatory effects) and transactivating (side effects) roles of GR, future studies targeting GR signaling temporally and spatially by generating genetically manipulated mouse models would enable us to tease apart the yin and the yang of GR signaling in a more physiologically relevant fashion. Ultimately, future synthesis of new glucocorticoids/drugs developed by applying the knowledge obtained from in vitro and in vivo studies, provide hope of a new era where the adverse effects of glucocorticoids are infinitesimal compared to their benefits.
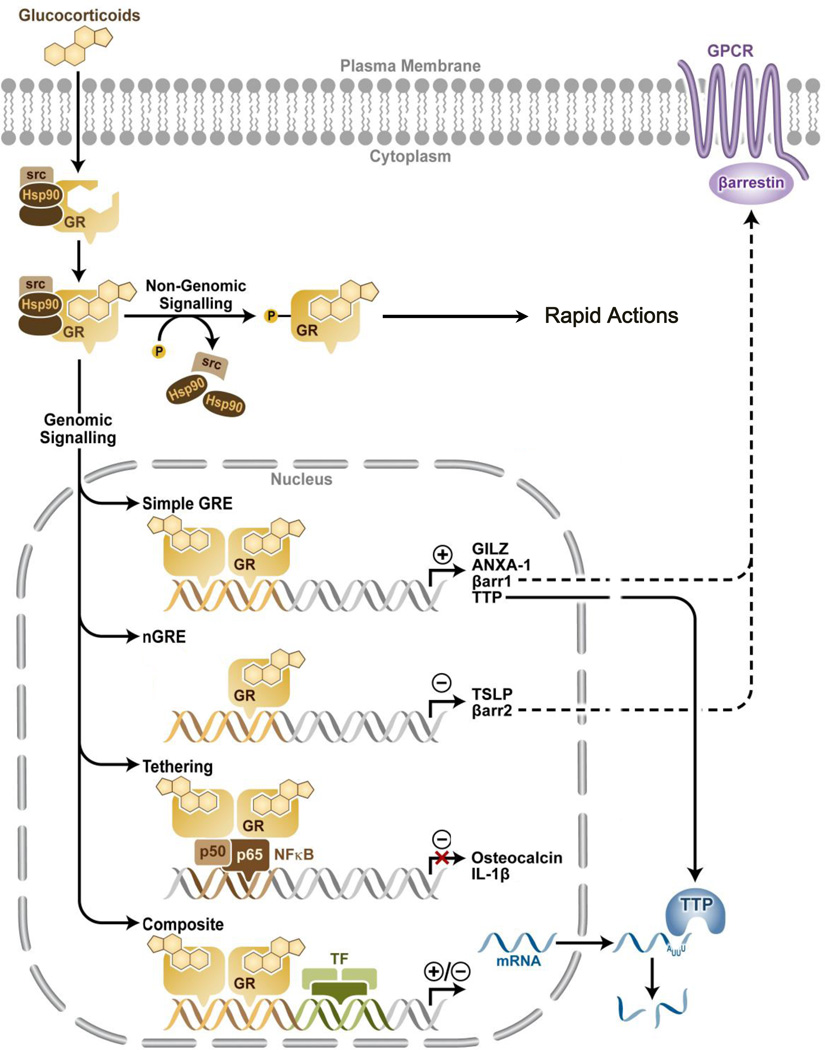
Figure 3.
Glucocorticoid Receptor Signaling. Upon binding glucocorticoids, cytoplasmic GR undergoes a confirmational change, becomes hyperphosphorylated (P), dissociates from accessory proteins, and translocates into the nucleus, where it can exert its actions through genomic mechanisms. Activated cytoplasmic GR is also known to exert its actions via non-genomic mechanisms. In the nucleus, GR enhances or represses transcription of target genes by direct binding to simple or negative GREs respectively, by tethering itself to other transcription factors, or in a composite manner by direct binding to GRE and interacting with other transcription factors. One of the mechanisms by which GR suppresses inflammation is by inducing TTP expression, which in turn binds to mRNA of pro-inflammatory gene expression and destabilizes them. GR modulates the gene expression of arrestins 1 & 2 to alter GPCR signaling.
Acknowledgements
We thank all the members of the Molecular Endocrinology group for critical reading of this manuscript. We would like to extend our apologies to those colleagues whose work we were unable to cite owing to space limitations. We would like to thank and acknowledge these individuals for all of their work that significantly contributed to our current understanding of GR action. This work was supported by the NIEHS Intramural Research Program of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biddie SC, et al. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology (Oxford) 2012;51:403–412. doi: 10.1093/rheumatology/ker215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134:54–67. doi: 10.1016/j.pharmthera.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–190. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttgereit F. A fresh look at glucocorticoids how to use an old ally more effectively. Bull NYU Hosp Jt Dis. 2012;70(Suppl 1):26–29. [PubMed] [Google Scholar]
- 5.Baschant U, et al. The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:645–655. doi: 10.1038/nrrheum.2012.166. [DOI] [PubMed] [Google Scholar]
- 6.Vilasco M, et al. Glucocorticoid receptor and breast cancer. Breast Cancer Res Treat. 2011;130:1–10. doi: 10.1007/s10549-011-1689-6. [DOI] [PubMed] [Google Scholar]
- 7.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anbalagan M, et al. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal. 2012;10:001. doi: 10.1621/nrs.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis-Tuffin LJ, et al. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto C, et al. Absence of glucocorticoid receptor-beta in mice. J Biol Chem. 1997;272:26665–26668. doi: 10.1074/jbc.272.42.26665. [DOI] [PubMed] [Google Scholar]
- 11.Schaaf MJ, et al. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology. 2008;149:1591–1599. doi: 10.1210/en.2007-1364. [DOI] [PubMed] [Google Scholar]
- 12.Beger C, et al. Expression and structural analysis of glucocorticoid receptor isoform gamma in human leukaemia cells using an isoform-specific real-time polymerase chain reaction approach. Br J Haematol. 2003;122:245–252. doi: 10.1046/j.1365-2141.2003.04426.x. [DOI] [PubMed] [Google Scholar]
- 13.Kino T, et al. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol Life Sci. 2009;66:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu I, et al. Selective glucocorticoid receptor translational isoforms reveal glucocorticoid-induced apoptotic transcriptomes. Cell Death Dis. 2013;4:453. doi: 10.1038/cddis.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang JC, et al. Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci U S A. 2004;101:15603–15608. doi: 10.1073/pnas.0407008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itani OA, et al. Glucocorticoids stimulate human sgk1 gene expression by activation of a GRE in its 5'-flanking region. Am J Physiol Endocrinol Metab. 2002;283:971–979. doi: 10.1152/ajpendo.00021.2002. [DOI] [PubMed] [Google Scholar]
- 17.Smoak K, Cidlowski JA. Glucocorticoids regulate tristetraprolin synthesis and posttranscriptionally regulate tumor necrosis factor alpha inflammatory signaling. Mol Cell Biol. 2006;26:9126–9135. doi: 10.1128/MCB.00679-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oakley RH, et al. Glucocorticoids regulate arrestin gene expression and redirect the signaling profile of G protein-coupled receptors. Proc Natl Acad Sci U S A. 2012;109:17591–17596. doi: 10.1073/pnas.1209411109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramamoorthy S, Cidlowski JA. Ligand-induced repression of the glucocorticoid receptor gene is mediated by an NCoR1 repression complex formed by long-range chromatin interactions with intragenic glucocorticoid response elements. Mol Cell Biol. 2013;33:1711–1722. doi: 10.1128/MCB.01151-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck IM, et al. Glucocorticoid receptor mutants: man-made tools for functional research. Trends Endocrinol Metab. 2011;22:295–310. doi: 10.1016/j.tem.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Meijsing SH, et al. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324:407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonard DM, O'Malley B W. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Surjit M, et al. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell. 2011;145:224–241. doi: 10.1016/j.cell.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Hudson WH, et al. The structural basis of direct glucocorticoid-mediated transrepression. Nat Struct Mol Biol. 2013;20:53–58. doi: 10.1038/nsmb.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayroldi E, et al. Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. Faseb J. 2012;26:4805–4820. doi: 10.1096/fj.12-216382. [DOI] [PubMed] [Google Scholar]
- 28.Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013 doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol. 2006;246:142–146. doi: 10.1016/j.mce.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Lee SR, et al. Non-genomic effect of glucocorticoids on cardiovascular system. Pflugers Arch. 2012;464:549–559. doi: 10.1007/s00424-012-1155-2. [DOI] [PubMed] [Google Scholar]
- 31.Bellavance MA, Rivest S. The neuroendocrine control of the innate immune system in health and brain diseases. Immunol Rev. 2012;248:36–55. doi: 10.1111/j.1600-065X.2012.01129.x. [DOI] [PubMed] [Google Scholar]
- 32.Galliher-Beckley AJ, et al. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011;31:4663–4675. doi: 10.1128/MCB.05866-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhavsar P, et al. Relative corticosteroid insensitivity of alveolar macrophages in severe asthma compared with non-severe asthma. Thorax. 2008;63:784–790. doi: 10.1136/thx.2007.090027. [DOI] [PubMed] [Google Scholar]
- 34.Mercado N, et al. Restoration of corticosteroid sensitivity by p38 mitogen activated protein kinase inhibition in peripheral blood mononuclear cells from severe asthma. PLoS One. 2012;7:41582. doi: 10.1371/journal.pone.0041582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnes PJ. Histone deacetylase-2 and airway disease. Ther Adv Respir Dis. 2009;3:235–243. doi: 10.1177/1753465809348648. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HN, et al. Endogenous glucocorticoids inhibit myocardial inflammation induced by lipopolysaccharide: involvement of regulation of histone deacetylation. J Cardiovasc Pharmacol. 2012;60:33–41. doi: 10.1097/FJC.0b013e3182567fef. [DOI] [PubMed] [Google Scholar]
- 37.Wallace AD, Cidlowski JA. Proteasome-mediated glucocorticoid receptor degradation restricts transcriptional signaling by glucocorticoids. J Biol Chem. 2001;276:42714–42721. doi: 10.1074/jbc.M106033200. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, DeFranco DB. Alternative effects of the ubiquitin-proteasome pathway on glucocorticoid receptor down-regulation and transactivation are mediated by CHIP, an E3 ligase. Mol Endocrinol. 2005;19:1474–1482. doi: 10.1210/me.2004-0383. [DOI] [PubMed] [Google Scholar]
- 39.Cole TJ, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 40.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 41.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Manwani N, et al. Reduced viability of mice with lung epithelial-specific knockout of glucocorticoid receptor. Am J Respir Cell Mol Biol. 2010;43:599–606. doi: 10.1165/rcmb.2009-0263OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambroggi F, et al. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- 45.Desrivieres S, et al. Glucocorticoid receptor (NR3C1) gene polymorphisms and onset of alcohol abuse in adolescents. Addict Biol. 2011;16:510–513. doi: 10.1111/j.1369-1600.2010.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller MB, Holsboer F. Mice with mutations in the HPA-system as models for symptoms of depression. Biol Psychiatry. 2006;59:1104–1115. doi: 10.1016/j.biopsych.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Chourbaji S, Gass P. Glucocorticoid receptor transgenic mice as models for depression. Brain Res Rev. 2008;57:554–560. doi: 10.1016/j.brainresrev.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Wei Q, et al. Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biol Psychiatry. 2012;71:224–231. doi: 10.1016/j.biopsych.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnett MG, et al. Behavioral insights from mouse models of forebrain--and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol. 2011;336:2–5. doi: 10.1016/j.mce.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kiernan DF, Mieler WF. The use of intraocular corticosteroids. Expert Opin Pharmacother. 2009;10:2511–2525. doi: 10.1517/14656560903160671. [DOI] [PubMed] [Google Scholar]
- 51.Edelman JL. Differentiating intraocular glucocorticoids. Ophthalmologica. 2010;224(Suppl 1):25–30. doi: 10.1159/000315158. [DOI] [PubMed] [Google Scholar]
- 52.de Smet MD. Corticosteroid intravitreal implants. Dev Ophthalmol. 2012;51:122–133. doi: 10.1159/000336330. [DOI] [PubMed] [Google Scholar]
- 53.Cubilla MA, et al. Mifepristone, a blocker of glucocorticoid receptors, promotes photoreceptor death. Invest Ophthalmol Vis Sci. 2013;54:313–322. doi: 10.1167/iovs.12-10014. [DOI] [PubMed] [Google Scholar]
- 54.Wenzel A, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001;42:1653–1659. [PubMed] [Google Scholar]
- 55.Pimenta E, et al. Adverse cardiovascular outcomes of corticosteroid excess. Endocrinology. 2012;153:5137–5142. doi: 10.1210/en.2012-1573. [DOI] [PubMed] [Google Scholar]
- 56.Fardet L, et al. Risk of cardiovascular events in people prescribed glucocorticoids with iatrogenic Cushing's syndrome: cohort study. Bmj. 2012;345:e4928. doi: 10.1136/bmj.e4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taves MD, et al. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–E24. doi: 10.1152/ajpendo.00100.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farman N, Rafestin-Oblin ME. Multiple aspects of mineralocorticoid selectivity. Am J Physiol Renal Physiol. 2001;280:F181–F192. doi: 10.1152/ajprenal.2001.280.2.F181. [DOI] [PubMed] [Google Scholar]
- 59.Nussinovitch U, et al. Glucocorticoids and the cardiovascular system: state of the art. Curr Pharm Des. 2010;16:3574–3585. doi: 10.2174/138161210793797870. [DOI] [PubMed] [Google Scholar]
- 60.Ren R, et al. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology. 2012;153:5346–5360. doi: 10.1210/en.2012-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zen M, et al. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev. 2011;10:305–310. doi: 10.1016/j.autrev.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 62.Cao Y, et al. Glucocorticoid receptor translational isoforms underlie maturational stage-specific glucocorticoid sensitivities of dendritic cells in mice and humans. Blood. 2013 doi: 10.1182/blood-2012-05-432336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Busillo JM, et al. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hermoso MA, et al. Glucocorticoids and tumor necrosis factor alpha cooperatively regulate toll-like receptor 2 gene expression. Mol Cell Biol. 2004;24:4743–4756. doi: 10.1128/MCB.24.11.4743-4756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J Steroid Biochem Mol Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 66.Saffar AS, et al. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr Drug Targets. 2011;12:556–562. doi: 10.2174/138945011794751555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 68.Kaur M, et al. T lymphocyte insensitivity to corticosteroids in chronic obstructive pulmonary disease. Respir Res. 2012;13:20. doi: 10.1186/1465-9921-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hakim A, et al. Corticosteroid resistance and novel anti-inflammatory therapies in chronic obstructive pulmonary disease: current evidence and future direction. Drugs. 2012;72:1299–1312. doi: 10.2165/11634350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Rose AJ, et al. Role of glucocorticoids and the glucocorticoid receptor in metabolism: insights from genetic manipulations. J Steroid Biochem Mol Biol. 2010;122:10–20. doi: 10.1016/j.jsbmb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Mueller KM, et al. Hepatic growth hormone and glucocorticoid receptor signaling in body growth, steatosis and metabolic liver cancer development. Mol Cell Endocrinol. 2012;361:1–11. doi: 10.1016/j.mce.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- 73.Silva EJ, et al. Innate immunity and glucocorticoids: potential regulatory mechanisms in epididymal biology. J Androl. 2011;32:614–624. doi: 10.2164/jandrol.111.013565. [DOI] [PubMed] [Google Scholar]
- 74.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD004454.pub2. CD004454. [DOI] [PubMed] [Google Scholar]
- 75.Khulan B, Drake AJ. Glucocorticoids as mediators of developmental programming effects. Best Pract Res Clin Endocrinol Metab. 2012;26:689–700. doi: 10.1016/j.beem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Saugstad OD, et al. Impact of antenatal glucocorticosteroids on whole-genome expression in preterm babies. Acta Paediatr. 2013 doi: 10.1111/apa.12166. [DOI] [PubMed] [Google Scholar]
- 77.Alexander N, et al. Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J Clin Endocrinol Metab. 2012;97:3538–3544. doi: 10.1210/jc.2012-1970. [DOI] [PubMed] [Google Scholar]
- 78.Claahsen-van der Grinten HL, et al. Congenital adrenal hyperplasia--pharmacologic interventions from the prenatal phase to adulthood. Pharmacol Ther. 2011;132:1–14. doi: 10.1016/j.pharmthera.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Reisch N, et al. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2009;94:1665–1670. doi: 10.1210/jc.2008-1414. [DOI] [PubMed] [Google Scholar]
- 80.Moradan S, Ghorbani R. Dexamethasone in unexplained infertility. Saudi Med J. 2009;30:1034–1036. [PubMed] [Google Scholar]
- 81.Hanna CW, et al. Genetic variation within the hypothalamus-pituitary-ovarian axis in women with recurrent miscarriage. Hum Reprod. 2010;25:2664–2671. doi: 10.1093/humrep/deq211. [DOI] [PubMed] [Google Scholar]
- 82.Whirledge S, et al. Glucocorticoids regulate gene expression and repress cellular proliferation in human uterine leiomyoma cells. Horm Cancer. 2012;3:79–92. doi: 10.1007/s12672-012-0103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henneicke H, et al. Corticosterone selectively targets endo-cortical surfaces by an osteoblast-dependent mechanism. Bone. 2011;49:733–742. doi: 10.1016/j.bone.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 84.Bultink IE, et al. Glucocorticoid-induced osteoporosis: an update on current pharmacotherapy and future directions. Expert Opin Pharmacother. 2013 doi: 10.1517/14656566.2013.761975. [DOI] [PubMed] [Google Scholar]
- 85.Jia D, et al. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weinstein RS, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramli ES, et al. Glycyrrhizic acid (GCA) as 11beta-hydroxysteroid dehydrogenase inhibitor exerts protective effect against glucocorticoid-induced osteoporosis. J Bone Miner Metab. 2012 doi: 10.1007/s00774-012-0413-x. [DOI] [PubMed] [Google Scholar]
- 88.Weinstein RS, et al. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanaoka BY, et al. Glucocorticoid effects on skeletal muscle: benefit and risk in patients with autoimmune inflammatory rheumatoid diseases. Expert Rev Clin Immunol. 2012;8:695–697. doi: 10.1586/eci.12.76. [DOI] [PubMed] [Google Scholar]
- 90.Menconi M, et al. Role of glucocorticoids in the molecular regulation of muscle wasting. Crit Care Med. 2007;35:S602–S608. doi: 10.1097/01.CCM.0000279194.11328.77. [DOI] [PubMed] [Google Scholar]
- 91.Hanaoka BY, et al. Implications of glucocorticoid therapy in idiopathic inflammatory myopathies. Nat Rev Rheumatol. 2012;8:448–457. doi: 10.1038/nrrheum.2012.85. [DOI] [PubMed] [Google Scholar]
- 92.Kuo T, et al. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci U S A. 2012;109:11160–11165. doi: 10.1073/pnas.1111334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Larsson L, et al. Acute quadriplegia and loss of muscle myosin in patients treated with nondepolarizing neuromuscular blocking agents and corticosteroids: mechanisms at the cellular and molecular levels. Crit Care Med. 2000;28:34–45. doi: 10.1097/00003246-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 94.Coenraads PJ. Hand eczema. N Engl J Med. 2012;367:1829–1837. doi: 10.1056/NEJMcp1104084. [DOI] [PubMed] [Google Scholar]
- 95.Goldminz AM, et al. NF-kappaB: An essential transcription factor in psoriasis. J Dermatol Sci. 2012 doi: 10.1016/j.jdermsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 96.Sevilla LM, et al. Epidermal Inactivation of the Glucocorticoid Receptor Triggers Skin Barrier Defects and Cutaneous Inflammation. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.281. [DOI] [PubMed] [Google Scholar]
- 97.Sanchis A, et al. Glucocorticoid receptor antagonizes EGFR function to regulate eyelid development. Int J Dev Biol. 2010;54:1473–1480. doi: 10.1387/ijdb.103071as. [DOI] [PubMed] [Google Scholar]
- 98.Sanchis A, et al. Keratinocyte-targeted overexpression of the glucocorticoid receptor delays cutaneous wound healing. PLoS One. 2012;7:e29701. doi: 10.1371/journal.pone.0029701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manenschijn L, et al. Clinical features associated with glucocorticoid receptor polymorphisms. An overview Ann N Y Acad Sci. 2009;1179:179–198. doi: 10.1111/j.1749-6632.2009.05013.x. [DOI] [PubMed] [Google Scholar]
- 100.Hauer D, et al. Relationship of a common polymorphism of the glucocorticoid receptor gene to traumatic memories and posttraumatic stress disorder in patients after intensive care therapy. Crit Care Med. 2011;39:643–650. doi: 10.1097/CCM.0b013e318206bae6. [DOI] [PubMed] [Google Scholar]
- 101.Ackermann S, et al. The BclI polymorphism of the glucocorticoid receptor gene is associated with emotional memory performance in healthy individuals. Psychoneuroendocrinology. 2013;38:1203–1207. doi: 10.1016/j.psyneuen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 102.Galecka E, et al. Single nucleotide polymorphisms of NR3C1 gene and recurrent depressive disorder in population of Poland. Mol Biol Rep. 2013;40:1693–1699. doi: 10.1007/s11033-012-2220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ross IL, et al. Investigation of glucocorticoid receptor polymorphisms in relation to metabolic parameters in Addison's disease. Eur J Endocrinol. 2013;168:403–412. doi: 10.1530/EJE-12-0808. [DOI] [PubMed] [Google Scholar]
- 104.van Rossum EF, et al. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab. 2004;89:4004–4009. doi: 10.1210/jc.2003-031422. [DOI] [PubMed] [Google Scholar]
- 105.Derijk RH, et al. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001;28:2383–2388. [PubMed] [Google Scholar]
- 106.van den Akker EL, et al. Glucocorticoid receptor gene and risk of cardiovascular disease. Arch Intern Med. 2008;168:33–39. doi: 10.1001/archinternmed.2007.41. [DOI] [PubMed] [Google Scholar]
- 107.Schacke H, et al. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 108.Stamer WD, et al. Unique response profile of trabecular meshwork cells to the novel selective glucocorticoid receptor agonist, GW870086X. Invest Ophthalmol Vis Sci. 2013;54:2100–2107. doi: 10.1167/iovs.12-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Balsalobre A, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 110.Charmandari E, et al. Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS One. 2011;6:e25612. doi: 10.1371/journal.pone.0025612. [DOI] [PMC free article] [PubMed] [Google Scholar]