Abstract
Several classes of drugs bind to the dopamine transporter (DAT) with high affinity, but some are weaker positive reinforcers than cocaine, suggesting that affinity for and occupancy of the DAT is not the only determinant of a drug’s reinforcing effectiveness. Other factors such as the rate of onset have been positively and strongly correlated with the reinforcing effects of DAT inhibitors in nonhuman primates. In the current studies, we examined the effects of acute systemic administration of cocaine and three cocaine analogs (RTI-150, RTI-177, and RTI-366) on binding to DAT in squirrel monkey brain using PET neuroimaging. During the PET scan, we also measured drug effects on dopamine (DA) levels in the caudate using in vivo microdialysis. In general, our results suggest a lack of concordance between drug occupancy at DAT and changes in DA levels. These studies also indicate that acute cocaine administration decreases the availability of plasma membrane DAT for binding, even after cocaine is no longer blocking DA uptake as evidence by a return to basal DA levels.
Keywords: in vivo microdialysis, monoamine, psychostimulant, trafficking, transporter
INTRODUCTION
Psychostimulant abuse and dependence continues to be a significant public health problem (SAMHSA, 2007). To date, no FDA-approved pharmacotherapy is available to treat those addicted to psychostimulant drugs such as cocaine and amphetamine (Carroll et al., 1999; Vocci et al., 2005). While these drugs increase levels of all three monoamine neurotransmitters (dopamine (DA), serotonin (5HT), and norepinephrine (NE)) in the brain, the increases in DA have been found to be largely responsible for the behavioral-stimulant and reinforcing effects of these drugs (Heikkila and Manzino, 1984; Reith et al., 1986; Ritz and Kuhar, 1989; Ritz et al., 1987; Wise and Bozarth, 1987). The dopamine transporter (DAT) is primarily responsible for clearing DA from the synapse and there is a positive correlation between the binding affinity of psychostimulants at DAT and their behavioral potency in rodents and nonhuman primates (Bergman et al., 1989; Ritz et al., 1987; Wilcox et al., 1999).
However, several drugs bind to the DAT with high affinity, but are weaker positive reinforcers than cocaine (Stafford et al., 2001; Tella et al., 1996; Wilcox et al., 2000; Woolverton et al., 2001), suggesting that DAT affinity is not the only determinant of reinforcing effectiveness. Thus, we need to consider the pharmacokinetic and pharmacodynamic interactions of these drugs with DAT. Earlier studies determined that the rate of onset is positively and strongly correlated with the reinforcing effects of DAT inhibitors in nonhuman primates (Kimmel et al., 2007). These studies suggest that the kinetics of drug binding to DAT affect the psychostimulant profile of DAT inhibitors. Positron emission tomography (PET) neuroimaging is a noninvasive method that can be used to determine DAT occupancy of a drug in mammalian species, including nonhuman primates. This technique has been used to show that DAT occupancy of local anesthetics correlates with peak increases in DA in the caudate of rhesus monkeys (Wilcox et al., 2005). In addition, high DAT occupancy by selective DAT inhibitors is highly correlated with reductions in cocaine self-administration in rhesus monkeys (Lindsey et al., 2004).
The objective of this study was to use PET to determine the DAT occupancy of behaviorally-active doses of four DAT inhibitors in the squirrel monkey brain. These particular drugs were chosen for the current study, as they are selective for the DAT (vs. SERT and NET) (Kuhar et al., 1999) and have varying rates of onset (Kimmel et al., 2001b; Kimmel et al., 2008; Kimmel et al., 2007). DAT occupancy of the drug was calculated using a single injection of the DAT specific radioligand [18F]FECNT and displacing the radioligand at equilibrium with unlabeled DAT inhibitors. We hypothesized that an [18F]FECNT displacement protocol could be used to investigate changes in DAT occupancy during the imaging session as drug is metabolized and eliminated from the brain. Simultaneous with the PET scan, microdialysis studies were conducted to detect changes in DA levels following drug administration. The latter studies corroborated the PET data, indicating that the drug had reached the brain regions of interest and that the drug was exerting its typical profile of neurochemical effects, as determined by earlier studies (Kimmel et al., 2001b; Kimmel et al., 2005; Kimmel et al., 2008; Kimmel et al., 2007).
MATERIALS AND METHODS
Subjects
Three adult male squirrel monkeys (Samiri sciureus) weighing 700–1200 g served as subjects. Animals lived in individual home cages and had daily access to food (Harlan Teklad monkey chow; Harlan Teklad, Madison, WI; fresh fruit and vegetables) and unlimited access to water. All monkeys had prior exposure to cocaine and other drugs with selective dopaminergic or glutamatergic activity in various behavioral studies. Animal use procedures were in strict accordance with the National Institutes of Health “Guide for Care and Use of Laboratory Animals” (Publication No. 85-23, revised 1985) and were approved by the Institutional Animal Care and Use Committee of Emory University.
Guide Cannulae Implantation
A stereotaxic apparatus was used to implant CMA/11 guide cannulae (CMA/Microdialysis, Acton, MA) bilaterally to target the caudate nucleus in a procedure described previously (Czoty et al., 2000). Anesthesia was initiated with Telazol (tiletamine hydrochloride and zolazepam hydrochloride, 3.0 mg) and atropine. Inhaled isoflurane (1.0–2.0%) was administered to maintain depth of anesthesia during the procedure. A stainless steel stylet was placed in each guide cannulae when not in use. Analgesics [Banamine (flunixin meglumine)] and antibiotics [Rocephin (ceftriaxone)] were prescribed as necessary by veterinary staff. Animals were closely monitored during recovery from anesthesia, and a minimum of two weeks elapsed before microdialysis experiments were performed.
PET imaging apparatus
PET neuroimaging was performed at the Yerkes National Primate Research Center on a microPET Focus 220 scanner (Siemens, Concorde Microsystems, Knoxville, TN). The PET ligand [18F]FECNT is an N-fluoroethyl nortropane derivative that is selective for the DAT (Goodman et al., 2000). Monkeys were initially anesthetized with ketamine and Telazol, intubated and maintained on 1–2% isoflurane for the duration of the procedure. An arm or leg was used for placement of an acute intravenous catheter through which to provide a sterile saline drip and administer the radioligand and test drugs. Subjects were positioned supine in the tomograph, and then fitted with pulse oximetry equipment and a rectal thermistor for physiological monitoring during the procedure. Approximately 5 mCi of [18F]FECNT was administered over five minutes to each animal concordant with the start of the PET imaging session. At 90 min, when binding had reached equilibrium, a bolus injection of drug was then administered intravenously to displace [18F]FECNT at the DAT. An additional 120 min of PET scanning was then acquired for a total of 210 minutes of PET imaging. All images were reconstructed with OSEM3D/MAP using measured attenuation correction and zoom factor 2. Image data were decay-corrected to the time of injection. Regions of interest were manually drawn on the late images fused with MRI over the caudate, putamen and cerebellum. The regions of interest were then applied to all images to obtain time-activity curves.
PET data analysis
Kinetic analysis of the time-activity data was performed with the General Reference Tissue Model (GRTM) (Votaw et al., 2002). This model is based on the simplified reference tissue model (SRTM) (Lammertsma and Hume, 1996), but allows for the case where the initial activity in the compartments is not zero. GRTM equations describe the data with parameters using the cerebellum as the reference region. The model parameters as described in (Votaw et al., 2002) are: R – the ratio of K1’s in the target and reference region, k2 - the rate that tracer leaves the extracellular compartment for the plasma, k3 - konBavail the DAT association rate times the number of available binding sites (Mintun et al., 1984), and k4 - the off rate from the DAT.
The PET scan data contain two phases: a baseline phase from 0 to 90 min and a drug challenge phase from 90 to 210 min. In this model, the assumption is that R, k2 and k4 remain constant during the baseline and drug challenge phases. It is postulated that the introduction of unlabeled drug competition at 90 min changes the number of available DAT binding sites for [18F]FECNT but not the association rate (kon) at these binding sites. Furthermore, we assume the affinity (Kd) of [18F]FECNT does not change with the introduction of the drug. Therefore, the non-displaceable binding potential, BPND, equal to k3/k4 = kon × Bavail/koff = Bavail/Kd, will reflect a change in the number of available DAT binding sites and can be calculated during the baseline phase as k3/k4 and the drug challenge phase as k3D/k4. Where k3D - kon*BavailD and BavailD is the change in the number of available DAT binding sites with the drug onboard. All rate constants are solved simultaneously using the numerical Runga Kutta method with adaptive step-size control (Press, 1989) by minimizing the area under the curved between model and measured data at each time point (Votaw et al., 2002). A model fit was determined to be acceptable when the fitted curve fell within the standard error of the sample mean (SEM), which are assumed to be normally distributed.
The DAT occupancy of each drug was calculated by dividing the non-displaceable binding potential during the baseline phase by the non-displaceable binding potential during the drug challenge phase (Innis et al., 2007),
In the model, the onset of occupancy begins at the time of the drug challenge phase and model assumes a single occupancy during the drug challenge phase.
Microdialysis procedures
CMA/11 dialysis probes with a shaft length of 14 mm and active dialysis membrane measuring 4 mm long and 0.24 mm diameter were flushed with artificial cerebrospinal fluid (1.0 mM Na2HPO4, 150 mM NaCl, 3 mM KCl, 1.3 mM CaCl2, 1.0 mM MgS04 and 0.15 mM ascorbic acid, final pH=7.4–7.56) for at least 20 min. Probes were inserted into the guide cannulae and connected to a Harvard PicoPlus microinfusion pump via FEP Teflon tubing. Probes were perfused with artificial cerebrospinal fluid at 2.0 μl/min for the duration of the experiment. Samples were collected in microcentrifuge tubes every 5 min and immediately refrigerated. Following an equilibration period of at least 60 min after the start of the PET scan, four consecutive 5-min samples were collected for determination of baseline DA concentration. Following collection of baseline samples, saline or a dose of a test drug was administered i.v. and 5-min samples were collected for an additional 120 min. Animals were tested a maximum of one time every other week, and both sites were accessed in each study. This regimen of repeated access has produced consistent responses to drug treatment without significant gliosis or compromised tissue integrity (Czoty et al., 2000).
Analysis of dialysates
High-performance liquid chromatography (HPLC) and electrochemical detection were used to quantify levels of DA (Kimmel et al., 2007). A small bore (3.2 mm × 150 mm, 3 micron) column (ESA, Inc., Chelmsford, MA) was used with mobile phase (MD-TM, ESA, Inc.) delivered by an ESA 582 solvent delivery pump at a flow rate of 0.6 ml/min. Samples (10 μl) were automatically mixed with 3 μl of ascorbate oxidase, and 5 μl of this mixture was injected into the HPLC system by an ESA Model 542 autosampler. An ESA dual-channel analytical cell (model 5040) and guard cell (model 5020, potential = 350 mV) and an ESA Coulochem III detector were used for electrochemical analysis. The potential of channel 1 was set to −150 mV for oxidation, while the potential of channel 2 was set to 275 mV for reduction. Chromatograms were generated and analyzed by EZChrom Elite software (version 3.1, Scientific Software, Pleasanton, CA), comparing the area under the curve of the experimental samples with that of standard solutions (0.5 – 25 nM DA). Basal levels of DA were between 3–5 nM, unadjusted for probe recovery, as reported in earlier studies (Czoty et al., 2000).
Drugs
3β-(4-methylphenyl)tropan-2β-carboxylic acid cyclobutyl ester hydrochloride (RTI-150) (Research Triangle Institute, Research Triangle, NC) and cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) were dissolved in 0.9% saline. 3β-(4-chlorophenyl)tropane-2β-(3-phenylisoxazol-5-yl) hydrochloride (RTI-177) and 3β-(4-chlorophenyl)tropane-2β-[3-(4-methylphenyl)isoxazol-5-yl] hydrochloride (RTI-336) (Research Triangle Institute, Research Triangle, NC) were dissolved in 2% final volume 0.1 N HCl and 98% final volume water. 0.9% saline was administered as a control in a volume comparable to the other drugs. Drug doses were determined as salts and administered through the intravenous catheter. The drug doses chosen for the displacement of [18F]FECNT produce peak behavioral-stimulant and neurochemical changes (Kimmel et al., 2007): cocaine (1.0 mg/kg), RTI-150 (0.3 mg/kg), RTI-336 (1.0 mg/kg), and RTI-177 (0.3 mg/kg). A 3 mL 0.9% saline flush was administered immediately following each drug injection.
RESULTS
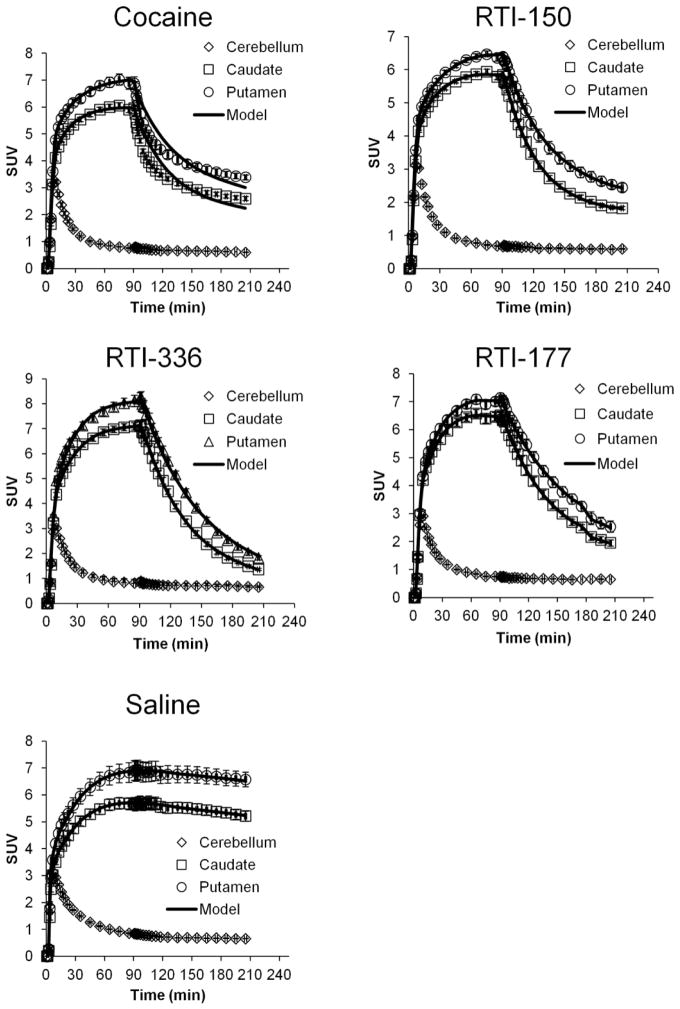
Examination of the time-activity curves indicated that the onset of drug occupancy at the DAT as measured by PET was immediate following intravenous infusion of all four drugs (Figure 1). These data indicate that all four drugs interact with the DAT in similar ways. Application of the GRTM displacement model to these data determined a single occupancy value in caudate of 0.75 +/− 0.05 for RTI-150, 0.96 +/− 0.02 for RTI-336, and 0.83 +/− 0.09 for RTI-177. Table 1 summarizes the measured fractional occupancy values for caudate and other regions. However, the GRTM model agreed poorly with the cocaine PET displacement experiments and appeared to over-estimate the occupancy of cocaine in caudate. The deviation of the measured data from the model suggests that the initially displaced [18F]FECNT is re-binding to the DAT in the later portion (150–210 min) of the drug displacement phase. These data were not reported for saline, as there was no observable DAT occupancy following saline infusion.
Figure 1.
Representitive time-activity curves of [18F]FECNT uptake in squirrel monkey caudate following i.v. infusion of cocaine, RTI-150, RTI-336, RTI-177, or saline. The drug challenge phase began 90 min post-injection of [18F]FECNT at which the drug was delivered by i.v. injection. The cerebellum was used as a reference region in the 5-parameter GRTM compartment model fit to estimate DAT density. Note that the caudate and putamen data for cocaine deviates from the model fit, while the data from the other three drugs do not.
Table 1.
Summary of dopamine transporter fractional occupancy in caudate and putamen of squirrel monkey (n=3).
| Compound | Dose mg/kg | Caudate | Putamen |
|---|---|---|---|
| RTI-150 | 0.3 | 0.85 ± 0.05 | 0.76 ± 0.05 |
| RTI-336 | 1.0 | 0.96 ± 0.02 | 0.97 ± 0.01 |
| RTI-177 | 0.3 | 0.83 ± 0.09 | 0.85 ± 0.10 |
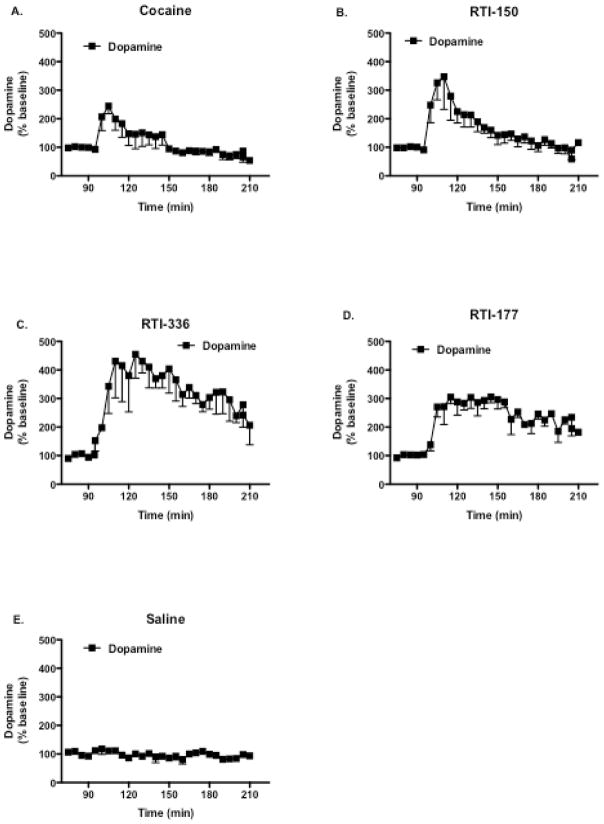
In contrast to the DAT occupancy data, the microdialysis data suggest that these drugs alter extracellular DA in different ways. Cocaine increased DA to a peak 245% of baseline levels 15 min after infusion, then DA declined to basal levels within 60 min post-injection. Infusion of RTI-150 increased DA to a peak of 350% of baseline levels 20 min later, and this increase declined to basal levels within 60 min after infusion. In contrast, RTI-336 slowly increased DA levels to a peak 450% of baseline levels 30 min following infusion. DA levels remained elevated for 60 min, and DA levels slowly declined to 200% of basal levels during the remaining 60 min of the imaging session with no indication of a further decline during the experimental session. RTI-177 increased DA levels to 300% of basal levels 15 min following drug infusion. DA levels remained elevated at this level for 60 min and then gradually decreased to 200% basal levels for the remaining 60 min of the imaging session with no indication of a further decline during the experimental session. Saline did not alter extracellular DA throughout the experimental session.
DISCUSSION
In this study, we determined the occupancy of three selective DAT inhibitors (RTI-150, RTI-336 and RTI-177) and cocaine in squirrel monkey caudate with the hypothesis that the previously observed differences in the neurochemistry of these drugs was a result of changes in drug occupancy at DAT over time. In the present study, all four drugs increased DA levels in the caudate to 250–400% of basal levels as measured by in vivo microdialysis, but the time course of their effects differed. These results corresponded well with our earlier observations in awake squirrel monkeys (Kimmel et al., 2007), indicating that the isoflurane anesthesia did not interfere with the effects of these drugs on extracelluar DA. The rate of onset of DAT occupancy of all four drugs was rapid and could not be resolved by the PET scanner. While the fast onset of occupancy of cocaine is supported by studies in rhesus monkeys showing that [11C]-labeled cocaine enters the brain rapidly following intravenous infusion (Kimmel et al., 2008), the current data contrasts with [11C]-labeled RTI-150, RTI-336, and RTI-177 which enter the brain more slowly. In addition, our earlier awake squirrel monkey (Kimmel et al., 2007) and rodent (Kimmel et al., 2001b) microdialysis studies suggest a faster brain entry of the challenge drug. However, these [11C]-labeled RTI studies do support a prolonged occupancy at DAT where binding of RTI-150, RTI-336 and RTI-177 peaked at 80, 50 and >90 min post-injection with a slow washout phase, respectively (Kimmel et al., 2008).
To the best of our knowledge, this is the first published study reporting the steady-state occupancy of a behaviorally active dose of RTI-150 in nonhuman primates. The in vivo microdialysis data showed that i.v. administration of RTI-150 rapidly increased DA levels, which then approached baseline DA levels by the end of the 2 hr observation period. However, the prolonged occupancy of DAT by RTI-150 suggests that DA levels should be elevated for a longer time, indicating a lack of concordance between the drug occupancy at DAT and the effects on extracelluar DA. This apparent discrepancy can be explained by reduced firing of the presynaptic neuron and/or to increased activation of presynaptic D2 autoreceptors, both of which would lead to decreased release of DA from the presynaptic neuron. As RTI-150 is a DAT inhibitor, its effects on extracelluar DA are impulse-dependent, similar to cocaine (Rudnick and Clark, 1993).
The calculated occupancies of RTI-336 and RTI-177 at the DAT correspond well with reported values of 90 +/− 5% for 1.07 mg/kg RTI-336 (Howell et al., 2007) and 73 +/−5% for RTI-177 (0.11 mg/kg) (Lindsey et al., 2004) in rhesus monkey, respectively. The earlier studies with [11C]-labeled RTI-336 and RTI-177 suggests that these drugs enter the brain slowly, whereas the current imaging data suggest that these drugs occupy DAT quickly and for a prolonged time. Despite the discrepancy between the rate of drug entry and occupancy at DAT, the prolonged occupancy of RTI-336 and RTI-177 at DAT agrees well with the extended elevation of extracellular DA observed using in vivo microdialysis.
The conflicting observations between rapid onset of occupancy in this work and our earlier studies with [11C]-labeled RTI may be due to a difference between tracer and drug kinetics. Other studies have shown that blockage of peripheral non-specific or specific binding sites by injecting cold carrier or competing ligand increases a drug’s free fraction in blood which has been shown to increase specific binding in receptors outside the brain (Breeman et al., 1995) as well as the rate of entry into brain (Huang et al., 2004; Malizia et al., 1997). A more complete profile of RTI kinetics could be obtained from a multiple injection study using increasing concentrations of cold-carrier with the [11C]-labeled injections. Thus the lack of such data is a limitation when interpreting the results of this work.
In contrast to RTI-150, RTI-336, and RTI-177, cocaine displacement of [18F]FENCT was poorly fit by the GRTM model, probably due to the short half-life of cocaine in brain. This allows for a proportion of previously unavailable DAT bound by cocaine earlier in the imaging session to become available for binding by [18F]FECNT later in the session. As measured by in vivo microdialysis, DA levels returned to basal values 60 min before the end of the imaging session, suggesting that cocaine was no longer binding to the DAT at levels that would produce these neurochemical effects. Based on these observations, we hypothesize that the binding of cocaine to DAT changes over the time course of the PET study due to cocaine metabolism and clearance from brain. Therefore, as the levels of cocaine decrease in brain, binding sites otherwise occupied by cocaine become available again for [18F]FECNT binding. Since the effects of cocaine are impulse-dependent, increases in DA would activate the autoreceptors, decreasing DA release, thereby attenuating the effects of cocaine, even if cocaine was still bound to DAT. In the current studies, we did not measure cocaine levels in brain, but previous studies have shown that cocaine is rapidly cleared from the nonhuman primate brain with a half-time of 45 min (Bradberry et al., 1993; Hurd and Herkenham, 1993). In addition, the short half-time of cocaine in non-human primate brain is supported by our studies in rhesus monkeys using radiolabeled cocaine, showing that cocaine is mostly cleared from the brain 90 min after administration (Kimmel et al., 2008).
That [18F]FECNT binding did not return to baseline levels late in the cocaine challenge suggests a change in the cell-surfaced expressed DAT equilibrium following acute cocaine administration. Previous animal and human studies have shown that chronic exposure to psychostimulants alter cell surface expression of DAT (Zahniser and Sorkin, 2009). While a number of studies support that amphetamine and other substrates for the DAT consistently decrease cell-surface expression of the DAT, fewer studies have examined the effects of DAT inhibitors on DAT expression (Schmitt and Reith, 2010). Studies conducted with DAT expressing heterologous cell lines show that cocaine increases cell-surface expression (Daws et al., 2002; Little et al., 2002), while others suggest that cocaine has no effect (Chi and Reith, 2003; Gorentla and Vaughan, 2005). Binding of [3H] WIN35,428 decreased in the caudate of rhesus monkeys self-administering a total of 4.5 mg/kg cocaine over 5 days, but [3H] WIN35,428 binding was increased in those animals self-administering a total of 431–588 mg/kg cocaine over a 2.5-year period (Letchworth et al., 2001), suggesting that the effects of acute cocaine on DAT expression differ from the effects of prolonged cocaine exposure. Validation of these putative models will require ex vivo visualization of DAT at the cell surface before and after acute drug challenge.
As the rate of DAT protein synthesis is relatively slow (2–3 days) (Kimmel et al., 2000; Kimmel et al., 2003), cells have to employ other strategies to regulate DAT protein expression on a short time scale (Schmitt and Reith, 2010). Two proposed ways for rapid regulation of DAT function are by a direct modification of single-transporter parameters, such as intrinsic substrate permeation efficacy or by affecting the trafficking of the protein, redistributing the DAT between the plasma membrane and intracellular compartments (Mortensen and Amara, 2003). The literature supports both processes, but the latter seems to be the primary mechanism for regulating DAT function (Gulley and Zahniser, 2003; Zahniser and Sorkin, 2004; Zahniser and Sorkin, 2009). Our studies seek to address this discrepancy using in vivo methods, which also allow us the advantage of measuring changes in cell surface DAT binding over long periods of time in order to determine the effects of repeated drug treatment or withdrawal periods. A limitation to these methods is that we will not be able to differentiate between the internalization of DAT and DAT that is expressed but does not bind to the radioligand. However, a recent study showed that the DAT of rats self-administering cocaine exhibited reduced sensitivity to cocaine without altering the ability of the DAT to transport DA and other substrates (Ferris et al., 2011). These results support a mechanism by which DAT function is regulated by exposure to DAT inhibitors and that cocaine binding and DA transport can be disassociated (Chen et al., 2005). In our studies, we indirectly assess DAT function by measuring changes in extracellular DA using in vivo microdialysis.
In summary, this PET neuroimaging study investigating DAT occupancy of cocaine and three analogs in squirrel monkeys study provided two important findings. One is that the occupancy of these drugs at DAT does not necessarily correspond directly with the rate of drug uptake in brain or with the observed changes in DA levels. The reason for this is unclear, but this finding supports the use of both neuroimaging and in vivo microdialysis techniques to elucidate the mechanism of action of drugs acting at DAT. The second important finding is that acute cocaine reduces the number of DAT available for [18F]FECNT binding.
Figure 2.
Time course of changes in DA levels, measured in caudate of squirrel monkeys (n=3) as determined by in vivo microdialysis. Cocaine (1.0 mg/kg), RTI-150 (0.3 mg/kg), RTI-336 (1.0 mg/kg), RTI-177 (0.3 mg/kg), or saline (equivalent volume) were administered i.v. 90 min following infusion of [18F]FECNT.
Acknowledgments
The authors thank Juliet Brown, Paul Chen, and Mi Zhou for their expert technical assistance in conducting this work. This research was supported by USPHS grants DA00517 (LLH), DA12514 (LLH), DA13326 (FIC), and DA010344 (LLH); RR00165 (Division of Research Resources, NIH); and Emory University Research Committee grant 281217, Emory University Research Council (URC) (HLK).
References
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Bradberry CW, Nobiletti JB, Elsworth JD, Murphy B, Jatlow P, Roth RH. Cocaine and Cocaethylene: Microdialysis Comparison of Brain Drug Levels and Effects on Dopamine and Serotonin. J Neurochem. 1993;60(4):1429–1435. doi: 10.1111/j.1471-4159.1993.tb03305.x. [DOI] [PubMed] [Google Scholar]
- Breeman WA, Kwekkeboom DJ, Kooij PP, Bakker WH, Hofland LJ, Visser TJ, Ensing GJ, Lamberts SW, Krenning EP. Effect of dose and specific activity on tissue distribution of indium-111-pentetreotide in rats. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1995;36(4):623–627. [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999;42(15):2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Chen R, Han DD, Gu HH. A triple mutation in the second transmembrane domain of mouse dopamine transporter markedly decreases sensitivity to cocaine and methylphenidate. J Neurochem. 2005;94(2):352–359. doi: 10.1111/j.1471-4159.2005.03199.x. [DOI] [PubMed] [Google Scholar]
- Chi L, Reith ME. Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther. 2003;307(2):729–736. doi: 10.1124/jpet.103.055095. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Justice JB, Jr, Howell LL. Cocaine-induced changes in extracellular dopamine determined by microdialysis in awake squirrel monkeys. Psychopharmacology. 2000;148:299–306. doi: 10.1007/s002130050054. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Moron JA, Kahlig KM, Shippenberg TS, Javitch JA, Galli A. Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun. 2002;290(5):1545–1550. doi: 10.1006/bbrc.2002.6384. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69(3):201–207. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, Camp VM, Malveaux E, Hoffman JM. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol. 2000;27(1):1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Gorentla BK, Vaughan RA. Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology. 2005;49(6):759–768. doi: 10.1016/j.neuropharm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. Eur J Pharmacol. 2003;479(1–3):139–152. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L. Behavioral properties of GBR12909, GBR13069, GBR13098: specific inhibitors of dopamine uptake. Eur J Pharmacol. 1984;103:241–248. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320(2):757–765. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Huang Y, Hwang DR, Bae SA, Sudo Y, Guo N, Zhu Z, Narendran R, Laruelle M. A new positron emission tomography imaging agent for the serotonin transporter: synthesis, pharmacological characterization, and kinetic analysis of [11C]2-[2-(dimethylaminomethyl)phenylthio]-5-fluoromethylphenylamine ([11C]AFM) Nucl Med Biol. 2004;31(5):543–556. doi: 10.1016/j.nucmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13(4):357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang S-C, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Carroll FI, Kuhar MJ. Dopamine transporter synthesis and degradation rate in rat striatum and nucleus accumbens using RTI-76. Neuropharmacology. 2000;39:578–585. doi: 10.1016/s0028-3908(99)00160-4. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Carroll FI, Kuhar MJ. Locomotor stimulant effects of novel phenyltropanes in the mouse. Drug Alcohol Depend. 2001a;65(1):25–36. doi: 10.1016/s0376-8716(01)00144-2. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Carroll FI, Kuhar MJ. Withdrawal from repeated cocaine alters dopamine transporter protein turnover in the rat striatum. J Pharmacol Exp Ther. 2003;304(1):15–21. doi: 10.1124/jpet.102.038018. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Ivy Carroll F, Kuhar MJ. Locomotor stimulant effects of novel phenyltropanes in the mouse. Drug Alcohol Depend. 2001b;65(1):25–36. doi: 10.1016/s0376-8716(01)00144-2. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Ewing SB, Votaw JR, Goodman MM, Carroll FI, Mello NK, Howell LL. Behavioral pharmacology and pharmacokinetics of cocaine analogs in rhesus monkeys. Waikoloa, HI: Nature Publishing Group; 2005. Dec 11–15, pp. S147–S148. [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90(3):453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O’Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86(1):45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug Alcohol Depend. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified Reference Tissue Model for PET Receptor Studies. Neuroimage. 1996;4(3):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309(3):959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Little KY, Elmer LW, Zhong H, Scheys JO, Zhang L. Cocaine induction of dopamine transporter trafficking to the plasma membrane. Mol Pharmacol. 2002;61(2):436–445. doi: 10.1124/mol.61.2.436. [DOI] [PubMed] [Google Scholar]
- Malizia AL, Melichar JM, Brown DJ, Gunn RN, Reynolds A, Jones T, Nutt DJ. Demonstration of clomipramine and venlafaxine occupation at serotonin reuptake sites in man in vivo. J Psychopharmacol. 1997;11(3):279–281. doi: 10.1177/026988119701100312. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15(3):217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479(1–3):159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Press WH. Numerical recipes: the art of scientific computing. xx. New York, N.Y: Cambridge University Press; 1989. p. 702. [Google Scholar]
- Reith MEA, Meisler BE, Sershen H, Lajtha A. Structural requirements for cocaine congeners to interact with dopamine and serotonin uptake sites in mouse brain and to induce stereotyped behavior. Biochem Pharmacol. 1986;35:1123–1129. doi: 10.1016/0006-2952(86)90148-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Kuhar MJ. Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther. 1989;248:1010–1017. [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144(3):249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- SAMHSA U. Results from the 2006 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of Applied Studies; 2007. [Google Scholar]
- Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Rice KC, Glowa JR. A comparison of cocaine, GBR 12909, and phentermine self-administration by rhesus monkeys on a progressive-ratio schedule. Drug Alcohol Depend. 2001;62(1):41–47. doi: 10.1016/s0376-8716(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16(23):7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162(8):1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Votaw JR, Howell LL, Martarello L, Hoffman JM, Kilts CD, Lindsey KP, Goodman MM. Measurement of dopamine transporter occupancy for multiple injections of cocaine using a single injection of [F-18]FECNT. Synapse. 2002;44(4):203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL. In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse. 2005;58(4):220–228. doi: 10.1002/syn.20199. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. Eur J Pharmacol. 1999;367(2–3):175–181. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: practical and theoretical concerns. Psychopharmacology (Berl) 2000;153(1):139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94(4):469–492. [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, Newman AH. Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology (Berl) 2001;154(4):375–382. doi: 10.1007/s002130000616. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47(Suppl 1):80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin Cell Dev Biol. 2009;20(4):411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]