Abstract
Purpose
To examine effects and mechanisms of transient activation of Hedgehog pathway on rescuing radiotherapy-induced hyposalivation in head and neck cancer survivors.
Experimental Design
Mouse salivary glands and cultured human salivary epithelial cells were irradiated by single 15Gy dose. Hedgehog pathway was transiently activated in mouse salivary glands by shortly over-expressing Sonic hedgehog (Shh) transgene or administrating Smoothened Agonist and in human salivary epithelial cells by infecting with adenovirus encoding Gli1. Activity of Hedgehog signaling was examined by expression of Ptch1-lacZ reporter and endogenous Hedgehog target genes. Salivary flow rate was measured following pilocarpine stimulation. Salivary stem/progenitor cells (SSPCs), parasympathetic innervation and expression of related genes were examined by flow cytometry, salisphere assay, IHC, quantitative RT-PCR, Western blot and ELISA.
Results
Irradiation does not activate Hedgehog signaling in mouse salivary glands. Transient Shh over-expression activated Hedgehog pathway in ductal epithelia and that after irradiation rescued salivary function in male mice, which is related with preservation of functional SSPCs and parasympathetic innervation. The preservation of SSPCs was likely mediated by rescue of signaling activities of Bmi1 and Chrm1/HB-EGF pathways. The preservation of parasympathetic innervation was related with rescue of expression of neurotrophic factors such as Bdnf and Nrtn. The expression of genes related with maintenance of salivary stem/progenitor cells and parasympathetic innervation in female salivary glands and cultured human salivary epithelial cells was similarly affected by irradiation and transient Hedgehog activation.
Conclusions
These findings suggest that transient activation of Hedgehog pathway has the potential to restore irradiation-induced salivary gland dysfunction.
Keywords: hyposalivation, Hedgehog signaling, radiotherapy, stem cells, parasympathetic innervation
Introduction
Head and neck cancers (HNC) account about 3.2% of all cancers in USA with about 342,550 survivors in 2012 (1, 2). Radiotherapy is the most common form of treatment for HNC, and nondiseased salivary glands are often exposed to radiotherapy. Due to the exquisite radiosensitivity of salivary glands, irreversible hyposalivation is common in long-term HNC survivors treated with radiotherapy even when various new techniques were applied to minimize the irradiation (IR) damage to salivary glands (3). Hyposalivation exacerbates dental caries and periodontal disease, and severely impair the quality of life of patients. The irreversible hyposalivation has been attributed to loss of functional salivary stem/progenitor cells (SSPCs) that normally continuously replenish aged saliva producing cells (4). Current treatments for IR-induced xerostomia can only temporarily relieve these symptoms. Several new approaches, including gene transfer and stem cell infusion, showed promises to restore salivary gland function by protection or regeneration of saliva-producing cells (5). However, little is known about the molecular regulation of SSPCs and functional restoration.
Hedgehog (Hh) signaling is required for salivary branching morphogenesis (6) and activated during regeneration of adult salivary glands (7). The Hh receptor Patched (Ptch) acts as a negative regulator of Smoothened (Smo) that blocks production of repressor forms of the Gli transcription factors (GliR) and promotes production of the activator forms (GliA). The binding of Hh ligands to Ptch unleashes Smo activity to tip the balance toward GliA that consequently activates expression of Hh target genes (8). Sonic Hedgehog (Shh), one major Hh ligand, is expressed in epithelium of embryonic salivary gland, and may act within the epithelium in a juxtacrine manner to promote proliferation and differentiation of epithelial cells (9). In submandibular glands (SMG) of adult mouse Hh signaling is marginal but activated significantly during functional regeneration after duct ligation (7). We report in this study that in mouse models IR does not activate Hh pathway in SMGs as occlusive damage does, and transient activation of Hh pathway by Shh over-expression in basal epithelia of male mice rescued IR-induced hyposalivation likely through perseveration of SSPCs and parasympathetic innervation. IR and transient Hh activation by other approach similarly affected the expression of genes essential for SSPCs maintenance and parasympathetic innervation in SMGs of female mice and cultured human salivary epithelial cells. These data indicated that transient activation of Hh pathway could potentially benefit salivary gland function following radiotherapy of head-and-neck cancer.
Materials and Methods
Mice
Hedgehog signaling activity was analyzed with B6;129-Ptch1tm1Mps/J (Ptch1-lacZ) mice (The Jackson Laboratory). Mice carrying tetO-Shh (10) and Krt5-rtTA (11) transgenes were placed on doxycycline (Dox) chow (6g/kg, Bio-serv) to induce Shh expression. Irradiation of mouse and measurement of stimulated saliva flow rate was as reported (11). All animal procedures were approved by TAMHSC and Scott & White Hospital IACUC.
Quantitative RT-PCR Analysis
Quantitative RT-PCR (qRT-PCR) was done as reported (7). qRT-PCR analysis for miRNAs was performed with TaqMan microRNA assays (Applied Biosystems) using U6 snRNA as the reference RNA.
ELISA and Western blot
Fresh SMG samples were homogenized with 40μl T-PRE reagent containing protease inhibitors (Pierce, USA) per mg followed by centrifugation at 10,000 g for 5 minutes to collect supernatant for ELISA and Western blot. The concentration of Bdnf and Nrtn in saliva and SMG samples was examined with ELISA kits (Insight Genomics and MyBioSource). Western blot was done as reported (7) with antibodies for Aqp5 (Abcam 1:5000), p21Waf1 (Millipore, 1:500) and GFRa2 (Abcam 1:1000).
Flow cytometry
The antibodies used were against c-kit or Sca-1 (BioLegend, 1:50), Bmi1 (Abcam, 1:100), Gli1 (Thermo, 1:100) or Chrm1 (ABBIOTEC, 1:100). For Bmi1 and Gli1 staining, cells were permeabilized with FIX & PERM® reagents (Life Technologies). Stained cells were analyzed on a Cytomics FC500 flow cytometer (Beckman Coulter) and data were processed using the manufacturer's software (CXP).
Histology and immunofluorescence staining
Frozen SMG sections were stained with Acetylcholinesterase (AChE) rapid staining kit (MBL, Japan) or an antibody against Chrm1 (1:2000, R&D Abs). AChE stain was quantified with NIS-Elements AR software (Nikon, Japan).
SAG treatment
Small molecule Hh agonist SAG (EMD) or vehicle were administrated into female Ptch1-lacZ mice by SMG cannulation (2μg/g) then followed by daily i.p. injection of 5μg/g for 3 days. SMGs samples were collected 1 day after last injection for X-gal staining and qRT-PCR analysis.
Isolation and treatment of human salivary epithelial cells
Healthy human salivary gland samples from patients (2 males and 2 female) with an age range of 25–61 years old were provided by the Cooperative Human Tissue Network (CHTN), Southern and Mid-Western Divisions, a National Cancer Institute supported resource. Human SMG epithelial cells were isolated as reported (12) and cultured in mammary epithelial medium CnT-27 (Zen-Bio). Passage 4 cells were treated with 15 Gy single dose IR, then some cells were infected with adenoviruses encoding human Gli1 or GFP (Applied Biological Materials Inc., Canada, MOI = 10) 3 days later. All cells were collected 7 days after IR for analysis.
Effects of transient Hh activation in SMGs on SCC VII tumors
The mouse SCC VII tumor model was established and some tumors were irradiated as reported (13). Adenoviral vectors encoding GFP or rat Shh (AdGFP or AdShh, Applied Biological Materials Inc., Canada) was delivered at 109 particles per SMG by retrograde ductal instillation on Day 0 (inoculation of 3 × 104 SCC VII cells) or Day 9 (3 × 105 cells). Mice were euthanized when tumor diameter was approximately 15 mm.
Statistics
All quantified data were analyzed using one-way ANOVA followed by Tukey's multiple-comparison test. Statistical analysis and graphical generation of data were done with GraphPad Prism software.
Results
Hh activity in SMGs after irradiation or transient Shh over-expression
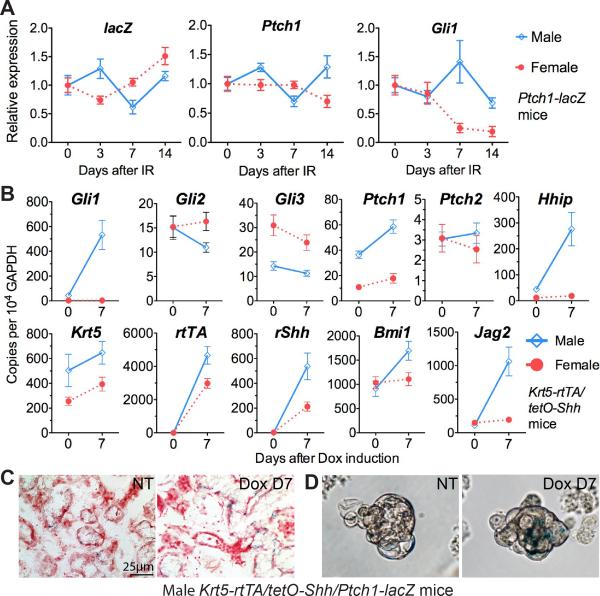
We reported previously that the Hh signaling is activated during functional regeneration of salivary gland after ligation of the main excretory ducts of SMGs (7). Using Ptch1-lacZ Hh reporter transgenic mice, we confirmed that the Hh activity is marginal in SMGs of adult mice but remarkably elevated in ductal epithelia after duct ligation (Fig. S1). To examine the effect of IR on Hh signaling in salivary gland, Ptch1-lacZ mice were treated with 15 Gy single dose IR in head and neck region, and SMGs were collected 3, 7 or 14 days after IR for qRT-PCR analysis and X-gal staining of lacZ activity. IR did not significantly affect the expression of reporter Ptch1-lacZ and endogenous Ptch1 gene in SMGs of both male and female mice (Fig. 1A and Fig. S1, P > 0.05, n=3). The expression of another Hh target gene Gli1 was not significantly affected by IR in male SMGs but was significantly decreased in female SMGs on Day 7 and Day 14 (Fig. 1A, P<0.05, n=3). These results indicated that IR does not activate Hh pathway in SMGs.
Fig. 1.
Effects of IR and Shh on Hh activity in SMGs. (A) SMG samples from Ptch1-lacZ Hh reporter transgenic mice were collected before (D0) or 3, 7 or 14 days after 15 Gy single dose IR, and analyzed with qRT-PCR for relative mRNA expression (n=3). (B) Krt5-rtTA/tetO-Shh mice were not treated (NT) or induced with Dox for 7 days, then sacrificed to collect SMGs for qRTPCR analysis of transgenes and genes related with Hh pathway (n=3). (C,D) SMGs of male Krt5-rtTA/tetO-Shh/Ptch1-lacZ mice were collected for X-gal staining on sections (C) or salispheres (D) without or with Dox induction for 7 days.
To modulate Hh pathway we generated Krt5-rtTA/tetO-Shh mice that overexpress Shh in basal epithelial cells including those in ducts of salivary glands upon Dox induction. 7 days of Dox induction significantly upregulated expression of Hh target genes Gli1, Ptch1, Hhip, Bmi1 and Jag2 in male SMGs not in female SMGs (Fig. 1B). The much higher expression level of Ptch1 compared to Ptch2 indicated that Ptch1 is the dominant Hh receptor in SMGs of both gender (Fig. 1B), whereas the Ptch1 expression in female SMGs is much lower than that in males before or after Dox induction (Fig. 1B). Similarly, although the expression of Gli2 and Smo in SMGs before induction (Fig. S6) was comparable between males and females, the expression of Gli1 with dominant activator activity (14) was much lower whereas the expression of Gli3 with dominant repressor activity (14) was much higher in females (Fig. 1B). In addition, the induction of Shh transgene in female SMGs is much less efficient likely due to lower expression of Krt5 and rtTA (Fig. 1B). All these factors likely contribute to the insufficient Hh activation by Shh in females SMGs. In SMGs of male Krt5-rtTA/tetO-Shh/Ptch1-lacZ, the expression of lacZ Hh reporter was marginal before Dox induction and was significantly elevated in ductal epithelia after 7 days of Dox induction (Fig. 1C). The upregulation of Ptch1-lacZ reporter and Hh target genes after transient Shh over-expression appeared comparable to that after duct ligation (Fig. S1 and Hai et al., 2010 Fig. 3C, about 2 folds for Ptch1 in both cases and 12 vs. 7 folds for Gli1), indicating that the overall Hh activation by transient Shh over-expression is comparable to that during functional SMG regeneration. SSPCs can be enriched by spherical culture to form salispheres (15). In salisphere cells from male Krt5-rtTA/tetO-Shh/Ptch1-lacZ mice, expression of lacZ Hh reporter was very weak without Dox induction, but was significantly increased in a subset of salisphere cells after 7 days of Dox induction (Fig. 1D). These data indicated that Hh pathway was efficiently activated in ductal epithelia and SSPCs in SMGs of male Krt5-rtTA/tetO-Shh mice upon Dox induction.
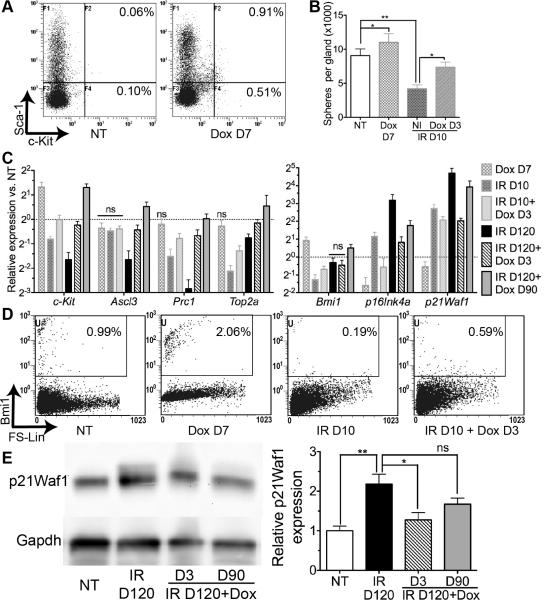
Fig. 3.
Transient Hh activation preserved functional SSPCs and the activity of Bmi1 pathway impaired by IR. (A) Flow cytometry analysis of c-Kit+/Sca-1+ cells in SMGs. (B) Salisphere numbers from each whole SMG collected 10 days after IR or 7 day after Dox treatment. (C) Expression of genes related with SSPCs and proliferation examined by qRT-PCR. (D) Flow cytometry analysis of Bmi1+ cells in SMGs . (E) Western blot and relative quantification of p21Waf1. n=3 for all studies. ns: not significant.
Rescue of IR-induced hyposalivation by transient Hh activation
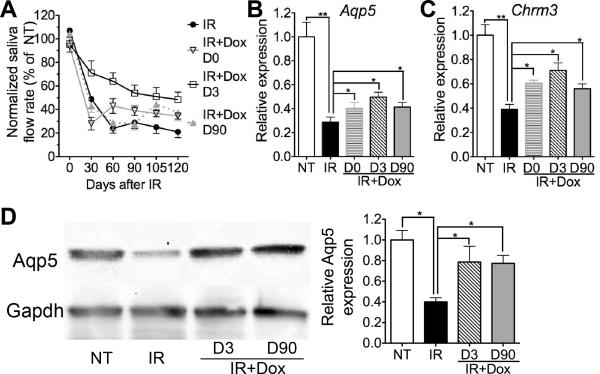
To evaluate the potential of transient activating Hh pathway in restoration of salivary gland function after IR, adult male Krt-rtTA/tetO-Shh mice were treated with 15 Gy single dose IR in head and neck region, then Shh expression was induced from Day 0, 3 or 90 after IR for 7 days. As expected, IR significantly reduced the saliva flow rate in mice throughout the observation period from Day 30 to 120 (Fig. 2A, P<0.05, n=5). Hh activation 3 or 90 days after IR both significantly improved the saliva flow rate compared to IR group throughout the observation period after Dox induction, whereas concurrent Hh activation improved the saliva flow rate only from Day 60 after IR to a much less extent than Hh activation from Day 3 (Fig. 2A, n=5). Consistently, expression of acinar markers Aquaporin5 (Aqp5) and cholinergic receptor muscarinic 3 (Chrm3) in SMGs was significantly down-regulated by IR on Day 120, but improved by transient Hh activation 0, 3 or 90 days after IR as indicated by qRT-PCR and Western blot, with highest improvement in the Day 3 group (Fig. 2B-D, n=3). 7 days of Dox treatment in female Krt-rtTA/tetO-Shh mice or male wild type C57BL/6 mice 3 or 90 days after IR have no significant effects on IR-induced decrease of saliva flow rate and expression of Aqp5 and Chrm3 (Fig. S2, P > 0.05, n=5), indicating that the rescue effects of Dox induction in male Krt-rtTA/tetO-Shh mice was mediated by efficient Hh activation.
Fig. 2.
Transient Hh activation rescued radiation-induced hyposalivation and expression of acinar markers. (A) The stimulated whole saliva flow rate in male Krt5-rtTA/tetO-Shh mice irradiated (IR) or IR and induced with Dox for 7 days starting from Day 0, 3 or 90 after IR (IR+Dox D0, D3 or D90) were standardized with the body weight and normalized to that of non-treated (NT) group (n = 5). (B-D) 120 days after IR, SMG samples were collected and analyzed with qRT-PCR (B, C) and Western blot (D) for expression of acini marker Aqp5 and Chrm3 normalized to Gapdh (n=3). *: P<0.05, **: P < 0.01.
Preservation of salivary stem/progenitor cells and Bmi1 signaling pathway after IR by transient Hh activation
SSPCs can be identified by expression of surface markers c-Kit and Sca-1 (16). Flow cytometry analysis found that the population of c-Kit+/Sca-1+ cells was very rare in non-treated SMGs, but was significantly expanded after 7 days of Hh activation (Fig. 3A, 0.85%±0.33% vs. 0.06%±0.02% in NT group, mean±SEM, P<0.05, n=3). However, 60 days after Hh activation, the percentage of c-Kit+/Sca-1+ cell population was not significantly affected compared to control mice (Fig. S3), indicating that the effect of Hh activation on expansion of SSPCs is transient. The rarity of c-Kit+/Sca-1+ cells in NT SMGs made it difficult to evaluate the effects of IR and transient Hh activation after IR on this cell population. Hence we examined the number of salisphere-forming cells per gland (Fig. 3B, n=3) that reflects the number of functional salivary stem/progenitor cells and was significantly reduced several days after IR (15). Shh over-expression for 7 days in male mice increased numbers of salisphere-forming cells per SMG significantly (P<0.05), consistent with the expansion of c-Kit+/Sca-1+ cell population. IR decreased salisphere numbers by Day 10 as expected, whereas Hh activation 3 days after IR significantly ameliorated IR-induced decrease of salisphere numbers (P<0.05). Consistently, as indicated by qRT-PCR analysis of SMG tissues (Fig. 3C, n=3), the expression of c-Kit mRNA was significantly upregulated by transient Hh activation, decreased by IR on both Day 10 and 120, recovered on Day 10 and 120 by transient Hh activation 3 days after IR, and upregulated on Day 120 by transient Hh activation 90 days after IR (P<0.05). On the other hand, the expression of salivary progenitor cell marker Ascl3 (17) was not significantly affected by Hh activation or by IR on Day 10, but was significantly decreased on Day 120 after IR, and recovered by transient Hh activation 3 or 90 days after IR (P<0.05). Consistent with the reported impairment of proliferation by IR (18), the expression of proliferation related genes Prc1 and Top2a (19) was significantly decreased by IR (P<0.05). Transient Hh activation did not significantly affect the expression of proliferation markers Prc1, Top2a and proliferative cell nuclear antigen (PCNA) in non-irradiated SMGs (Fig. 3C and Fig. S4A, P>0.05), but significantly recovered the expression of Prc1 and Top2a decreased by IR (Fig. 3C, P<0.05).
IR-induced apoptosis in salivary glands peaked on Day 2 to 3 after IR and decreased significantly thereafter (11)(20), which may contribute to SSPCs loss. Hh signaling is anti-apoptotic in most circumstances (21). However, Tunel assay (Fig. S4B) indicated that on Day 7 and 11 after IR, Hh activation from Day 3 did not significantly affect the IR-induced apoptosis, suggesting that the beneficial effects of post-IR transient Hh activation is not likely related with inhibition of apoptosis. Interestingly, although concurrent transient Hh activation (Day 0-7) significantly inhibited apoptosis on Day 3, it significantly increased apoptosis on Day 7 and 11, which may contribute to the much less improvement of salivary function in this group. The delayed increase of apoptosis on Day 7 and 11 in concurrent Hh activation group might be related with increased Ptch1 expression that is pro-apoptotic in the absence of Hh ligands (22) and decreased expression of anti-apoptosis protein p21Waf1 (23) after Hh activation as shown below.
Hh target gene Bmi1 is related with maintenance of various adult epithelial stem cells through repression of cyclin dependent kinase (CDK) inhibitors p16Ink4a/p19Arf and p21Waf1 (24-26). As indicated by qRT-PCR analysis (Fig. 3C, n=3), the expression of Bmi1 mRNA in SMGs was significantly upregulated by transient Hh activation, decreased by IR on Day 10 but not on Day 120, and recovered by transient Hh activation 3 days after IR (P<0.05). Meanwhile, the expression of p16Ink4a and p21Waf1 mRNA was regulated oppositely to that of Bmi1 by Hh activation and IR, and remained at high level on Day 120 after IR (P<0.05). Flow cytometry analysis (Fig. 3D, n=3) confirmed that Bmi1+ cell population in SMGs was significantly increased after 7 days of Hh activation (1.95%±0.23% vs. 1.02%±0.07% in NT group, P<0.05), decreased on Day 10 after IR (0.22%±0.06%, P<0.05 vs. NT), and preserved by Hh activation 3 days after IR (0.74%±0.22%, P > 0.05 vs. NT). Western blot confirmed that the expression of p21Waf1 in SMGs was significantly increased by IR on Day 120 but reversed by transient Hh activation 3 or 90 days after IR (Fig. 3E, P<0.05, n=3). Flow cytometry also indicated that Bmi1 is expressed in a sub-population of c-Kit+ salivary stem/progenitor cells that is expanded by Hh activation (Fig. S5). These data indicated that the rescue of SSPC maintenance after IR by Hh activation is related with Bmi1 pathway.
Preservation of parasympathetic innervation, Chrm1/HB-EGF signaling and expression of neurotrophic factors after IR by transient Hh activation
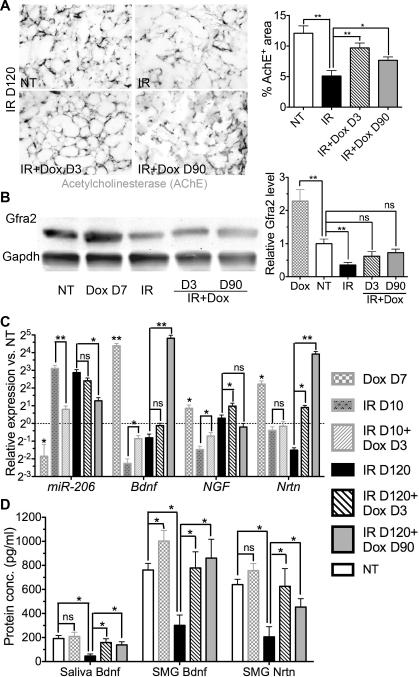
Parasympathetic innervation is essential for both organogenesis and regeneration of salivary glands after duct ligation (27, 28), whereas IR reduced parasympathetic innervation in salivary glands of both human adults and mouse embryos (29). By examining the activity or expression of two markers of parasympathetic nerve, Acetylcholinesterase (AChE) and glial cell line derived neurotrophic factor family receptor α2 (GFRα2) (Fig. 4A, B, n=3), we confirmed that IR impaired parasympathetic innervation similarly in adult mouse SMGs on Day 120 (P<0.01). The impairment of AChE activity by IR was significantly ameliorated by transient Hh activation 3 or 90 days after IR (P<0.05), whereas GFRα2 expression was remarkably increased by transient Hh activation alone (P<0.01) and was not significantly decreased in SMGs underwent transient Hh activation 3 or 90 days after IR compared to that in NT group (P>0.05). These data indicated that the parasympathetic innervation is preserved by transient Hh activation after IR.
Fig. 4.
Transient Hh activation preserved parasympathetic innervation and the expression of neurotrophic factors impaired by IR. (A) AChE staining on SMG sections and the quantification of staining intensity. (B) Western blot and relative quantification of GFRα2. (C) Expression of neurotrophic factors and miR-206 examined by qRT-PCR. (D) Concentrations of Bdnf in whole saliva or SMG homogenates and that of SMG Nrtn detected by ELISA. n=3 for all studies.
Hh signaling is known to promote innervation or regeneration of various peripheral nerves via maintenance of nerve structure (30), induction of angiogenesis (31), or induction of neurotrophic factors such as Brain-derived neurotrophic factor (Bdnf) via repression of miR-206 or other unknown mechanisms (32, 33). Bdnf, Nerve growth factor (Ngf) and Neurturin (Nrtn) are expressed in salivary glands (34) and are essential for parasympathetic innervation in embryonic mouse SMGs (29) or other tissues (35, 36). As indicated by qRT-PCR (Fig. 4C, n=3), transient Hh activation significantly increased miR-206 expression and decreased Bdnf expression, whereas IR significantly increased miR-206 expression and decreased Bdnf expression on both Day 10 and 120, and transient Hh activation after IR significantly reversed the effects of IR on expression of miR-206 and Bdnf on Day 7 or 30 after Hh activation (P<0.05). The expression of Ngf and Nrtn was only significantly decreased by IR on Day 10 or Day 120 respectively (P<0.05). Similar to Bdnf, the expression of Ngf and Nrtn was significantly upregulated by transient Hh activation, and restored or elevated by transient Hh activation after IR on Day 10 or Day 120 respectively (P<0.05). ELISA confirmed that the Bdnf level in SMG homogenates was significantly increased by transient Hh activation, whereas the levels of Bdnf and Nrtn in SMG homogenates and whole saliva Bdnf were significantly decreased on Day 120 after IR and restored by transient Hh activation either 3 or 90 days after IR (Fig. 4D, P<0.05, n=3).
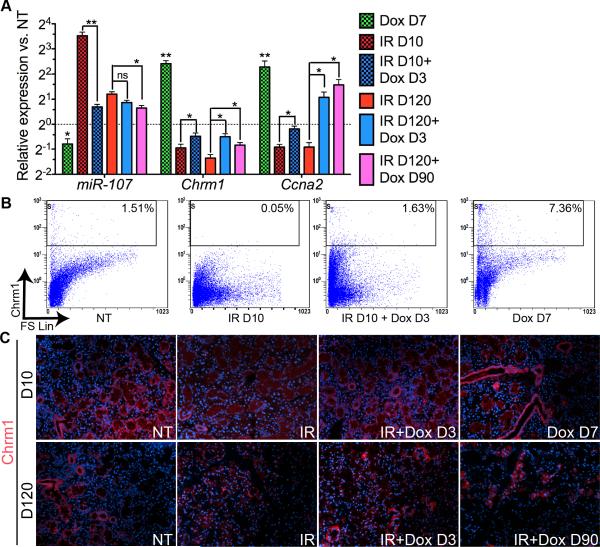
During salivary organogenesis, parasympathetic innervation signals via cholinergic receptor muscarinic 1 (Chrm1) to promotes epithelial morphogenesis and proliferation of progenitor cells by transactivating Heparin-Binding Epidermal Growth Factor (HB-EGF) pathway (27), which activates expression of genes related with cell cycle progression such as cyclin A2 (Ccna2) (37). Chrm1 expression is repressed by miR-107 (38) that is upregulated by IR (39) but decreased in cells expressing high level of Shh (40). With qRT-PCR analysis (Fig. 5A, n=3), we found that in mouse SMGs the expression of miR-107 was significantly decreased by transient Hh activation and increased by IR as expected and recovered by transient Hh activation after IR within 7 or 30 days (P<0.05); consistently, the expression of Chrm1 and Ccna2 was significantly increased by transient Hh activation, decreased by IR, and recovered by transient Hh activation after IR (P<0.05). Flow cytometry assay (Fig. 5B, n=3) indicated that the proportion of Chrm1+ cells in SMGs was significantly increased after transient Hh activation (6.79%±1.55% vs. 1.63%±0.24% in NT group, P<0.05), decreased on Day 10 after IR (0.07%±0.03%, P<0.05 vs. NT), and preserved by Hh activation 3 days after IR (1.88%±0.21%, P>0.05 vs. NT). Immunofluorescent staining for Chrm1 (Fig. 5C) indicated that in non-treated SMGs Chrm1 is expressed weakly in acinar structures and strongly in ductal structures, IR significantly downregulated Chrm1 expression particularly in ductal structures, whereas transient Hh activation after IR restored Chrm1 expression. These data suggested Chrm1/HB-EGF signaling activity was preserved by transient Hh activation after IR, which may contribute to preservation of functional salivary stem/progenitor cells in combination of preserved parasympathetic innervation.
Fig. 5.
Transient Hh activation preserved the activity of Chrm1 pathway impaired by IR. (A) Expression of miR-107, Chrm1 and Ccna2 examined by qRT-PCR (n=3). (B) Flow cytometry analysis of Chrm1+ cells in SMGs. (C) Immunofluorescence staining of Chrm1.
Effects of IR and transient Hh activation on SMGs of female mice and human salivary epithelial cells
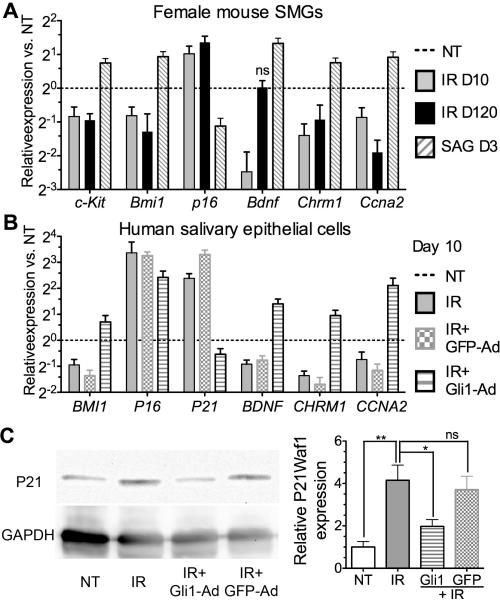
Sexual dimorphism is well recognized in salivary glands of rodents and the SMGs of male rodents contain a unique structure, granular convoluted tubule (GCT), that is not present in SMGs of human or female rodents (41). To determine whether the data above from male mice are gender-specific, we examined effects of IR and transient Hh activation with a small molecule Hh agonist SAG (Smoothened Agonist) in SMGs of female mice based on the comparable expression of Smoothened in SMGs between males and females (Fig. S6). As indicated by qRTPCR (Fig. 6A, n=3), in female SMGs IR affected the expression of genes related with SSPCs maintenance (c-Kit, Bmi1, p16, Chrm1 and Ccna2) on Day 10 and 120 and gene essential for parasympathetic innervation (Bdnf) on Day 10 after IR in a manner similar to that in male SMGs (P<0.05). Local delivery of SAG by cannulation efficiently activated Hh pathway in ductal SMG epithelial cells of female mice as indicated by induction of Ptch1-lacZ Hh reporter (Fig. S6). Similar to that in male SMGs transiently over-expressing Shh, qRT-PCR indicated that SAG treatment significantly upregulated expression of c-kit, Bmi1, Bdnf, Chrm1 and Ccna2, and decreased p16 expression (P<0.05).
Fig. 6.
Effects of IR and transient Hh activation on gene expression in SMGs of female mice and human salivary epithelial cells. (A,B) Gene expression examined by qRT-PCR in SMGs of female C57BL/6 mice 10 or 120 days after IR or in SMGs of female Ptch1-lacZ mice treated with SAG for 3 days (A, n=3), and in human salivary epithelial cells 10 days after IR with or without infection of Gli1 or GFP adenoviruses (B, n = 4). (C) Western blot and relative quantification of human P21 (n = 4).
To examine effects of IR and transient Hh activation on human salivary glands, we isolated human salivary epithelial cells from biopsy samples. These cells showed a typical cobblestone morphology of epithelial cells in culture and most cells express epithelial marker Krt14 and/or salivary glandular cell marker Aqp5 (Fig. S7). Similar to that in mouse SMGs, qRT-PCR (Fig. 6B, n = 4) indicated that in human salivary epithelial cells collected on day 10 after IR the mRNA expression of BMI1, BDNF, CHRM1 and CCNA2 was significantly decreased, whereas that of P16 and P21 was significantly increased (P<0.05). These IR-caused changes of gene expression was not significantly affected by infection with control adenovirus encoding GFP (GFP-Ad) (P>0.05), but was significantly reversed by infection with adenovirus encoding Gli1 (Gli1-Ad) 3 days after IR (P<0.05). Western blot assay confirmed that the expression of P21Waf1 protein was upregulated by IR and this upregulation was not affected by GFP-Ad infection but repressed by Gli1-Ad infection (Fig. 6C, P<0.05, n = 4).
Taken together, these data indicated that effects of IR and transient Hh activation on SMGs of female mice and cultured human salivary epithelial cells are similar to that in male mice, and suggested that transient activation of Hh pathway might restore the expression of genes related with SPSC maintenance and parasympathetic innervation impaired by IR to rescue IR-induced hyposalivation in human.
Effects of Shh gene transfer within SMGs on solid tumor
Since activation of Hh pathway in head and neck cancer is linked to poorer outcomes (42), it is important to determine whether transient Hh activation within salivary gland can affect the Hh activity and growth of such pre-existed cancers. Squamous cell carcinoma (SCC) is the most common head and neck cancer, so we used the mouse SCC VII tumor model to test this possibility. Infecting SCC VII cells in vitro with adenovirus encoding rat Shh (AdShh) significantly increased the expression of Hh target genes Gli1 and Ptch1 in 7 days (Fig. S8A), indicating that these cells are Hh-responsive. Retrograde delivery of AdShh into SMGs of mice carrying subcutaneous SCC VII tumors significantly increased expression of rShh transgene and Hh target genes Gli1 and Ptch1 in SMGs in 7 days (n=4, P<0.05), but had no significant effect on expression of these genes in tumors at this time point and on tumor growth with or without IR of tumor throughout the observation period (Fig. S8B-D, P>0.05). Similar results were found when AdShh was delivered into SMGs right after subcutaneous inoculation of 10 times fewer SCC VII cells (Fig. S8E). These data indicated that local Hh activation could be achieved in SMGs without promoting growth of pre-existed tumors outside of salivary glands.
Discussion
The loss of functional salivary stem/progenitor cells is believed to be a major cause of IR-induced hyposalivation (4). We reported here that transient Hh activation promotes SSPC expansion without IR and rescues SSPC maintenance after IR. Hh signaling regulates adult stem/progenitor cells upstream of Bmi1 pathway in other epithelial tissues (24)(43). Bmi1 is a transcriptional repressor that regulates stem cell self-renewal through repression of important cell cycle regulatory genes including p16INK4A, p19ARF and p21Waf1 (24-26). Our data indicated that in salivary glands the expression of Bmi1 and its target genes is highly related with changes of functional SSPC population caused by IR and transient Hh activation, suggesting that Bmi1 pathway may mediate the effect of Hh activation on SSPC maintenance. In human salivary glands (29). Hh signaling can protect and promote recovery of various peripheral nerves from injuries (30-32). We showed here that in adult mouse SMGs the expression of multiple neurotrophic factors, the parasympathetic innervation, and the Chrm1/HBEGF signaling pathway connecting the parasympathetic innervation to maintenance of stem/progenitor cells were significantly impaired by IR and rescued by transient Hh activation after IR, which may also contribute to IR-induced hyposalivation and the rescue by Hh activation. The mechanisms of regulation by IR and Hh activation on the expression of Bdnf and Chrm1 were related with miR-206 and miR-107 respectively, but other mechanisms and those on the expression of Ngf and Nrtn remain to be explored.
Although it was reported that aberrant regulation of Hh signaling is related with tumorigenesis of Head and Neck cancer (44), 7 days induction of Shh over-expression in basal epithelia in current study led to Hh activation comparable to that after duct ligation and did not result in any detectable tumor in mice up to 4 months after induction. Prolonged Hh activation for 15 weeks in salivary glands by Gli1 transgene resulted in hyperplasia, but these lesions regressed after withdrawal of transgene expression and become histologically normalized (45). These data indicated that the probability of inducing cancer in adult salivary glands by transient Hh activation at near-physiological level is likely very rare. For potential application in head and neck cancer survivors, great cautions should be taken to only activate Hh pathway in salivary gland to eliminate the risk of prompting growth, relapse or metastasis of pre-existed cancers. Advantageously, salivary glands are well encapsulated and local gene/protein/drug delivery can be easily achieved by cannulation via the orifices in the mouth. Previous report and our preliminary data have indicated that transgenes delivered by this method would not significantly increase levels of transgenes and/or protein products systemically and the growth of pre-existed tumors (13).
Interestingly, in SMGs of female mice transient Shh over-expression failed to activate Hh pathway efficiently and rescue the IR-induced hyposalivation, which is related to the lower expression of both endogenous components of Hh pathway and Shh transgene. For future study with female mice and probably other animal models, such gender differences should be considered and higher dose or alternative agonist may be needed to achieve efficient Hh activation in female salivary glands. In SMGs of female mice and human salivary epithelial cells from female patients, transient Hh activation was achieved with other approaches and similarly regulated expression of genes related with SSPCs and parasympathetic innervation, suggesting that the transient Hh activation may also rescue IR-induced hypofunction in females. Even in the worst scenario that this strategy only works in males, it will still be of significant importance since about 2/3 of head and neck patients are male (1, 2).
In summary, we have been able to rescue the salivary gland function by transient activation of Hh signaling in a mouse model. The mechanism is related with preservation of functional stem/progenitor cells and parasympathetic innervation. We observed similar rescue effects on expression of genes related to both aspects in cultured human salivary epithelial cells, suggesting the potential of transient Hh activation in treating IR-induced hyposalivation in human patients.
Supplementary Material
Translational relevance.
Irreversible hyposalivation is common in Head and Neck cancer survivors treated with radiotherapy even when various new techniques were applied to minimize the irradiation damage to salivary glands. Current treatments for irradiation-induced hyposalivation can only temporarily relieve xerostomia symptoms. The morbidity is caused by loss of functional epithelial stem/progenitor cells. Recent studies indicated that parasympathetic innervation is also essential for regeneration of salivary glands and impaired by radiotherapy. This manuscript reports on a novel finding that transient activation of Hedgehog pathway after irradiation rescued salivary gland dysfunction by preserving both salivary stem/progenitor cells and parasympathetic innervation in a mouse model. Moreover, in cultured human salivary gland epithelial cells, transient Hedgehog activation after irradiation rescued expression of genes essential for maintenance of salivary stem/progenitor cells and parasympathetic innervation similarly as in mice. These findings suggested that targeting Hedgehog pathway is promising to treat irradiation-induced hyposalivation.
Acknowledgments
We sincerely thank Drs. Adam Glick of Pennsylvania State University, Jeff Whitsett of Cincinnati Children's Hospital Medical Center, and Changyu Zheng of NIH for providing Krt5-rtTA mice, tetO-Shh mice and SCC VII cells respectively.
Grant Support: This study was supported by NIH/NIDCR 1RC1DE020595-01 (FL) and 1R01DE022975-01 (FL).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that they have no competing interests.
Author contributions: The study was conceived and supervised by FL; BH and LQ performed most of the experiments; ZY contributed with mice breeding and genotyping; QZ, XT, LS, and YZ contributed with biochemical and cell culture assays; SK and DR performed IR; FL wrote the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:1061–79. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 4.Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62:1187–94. doi: 10.1016/j.ijrobp.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983–91. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haara O, Fujimori S, Schmidt-Ullrich R, Hartmann C, Thesleff I, Mikkola ML. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development. 2011;138:2681–91. doi: 10.1242/dev.057711. [DOI] [PubMed] [Google Scholar]
- 7.Hai B, Yang Z, Millar SE, Choi YS, Taketo MM, Nagy A, et al. Wnt/beta-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev. 2010;19:1793–801. doi: 10.1089/scd.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–12. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaskoll T, Leo T, Witcher D, Ormestad M, Astorga J, Bringas P, Jr., et al. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn. 2004;229:722–32. doi: 10.1002/dvdy.10472. [DOI] [PubMed] [Google Scholar]
- 10.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 11.Hai B, Yang Z, Shangguan L, Zhao Y, Boyer A, Liu F. Concurrent transient activation of Wnt/beta-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys. 2012;83:e109–16. doi: 10.1016/j.ijrobp.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaniv A, Neumann Y, David R, Stiubea-Cohen R, Orbach Y, Lang S, et al. Establishment of Immortal Multipotent Rat Salivary Progenitor Cell Line Toward Salivary Gland Regeneration. Tissue Eng Part C Methods. 2010 doi: 10.1089/ten.TEC.2010.0228. [DOI] [PubMed] [Google Scholar]
- 13.Zheng C, Cotrim AP, Rowzee A, Swaim W, Sowers A, Mitchell JB, et al. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res. 2011;17:2842–51. doi: 10.1158/1078-0432.CCR-10-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahmane N, Lee J, Robins P, Heller P, Ruiz i Altaba A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–81. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 15.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells. 2008;26:2595–601. doi: 10.1634/stemcells.2007-1034. [DOI] [PubMed] [Google Scholar]
- 16.Hisatomi Y, Okumura K, Nakamura K, Matsumoto S, Satoh A, Nagano K, et al. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39:667–75. doi: 10.1002/hep.20063. [DOI] [PubMed] [Google Scholar]
- 17.Arany S, Catalan MA, Roztocil E, Ovitt CE. Ascl3 knockout and cell ablation models reveal complexity of salivary gland maintenance and regeneration. Dev Biol. 2011;353:186–93. doi: 10.1016/j.ydbio.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limesand KH, Avila JL, Victory K, Chang HH, Shin YJ, Grundmann O, et al. Insulin-like growth factor-1 preserves salivary gland function after fractionated radiation. Int J Radiat Oncol Biol Phys. 2010;78:579–86. doi: 10.1016/j.ijrobp.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson EC, Doser TA, Cepurna WO, Dyck JA, Jia L, Guo Y, et al. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Invest Ophthalmol Vis Sci. 2011;52:504–18. doi: 10.1167/iovs.10-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avila JL, Grundmann O, Burd R, Limesand KH. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 2009;73:523–9. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podlasek CA. Sonic hedgehog, apoptosis, and the penis. J Sex Med. 2009;6(Suppl 3):334–9. doi: 10.1111/j.1743-6109.2008.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thibert C, Teillet MA, Lapointe F, Mazelin L, Le Douarin NM, Mehlen P. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301:843–6. doi: 10.1126/science.1085405. [DOI] [PubMed] [Google Scholar]
- 23.Romanov VS, Pospelov VA, Pospelova TV. Cyclin-dependent kinase inhibitor p21(Waf1): contemporary view on its role in senescence and oncogenesis. Biochemistry Biokhimiia. 2012;77:575–84. doi: 10.1134/S000629791206003X. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subkhankulova T, Zhang X, Leung C, Marino S. Bmi1 directly represses p21Waf1/Cip1 in Shh-induced proliferation of cerebellar granule cell progenitors. Mol Cell Neurosci. 2010;45:151–62. doi: 10.1016/j.mcn.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Yadirgi G, Leinster V, Acquati S, Bhagat H, Shakhova O, Marino S. Conditional activation of Bmi1 expression regulates self-renewal, apoptosis, and differentiation of neural stem/progenitor cells in vitro and in vivo. Stem Cells. 2011;29:700–12. doi: 10.1002/stem.614. [DOI] [PubMed] [Google Scholar]
- 27.Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–7. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32:1091–101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sekiguchi H, Ii M, Jujo K, Renault MA, Thorne T, Clarke T, et al. Estradiol triggers sonic-hedgehog-induced angiogenesis during peripheral nerve regeneration by downregulating hedgehog-interacting protein. Lab Invest. 2012;92:532–42. doi: 10.1038/labinvest.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radzikinas K, Aven L, Jiang Z, Tran T, Paez-Cortez J, Boppidi K, et al. A Shh/miR-206/BDNF Cascade Coordinates Innervation and Formation of Airway Smooth Muscle. J Neurosci. 2011;31:15407–15. doi: 10.1523/JNEUROSCI.2745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bond CW, Angeloni N, Harrington D, Stupp S, Podlasek CA. Sonic hedgehog regulates brain-derived neurotrophic factor in normal and regenerating cavernous nerves. J Sex Med. 2013;10:730–7. doi: 10.1111/jsm.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saruta J, Fujino K, To M, Tsukinoki K. Expression and localization of brain-derived neurotrophic factor (BDNF) mRNA and protein in human submandibular gland. Acta histochemica et cytochemica. 2012;45:211–8. doi: 10.1267/ahc.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bella AJ, Lin G, Tantiwongse K, Garcia M, Lin CS, Brant W, et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J Sex Med. 2006;3:815–20. doi: 10.1111/j.1743-6109.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 36.Hazari MS, Pan JH, Myers AC. Nerve growth factor acutely potentiates synaptic transmission in vitro and induces dendritic growth in vivo on adult neurons in airway parasympathetic ganglia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L992–1001. doi: 10.1152/ajplung.00216.2006. [DOI] [PubMed] [Google Scholar]
- 37.Tanida S, Kataoka H, Mizoshita T, Shimura T, Kamiya T, Joh T. Intranuclear translocation signaling of HB-EGF carboxy-terminal fragment and mucosal defense through cell proliferation and migration in digestive tracts. Digestion. 2010;82:145–9. doi: 10.1159/000310903. [DOI] [PubMed] [Google Scholar]
- 38.Scarr E, Craig JM, Cairns MJ, Seo MS, Galati JC, Beveridge NJ, et al. Decreased cortical muscarinic M1 receptors in schizophrenia are associated with changes in gene promoter methylation, mRNA and gene targeting microRNA. Translational psychiatry. 2013;3:e230. doi: 10.1038/tp.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhry MA, Omaruddin RA, Brumbaugh CD, Tariq MA, Pourmand N. Identification of radiation-induced microRNA transcriptome by next-generation massively parallel sequencing. Journal of radiation research. 2013 doi: 10.1093/jrr/rrt014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo G, Long J, Cui X, Xiao Z, Liu Z, Shi S, et al. Highly lymphatic metastatic pancreatic cancer cells possess stem cell-like properties. Int J Oncol. 2013;42:979–84. doi: 10.3892/ijo.2013.1780. [DOI] [PubMed] [Google Scholar]
- 41.Pinkstaff CA. Salivary gland sexual dimorphism: a brief review. Eur J Morphol. 1998;36(Suppl):31–4. [PubMed] [Google Scholar]
- 42.Chung CH, Dignam JJ, Hammond ME, Klimowicz AC, Petrillo SK, Magliocco A, et al. Glioma-associated oncogene family zinc finger 1 expression and metastasis in patients with head and neck squamous cell carcinoma treated with radiation therapy (RTOG 9003). J Clin Oncol. 2011;29:1326–34. doi: 10.1200/JCO.2010.32.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidel K, Ahn CP, Lyons D, Nee A, Ting K, Brownell I, et al. Hedgehog signaling regulates the generation of ameloblast progenitors in the continuously growing mouse incisor. Development. 2010;137:3753–61. doi: 10.1242/dev.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavicchioli Buim ME, Gurgel CA, Goncalves Ramos EA, Lourenco SV, Soares FA. Activation of sonic hedgehog signaling in oral squamous cell carcinomas: a preliminary study. Hum Pathol. 2011;42:1484–90. doi: 10.1016/j.humpath.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Fiaschi M, Kolterud A, Nilsson M, Toftgard R, Rozell B. Targeted Expression of GLI1 in the Salivary Glands Results in an Altered Differentiation Program and Hyperplasia. Am J Pathol. 2011;179:2569–79. doi: 10.1016/j.ajpath.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.