SUMMARY
UHRF1 is an essential regulator of DNA methylation that is highly expressed in many cancers. Here, we use transgenic zebrafish, cultured cells and human tumors to demonstrate that UHRF1 is an oncogene. UHRF1 overexpression in zebrafish hepatocytes destabilizes and delocalizes DNMT1, causes DNA hypomethylation and Tp53-mediated senescence. Hepatocellular carcinoma (HCC) emerges when senescence is bypassed. tp53 mutation both alleviates senescence and accelerates tumor onset. Human HCCs recapitulate this paradigm, as UHRF1 overexpression defines a subclass of aggressive HCCs characterized by genomic instability, TP53 mutation and abrogation of the TP53-mediated senescence program. We propose that UHRF1 overexpression is a mechanism underlying DNA hypomethylation in cancer cells and that senescence is a primary means of restricting tumorigenesis due to epigenetic disruption.
INTRODUCTION
The expression of genes that encode readers and writers of the epigenetic code are widely deregulated across cancer types (You and Jones, 2012). This contributes to the massive gene expression changes and remodeling of the epigenetic landscape, a characteristic of many types of cancer. In particular, loss of global DNA methylation is a hallmark of cancer cells.
DNA hypomethylation contributes to oncogenesis through multiple mechanisms, including chromosomal instability (Eden et al., 2003; Karpf and Matsui, 2005), derepression of imprinted genes (Berdasco and Esteller, 2010; Jirtle, 2004; Li et al., 1993), retrotransposon activation (Gaudet et al., 2004; Howard et al., 2008; Jackson-Grusby et al., 2001; Sharif et al., 2007) and aberrant gene expression, including induction of oncogenes (Cheah et al., 1984). Many studies have documented that expression of the core factors required for maintenance DNA methylation – i.e. DNA methyltransferase 1 (DNMT1) and ubiquitin-like with PHD and RING finger domains 1 (UHRF1) (Babbio et al., 2012; Jin et al., 2009; Unoki et al., 2010; Wang et al., 2012) – are significantly altered across cancer types. However, whether changes in the expression of these key factors are sufficient to alter the cancer cell methylome and drive carcinogenesis is unknown. Moreover, the mechanism by which DNA methylation is lost in cancer cells is poorly understood.
The cellular response to DNA hypomethylation varies by cell type, physiological context and degree of hypomethylation. In some cells, DNA hypomethylation induces tumor suppressive mechanisms, including apoptosis (Anderson et al., 2009; Biniszkiewicz et al., 2002; Chen et al., 2007; Jackson-Grusby et al., 2001) or senescence (Decottignies and d’Adda di Fagagna, 2011; Fairweather et al., 1987), whereas in other cells it blocks differentiation of progenitor cells (Rai et al., 2010) or causes cancer (Gaudet et al., 2003; Yamada et al., 2005). In part, the cellular response to loss of DNA methylation is dictated by the genomic region affected: hypomethylation of gene regulatory regions, such as promoters, can de-repress gene expression but hypomethylation of repetitive elements can reduce heterochromatin formation, promote recombination and genomic instability.
UHRF1 plays an essential role in DNA methylation by both recognizing hemimethylated DNA generated during DNA replication and then recruiting DNMT1 to ensure faithful maintenance of DNA methylation patterns in daughter cells (Arita et al., 2008; Avvakumov et al., 2008; Bostick et al., 2007; Hashimoto et al., 2008; Liu et al., 2013; Nishiyama et al., 2013; Sharif et al., 2007). Consequently, UHRF1 depletion results in global DNA hypomethylation (Bostick et al., 2007; Feng et al., 2010; Sharif et al., 2007; Tittle et al., 2011). Conversely, UHRF1 may also limit DNA methylation by targeting DNMT1 for ubiquitin-mediated degradation (Du et al., 2010; Qin et al., 2011) or by delocalizing DNMT1 (Sharif et al., 2007). How UHRF1 overexpression impacts the methylome is not known.
We previously reported that uhrf1 mutation in zebrafish blocks liver outgrowth in embryos and regeneration in adults (Sadler et al., 2007) and that depleting UHRF1 from cancer cells induces apoptosis (Tien et al., 2011). Here, we tested the hypothesis that UHRF1 overexpression would alter global DNA methylation, and promote hepatocellular carcinoma (HCC).
RESULTS
High UHRF1 expression causes DNA hypomethylation
UHRF1 is required for DNA methylation, as it recruites DNMT1 to hemimethylated DNA during DNA replication (Bostick et al., 2007; Feng et al., 2010; Liu et al., 2013; Nishiyama et al., 2013; Sharif et al., 2007). Paradoxically, UHRF1 also serves as an ubiquitin ligase that targets DNMT1 for degradation (Du et al., 2010; Qin et al., 2011). To determine how UHRF1 overexpression impacts DNA methylation, we generated transgenic zebrafish expressing human UHRF1 fused to GFP (hsa.UHRF-GFP) under the hepatocyte specific fabp10 promoter (Tg(fabp10:hsa.UHRF1-GFP) (Chu et al., 2012)).
Human and zebrafish UHRF1 are 66% identical and the ability of human UHRF1 to rescue zebrafish embryos depleted of uhrf1 (VJ and KCS, unpublished and (Chu et al., 2012)) indicates they are functional orthologs. Expression is first detected in hepatocytes on 3 dpf and 5 dpf, nuclear UHRF1-GFP is easily detected at variable levels in hepatocytes (Figure S1A and (Chu et al., 2012)). We isolated an allelic series of Tg(fabp10:hsa.UHRF1-GFP) transgenics expressing a range of UHRF1 levels (Figure S1A–B; hereafter referred to UHRF1-GFP High, Medium and Low).
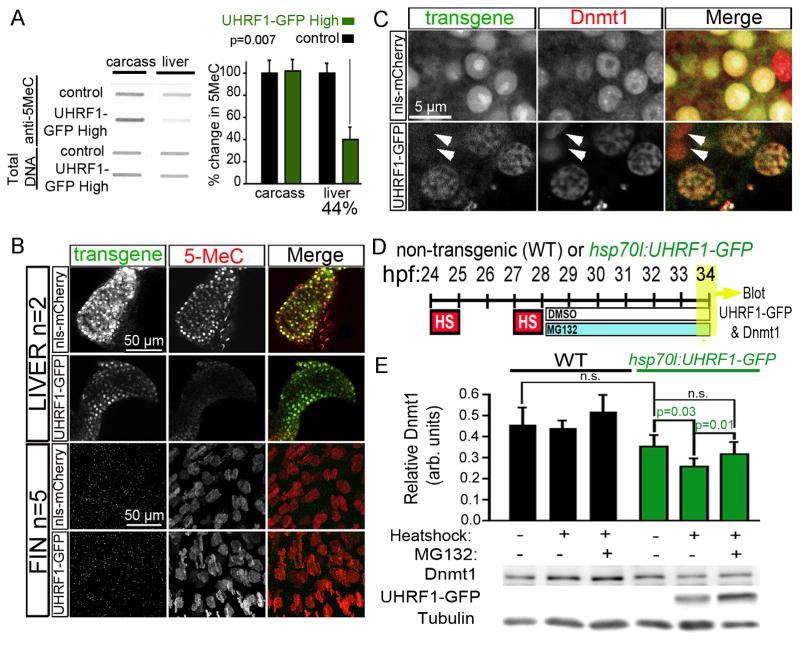
We probed genomic DNA isolated from the liver of 5 dpf larvae from each line with an antibody specific for 5-methyl cytosine (5MeC) to assess DNA methylation and found significant hypomethylation only in the liver of UHRF1-GFP High larvae (38% of controls; p=0.007; Figure 1A and Figure S1C). 5MeC immunofluorescence showed DNA methylation as uniformly distributed in the nuclei of hepatocytes from control larvae that express nuclear-localized fluorescent mCherry under the fabp10 promoter (Tg(fabp10:nls-mCherry); abbreviated to nls-mCherry) but only dim staining in hepatocytes of UHRF1-GFP High larvae (Figure 1B). Since Equivalent levels of 5MeC were detected in the liverless carcasses (Figure 1A) or fins (Figure 1B), we conclude that DNA hypomethylation was specific to the liver.
Figure 1. High UHRF1 expression causes global DNA hypomethylation.
(A) 5MeC levels and total DNA stained with methylene blue were measured in 5 dpf control and UHRF1-GFP High livers (n=4) and liver-less carcasses (n=3). The ratio of 5MeC to total DNA was averaged and normalized to controls. Student’s T-test was used to determine p values. (B) Confocal stacks of livers (top) and fins (bottom) from 5 dpf nls-mCherry and UHRF1-GFP High larvae stained with anti-5MeC. Since a hepatocyte specific promoter was used for transgenesis, there was no transgene expression in the fin. (C) Dnmt1 is uniform in the hepatocyte nucleus of 4 dpf nls-mCherry larvae but is found in GFP-containing punctae in UHRF1-GFP High hepatocytes. Arrows point to cells that do not express GFP and have Dnmt1 distribution pattern similar to controls. (D) Tg(hsp70I:UHRF1-EGFP) and non-transgenic controls were heat shocked at 37° C for 1 hour at 24 and 27 hpf, treated with 10 μM MG132 or DMSO at 28 hpf and collected at 34 hpf for immunoblotting. (E) Dnmt1 levels normalized to tubulin were averaged from 6 experiments. Student’s T-test was used to determine p values; n.s.: not significant; error bars represent SD. See also Figure S1.
We hypothesized that UHRF1-induced DNA hypomethylation could be caused by mislocalization or destabilization of Dnmt1. Immunofluorescence revealed uniform Dnmt1 distribution in the nucleoplasm of nls-mCherry hepatocytes (Figure 1C) but in UHRF1-GFP high hepatocytes, it was concentrated in nuclear foci that contained UHRF1-GFP (Figure 1C). Interestingly, the range of UHRF1-GFP expression within cells of the same liver revealed that in cells with high GFP, Dnmt1 was dim and punctate, but levels and distribution pattern was similar to control hepatocytes in those cells with low or no GFP (arrows, Figure 1C). Thus, high expression of UHRF1 co-localizes with Dnmt1 and redistributes it to intra-nuclear structures reminiscent of senescence associated heterochromatin foci (Di Micco et al., 2011).
Endogenous Dnmt1 in the liver was below the levels detectable by immunoblotting (not shown), so we used a transgenic line expressing UHRF1-GFP under the inducible hsp70l promoter (Chu et al., 2012) to quantitatively assess the impact of UHRF1 overexpression on Dnmt1 stability at a developmental time when we could easily detect Dnmt1 levels by immunoblotting. We optimize a heat shock protocol that maximized UHRF1-GFP expression in Tg(hsp70l:UHRF1-GFP) larvae (Figure 1D) and found that UHRF1-GFP overexpression reduced Dnmt1 levels by 27% compared to non-heat-shocked transgenics (p=0.03; Figure 1E). Treatment with a non-toxic dose of the proteasome inhibitor, MG132, prevented the decrease in Dnmt1 induced by UHRF1 overexpression (p=0.01; Figure 1E). Neither heat-shock nor MG132 significantly affected Dnmt1 protein levels in non-transgenic controls (Figure 1E). Thus, both Dnmt1 delocalization and destabilization could account for DNA hypomethylation caused by UHRF1 overexpression.
DNA hypomethylation caused by UHRF1 overexpression reduces liver size
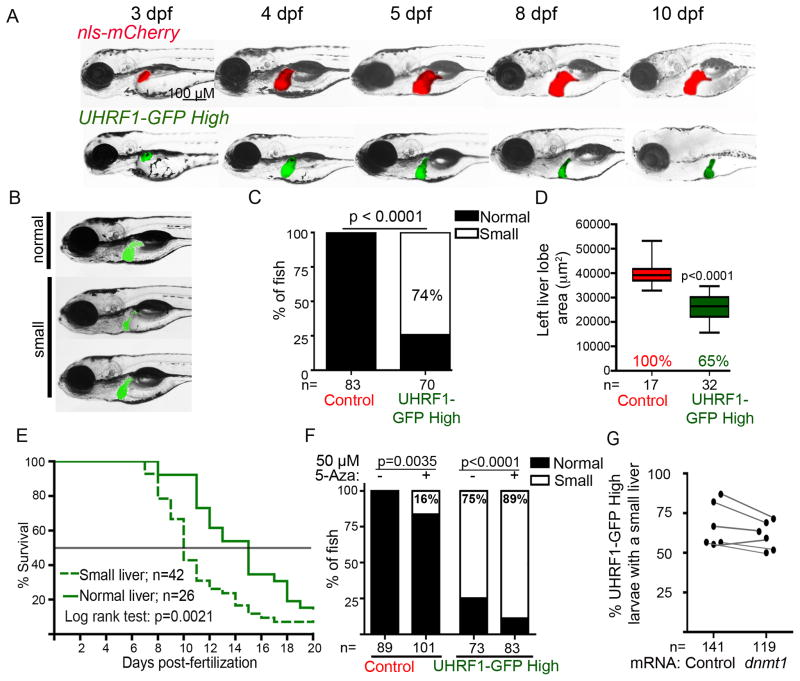
Uhrf1 in zebrafish is required for hepatic outgrowth and liver regeneration (Sadler et al., 2007). To determine if UHRF1 overexpression in hepatocytes affected hepatic outgrowth, we assessed liver size all transgenic lines (Figures 2A–C and S2A). Low and Medium expressing lines had no gross changes in liver size (Figure S2A), but 74% of UHRF1-GFP High larvae on 5 dpf had small livers (Figure 2C), with the median area of the left liver lobe reduced by 35% compared to controls (p<0.001; Figures 2D, S2A), without reduction of total fish size (Figure S2B).
Figure 2. UHRF1-induced hypomethylation reduces liver size.
(A) Individual larvae were imaged daily from 3–10 dpf. (B) 5 dpf UHRF1-GFP High larvae display a range of liver sizes scored as “normal” or “small”‘ (C) 3 clutches were scored according to criteria in B; n= number of larvae. Fisher’s exact test was used to determine p value. (D) The area of the left liver lobe was measured in 5 dpf fish from 2 clutches. Boxes represent 75th and 25th percentile, horizontal line is the median and whiskers mark lowest and highest values. Student’s T-test was used to determine p value. (E) UHRF1-GFP High larvae were sorted by liver size on 5 dpf and tracked daily for survival to 20 dpf. Data are pooled from three clutches. (F) UHRF1-GFP High and control larvae were treated with 50 μM 5-Aza from 2.5–5 dpf and scored for liver size in 6 clutches. Fisher’s exact test was used to determine p values. (G) UHRF1-GFP High embryos were injected with mRNA encoding dnmt1 or Mpi before 1 hpf. The percent of fish with a normal liver size was scored at 5 dpf in 6 clutches. See also Figure S2.
Larvae with small livers appeared sick (see 10 dpf larvae in Figure 2A), and only 20% of UHRF1-GFP High fish survived to 20 dpf (Figure S2C and Figure 2E). Moreover, UHRF1-GFP High larvae with the “microliver” phenotype on 5 dpf had significantly higher mortality by 10 dpf (67%) than those that started with a normal sized liver (7%; Figure 2E). Thus, high UHRF1 expression in hepatocytes causes DNA hypomethylation, microliver and larval death.
We next investigated the relationship between DNA hypomethylation and the microliver phenotype by asking whether further reducing DNA methylation could enhance this phenotype or restoring Dnmt1 could suppress it. We previously reported that exposing embryos to 50 μM of the Dnmt1 inhibitor, 5-azacytidine (5-Aza) from 0–5 dpf caused a profoundly small liver and DNA hypomethylation (Mudbhary and Sadler, 2011). By restricting the 5-Aza exposure time to 2.5–5 dpf, we reduced DNA methylation in the liver of control larvae by 40% (Figure S1C) which induced a moderately small liver in 16% of larvae (p=0.0035; Figure 2F). This same treatment of UHRF1-GFP High larvae significantly increased the percent with small livers (p<0.0001; Figure 2F). While this could be attributed to DNA damage caused by 5-Aza, our finding that injecting UHRF1-GFP High embryos with mRNA encoding Dnmt1 modestly suppressed the percent of fish with a small liver (Figure 2G) and increased the average size of the left liver lobe by 10–15% (Figure S2D) suggests that hypomethylation, at least in part, contributes to the small liver phenotype of UHRF1-GFP High larvae.
UHRF1 overexpression triggers Tp53-mediated senescence
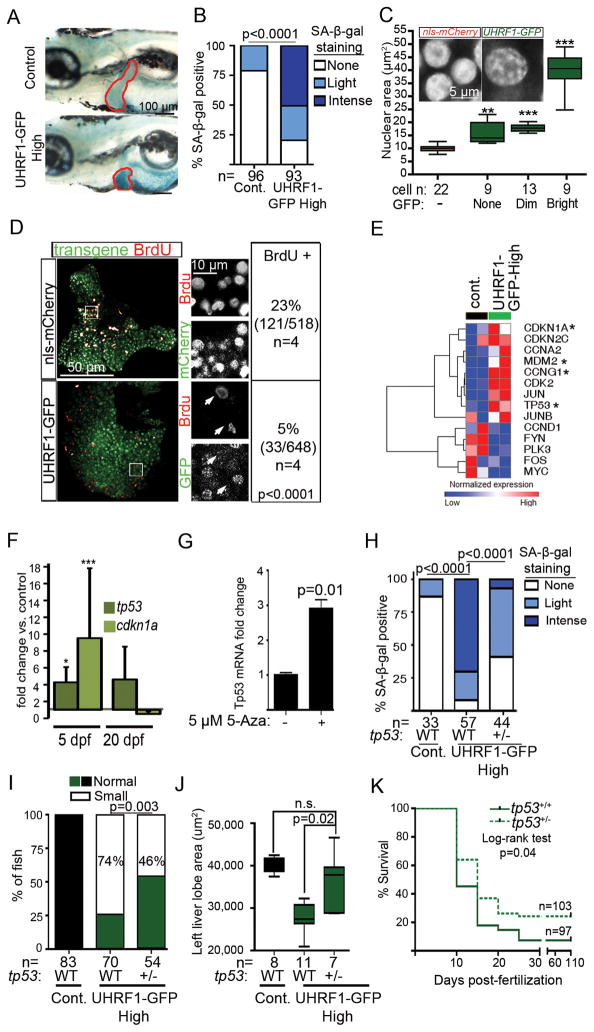
DNA hypomethylation can induce apoptosis, but we found no TUNEL positive cells on 5 dpf of UHRF1-GFP High livers (Figure S3A). However, senescence associated β-galactosidase (SA-β-gal) staining was detected throughout the liver of most UHRF1-GFP High 5 dpf larvae (n=93; Figure 3A–B), but not other transgenic lines (Figure S3B). Additionally, the DNA in control hepatocytes was evenly distributed throughout the uniformly sized nuclei compared to the large nuclei where DNA resembled senescence associated heterochromatic foci (inset, Figure 3C). Strikingly, the largest hepatocyte nuclei also had the brightest GFP (Figure 3C), suggesting the effect of UHRF1 overexpression cell autonomously affected nuclear morphology.
Figure 3. UHRF1 overexpression in hepatocytes induces Tp53-mediated senescence.
(A) Intense senescence associated β-galactosidase (SA-β-gal) staining was detected in the liver (outlined) of 5 dpf UHRF1-GFP High larvae compared to light or no staining in controls. (B) 5 dpf fish from 5 clutches were scored for hepatic SA-β-gal staining. *** indicates p<0.0001 by Fisher’s exact test. (C) Nuclear size was measured in hepatocytes of a single control or UHRF1-GFP High 5 dpf liver and cells were stratified according to GFP expression. Inset shows confocal stack of the DNA organized into foci. ** and *** indicates p<0.001 or 0.0001, respectively compared to nuclear size in nls-mCherry larvae. (D) BrdU positive cells and the total number of transgene-expressing hepatocytes in nls-mCherry and UHRF1-GFP High larvae (Bottom) 5 dpf larvae. A Fisher’s exact test was used to calculate p value. In nls-mCherry larvae, most BrdU positive cells also express the transgene whereas the BrdU positive cells in UHRF1-GFP High livers did not express GFP (white arrows in magnified regions which are marked by the white box). (E) Heatmap of log2 values from RNAseq shows cell cycle regulators are down and Tp53 target genes (marked by *) are up in UHRF1-GFP High 5 dpf livers. (F) tp53 and cdkn1a mRNA expression were induced on 5 dpf and down regulated on 20 dpf in UHRF1-GFP High livers. * indicates p=0.05; *** indicates p=0.001 calculated by 1 sample Student’s T-test. Error bars represent SD. (G) 5-Aza induces Tp53 expression in primary mouse hepatocytes. Student’s T-test was used to determine p value with SD indicated by the error bars across 3 replicates. tp53+/− in UHRF1-GFP High larvae significantly reduced SA-β-gal staining in the liver (2 clutches) (H), and increased the percent of larvae with normal liver size (I), the area of the left liver lobe (J), and survival at 5 dpf (K). p values were calculated with a Fisher’s test with Freeman-Halton extension (H), Fisher’s exact test (I), and Student’s T-test. Boxes represent 75th and 25th percentile, horizontal line is the median and whiskers mark lowest and highest values. (J). See also Figure S3.
Senescent cells do not divide, and we found significantly less BrdU incorporation in the liver of 5 dpf UHRF1-GFP High larvae compared to controls (p<0.0001; Figure 3D). Interestingly, in nls-mCherry larvae, most BrdU incorporation was detected in hepatocytes that express nls-mCherry, but the only BrdU positive cells in UHRF1-GFP high larvae were negative for GFP (see inset in Figure 3D). RNAseq revealed some pro-proliferative genes (ccnd1 and myc) downregulated (Figure 3E), lending further support to the conclusion that senescence is the primary response to high UHRF overexpression in hepatocytes during hepatic outgrowth.
Tp53 is a key mediator of senescence caused by DNA damage and oncogenic stress (Di Micco et al., 2011; McDuff and Turner, 2011; Ventura et al., 2007; Xue et al., 2007). RNAseq (Figure 3E) and qPCR analysis (Figure 3F) show that tp53 and its target genes, especially cdkn1a, are significantly induced in the liver of 5 dpf UHRF1-GFP High larvae, but then return to baseline by 20 dpf (Figure 3F). 5-Aza treatment of primary mouse hepatocytes induced Tp53 expression (Figure 3G), similar to the effects of 5-Aza treatment or Dnmt1 depletion in other cell types (Jackson-Grusby et al., 2001; Karpf et al., 2001) and in zebrafish (VJ and KCS, not shown). However, since no significant alteration in methylation of the tp53 promoter was detected in these models (not shown), we hypothesize that DNA methylation does not directly regulate Tp53 expression. Instead, DNA hypomethylation may induce tp53 by an indirect mechanism, such as increased DNA damage or genomic instability.
A direct role for Tp53 in the phenotype of UHRF1-GFP High larvae was demonstrated by removing one copy of tp53. This reduced the incidence and intensity of SA-β-gal staining in the liver (Figure 3H), increased liver size (Figure 3I–J) and reduced mortality (Figure 3K). We thus propose a model whereby high UHRF1 causes DNA hypomethylation, induces Tp53-mediated senescence and prevents expansion of the hepatic bud, resulting in hepatic insufficiency and larval death.
UHRF1 overexpression induces liver cancer in zebrafish
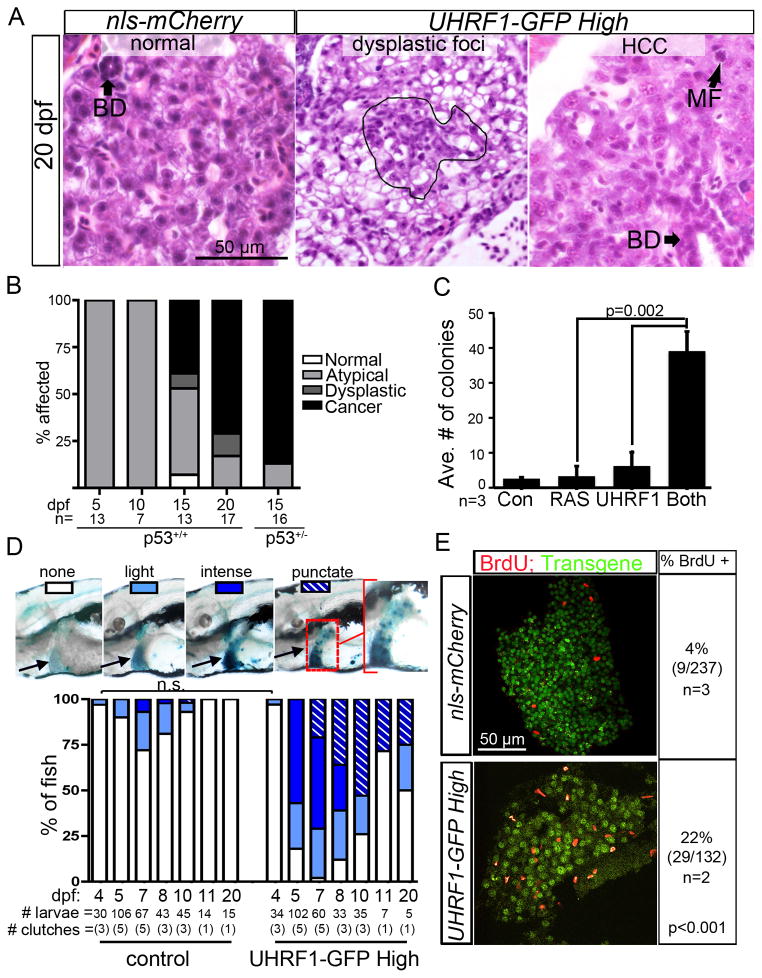
To determine if UHRF1 overexpression was sufficient to cause HCC, 281 control and UHRF1-GFP transgenics were collected between 5–300 dpf, serial sectioned and analyzed for atypical cells, dysplastic foci and HCC using histological criteria devised by two expert pathologists (RTB and MIF) based on disrupted tissue architecture, cell size, shape, nuclear structure and presence of mitotic figures (Figure S4A–B). Evidence of increased hepatocyte proliferation was detected in all UHRF1 overexpressing lines on 20 and 40 dpf (Figure S4C), but this was insufficient to cause HCC, as UHRF1-GFP Low fish were tumor free at all time points (Table 1). In contrast, UHRF1-GFP High fish developed atypical hepatocytes as early as 5 dpf, with an 8% incidence of dysplastic foci and 46% incidence of HCC by 15 dpf. On 20 dpf, 76% of fish had HCC (Figure 4A–B, Table 1). UHRF1-GFP Medium fish also developed atypical hepatocytes and dysplastic foci at young ages, and one large HCC was detected in a 60 dpf fish (Table 1). In a classical transformation assay using NIH-3T3, we found that UHRF1 cooperated with RAS to promote growth on soft agar (Figure 4C). These conclusively demonstrate that UHRF1 is an oncogene.
Table 1.
HCC onset and incidence in Tg(fabp10:nls-mCherry) and Tg(fabp10:UHRF1-GFP) zebrafish.
| transgene | dpf: | 5 | 10 | 15 | 20 | 25 | 30 | 40 | 50 | 60 | 90 | 180 | 300 | Total N = 281 | CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nls-mCherry | Normal | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 65 | 100% | ||
| Atypical cells | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0 | 0% | |||
| Dysplastic foci | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0 | 0% | |||
| Tumor | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0 | 0% | |||
|
| |||||||||||||||
| n = | 8 | 8 | 8 | 11 | nd | nd | 5 | 5 | 5 | 5 | 5 | 5 | 65 | ||
|
| |||||||||||||||
| UHRF1-GFP Low | Normal | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 60 | 100% | ||||
| Atypical cells | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0 | 0% | |||||
| Dysplastic foci | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0 | 0% | |||||
| Tumor | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0 | 0% | |||||
|
| |||||||||||||||
| n = | 7 | 9 | 10 | 12 | nd | nd | 4 | nd | nd | 6 | 6 | 6 | 60 | ||
|
| |||||||||||||||
| UHRF1-GFP Medium | Normal | 90% (9) | 100% (11) | 50% (3) | 28% (4) | 66% (4) | 80% (4) | 50% (4) | 50% (5) | 81% (13) | 57 | 66% | |||
| Atypical cells | 10% (1) | 0% | 50% (3) | 57% (8) | 33% (2) | 20% (1) | 50% (4) | 20% (2) | 44% (7) | 28 | 32% | ||||
| Dysplastic foci | 0% | 0% | 0% | 7% (1) | 0% | 0% | 25% (2) | 30% (3) | 0% | 6 | 7% | ||||
| Tumor | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 6% (1) | 1 | 1% | ||||
|
| |||||||||||||||
| n = | 10 | 11 | 6 | 14 | 6 | 5 | 8 | 10 | 16 | 86 | |||||
|
| |||||||||||||||
| UHRF1-GFP High | Normal | 0% | 0% | 7% (1) | 0% | 0% | 1 | 2% | |||||||
| Atypical cells | 100% (13) | 100% (7) | 46% (6) | 17% (3) | 75% (3) | 36 | 61% | ||||||||
| Dysplastic foci | 0% | 0% | 8% (1) | 12% (2) | 25% (1) | 4 | 7% | ||||||||
| Tumor | 0% | 0% | 46% (6) | 76% (13) | 0% | 18 | 30% | ||||||||
|
| |||||||||||||||
| n = | 13 | 7 | 13 | 17 | 4 | 54 | |||||||||
|
| |||||||||||||||
| UHRF1-GFP High/ p53+/− | Normal | 0% | 0 | 0% | |||||||||||
| Atypical cells | 13% (2) | 2 | 13% | ||||||||||||
| Dysplastic foci | 0% | 0 | 0% | ||||||||||||
| Tumor | 88% (14) | 14 | 88% | ||||||||||||
|
| |||||||||||||||
| n = | 16 | 16 | |||||||||||||
The incidence (percent) and absolute number of fish (parenthesis) with cancer-relevant histological phenotypes were scored in a total of 281 fish from 4 transgenic lines. The total n for each time point is indicated. nd: not done. Some fish were diagnosed with more than one lesion. CI: Cumulative incidence.
Figure 4. UHRF1 is an oncogene.
(A) Atypical cells, dysplastic foci (outlined) and HCC are apparent in H&E stained UHRF1-GFP High livers. BD: bile duct, MF: mitotic figure. (B) Incidence of normal and atypical hepatocytes, dysplastic foci and cancer in the liver of UHRF1-GFP High fish on WT or tp53+/− background. (C) NIH-3T3 cell growth in soft agar is enhanced when UHRF1 overexpression is combined with RAS (n=3). p value was calculated by Student’s T-test and error bars represent the SD. (D) Hepatic SA-β-gal staining patterns in UHRF1-GFP High larvae changes as fish age. Images of 8 dpf larvae illustrate the SA-β-gal staining patterns that were scored in the time course shown in the graph. A significant increase in the number of UHRF1-GFP High fish with intense or punctate SA-β-gal compared to controls at all time points (p<0.01 by Fisher’s exact test) except at 4 dpf; not significant=n.s. (E) BrdU incorporation in the liver on 11 dpf is 5 times higher in UHRF1-GFP High fish than in controls. Total # of cells counted is indicated with n= # of clutches assessed. Fisher’s exact test was used to calculate p value. See also Figure S4.
We found that UHRF1-GFP High larvae older than 8 dpf had a lower incidence and intensity of hepatic SA-β-gal staining (Figure 4D). Interestingly, in many of these fish, intense staining was distributed in a punctate pattern. By 20 dpf, 75% of fish had either punctate or no staining (Figure 4D), which is a striking correlation with the 70% incidence of HCC at this time point. This was mirrored by increased proliferation of liver cells, detected by increased BrdU incorporation in UHRF1-GFP High livers (22% vs. 4% in controls) at 11 dpf (p<0.001; Figure 4E), and higher PCNA staining 20 and 40 dpf in all lines (Figure S4C). Loss of senescence was not attributed to reestablishment of DNA methylation or transgene silencing, as reduced 5MeC staining persisted in tumor cells (Figure S4D) and transgene expression was detectable in all 20 dpf fish (Figures S4E–F), albeit reduced from levels detected in 5 dpf livers.
We asked whether Tp53 epistatically interacted with UHRF1 overexpression to contribute to HCC by removing one copy of tp53 (Berghmans et al., 2005). The liver appeared normal in 13 tp53+/+ fish without transgene expression (not shown), but in UHRF1-GFP High fish, tumor incidence on 15 dpf increased from 50% in WT to 87% in tp53+/− fish (Figure 4B). Interestingly, in a single Tg(fabp10:UHRF1-GFPHIGH);p53+/−, we found a tumor with immature cells resembling a cholangiocarcinoma. Thus, tp53 functions to suppress tumor formation and may alter the spectrum of tumors caused by UHRF1 overexpression.
UHRF1 is upregulated in human HCC
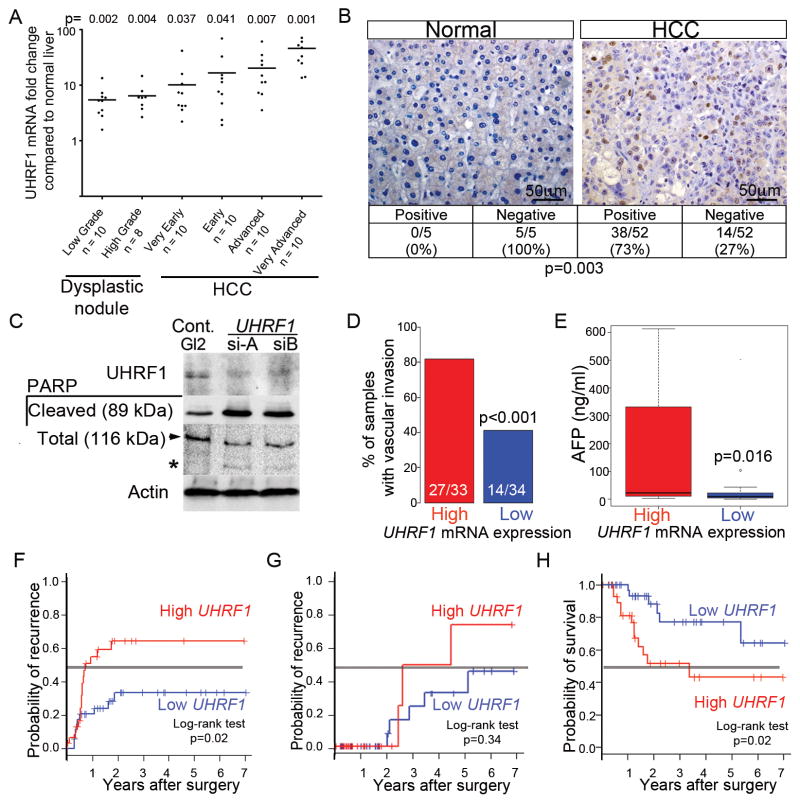
We next investigated the relevance of UHRF1 expression in human HCC. We assessed UHRF1 expression by qPCR in 16 normal liver samples and in 2 cohorts of patients with dysplastic nodules or HCC: the 1st cohort of 58 patients had hepatitis C infection (Figure 5A; (Wurmbach et al., 2007)) and the 2nd cohort of 69 patients had HBV, alcohol and other etiologies (Figure S5A; (Villanueva et al., 2008)). Additional publically available transcriptome data sets from three other cohorts of HCCs (Figure S5B) and from lung, gastric, colorectal and breast cancer (Figure S5C) were also analyzed. All showed elevated UHRF1 in tumors, with expression elevated >2-fold compared to controls in 17/18 dysplastic foci and 104/109 HCCs (Figures 5A and S5A). An average of 20- and 46-fold overexpression of UHRF1 was detected in advanced and very advanced HCCs. UHRF1 protein was barely detectable in 5 normal liver samples but highly expressed in 38/52 (73%) of the tumors analyzed for UHRF1 mRNA in Figure 5A (p<0.003; Figure 5B). Thus, UHRF1 is overexpressed in HCCs of diverse etiologies as well as in other tumor types, and high UHRF1 expression is significantly associated with the most advanced tumors.
Figure 5. UHRF1 mRNA and protein are overexpressed in HCC.
(A) UHRF1 detected by qRT-PCR in 18 pre-neoplastic lesions and 40 HCCs from HCV infected patients compared to expression in 9 normal livers. Horizontal line indicates median. (B) Immunohistochemistry for UHRF1 protein (brown) was evaluated in 52 of the same HCCs examined in A plus 5 normal liver samples. Fisher’s exact test was used to calculate p value. 71 of the HCV-associated HCCs analyzed by qPCR were grouped into high (n=35) and low (n=36) UHRF1 expressing tumors based on the median log2-fold change of 3.64. (C) HepG2 cells transfected with control siRNA (GL2) or two different siRNAs targeting UHRF1 described in (Tien et al., 2011) were blotted for UHRF1, cleaved and total PARP (arrow indicates full length, * indicates cleaved protein). (D) Vascular invasion (33 high and 34 low tumors, 4 missing values), (E) serum AFP (29 UHRF1-high and 29 low tumors, 13 missing values), (F) early (<2 years) and (G) late (>2 years; 32 UHRF1-high and 35 low tumors, 4 missing values) tumor recurrence and (H) overall survival after surgery (32 high and 35 low tumors, 4 missing values) were stratified according to UHRF1-expression. Continuous and categorical variables were assessed by Wilcoxon rank-sum test and Fisher’s exact test, respectively. Clinical outcome difference was evaluated by log-rank test. In box and whisker plots, boxes represent the 75th and 25th percentiles, the whiskers represent the most extreme data points within interquartile range x 1.5, and the horizontal bar represents the median. See also Figure S5.
Targeting UHRF1 in liver cancer cells (HepG2) using siRNA induced PARP cleavage (Figure 5C) and other markers of apoptosis (not shown), similar to results obtained using another HCC cell line (Hep3B; not shown) and colon cancer cells (Tien et al., 2011). Thus, blocking UHRF1 cancers such as HCC with high UHRF1 expression could be an effective means to induce tumor cell death.
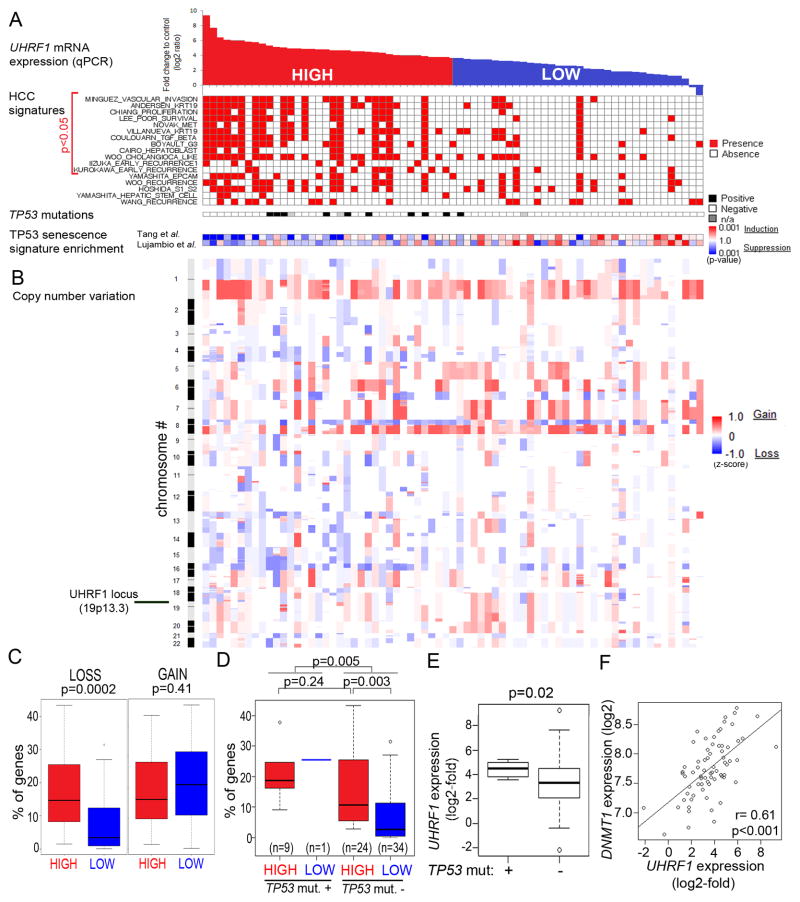
Of the 109 HCCs analyzed for UHRF1 expression by qPCR, 71 had multiple clinical and genomics parameters available (Chiang et al., 2008). These were rank-ordered based on UHRF1 expression determined by qPCR (Figure 6A). Tumors with expression above and equal to or below the median log2-fold change value of 3.64 were designated as “high” (n=35) and “low” (n=36), respectively (Figure 6A). High UHRF1 expressing tumors were associated with signs of poor clinical outcome: 80% had microvascular invasion (Figure 5D) and significantly higher alpha-feto protein (AFP) levels (Figure 5E), although AFP levels alone did not cooperate with UHRF1 to predict survival (not shown). Importantly, high UHRF1 expression significantly correlated with early (<2 years; Figure 5F), but not late (Figure 5G) tumor recurrence and was inversely correlated with survival (Figure 5H). This suggests that high UHRF1 expression predicted recurrence of the primary tumor, causing decreased survival (Villanueva et al., 2011). Molecular signatures of aggressive HCC tumors compiled from several previous studies (Table S1) were concordantly and significantly enriched in high UHRF1 expressing tumors (Figure 6A). Moreover, high UHRF1 expressing tumors were distinguished by induction of pathways that drive the cell cycle, DNA replication and repair (Figure S6A, Tables S2 and S3). UHRF1 overexpression also significantly correlated with advanced stage prostate cancer, but not lung or colon (Figure S5D–G).
Figure 6. High UHRF1 expression defines a subclass of tumors with inactivated tp53, repression of senescence and chromosomal instability.
(A) The 71 human HCC tumors analyzed in Figure 5C–G were rank-ordered according to UHRF1 expression by qPCR and classified as high (<median, red; n=35) or low (≥median, blue; n=36). The presences of aggressive human HCC gene signatures from published studies and of TP53 inactivating mutations are indicated by red and black boxes, respectively. TP53-mediated senescence gene signatures (Lujambio et al., 2013; Tang et al., 2007) are displayed as a range from repressed (blue) to activated (red). (B) Genome-wide profile of DNA copy number variation was obtained from GEO geneset GSE9829. (C) Proportion of genes with DNA copy number loss and gain in tumors according to UHRF1 expression. (D) Proportion of genes with DNA copy number loss according to UHRF1 expression and TP53 mutation status. (E) UHRF1 expression is significantly higher in tumors with TP53 mutations. (F) DNMT1 expression by microarray analysis is significantly correlated with UHRF1 expression assessed by qPCR in HCCs. In box and whisker plots, boxes represent the 75th and 25th percentiles, the whiskers represent the most extreme data points within interquartile range x 1.5, and the horizontal bar represents the median. See also Figure S6 and Tables S1-S6.
High UHRF1 expression delineates a subclass of HCCs that have downregulated TP53-mediated senescence
Our zebrafish studies demonstrated that bypass of Tp53-induced senescence is required for UHRF1 to act as an oncogene. Our analysis of human HCCs indicates that a similar paradigm occurs in these samples. First, inactivating mutations in TP53 significantly correlated with high UHRF1 expression (Figure 6A). Second, many of the core enriched genes high UHRF1 expressing tumors are regulated by TP53 in senescent fibroblasts (Table S4 and (Tang et al., 2007), and high UHRF1 expressing tumors have downregulated the gene expression signature derived from TP53-induced senescence in fibroblasts (Table S5, Figure S6B; (Tang et al., 2007)) or in hepatic stellate cells (Table S6, Figure S6C; (Lujambio et al., 2013)). Fourth, significant correlation between UHRF1 overexpression, TP53 mutation and genome integrity in human HCCs indicates that these pathways act together: high UHRF1 expression (Figure 6B–C) and TP53 mutation (Figure 6D) are independently correlated with chromosomal loss but tumors that have both features display even more chromosomal loss (Figure 6D). Finally, UHRF1 expression was significantly higher in tumors with TP53 mutation (Figure 6E). UHRF1 overexpression did not correlate with copy number variation at the UHRF1 locus (Figure S6D) suggesting that a different mechanism drives UHRF1 overexpression.
Genome wide DNA hypomethylation is found in most HCCs (Calvisi et al., 2007) and this rendered it difficult to correlate methylome changes with UHRF1 expression. However, DNMT1 expression was directly correlated with UHRF1 expression in HCC samples (Figure 6F). This may be a consequence of the high proliferation rate in these tumors or an induction of the methylation machinery to compensate for hypomethylation. Together, these data indicate that high UHRF1 expression in HCC defines a subset of aggressive tumors that have inactivated the TP53 induced senescence program, suggesting that in humans, as observed in zebrafish, TP53 acts as a tumor suppressor to restrict the oncogenic potential of UHRF1.
DISCUSSION
We show that UHRF1 overexpression is sufficient to cause two oncogene-associated phenotypes: senescence and cancer. This defines UHRF1 as an epigenetic regulator that can cause cancer when overexpressed and is thus an oncogene. We hypothesize that UHRF1 overexpression is one mechanism by which cancer genomes can become hypomethylated, either via UHRF1-mediated Dnmt1 ubiquitination and degredation (Du et al., 2010; Qin et al., 2011) or by redistribution and/or sequestration of Dnmt1 away from DNA. Excess UHRF1 might also sequester the DNMT1 deubiquitinating enzyme, USP7 (Qin et al., 2011) to further promote DNMT1 ubiquitination and degradation.
The precise mechanism by which DNA hypomethylation causes cancer remains elusive. Both apoptosis and senescence serve to limit the propagation of cells with aberrant DNA methylation (Decottignies and d’Adda di Fagagna, 2011; Fairweather et al., 1987), however, once epigenetically altered cells escape these tumor suppressive mechanisms, they likely accumulate genetic lesions which predispose to cancer. Indeed, chromosomal instability and mitotic catastrophe occur following DNMT1 depletion (Chen et al., 2007; Karpf and Matsui, 2005; Weber and Schubeler, 2007) and transposons, which are heavily methylated in normal cells, could become activated and cause genomic instability upon UHRF1-induced DNA hypomethylation. Additionally, hypomethylated DNA is more likely to assume an open chromatin conformation, which may promote oncogene expression, although this possibility has not been fully evaluated. While the oncogenic role of UHRF1 could also be mediated by the impact of UHRF1 on other epigenetic marks or on DNA replication (Taylor et al., 2013), our data combined with findings from others (Eden et al., 2003; Gaudet et al., 2003) suggests that DNA hypomethylation is a likely mechanism driving UHRF1-mediated transformation.
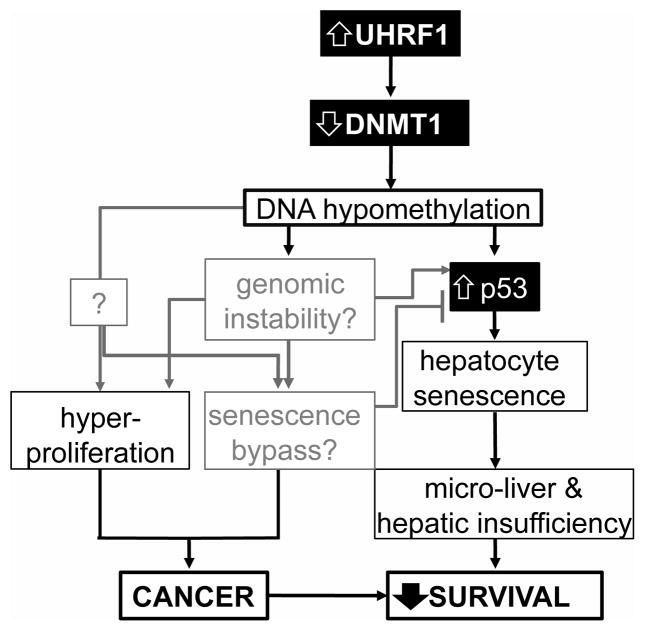
Our working model (Figure 7) proposes that UHRF1 overexpression causes DNA hypomethylation by reducing Dnmt1 levels and its access to DNA. Tp53 is induced in response to either genomic instability or some unidentified direct mechanism, and causes hepatocyte senescence, which prevents hepatic expansion and results in larval death from hepatic insufficiency. We propose that Tp53 inactivation and senescence bypass allows for the unhindered proliferation and malignant transformation. Identifying the mechanism by which this tumor suppressive mechanism is overcome remains a central, unanswered question in cancer biology.
Figure 7. Model of the relationship between UHRF1 overexpression, DNA hypomethylation, Tp53 mediated senescence, cancer and survival.
Factors investigated in this study are in solid black boxes with black lines indicating the correlations demonstrated in this work and gray lines indicating relationships that are speculative. Senescence reduces liver size and function and reduces larval survival while cancer occurs when senescence is bypassed and also reduces survival.
Studies in a mouse liver cancer model show that Tp53 reactivation causes senescence, and these cells are then cleared by the immune system (Xue et al., 2007) and the liver is then repopulated with senescence-resistant tumor forming cells. Our finding that senescence decreases and BrdU incorporation increases in UHRF1-GFP high livers over time suggests a similar process at play. Moreover, BrdU incorporation in primarily GFP negative cells suggests the expansion of either immune cells or immature hepatic progenitors in response to senescent hepatocytes. The finding of a cholangiocarcinoma in a UHRF1-GFP High/p53+/− fish may indicate that a bipotential progenitor cell could be the tumor forming cell in this model. Additionally, our data indicate that there is a threshold effect of UHRF1 expression, in which the highest expressing cells undergo senescence and neighboring hepatocytes expressing UHRF1-GFP at levels below those detectable via microscopy undergo unhindered expansion.
HCC is the third cause of cancer-related deaths globally (Llovet et al., 2003), yet curative therapies are limited, with Sorafanib as the only systemic therapy available for advanced cases (Llovet et al., 2008). Thus, there is an urgent and unmet need for novel therapies. HCC, like other cancers, is characterized by global DNA hypomethylation (Calvisi et al., 2007; Pogribny and Rusyn, 2012; Tischoff and Tannapfe, 2008), and high UHRF1 expression could be the cause. Our finding that UHRF1 depletion in HCC and other types of cancer cells causes apoptosis (Tien et al., 2011) presents UHRF1 as an attractive target for cancer therapy, whereby blocking UHRF1 could trigger massive epigenetic changes incompatible with cell survival or could reset the cancer cell methylome.
EXPERIMENTAL PROCEDURES
Zebrafish maintenance and generation of transgenics
Zebrafish were maintained on a 14:10 hour light:dark cycle at 28°C. mRNA encoding zebrafish Dnmt1 (Rai et al., 2006) or mannose phosphate isomerase (Mpi; (Chu et al., 2013) as a control was injected into embryos just after fertilization.
Tg(fabp10:nls-mCherry) fish expressing nls-mCherry exclusively in hepatocytes were generated using Gateway cloning (Invitrogen) to produce vectors with tol2 transposon sites (Kwan et al., 2007). Tg(hsp70l:hsa.UHRF1-GFP) and Tg(fabp10:hsa.UHRF1-GFP) were described in (Chu et al., 2012). The high, medium and low expressing alleles are listed in ZFIN.org with superscripts mss1a, mss1b and mss1c, respectively. Transgenics were outcrossed to Tab14 (WT) or tp53−/− fish (Berghmans et al., 2005).
Tg(hsp70l:UHRF1-GFP) embryos were heat-shocked at 37°C for 1 hour at 24 and 27 hours post fertilization (hpf). At 28 hpf, embryos were sorted visually for GFP expression and incubated with either 10 μM MG132 or DMSO and collected for immunoblotting at 34 hpf. 5-Aza (50 μM) was added to larvae from 2.5–5 dpf. The Mount Sinai Institutional Animal Care and Use Committee approved all protocols. Nomenclature guidelines for the species under discussion were followed, and when no species is specified, human nomenclature was used.
Gene expression analysis
RNA was isolated from a pool of at least 10 livers from 5 dpf fish and from 1–5 livers from 20 dpf fish using the RNeasy mini-kit (Qiagen). cDNA was prepared by polyA priming using qScript SuperMix (Quanta). qRT-PCR analysis was performed in the Light Cycler 480 (Roche) using gene-specific primers (see supplemental materials) and PerfeCTa SYBRGreen FastMix (Quanta). Ct values from triplicate reactions were averaged and 2-Ct(target)/2-Ct(reference) was used to calculate expression, with rpp0 and cyclophilin A used as references genes zebrafish and mouse samples, respectively.
RNAseq analysis was carried out on RNA from pools of 50 livers dissected from 2 clutches of 5 dpf UHRF1-GFP High and nls-mCherry larvae, described in Supplemental Material.
Histology
Fish younger than 20 dpf were fixed overnight at 4°C in 4% paraformaldehyde (PFA) and older fish were fixed for 2–5 days at room temperature in Bouins fixative. 4 μM serial sections of paraffin embedded fish were stained with hematoxylin and eosin as described (Imrie and Sadler, 2010) and imaged on an Olympus BX41 Clinical Microscope equipped with a Nikon DS-Ri1 digital camera. Histological criteria used for scoring tumors are described in Figure S4A–B.
Immunoblotting
Lysates prepared from 15 embryos dissolved in 150 μl protein lysis buffer were homogenized by sonication. 1 embryo equivalent was loaded per lane of an 8 or 12% SDS gel, transferred to nitrocellulose and blotted. HepG2 cell lysates were prepared as described (Tien et al., 2011).
Antibodies
Antibodies recognizing DNMT1 (1:1000 for blotting, 1:10 for immunofluorescence, Santa Cruz), UHRF1 (immunoblotting: 1:1000; BD Biosciences. Immunohistochemistry: 1:50; ab57083; Abcam), Tubulin (1:5000 DSHB), p89 PARP and total PARP (1:1000; Cell Signaling) and b-actin (1:2000; Sigma), 5MeC (1:500; Eurogentec), BrdU (1:200; BD Bioscences) and anti-rabbit or mouse conjugated to Alexa 555 or Alexa 488 (1:100; Invitrogen) were diluted in 10% FBS or 2% BSA in 1% Triton in PBS (PBST).
Zebrafish staining
Larvae fixed in 4% PFA were washed in PBST and stained using the Senescence β-Galactosidase Staining Kit (Cell Signaling) or stained with CY3-streptavadin (CY3-SA; 1:300; Sigma) as described (Sadler et al., 2005). The left liver lobe area was measured using ImageJ.
Immunofluorescence was carried out on whole fish or on livers dissected from fixed larvae. BrdU was added to larvae water (10 mM) for 4–6 hours followed by immediate fixation, dehydration in methanol, rehydration to PBST and permeablization with 10ug/ml Proteinase K. DNA was denatured in 1 or 2N HCl, renatured and blocked in 10% FBS or 1% BSA in PBST prior to immunofluorescence. DNA was stained with Hoechst 33342 (Sigma). A Leica SP5 DM confocal microscope was used for imaging.
Slot blots
Genomic DNA was denatured in 0.4M NaOH at 95°C for 10 minutes, neutralized in an equal volume of cold 2M ammonium acetate and 100 ng DNA was blotted in duplicate onto nitrocellulose membrane using a slot blot apparatus and washed in 2x SSC and vacuum baked at 80°C for 2 hours. Half was stained with 0.2% methylene blue in 0.3 M NaOAc and the other with anti-5MeC followed by HRP-conjugated anti-mouse (1:2000) and visualized by Chemilumiescence (Roche). Image J was used to quantify 5MeC and methylene blue intensity and 5MeC levels were determined by normalizing to total DNA.
Cell culture
Primary mouse hepatocytes were isolated and plated in triplicate at 50% confluency then treated with 5 μm 5-Aza or DMSO for 24 hours and RNA was isolated.
NIH-3T3 cells were transfected in triplicate with pCDNA3.1 lacking an insert (control) or containing human RAS, UHRF1 or both and were re-transfected 24 hours later. 48 hours after the second transfection, 700 mg /ml Neomycin was added for 7 days. 10,000 cells per condition were plated on 0.3% soft agar layered on top of 0.6% soft agar. Media was changed every 3rd day for 2 weeks and plates were stained with 0.005% crystal violet and colony number was counted.
HepG2 cells cultured in DMEM supplemented with 10% (v/v) FBS and 5% (w/v) penicillin-streptomycin were transfected twice, 24 hours apart, with 20 nM siRNA targeting firefly luciferase (GL2; Dhamarcon) or UHRF1 targeting si-A and si-B using RNAiMAX (Invitrogen) as described (Tien et al., 2011).
Human tissue samples
Pathologically staged human tumors, dysplastic foci and normal liver samples were obtained from the HCC Genomic Consortium (Mount Sinai Hospital, NY; Instituto Nazionale dei Tumori, Milan and Hospital Clinic, Barcelona). The study was approved by the IRB of each institution and informed consent was obtained from all participants. TaqMan Probes were used to analyze UHRF1 expression by qPCR as described (Villanueva et al., 2008). Samples were grouped into a training set (9 normal liver, 18 low- and high-grade dysplastic nodules, and 40 pathologically staged HCCs; (Llovet et al., 2006; Wurmbach et al., 2007)) and a validation set (7 normal liver and 69 HCC; (Villanueva et al., 2008)). Integrative genomic and clinical data analysis was performed on 71 of the hepatitis C-related, surgically treated HCC patients (NCBI Gene Expression Omnibus, accession numbers GSE9829 and GSE44970). Liver pathologist, ST, scored UHRF1 staining of 52 paraffin embedded tumors from the training set.
Bioinformatics and statistical analysis
71 patients with HCC were grouped based on the median expression level of UHRF1 in the tumors determined by qPCR (UHRF1-high; n=35 or low; n=36). Presence of aggressive human HCC gene signatures (Table S1) were evaluated in the transcriptome dataset using nearest template prediction method (Hoshida, 2010) implemented by the GenePattern genomic analysis toolkit (www.broadinstitute.org/genepattern) based on prediction confidence p<0.05. The senescence-related TP53 target genes were obtained from Molecular Signature Database (MSigDB, www.broadinstitute.org/msigdb, TANG_SENESCENCE_TP53_TARGETS_UP and _DN; (Tang et al., 2007)) and from NCBI Gene Expression Omnibus (GSE39469; (Lujambio et al., 2013)). Mouse genes from normalized microarray data were converted to human orthologs based on a mapping table (www.informatics.jax.org). Differentially expressed genes between senescent and proliferative cells were identified by using Bayesian T-test implemented in Cyber-T software (molgen51.biol.rug.nl/cybert) at the significance threshold of Posterior Probability of Differential Expression >0.998 after excluding less variable genes with coefficient of variation ≤ 0.1 across the samples. Expression pattern of each signature was assessed by nearest template prediction algorithm. Significance of induction or suppression of each signature was quantitatively measured by nominal p-value of cosine distance. Pearson correlation test determined the significance between induction/suppression of gene signatures with UHRF1 mRNA expression level as measured by qPCR. Statistical difference between groups was assessed by Wilcoxon rank-sum test and Fisher’s exact test for continuous and categorical variables, respectively. Bonferroni correction for multiple hypothesis testing was applied when appropriate.
Survival analysis was performed by using Kaplan-Meier estimator and log-rank test. All analyses were performed using R statistical package (www.r-project.org) and GenePattern genomic analysis tool kit (www.broadinstitute.org/genepattern). Gene expression levels and liver size were compared using Student’s T or Mann Whitney U tests
Supplementary Material
Highlights.
UHRF1 overexpression induces DNA hypomethylation
Liver specific overexpression of UHRF1 in zebrafish causes senescence and HCC
Human HCCs with high UHRF1 are aggressive and inactivate TP53-mediated senescence
Our studies in zebrafish and human tumors identify UHRF1 as an oncogene in HCC
SIGNIFICANCE.
Global DNA hypomethylation occurs in most types of cancer and can induce genomic instability and widespread changes in gene expression. UHRF1 is a key regulator of DNA methylation, and here we show that UHRF1 overexpression causes HCC in zebrafish without any other genetic alteration, demonstrating that it is an oncogene. High UHRF1 expression causes DNA hypomethylation and tp53-mediated senescence, which serves to restrict transformation of UHRF1 overexpressing cells. High UHRF1 expression in human HCC correlates with a poor prognosis, genomic instability, TP53 mutation and repression of the TP53-mediated senescence program. We conclude that UHRF1 is an oncogene that promotes widespread DNA hypomethylation, an epigenetic hallmark of cancer cells, and that UHRF1 overexpression drives tumorigenesis when senescence is bypassed.
Acknowledgments
Support was provided by The Breast Cancer Alliance and the March of Dimes (to KCS), a Pilot Project from the DFCI NCI Cancer Center (5P30CA006516-45; to CU), the NIH (5R01DK080789-02 to CU and KCS, 1R01DK099558 to YH, 1R01DK076986 to JML, F30DK094503 to VJ and T32CA078207-14 for YC), the European Commission 7th Framework Programme FP7-Health 2010 (Heptromic #259744 to YH and JML), The Samuel Waxman Cancer Research Foundation, The Spanish National Health Institute (SAF-2010-16055) and the Asociación Española Contra el Cáncer (JML). Liz Loughlin, Brandon Kent, Meghan Walsh, Alex Mir, Laia Cabellos, Helena Cornellà Vives and Sara Toffanin provided expert technical assistance. We are grateful to Sam Sidi for tp53−/− fish, stimulating discussions and critical reading of the manuscript.
Footnotes
Accession Number
Data from RNAseq was deposited with accession number GSE52605.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RM, Bosch JA, Goll MG, Hesselson D, Dong PD, Shin D, Chi NC, Shin CH, Schlegel A, Halpern M, Stainier DY. Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita K, Ariyoshi M, Tochio H, Nakamura Y, Shirakawa M. Recognition of hemi-methylated DNA by the SRA protein UHRF1 by a base-flipping mechanism. Nature. 2008;455:818–821. doi: 10.1038/nature07249. [DOI] [PubMed] [Google Scholar]
- Avvakumov GV, Walker JR, Xue S, Li Y, Duan S, Bronner C, Arrowsmith CH, Dhe-Paganon S. Structural basis for recognition of hemi-methylated DNA by the SRA domain of human UHRF1. Nature. 2008;455:822–825. doi: 10.1038/nature07273. [DOI] [PubMed] [Google Scholar]
- Babbio F, Pistore C, Curti L, Castiglioni I, Kunderfranco P, Brino L, Oudet P, Seiler R, Thalman GN, Roggero E, et al. The SRA protein UHRF1 promotes epigenetic crosstalks and is involved in prostate cancer progression. Oncogene. 2012 doi: 10.1038/onc.2011.641. [DOI] [PubMed] [Google Scholar]
- Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci U S A. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biniszkiewicz D, Gribnau J, Ramsahoye B, Gaudet F, Eggan K, Humpherys D, Mastrangelo MA, Jun Z, Walter J, Jaenisch R. Dnmt1 overexpression causes genomic hypermethylation, loss of imprinting, and embryonic lethality. Mol Cell Biol. 2002;22:2124–2135. doi: 10.1128/MCB.22.7.2124-2135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostick M, Kim JK, Esteve PO, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Gorden A, Farina M, Lee JS, Conner EA, Schroeder I, Factor VM, Thorgeirsson SS. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J Clin Invest. 2007;117:2713–2722. doi: 10.1172/JCI31457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah MS, Wallace CD, Hoffman RM. Hypomethylation of DNA in human cancer cells: a site-specific change in the c-myc oncogene. J Natl Cancer Inst. 1984;73:1057–1065. [PubMed] [Google Scholar]
- Chen T, Hevi S, Gay F, Tsujimoto N, He T, Zhang B, Ueda Y, Li E. Complete inactivation of DNMT1 leads to mitotic catastrophe in human cancer cells. Nat Genet. 2007;39:391–396. doi: 10.1038/ng1982. [DOI] [PubMed] [Google Scholar]
- Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Sole M, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Loughlin EA, Gaur NA, Senbanerjee S, Jacob V, Monson C, Kent B, Oranu A, Ding Y, Ukomadu C, Sadler KC. UHRF1 phosphorylation by cyclin A2/cyclin-dependent kinase 2 is required for zebrafish embryogenesis. Mol Biol Cell. 2012;23:59–70. doi: 10.1091/mbc.E11-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Mir A, Gao N, Rosa S, Monson C, Sharma V, Steet R, Freeze HH, Lehrman MA, Sadler KC. A zebrafish model of congenital disorders of glycosylation with phosphomannose isomerase deficiency reveals an early opportunity for corrective mannose supplementation. Dis Model Mech. 2013;6:95–105. doi: 10.1242/dmm.010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decottignies A, d’Adda di Fagagna F. Epigenetic alterations associated with cellular senescence: a barrier against tumorigenesis or a red carpet for cancer? Semin Cancer Biol. 2011;21:360–366. doi: 10.1016/j.semcancer.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Di Micco R, Sulli G, Dobreva M, Liontos M, Botrugno OA, Gargiulo G, dal Zuffo R, Matti V, d’Ario G, Montani E, et al. Interplay between oncogene-induced DNA damage response and heterochromatin in senescence and cancer. Nat Cell Biol. 2011;13:292–302. doi: 10.1038/ncb2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Song J, Wang Y, Zhao Y, Guda K, Yang S, Kao HY, Xu Y, Willis J, Markowitz SD, et al. DNMT1 stability is regulated by proteins coordinating deubiquitination and acetylation-driven ubiquitination. Sci Signal. 2010;3:ra80. doi: 10.1126/scisignal.2001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden A, Gaudet F, Waghmare A, Jaenisch R. Chromosomal instability and tumors promoted by DNA hypomethylation. Science. 2003;300:455. doi: 10.1126/science.1083557. [DOI] [PubMed] [Google Scholar]
- Fairweather DS, Fox M, Margison GP. The in vitro lifespan of MRC-5 cells is shortened by 5-azacytidine-induced demethylation. Exp Cell Res. 1987;168:153–159. doi: 10.1016/0014-4827(87)90424-1. [DOI] [PubMed] [Google Scholar]
- Feng S, Cokus SJ, Zhang X, Chen PY, Bostick M, Goll MG, Hetzel J, Jain J, Strauss SH, Halpern ME, et al. Conservation and divergence of methylation patterning in plants and animals. Proc Natl Acad Sci U S A. 2010;107:8689–8694. doi: 10.1073/pnas.1002720107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- Gaudet F, Rideout WM, 3rd, Meissner A, Dausman J, Leonhardt H, Jaenisch R. Dnmt1 expression in pre- and postimplantation embryogenesis and the maintenance of IAP silencing. Mol Cell Biol. 2004;24:1640–1648. doi: 10.1128/MCB.24.4.1640-1648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H, Horton JR, Zhang X, Bostick M, Jacobsen SE, Cheng X. The SRA domain of UHRF1 flips 5-methylcytosine out of the DNA helix. Nature. 2008;455:826–829. doi: 10.1038/nature07280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshida Y. Nearest template prediction: a single-sample-based flexible class prediction with confidence assessment. PLoS One. 2010;5:e15543. doi: 10.1371/journal.pone.0015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard G, Eiges R, Gaudet F, Jaenisch R, Eden A. Activation and transposition of endogenous retroviral elements in hypomethylation induced tumors in mice. Oncogene. 2008;27:404–408. doi: 10.1038/sj.onc.1210631. [DOI] [PubMed] [Google Scholar]
- Imrie D, Sadler KC. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev Dyn. 2010;239:3013–3023. doi: 10.1002/dvdy.22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nat Genet. 2001;27:31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- Jin W, Chen L, Chen Y, Xu SG, Di GH, Yin WJ, Wu J, Shao ZM. UHRF1 is associated with epigenetic silencing of BRCA1 in sporadic breast cancer. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0652-2. [DOI] [PubMed] [Google Scholar]
- Jirtle RL. IGF2 loss of imprinting: a potential heritable risk factor for colorectal cancer. Gastroenterology. 2004;126:1190–1193. doi: 10.1053/j.gastro.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Karpf AR, Matsui S. Genetic disruption of cytosine DNA methyltransferase enzymes induces chromosomal instability in human cancer cells. Cancer Res. 2005;65:8635–8639. doi: 10.1158/0008-5472.CAN-05-1961. [DOI] [PubMed] [Google Scholar]
- Karpf AR, Moore BC, Ririe TO, Jones DA. Activation of the p53 DNA damage response pathway after inhibition of DNA methyltransferase by 5-aza-2′-deoxycytidine. Mol Pharmacol. 2001;59:751–757. [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Li E, Beard C, Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993;366:362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Liu X, Gao Q, Li P, Zhao Q, Zhang J, Li J, Koseki H, Wong J. UHRF1 targets DNMT1 for DNA methylation through cooperative binding of hemi-methylated DNA and methylated H3K9. Nat Commun. 2013;4:1563. doi: 10.1038/ncomms2562. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Chen Y, Wurmbach E, Roayaie S, Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A, et al. A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis. Gastroenterology. 2006;131:1758–1767. doi: 10.1053/j.gastro.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, Lowe SW. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuff FK, Turner SD. Jailbreak: oncogene-induced senescence and its evasion. Cell Signal. 2011;23:6–13. doi: 10.1016/j.cellsig.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Mudbhary R, Sadler KC. Epigenetics, development, and cancer: Zebrafish make their mark. Birth Defects Res C Embryo Today. 2011;93:194–203. doi: 10.1002/bdrc.20207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Yamaguchi L, Sharif J, Johmura Y, Kawamura T, Nakanishi K, Shimamura S, Arita K, Kodama T, Ishikawa F, et al. Uhrf1-dependent H3K23 ubiquitylation couples maintenance DNA methylation and replication. Nature. 2013;502:249–253. doi: 10.1038/nature12488. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Rusyn I. Role of epigenetic aberrations in the development and progression of human hepatocellular carcinoma. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Leonhardt H, Spada F. Usp7 and Uhrf1 control ubiquitination and stability of the maintenance DNA methyltransferase Dnmt1. J Cell Biochem. 2011;112:439–444. doi: 10.1002/jcb.22998. [DOI] [PubMed] [Google Scholar]
- Rai K, Nadauld LD, Chidester S, Manos EJ, James SR, Karpf AR, Cairns BR, Jones DA. Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol Cell Biol. 2006;26:7077–7085. doi: 10.1128/MCB.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K, Sarkar S, Broadbent TJ, Voas M, Grossmann KF, Nadauld LD, Dehghanizadeh S, Hagos FT, Li Y, Toth RK, et al. DNA demethylase activity maintains intestinal cells in an undifferentiated state following loss of APC. Cell. 2010;142:930–942. doi: 10.1016/j.cell.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development. 2005;132:3561–3572. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- Sadler KC, Krahn KN, Gaur NA, Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proc Natl Acad Sci U S A. 2007;104:1570–1575. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, Shinga J, Mizutani-Koseki Y, Toyoda T, Okamura K, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- Tang X, Milyavsky M, Goldfinger N, Rotter V. Amyloid-beta precursor-like protein APLP1 is a novel p53 transcriptional target gene that augments neuroblastoma cell death upon genotoxic stress. Oncogene. 2007;26:7302–7312. doi: 10.1038/sj.onc.1210542. [DOI] [PubMed] [Google Scholar]
- Taylor EM, Bonsu NM, Price RJ, Lindsay HD. Depletion of Uhrf1 inhibits chromosomal DNA replication in Xenopus egg extracts. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien AL, Senbanerjee S, Kulkarni A, Mudbhary R, Goudreau B, Ganesan S, Sadler KC, Ukomadu C. UHRF1 depletion causes a G2/M arrest, activation of DNA damage response and apoptosis. Biochem J. 2011;435:175–185. doi: 10.1042/BJ20100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischoff I, Tannapfe A. DNA methylation in hepatocellular carcinoma. World J Gastroenterol. 2008;14:1741–1748. doi: 10.3748/wjg.14.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittle RK, Sze R, Ng A, Nuckels RJ, Swartz ME, Anderson RM, Bosch J, Stainier DY, Eberhart JK, Gross JM. Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens. Dev Biol. 2011;350:50–63. doi: 10.1016/j.ydbio.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoki M, Daigo Y, Koinuma J, Tsuchiya E, Hamamoto R, Nakamura Y. UHRF1 is a novel diagnostic marker of lung cancer. Br J Cancer. 2010;103:217–222. doi: 10.1038/sj.bjc.6605717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, Tovar V, Roayaie S, Minguez B, Sole M, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. 1983 e1971–1911. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A, Hoshida Y, Battiston C, Tovar V, Sia D, Alsinet C, Cornella H, Liberzon A, Kobayashi M, Kumada H, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140:1501–1512.e1502. doi: 10.1053/j.gastro.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Yang YZ, Shi CZ, Zhang P, Moyer MP, Zhang HZ, Zou Y, Qin HL. UHRF1 Promotes Cell Growth and Metastasis Through Repression of p16(ink4a) in Colorectal Cancer. Ann Surg Oncol. 2012 doi: 10.1245/s10434-011-2194-1. [DOI] [PubMed] [Google Scholar]
- Weber M, Schubeler D. Genomic patterns of DNA methylation: targets and function of an epigenetic mark. Curr Opin Cell Biol. 2007;19:273–280. doi: 10.1016/j.ceb.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Wurmbach E, Chen YB, Khitrov G, Zhang W, Roayaie S, Schwartz M, Fiel I, Thung S, Mazzaferro V, Bruix J, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45:938–947. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Jackson-Grusby L, Linhart H, Meissner A, Eden A, Lin H, Jaenisch R. Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci U S A. 2005;102:13580–13585. doi: 10.1073/pnas.0506612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell. 2012;22:9–20. doi: 10.1016/j.ccr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.