Abstract
A model of the alignment of neurosteroids and ent-neurosteroids at the same binding site on γ-aminobutyric acid type A (GABAA) receptors was evaluated for its ability to identify the structural features in ent-neurosteroids that enhance their activity as positive allosteric modulators of this receptor. Structural features that were identified included: 1) a ketone group at position C-16; 2) an axial 4α-OMe group and 3) a C-18 methyl group. Two ent-steroids were identified that were more potent than the anesthetic steroid alphaxalone in their threshold for and duration of loss of the righting reflex in mice. In tadpoles, loss of righting reflex for these two ent-steroids occurs with EC50 values similar to those found for allopregnanolone. The results indicate that ent-steroids have considerable potential to be developed as anesthetic agents as and drugs to treat brain disorders that are ameliorated by positive allosteric modulators of GABAA receptor function.
INTRODUCTION
Currently, there is considerable interest in neurosteroid physiology and in the development of neurosteroid analogues for the treatment of a variety of disorders of central nervous system (CNS) function and as new intravenous general anesthetics.1-4 The vast majority of the medicinal chemistry done in the field of neuroactive steroids is focused on developing new neurosteroid analogues having the absolute configuration of naturally-occurring steroids.5 The potential of ent-neurosteroids, steroids with an absolute configuration opposite to that of naturally-occurring steroids, to be developed for these purposes has not been addressed even though ent-steroids could have a different spectrum of actions in the CNS and different routes of metabolism.
As an initial approach to evaluating the potential of ent-steroids as new anesthetic agents and CNS drugs, we have been engaged for a number of years in exploring the molecular basis for the enantioselectivity of neurosteroid action at γ-aminobutyric acid type A (GABAA) receptors.6-10 Androsterone (1), a naturally occurring neurosteroid, is a weak potentiator of γ-aminobutyric acid (GABA)-mediated chloride currents at GABAA receptors (Chart 1).11,12 We reported previously that its enantiomer, ent-androsterone (ent-1), is a more effective positive allosteric modulator of this ion channel than steroid 1.9 In a subsequent study of 7-OBn and 11-OBn substituted analogues of compounds 1 and ent-1 we provided evidence in support of the hypothesis that one enantiomer of androsterone is inverted relative to the other in the neurosteroid modulation site on the GABAA receptor.13 We report herein a continuation of these enantioselectivity studies with the immediate goal of learning more about the SAR of ent-steroid modulators of GABAA receptors and the long term goal of identifying new ent-steroids with therapeutic potential.
Chart 1.
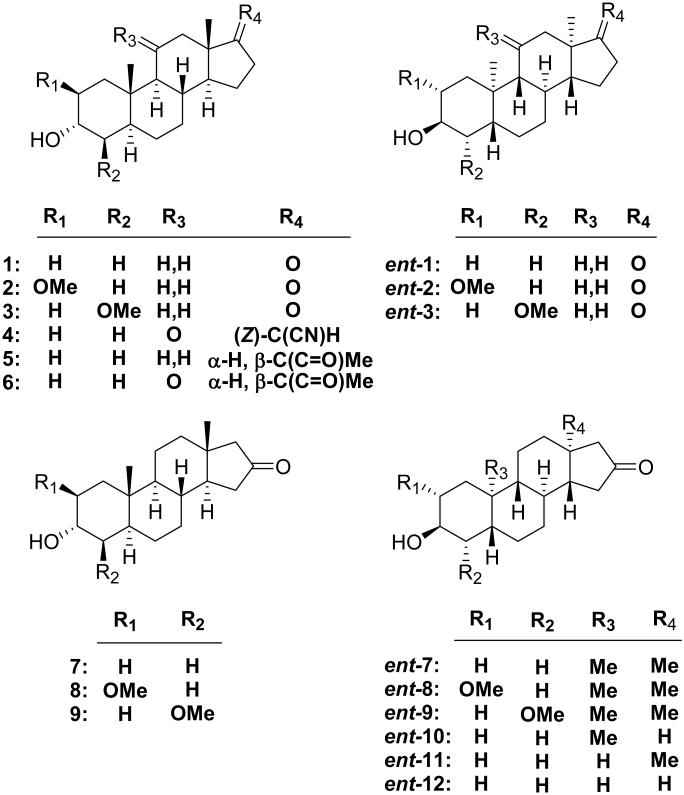
A heuristic model for the alignment of the enantiomers of androsterone at the neurosteroid binding site in the GABAA receptor has been used to select the ent-steroids chosen for synthesis and evaluation (Figure 1, panel A). The model posits that the A-rings of both enantiomers are identically located in the neurosteroid binding site as inferred from our previous study.13 This model, when considered in the context of what has been shown in previous studies of steroid modulators of GABAA receptors, was then used to make the following series of predictions for the activity of new ent-steroid analogues.
Figure 1.
Molecular modeling. (A) Proposed model of the alignment of enantiomers 1 and ent-1 at the neursosteroid modulation site on GABAA receptors. (B) Proposed model of the alignment of compounds 4 and ent-1 at the same site. Aligments were obtained by superimposing O-3, C-3, C-13 and C-18 for each pair of compounds. Additional views of the superimpositions from different perspectives are presented in the Supplemental Materials along with a view showing the superimposition of steroid 5 and analogue ent-9 (Figure S1).
First, previous structure–activity (SAR) studies of steroid anesthetics acting at GABAA receptors have shown that analogues with 2β-substituents (e.g., 2), but not 4β-substituents (e.g., 3), are highly active.14 Thus, as a consequence of the inverted orientation of compound ent-1 relative to 1, the model predicts that introducing a 2α-OMe group as a modification of compound ent-1 will yield inactive analogue ent-2, whereas modification with a 4α-OMe group will give active analogue ent-3.
Second, the model places the oxygens in the ketone groups at C-17 in the androsterone enantiomers in nearly the same location. However, results obtained with highly active anesthetic steroid 4 suggest that the C-17 position may not be the optimal location for the carbonyl group in ent-steroids.15 An alignment of the A-rings of steroid 4, a synthetic analogue of endogenous neurosteroid allopregnanolone (5), with compound ent-1suggests that the carbonyl group would be better placed at C-16 (Figure 1, panel B). This prediction, along with that of an expected loss of activity for the corresponding 16-ketosteroids was evaluated using the enantiomer pairs (7–9 and ent-7–ent-9).
Third, the model predicts that the C-18 and C-19 methyl groups are similarly located in the neurosteroid binding site. Either, both, or neither of these methyl groups may be important for orientation/activity of the enantiomers in the neurosteroid binding site. Previous studies have shown that the C-18 Me group can affect the number of components in TBPS binding curves (one vs. two), as well as the kinetic modes of action.16,17 This predicts that the activity of analogues of ent-1 without the C-18 methyl group will have their activities altered more than ent-1 analogues lacking the C-19 methyl group. This prediction was tested with compounds ent-7 and ent-10–ent-12.
Herein, we report results with novel steroid and ent-steroid analogues that are consistent with all three of these predictions. In addition, we have identified ent-steroids (ent-7 and ent-9) with potencies comparable to those of the endogenous neurosteroid 5 and higher than those of the anesthetic steroid alphaxalone (6).
CHEMISTRY
Since the synthetic pathways to either enantiomer of the compounds described in Schemes 1-3 are identical, only those for the ent-steroids are discussed here. Physical and spectroscopic details for the corresponding steroids, when prepared, are given immediately after these data are reported for the corresponding ent-steroids in the Experimental Section.
Scheme 1.
aReagents: (a) Li(t-OBu)3AlH, THF, –40 °C (82%); (b) MeSO2Cl, Et3N, CH2Cl2 (100%); (c) LiBr, DMF, 125 °C (87%, Δ2 & Δ3, 5:1); (d) 30% H2O2, HCO2H, CH2Cl2 (100%, 2,3 & 3,4 epoxides); (e) H2SO4, MeOH (62%).
Scheme 3.
aReagents: (a) i) C6H5CHO, EtOH, KOH; ii) NaBH4, CeCl3, EtOH, 0 °C; iii) Ac2O, Et3N, DMAP (91–97% overall); (b) i) O3, MeOH/EtOAc, −78 °C, ii) Me2S (82–98% overall); (c) i) Jones reagent; ii) SmI2,THF (under N2) (70–94%); (d) K2CO3, MeOH/H2O (93-98%).
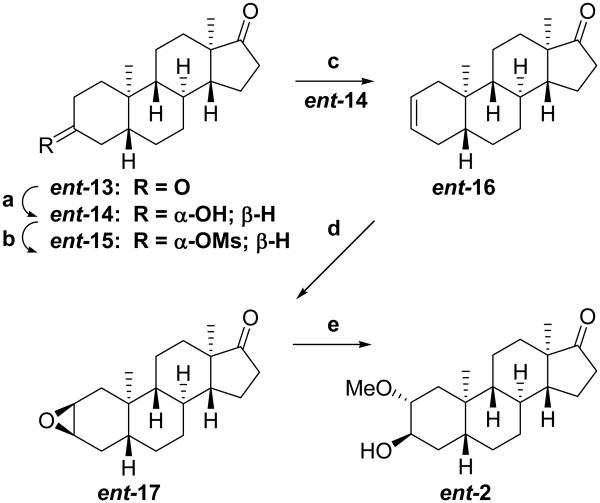
The synthesis of compound ent-1 was described previously.9 The synthesis of ent-2 is shown in Scheme 1. Starting material ent-13 was prepared as previously reported.9 Regio- and stereoselective reduction of this compound’s 3-ketone group with Li(t-OBu)3AlH yielded after workup predominately product ent-14 (82%). Mesylation of the resultant 3α-hydroxyl group gave the corresponding mesylate ent-15 (100%), and elimination of the mesylate group gave predominately the Δ2-olefinic product ent-16 (87%). Epoxidation of the double bond using HCOOH/H2O2 gave epoxide ent-17 (100%), and opening of the epoxide with H2SO4/MeOH gave ent-2 (62%).
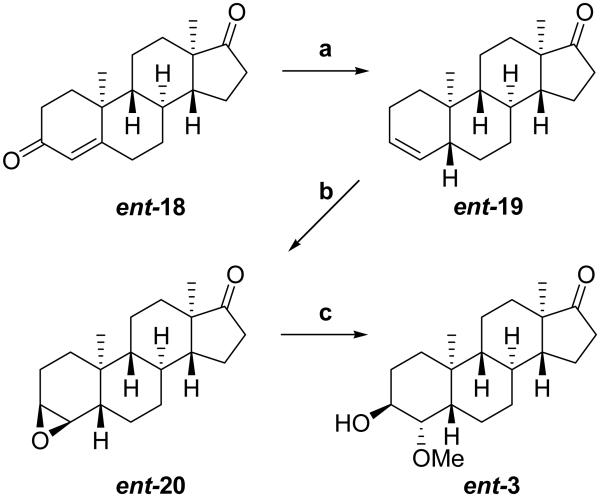
The synthesis of ent-3 is shown in Scheme 2. Starting material ent-18 was prepared as previously reported.7 Treatment of the starting material with Zn powder in HOAc gave the (5α)- and (5β)-Δ3-olefinic products in about a 1:1 ratio as determined by NMR. Fractional recrystallization gave the pure product ent-19 (43%). Epoxidation of the double bond using HCOOH/H2O2 gave epoxide ent-20 (84%), and opening of the epoxide with H2SO4/MeOH gave ent-3 (78%).
Scheme 2.
aReagents: (a) Zn dust, AcOH (43%); (b) 30% H2O2, HCO2H, CH2Cl2 (84%); (c) H2SO4, MeOH (78%).
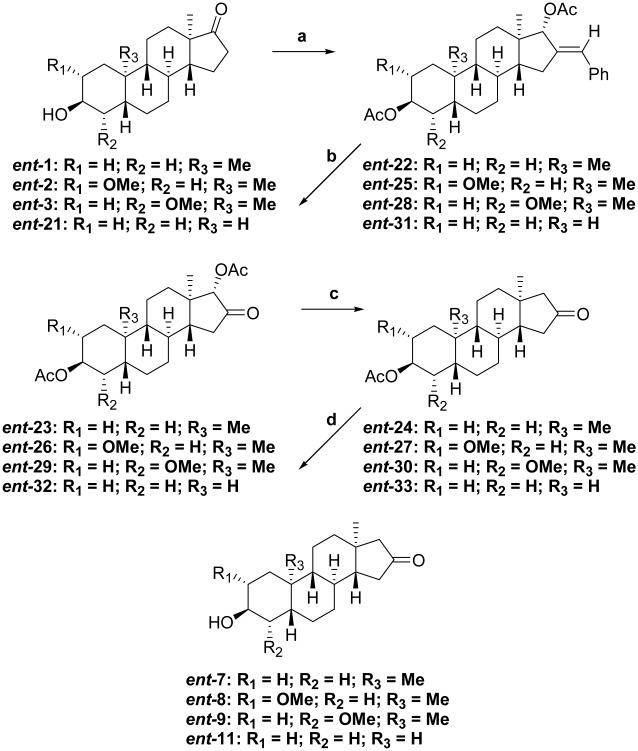
In Scheme 3, we adapted a route that we used previously to convert a steroid 3-ketone group to a 2-ketone group18 to transform the17-ketone groups of ent-1–ent-3 and ent-21 to the 16-ketone groups of ent-7–ent-9 and ent-11, respectively. Each starting material was condensed with benzaldehyde, reduced to a diol using NaBH4 /CeCl3 and acetylated using AcOAc/pyr/DMAP to give products ent-22 (91%), ent-25 (95%), ent-28 (97%), and ent-31 (91%). The 16-ketone groups were then formed by ozonolysis of the exocyclic double bonds at the 16-position to give products ent-23 (82%), ent-26 (90%), ent-29 (87%), and ent-32 (98%), respectively. In the next step, the 17α-acetate groups were removed using SmI2 in THF. This reaction also results in partial reduction of the 16-ketone groups. The crude 16-ketone, 16-alcohol product mixtures were treated with Jones reagent to give the isolated products ent-24 (94%), ent-27 (94%), ent-30 (84%), and ent-33 (70%), respectively. Saponification of the 3β-acetate groups gave the target compounds ent-7 (93%), ent-8 (97%), ent-9 (94%), and ent-11 (98%), respectively.
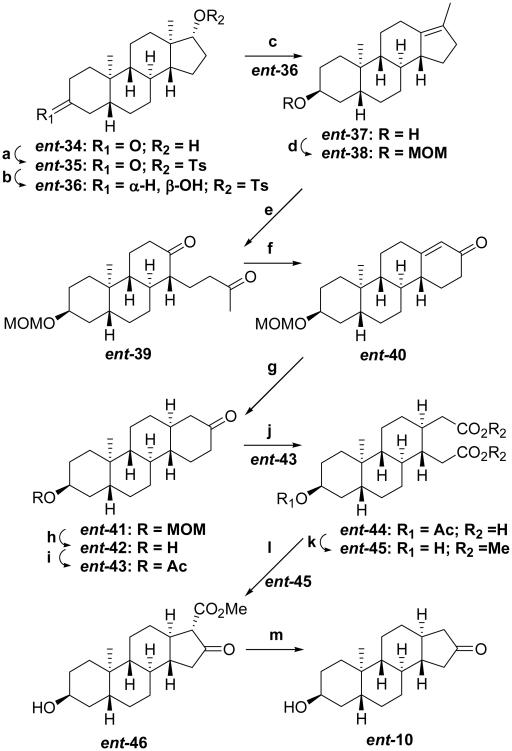
The synthetic routes to the 18-nor compound ent-10 (Scheme 4) and 18,19-dinor compound ent-12 (Scheme 5) are largely derived from methods developed previously to prepare chrysene enantiomers from 19-nortestosterone or to convert 19-nortestosterone into ent-19-nortestosterone.19,20 Starting material ent-34 was prepared as described previously,9 and converted to the 17α-tosylate ent-35 (91%). Stereoselective reduction of the 3-ketone group of ent-35 was achieved using K(s-Bu)3BH to yield product ent-36 (71%) containing the 3β-hydroxyl group. Rearrangement of compound ent-36 to product ent-37 (91%) was achieved using MeMgBr in refluxing toluene. Compound ent-37 is not very soluble in the solvents used for ozonolysis of the compound’s double bond, so it was converted to the MOM protected compound ent-38 (90%) and then converted by ozonolysis to the diketone ent-39 (65%). Aldol condensation of compound ent-39 using NaOH/aqueous MeOH gave enone ent-40 (75%). Li/liq. NH3 of this enone gave product ent-41 (72%).
Scheme 4.
aReagents: (a) p-TsCl, pyr, 40 °C (91%); (b) i) K(s-Bu)3BH, THF, −78 °C; ii) NaOH, 30% H2O2 (71% overall); (c) MeMgBr, toluene, reflux (91%); (d) ClCH2OMe, (i-Pr)2EtN, CH2Cl2, 0 °C (90%); (e) O3, CH2Cl2, −78 °C; ii) Zn dust, AcOH (65% overall); (f) NaOH, MeOH/H2O (75%); (g) Li/liq. NH3, THF (72%); (h) 6 N HCl, MeOH (92%); (i) Ac2O, Et3N, DMAP (95%); (j) CrO3, AcOH, 60 °C; (k) AcCl, MeOH (51%, steps j & k); (l) NaOMe, THF, reflux (71%); (m) LiCl, DMF, 160 °C (86%).
Scheme 5.
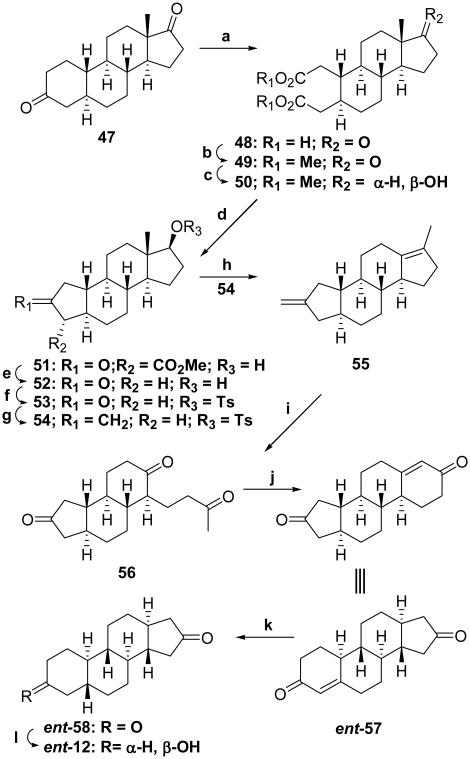
aReagents: (a) CrO3, AcOH, 60 °C; (b) AcCl, MeOH (61%,steps a & b); (c) NaBH4, EtOH, 0 °C (74%); (d) NaOMe, THF under N2, 100 °C (81%); (e) LiCl, DMF, 160 °C (85%); (f) p-TsCl, pyr, DMAP, 65 °C (91%); (g) n-BuLi, MeP(Ph)3Br, C6H6/THF under N2 (80%); (h) MeMgBr, toluene under N2, 100–115 °C (90%); (i) O3, CH2Cl2, −78 °C; ii) Zn dust, AcOH (51% overall); (j) NaOH, MeOH/H2O (82%); (k) Li/liq. NH3, THF (63%); (l) K(s-Bu)3BH, THF, −78 °C; ii) NaOH, 30% H2O2 (70%).
At this point, the MOM protecting group at the 3-position, which was chosen for it’s stability in the Li/liq. NH3 reduction step, had to be exchanged for an acetate group because the MOM group would not survive the harsh conditions of a subsequent CrO3 oxidation step. The MOM group in compound ent-41 was removed using 6 N HCl/MeOH, and the resultant hydroxyl group in compound ent-42 (92%) was acetylated using AcOAc/pyr/DMAP to yield the 3-acetate ent-43 (95%).
CrO3/AcOH oxidation of compound ent-43 cleaved the D-ring to yield crude dicarboxylic acid ent-44, which was isolated after esterification using AcCl/MeOH as diester ent-45 (51%). Dieckmann condensation using NaOMe/THF of compound ent-45 yielded the D-ring β-ketoester ent-46 (71%) as the only condensation product. An analogous oxidation, esterification, ring closure sequence has been reported previously for (5α)-3-ketosteroids.21-23 In the cited study, the hydrogen at the ring fusion and the carbomethoxy group in the product were found to be syn to each other. For this reason, we assigned the stereochemistry of the 17-carbomethoxy group in product ent-46 the α-configuration. Decarbomethoxylation using LiCl in non-dried DMF gave the desired analogue ent-10 (86%). Using carefully dried DMF gave lower yields.
For the synthesis of ent-12, steroid 47 was used as starting material.24 CrO3/AcOH oxidation of steroid 47 cleaved the A-ring to yield crude 2,3-secosteroid 48 (81%), which was purified and isolated after esterification using AcCl/MeOH as the diester 49 (61%). The 17-ketone group of 2,3-secosteroid 49 was then reduced using NaBH4 to yield product 50 (74%). Dieckmann condensation of compound 50 using NaOMe/THF yielded the A-norsteroid 51 (81%) as the only condensation product and the stereochemistry of the 3-carbomethoxy group was assigned, by literature analogy,21-23 the α-configuration. Decarbomethoxylation using LiCl in non-dried DMF gave A-norsteroid 52 (85%). This part of the synthetic sequence has the effect of converting the A-ring of a steroid into what will become the D-ring of an ent-steroid.
The last part of the synthesis of compound ent-12 involves converting the D-ring of A-norsteroid 52 into the A-ring of ent-steroid ent-57. Compound 52 was converted into the tosylate 53 (91%), and the A-ring ketone ring of this tosylate was converted into the vinylidene group of A-norsteroid 54 (80%). Treatment of compound 53 with MeMgBr in refluxing toluene gave the di-olefin 55 (90%). Ozonolysis of compound 55 regenerates the ketone group from the vinylidene group and cleaves the endocyclic double bond to form trione 56 (51%). Aldol condensation of trione 56 completes the 19-norsteroid to ent-18,19-dinorsteroid transformation yielding product ent-57 (82%). Li/liq. NH3 reduction of the double bond in enone ent-57 yields product ent-58 (63%), and regio- and stereoselective reduction of the 3-ketone group with K(s-Bu)3BH yields target compound ent-12 (70%).
[35S]TBPS DISPLACEMENT RESULTS
The compounds shown in Chart 1 were evaluated as noncompetitive displacers of [35S]TBPS from the picrotoxin binding site on the heterogeneous GABAA receptors found in rat brain membranes (Table 1; Supplemental Figure S2 shows each analogue in the alignment orientations shown in Figure 1 along with the IC50 values ). The results demonstrate first, for reference, that the endogeneous neurosteroid 5 (IC50 = 74 ± 7 nM) and compounds ent-7 and ent-9 (IC50 = 81 ± 8 and 83 ± 11 nM, respectively) are the most potent displacers of [35S]TBPS. These three compounds are all more potent than the clinically used anesthetic steroid 6 (IC50 = 226 ± 24 nM).
Table 1.
Inhibition of [35S]TBPS Binding by Steroids 1-9 and Steroid Enantiomers ent-1-ent-3 and ent-7-ent-12a
| compd | IC50(nM) | nHill |
|---|---|---|
| 1 b | 414 ± 127 | 0.89 ± 0.20 |
| ent-1 b | 311 ± 36 | 1.00 ± 0.10 |
| 2 | 2,190 ± 420 | 1.26 ± 0.24 |
| ent-2 | 6,490 ± 3,330 | 1.10 ± 0.33 |
| 3 | >30,000c | – |
| ent-3 | 198 ± 25 | 0.98 ± 0.11 |
| 4 d | 128 ± 11 | 1.44 ± 0.15 |
| 5 e | 74 ± 7 | 0.89 ± 0.06 |
| 6f | 226 ± 24 | 1.10 ± 0.11 |
| 7 | 895 ± 49 | 1.03 ± 0.05 |
| ent-7 | 81 ± 8 | 0.92 ± 0.07 |
| 8 | 1,270 ± 162 | 1.13 ± 0.14 |
| ent-8 | 3,030 ± 430 | 1.12 ± 0.13 |
| 9 g | 6,680 ± 2,180 | 1.56 ± 0.51 |
| ent-9 | 83 ± 11 | 1.15 ± 0.15 |
| ent-10 | 1,520 ± 230 | 1.06 ± 0.14 |
| ent-11 | 238 ± 21 | 0.86 ± 0.06 |
| ent-12 h | 2,020 ± 550 | 1.05 ± 0.23 |
The results presented are from duplicate experiments performed in triplicate. Error limits are calculated as the standard error of the mean. Unless noted otherwise, compounds inhibited binding of [35 S]TBPS by ≥ 95%.
Literature values.9
A binding curve could not be calculated. Maximum displacement at 30 μM was 45%.
Literature values.15
Literature values.46
Literature values.48
Inhibition was partial at 30 μM. Maximal displacement for the calculated binding curve was 65%.
Inhibition was partial at 30 μM. Maximal displacement for the calculated binding curve was 88%.
Comparison of the IC50 values for the steroid pair 1 and 7 shows that moving the D-ring ketone group from position C-17 to C-16 decreases displacement potency by about two-fold. By contrast, this same structural modification for the compound pair ent-1 and ent-7 increases displacement potency by about four-fold.
Introduction of an axial 2-OMe group lowers displacement potency for steroids with a 17-ketone group (compare steroids 1 and 2) by about five-fold, but for steroids with a 16-ketone group (compare steroids 7 and 8) the substituent lowers displacement potency only slightly (< two-fold). An axial 2-OMe substituent causes a much larger decrease in displacement potency for the corresponding ent-steroid pairs. For the pair ent-1 and ent-2, the effect is about 21-fold. For the pair ent-7 and ent-8, the effect is about 37-fold.
Addition of an axial 4-OMe group yields steroid analogues that are weak displacers of [35S]TBPS. Steroids 3 (17-ketone) and 9 (16-ketone) displaced only 45% and 65% of bound [35S]TPBS at the highest concentration tested (3 μM), respectively. An opposite effect on displacement potency was found for the axial 4-OMe substituent in the enantiomeric compounds ent-3 and ent-9. For the 17-ketone pair ent-1 and ent-3, the axial 4-OMe group increased displacement potency by about two-fold and for the 16-ketone pair ent-7 and ent-9 there was no significant effect of the axial 4-OMe group on displacement potency.
In summary, an axial 2-OMe substituent has a modest negative effect on displacement potency for steroids having a 17-ketone group and little, if any, effect on steroids with a 16-ketone group. A large negative effect of this substituent on the IC50 value is found for the corresponding ent-steroids having the D-ring ketone group in either position. An axial 4-OMe substituent has largely opposite effects on displacement potency. For the steroids, regardless of the position of the D-ring ketone group, displacement potency is greatly reduced by this substituent. For the corresponding ent-steroids, the axial 4-OMe substituent has little, if any, effect on displacement potency.
Compounds ent-10–ent-12 were evaluated to assess the relative importance of the C-18 and C-19 Me groups for the high displacement potency found for compound ent-7. Compound ent-10, which lacks the C-18 Me group, is about a 19-fold weaker displacer of [35S]TBPS than compound ent-7. Compound ent-11, which lacks the C-19 Me group, is only about a three-fold weaker displacer of [35S]TBPS than compound ent-7. Compound ent-12, which lacks both C-18 and C-19 Me groups, has an IC50 value that is essentially the same as that of compound ent-10. These results indicate that in this series of ent-steroids the C-18 Me group has a substantially larger effect on [35S]TBPS displacement potency than does the C-19 Me group. This difference was anticipated based on previous results obtained from similar studies carried out on steroids and discussed in the Introduction. The effect of the C-18 Me group on kinetic processes affecting the magnitude of potentiation of whole cell GABA currents is reported in the next section.
ELECTROPHYSIOLOGY RESULTS
The compounds shown in Chart 1 were initially evaluated for their ability to potentiate chloride currents elicited by 2 μM GABA (a concentration that on average gates ~4% of maximal GABA response) at rat α1β2γ2L type GABAA receptors expressed in Xenopus laevis oocytes (Table 2). The results demonstrate that compounds with a low IC50 value for [35S]TBPS displacement tended to exhibit detectable potentiation of GABAA receptor currents at 100 nM, the lowest concentration tested. All active compounds exhibited concentration dependent potentiation, and the weakest compounds failed to exhibit potentiation even at the highest concentration tested (10 μM), suggesting their qualitative inactivity The results shown were obtained on different batches of oocytes and reported potentiation values do not account for minor variations in GABA EC50 and other potential variables (e.g., different extent of receptor phosphorylation) that may quantitatively affect GABA and neurosteroid responsiveness. Therefore, only qualitative comparisons of results for the different analogues can be made for the results reported in Table 2.
Table 2.
Modulation of Rat α1β2γ2L GABAa Receptor Function by Steroids 1-9 and Steroid Enantiomers ent-1–ent-3 and ent-1–ent-12
| compd | oocyte electrophysiologya | |||
|---|---|---|---|---|
| 0.1 μM | 1 μM | 10 μM | (gating) 10 μM | |
| 1 b | 0.97 ± 0.02 | 1.41. ± 0.01 | 5.44 ± 0.19 | 0.02 ± 0.01 |
| ent-1 b | 1.27 ± 0.29 | 3.66 ± 0.89 | 18.87 ± 2.38 | 0.03 ± 0.21 |
| 2 | 0.84 ± 0.01 | 1.04 ± 0.04 | 3.39 ± 0.32 | 0.08 ± 0.07 |
| ent-2 | 0.93 ± 0.04 | 0.93 ± 0.10 | 1.53 ± 0.11 | −0.01 ±0.01 |
| 3 | 1.08 ± 0.21 | 1.01 ± 0.14 | 0.91 ± 0.07 | 0.12 ± 0.17 |
| ent-3 | 1.21 ± 0.02 | 3.67 ± 0.33 | 12.21 ± 1.26 | 0.05 ± 0.03 |
| 4 c | 1.17 ± 0.04 | 5.06 ± 0.60 | 21.51 ± 7.07 | 0.17 ± 0.00 |
| 5 d | 1.26 ± 0.14 | 3.89 ± 1.34 | 9.65 ± 3.87 | 0.37 ± 0.07 |
| 6 e | 2.91 ± 0.57 | 4.70 ± 1.11 | 19.64 ± 4.04 | 0.11 ± 0.02 |
| 7 | 1.01 ± 0.16 | 1.38 ± 0.13 | 9.98 ± 3.09 | −0.02 ±0.02 |
| ent-7 | 1.42 ± 0.02 | 7.72 ± 0.39 | 34.70 ± 1.69 | 0.15 ± 0.04 |
| 8 | 0.92 ± 0.04 | 1.23 ± 0.09 | 4.48 ± 0.90 | 0.01 ± 0.00 |
| ent-8 | 0.91 ± 0.01 | 1.03 ± 0.03 | 2.95 ± 0.24 | 0.01 ± 0.01 |
| 9 | 0.88 ± 0.08 | 0.72 ± 0.02 | 0.69 ± 0.01 | 0.02 ± 0.01 |
| ent-9 | 1.41 ± 0.07 | 6.48 ± 0.40 | 16.00 ± 2.21 | 0.08 ± 0.03 |
| ent-10 | 0.93 ± 0.02 | 1.13 ± 0.02 | 3.56 ± 0.15 | 0.01 ± 0.02 |
| ent-11 | 0.97 ± 0.02 | 2.43 ± 0.02 | 10.52 ± 0.61 | 0.02 ± 0.01 |
| ent-12 | 0.80 ± 0.02 | 0.76 ± 0.06 | 1.65 ± 0.13 | 0 ± 0.02 |
The GABA concentration used for the control response was 2 μM. Each compound was evaluated on at least four different oocytes at the concentrations indicated, and the results reported are the ratio of currents measured in the presence/absence of added compound. Gating represents direct current gated by 10 μM compound in the absence of GABA, and this current is reported as the ratio of compound-only current/2 μM GABA current. Error limits are calculated as standard error of the mean (N ≥ 4).
Literature values. 9
Literature values. 15
Literature values. 46
Literature values. 48
In general, results found in electrophysiological evaluations presented in Table 2 are congruent with results from [35S]TBPS binding studies presented in Table 1. In Table 2, steroids 1 (17-ketone) and 7 (16-ketone) are qualitatively similar to each other, and both are modest enhancers of currents. Results in Table 2 for the axial 2-OMe substituted compounds suggest that this substituent has a small negative effect on the concentration dependent potentiation caused by the steroids (compare steroids 1 and 2; 7 and 8) and a large negative effect on potentiation effects of the corresponding ent-steroids (compare compounds ent-1 and ent-2; ent-7 and ent-8). For steroid analogues 3 and 9, which have the axial 4-OMe substituent, the results in Table 2 indicate that this substituent eliminates potentiation of currents by the compounds at concentrations up to 10 μM. For analogues ent-3 and ent-9, this substituent also qualitatively diminishes potentiation of current (compare analogues ent-1 and ent-3; ent-7 and ent-9). However, both of these ent-steroids still show a concentration dependent increase in potentiation, an effect not seen with steroids 3 and 9.
Comparison of results in Table 2 for ent-16-ketosteroid analogues ent-7 and ent-10–ent-12 shows that absence of the C-18 Me group (ent-10) has a larger negative impact on potentiation than does absence of the C-19 Me group (ent-11). The analogue without the C-18 and C-19 Me groups (ent-12) has little potentiating activity at concentrations up to 10 μM.
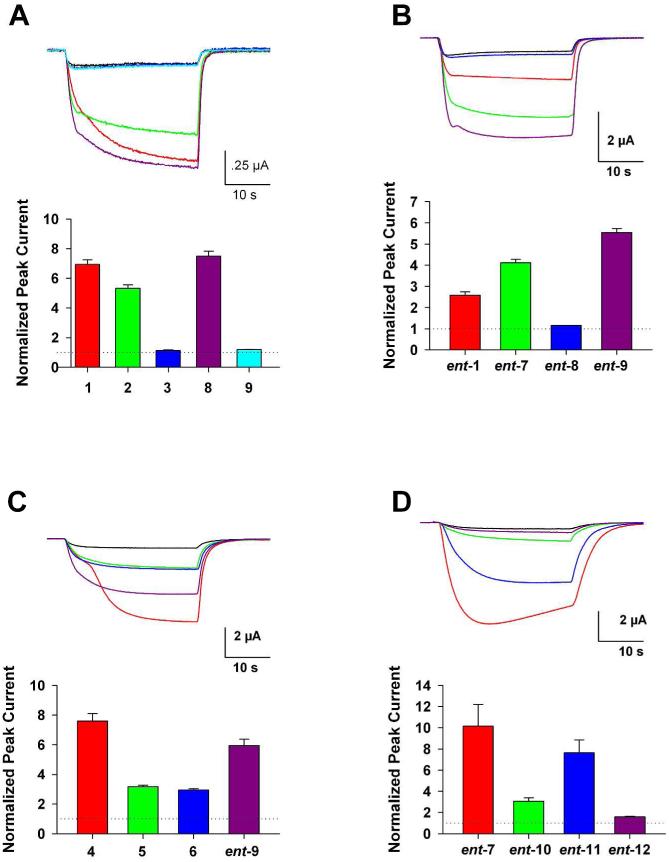
For quantitative purposes, several direct comparisons of potentiating effects of selected compounds at a fixed concentration on the same oocyte were made (Figure 2, panels A–D). In Figure 2 (panel A), the potentiation caused by 17-ketosteroids 1–3 and 16-ketosteroids 8 and 9 at 5 μM are compared. The minor effects on potentiation caused by the axial 2-OMe substituents (steroids 2 and 8), and the major negative effects of the axial 4-OMe groups (steroids 3 and 9) on potentiation are readily apparent when the potentiating effects of the four analogues are compared with that of reference steroid 1.
Figure 2.
Side-by-side, equimolar comparison of structurally related compounds in Xenopus oocytes expressing wild-type α1β2γL GABAA receptors. The traces in the top panels of (A–D) represent current responses of oocytes to 0.5 μM GABA alone (smallest, black trace) and GABA plus the color-indicated compound at 5 μM (A, D), or 0.5 μM (B, C). Bar graphs indicate normalized peak current relative to the response to GABA alone.
In Figure 2 (panel B), quantitative comparisons for selected ent-steroids (0.5 μM) are shown. Changing the position of the C-17 ketone (ent-1) group to C-16 (ent-7) enhances potentiation. The enhancement obtained by moving the position of the ketone group is eliminated by the addition of an axial 2-OMe group (ent-8) and further augmented by the addition of an axial 4-OMe group (ent-9).
In Figure 2 (panel C), a quantitative comparison of the potentiating effects of analogue ent-9 with those of the reference steroids 4-6 on the same oocyte at 0.5 μM is shown. Analogue ent-9 had higher activity than either the reference anesthetic steroid 6 or its C-17 vinylcyano analogue steroid 4, and had an activity very similar to that of the more active endogenous neurosteroid 5.
In Figure 2 (panel D), a quantitative comparison of the series of ent-16-ketosteroid analogues with the different pattern of C-18 and C-19 Me group substitutions is shown. The results from direct comparisons of 5 μM compound agree with the conclusion made from the qualitative results presented in Table 2. Absence of the C-18 Me group yields compounds with lower activity than those having this group whether or not the C-19 Me group is present.
To verify that the effects of the two most active ent-16-ketosteroids (ent-7 and ent-9) and endogenous neurosteroid 5 were mediated by the same site and similar mechanisms, we examined the effects of mutations that in previous studies had been shown to affect receptor modulation by steroids. These studies utilized mutated rat α1β2γ2L receptors expressed in HEK 293 cells. Steroid 5 potentiation of rat α1β2γ2L receptors was previously shown to be drastically reduced in the α1(Q241L)β2γ2L mutant, possibly due to the Leu residue being unable to form a hydrogen bond with the steroid molecule.25,26 Potentiation by compounds ent-7 and ent-9 was also reduced in this mutated receptor (Table 3). Receptors containing α1(S240L)β2γ2L subunits that abolish potentiation by some 5β-reduced steroids, but in which potentiation by steroid 5 is maintained,25 are also potentiated by both ent-steriods (Table 3). The third mutated receptor on which the compounds were tested is α1(W245L)β2γ2 receptor. This mutated receptor is not potentiated by steroids, and it has been proposed that this mutation affects a general transduction element required for steroid-induced potentiation.25 Neither analogue ent-7 nor analogue ent-9 potentiates receptors carrying this mutation (Table 3). Thus, these studies with mutated receptors are consistent with all three compounds (ent-7, ent-9 and 5) acting at the same potentiation sites.
Table 3.
Modulation of Rat Wild-Type and Mutant α1β2γ2L GABAA Receptor Function by Steroid Enantiomers ent-9 and ent-7a
| Receptor | ent-9 | ent-7 |
|---|---|---|
| α 1 β 2 γ 2L | 9.2 ± 5.2** | 7.8 ± 3.9** |
| α1(Q241L)β2γ2L | 1.3 ± 0.1**, ** | 1.3 ± 0.3*, ** |
| α1(S240L)β2γ2L | 9.4 ± 2.9**, # | 7.6 ± 3.8*, # |
| α1(W245L)β2γ2L | 0.9 ± 0.1#, ** | 1.0 ± 0.1 #,** |
The data give the mean (±S.D.) effect of 20 μM ent-9 or ent-7 on peak currents elicited by GABA at a concentration (5 μM for wild-type; 10 μM for mutants) producing 11-17% of maximal current. Number of cells was 4-7 at each receptor-compound combination. Receptors were expressed in HEK 293 cells. Statistical analysis applies to comparison with no effect (using paired two-sample t-test) and comparison with potentiation observed at the wild-type receptor (ANOVA with Bonferroni post-hoc correction). (
, not significant;
, p<0.05;
, p<0.01)
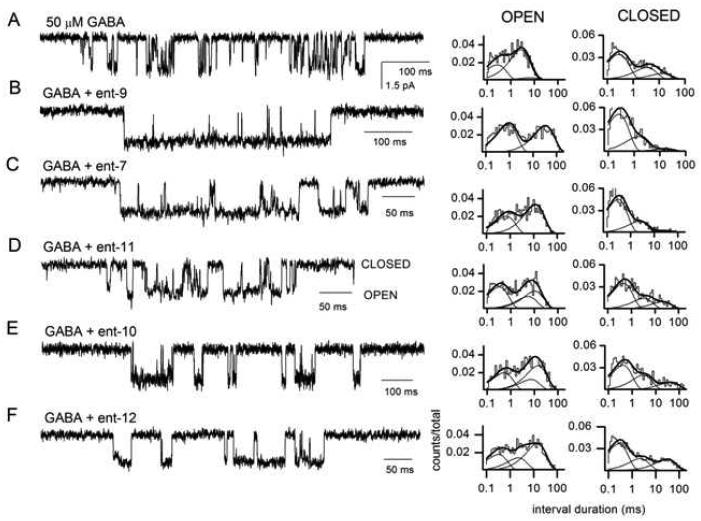
Five ent-16-ketosteroids were examined in greater detail to determine how single-channels kinetics are affected by the axial 4-OMe substituent (ent-9) or by the different C-18 and/or C-19 Me substituents (ent-7 and ent-10–ent-12). These studies were performed using rat wild type α1β2γ2L receptors expressed in HEK 293 cells.
Previous single-channel studies have demonstrated that receptors activated by high concentrations of GABA produce clusters of single-channel openings, i.e., episodes of intense activity separated by long quiescent periods.27 Coapplication of neurosteroids with GABA elicits specific changes in the intracluster open and closed time distributions. For example, in the presence of steroid 5, the changes are: an increase in the mean duration of the longest-lived open state, an increase in the fraction of events in this open state, and a decrease in the fraction of events in the longest intracluster closed state.28 Table 4 reports a statistical analysis of these parameters in the presence of the five novel ent-16-ketosteroids. Sample single-channel traces along with the open and closed time histograms for channel events in the corresponding patches are shown in Figure 3.
Table 4.
Summary of Results on the Effects of ent-9, ent-7, ent-11, ent-10, ent-12 on Single-Channel Currents from Rat α1β2γ2L GABAA Receptorsa
| compd | OT long (ms) | Fraction OT long | Fraction CT long |
|---|---|---|---|
| Controlb
(no steroid) |
6.3 ± 2.6 | 0.13 ± 0.05 | 0.29 ± 0.06 |
| ent-9 | 17.7 ± 6.6*** | 0.53 ± 0.03*** | 0.06 ± 0.03*** |
| ent-7 | 12.4 ± 3.4 | 0.57 ± 0.05*** | 0.05 ± 0.01*** |
| ent-11 | 9.8 ± 3.0 | 0.20 ± 0.08 | 0.11 ± 0.05*** |
| ent-10 | 16.1 ± 3.9* | 0.53 ± 0.06*** | 0.21 ± 0.04 |
| ent-12 | 11.5 ± 3.3 | 0.49 ± 0.04*** | 0.27 ± 0.04 |
The rat wild-type α1β2γ2L GABAA receptor was activated by 50 μM GABA in the absence (control) or presence of 20 μM ent-9, ent-7, ent-11, ent-10, or ent-12. Number of patches was 4-7. The intracluster open time histograms were best-fitted to the sums of three (control, ent-11, ent-10, and ent-12) or two exponentials (ent-9 and ent-7). The intracluster closed time histograms were fitted to the sums of three exponentials. The data give the mean duration and relative frequency (fraction) of the longest-lived intracluster open time component and the relative frequency (fraction) of the longest-lived intracluster closed-time component. Previous work has shown that potentiation by steroids is mediated by changes in was conducted using ANOVA these three parameters. 16, 30 Statistical analysis was conducted using ANOVA with Bonferroni post-hoc correction. The significance level applies to comparison to control condition.
, P < 0.05;
, P < 0.001.
Non-significant changes are not marked.
The control data are from our previous publication. 49
Figure 3.
Exposure to compounds ent-9, ent-7, ent-11, ent-10, or ent-12 modulates single-channel currents from wild-type α1β2γ2 GABAA receptors. Sample single-channel currents and the respective open and closed time histograms in the presence of 50 μM GABA (A), GABA + 20 μM ent-9 (B), GABA + 20 μM ent-7 (C), GABA + 20 μM ent-11 (D), GABA + 20 mM ent-10 (E), or GABA + 20 μM ent-12 (F). Channel openings are shown as downward deflections. In the presence of GABA alone, the open times were 0.27 ms (35%), 2.4 ms (53%), and 4.6 ms (11%), and the closed times were 0.22 ms (53%), 1.4 ms (24%), and 8.9 ms (22%). In the presence of GABA + ent-9, the open times were 0.80 ms (52%), and 30.3 ms (48%), and the closed times were 0.24 ms (67%), 1.4 ms (28%), and 14.3 ms (6%). In the presence of GABA + ent-7, the open times were 0.64 ms (39%), and 10.9 ms (61%), and the closed times were 0.27 ms (71%), 2.1 ms (24%), and 25.0 ms (5%). In the presence of GABA + ent-11, the open times were 0.30 ms (43%), 5.0 ms (24%), and 9.7 ms (33%), and the closed times were 0.43 ms (59%), 3.0 ms (24%), and 19.6 ms (17%). In the presence of GABA + ent-10, the open times were 0.48 ms (33%), 6.6 ms (21%), and 13.2 ms (46%), and the closed times were 0.38 ms (52%), 2.8 ms (31%), and 38.5 ms (16%). In the presence of GABA + ent-12, the open times were 0.29 ms (29%), 2.1 ms (23%), and 13.2 ms (48%), and the closed times were 0.25 ms (54%), 2.0 ms (24%), and 27.8 ms (22%). The summary of averaged data from multiple patches is given in Table 4.
Comparison of compounds ent-7 and ent-, compounds containing the C-18 and C-19 Me groups, reveals that the major effect of the axial 4-OMe substituent (ent-9) is to increase the mean lifetime of the longest open state. Both compounds increase the prevalence of long open events and decrease the prevalence of long closed events.
Comparison of results for compounds ent-7 and ent-10 allows the effect of the C-18 Me group to be determined. Both analogues increase the fraction of the longest open state. By contrast, analogue ent-7, but not analogue ent-10 (the analogue without the C-18 Me group), decreases the prevalence of the longest closed state. Both compounds prolong the mean duration of the longest state but the effect reaches significance only in the presence of analogue ent-10.
Comparison of results for compounds ent-7 and ent-11 allows the effect of the C-19 Me group on single-channel properties to be determined. Both compounds decrease the fraction of dwells in the longest closed state. However, analogue ent-7 (the compound with the C-19 Me group), unlike analogue ent-11 (the compound without the C-19 Me group), increases the fraction of dwells in the longest open state. Overall, the results suggest that single-channel properties are affected in different ways by each Me group. The C-19 Me group has its largest effect on the prevalence of the longest open event and the C-18 Me group has its largest effect on the prevalence of the long-lived closed state.
When neither Me group is present (ent-12), the sole kinetic effect of the compound is an increase in the fraction of the longest open state. This suggests that the increase in this kinetic parameter is not controlled solely by the presence of a C-19 Me substituent. In fact, previous studies have found that even steroid analogues with other ring systems such as cyclopenta[b]phenanthrenes and cyclopenta[b]anthracenes can increase the prevalence of long openings,29 thus indicating that this single-channel parameter is sensitive to a wide range of structural variations.
TADPOLE LOSS OF RIGHTING REFLEX (LRR) AND LOSS OF SWIMMING (LSR) RESULTS
The anesthetic effects of the compounds in tadpoles are summarized in Table 5. Comparison of EC50 values for the steroid pair 1 and 7 shows that moving the D-ring 17-ketone group from position C-17 to C-16 has no significant effect on tadpole LRR, but lowers LSR since only steroid 7 has an EC50 value below 10 μM. This same structural modification for the compound pair ent-1 and ent-7 led to the latter having lower EC50 values for both LLR and LSR (~three fold in both cases), results consistent with the same trend found in binding data reported in Table 1.
Table 5.
Effects of Steroids 1–9 and Steroid Enantiomers ent-1–ent-3 and ent-1–ent-12 on Tadpole Righting and Swimming Reflexesa
| compd | tadpole LRR EC50 (μM) |
tadpole LRR nHill |
Tadpole LSR EC50 (μM) |
Tadpole LSR nHillb |
|---|---|---|---|---|
| 1 c | 3.38 ± 0.90 | −2.83 ±2.66 | Noned | – |
| ent-1 c | 1.42 ± 0.18 | −2.17 ±0.48 | 5.48 ± 0.12 | −33 ± 0 |
| 2 | 5.09 ± 2.43 | −1.29 ± 1.22 | >10 | – |
| ent-2 | 2.39 ± 0.28 | −2.10 ±0.42 | >10 | – |
| 3 | None | – | None | – |
| ent-3 | 0.59 ± 0.07 | −1.84 ± 0.32 | 2.94 ± 0 | −20 ± 1 |
| 4 e | 1.44 ± 0.20 | −2.84 ± 0.77 | 5.48 ± 0.20 | −33 ± 0 |
| 5 f | 0.42 ±0.04 | −1.83 ± 0.32 | 5.5 ± 0.5 | −7.5 ± 1.1 |
| 6 g | 1.12 ± 0.14 | −3.38 ± 2.28 | 5.48 ± 0.11 | −34 ± 0 |
| 7 | 3.60 ± 1.52 | −2.74 ± 3.25 | 7.54 ± 0 | −30 ± 0 |
| ent-7 | 0.55 ± 0.09 | −2.90 ± 0.07 | 1.73 ± 0.04 | −37 |
| 8 | 3.15 ± 0.30 | −2.55 ± 0.78 | 9.29 ± 0.03 | −15 ± 1 |
| ent-8 | 2.91 ± 0.36 | −2.37 ± 0.80 | 10.3 ± 0 | −21 ± 0 |
| 9 | 3.15 ± 0.25 | −2.60 ± 0.70 | 13.5 ± 0 | −74 ± 0 |
| ent-9 | 0.34 ± 0.04 | −2.75 ± 1.13 | 1.73 ± 0.03 | −36 ± 0 |
| ent-10 | 3.64 ± 2.17 | −4.32 ± 11.8 | None | – |
| ent-11 | 1.16 ± 0.21 | −4.03 ± 4.06 | 5.48 ± 0.12 | −33 ± 0 |
| ent-12 | None | – | None | – |
The methods are as reported previously.46 Error limits are calculated as the standard error of the mean (N = 10 or more animals at each of five or more different concentrations).
LSR typically has a very steep concentration–response curve. The reported nHill values vary widely depending on how many animals had LSR at the concentration (typically, 3 μM) preceding the concentration (typically, 10 μM) that produced LSR in all animals. No SAR conclusions were based on these slope values.
Literature values.9
“None” indicates that all animals had a swimming response at 10 μM test compound.
Literature values. 15
Literature values. 46
Literature values. 48
For the 17-ketosteroids, the axial 2-OMe group had a minor negative effect on potency for either LRR or LSR (compare steroids 1 and 2). This substituent was without effect on LRR or LSR for steroids with a 16-ketone group (compare steroids 7 and 8). All four of these steroids have very similar LRR and LSR activity profiles. For the ent-17-ketosteroid pair ent-1 and ent-2, the latter compound has a higher LRR EC50 value (~two fold) and the LSR EC50 value is raised above 10 μM. For the enantiomer pair ent-7 and ent-8, the effect of the axial 2-OMe group is very large. This substituent raises both the LRR and LSR EC50 values by ~five fold. No inconsistencies were found for the axial 2-OMe substituent effects between the binding (Table 1.) and tadpole reflex results.
The steroids 3 (17-ketone) and 9 (16-ketone) with the axial 4-OMe substituent have low activity as anesthetics in tadpoles. Based on the high IC50 values found in the [35S]TBPS displacement experiments (Table 1), the result that steroid 9 had any effect on either LRR or LSR at concentrations below 30 μM is somewhat surprising. Yet again, the axial 4-OMe substituent in the enantiomeric compounds ent-3 and ent-9 produced analogues with high activity. The EC50 values for both LRR and LSR are the lowest values observed and correlate with the low IC50 values for [35S]TBPS displacement (Table 1). Both EC50 values are lower than those found for anesthetic steroid 6 and are comparable to those found for the potent endogenous neurosteroid 5. Thus, effects of the axial 2-OMe or 4-OMe groups in the LRR and LSR bioassay generally maintain the pattern wherein these substituents have opposite effects in the steroid and ent-steroid series.
The contribution that the C-18 and C-19 Me groups have on potency for LRR and LSR of compound ent-7 was determined by comparing the EC50 values of this compound with those of compounds ent-10–ent-12. Compound ent-10, which lacks the C-18 Me group, has a higher LRR EC50 value than compound ent-7 by about seven-fold and does not cause LSR at concentrations up to 10 μM. Compound ent-11, which lacks the C-19 Me group, is about two-fold less potent at causing LRR and three-fold less potent at producing LSR than compound ent-7. Compound ent-12, which lacks both the C-18 and C-19 Me groups, produces neither LRR nor LSR at concentrations up to 10 μM. Overall, these results indicate that in this series of ent-steroids the C-18 Me group has a major effect on LRR and LSR whereas the C-19 Me group has a minor effect.
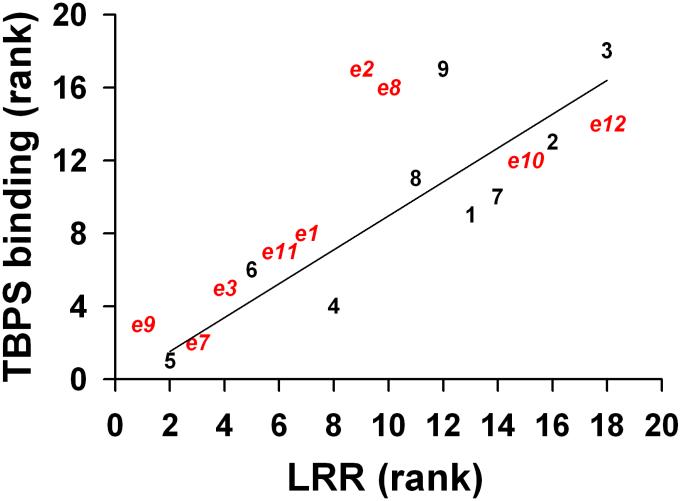
Figure 4 shows a rank order correlation for the [35S]TBPS IC50 and tadpole LRR EC50 values for all 18 compounds. The Pearson correlation coefficient is 0.81 indicative of a strong correlation between these parameters. The greatest outliers on the plot are compounds ent-2, ent-9 and 8 all of which were more potent at causing LRR than expected from their [35S]TBPS IC50 values.
Figure 4.
Correlations between rank order LRR anesthetic effects in tadpoles and TBPS displacement potency. The highest potency compound was assigned the rank of 1 and the least potent compound was assigned the rank of 18. For the least potent anesthetic compounds 3 and ent-12, their weak activity did not allow determination of the LLR EC50 value. These compounds were arbitrarily assigned the two highest LRR rankings of 18 and 17, respectively. Red symbols represent enantiomers (“e”). Black symbols represent natural compounds. Pearson r value of a linear regression (solid line) through all points was 0.81 (p<0.05).
ANESTHESIA IN MICE RESULTS
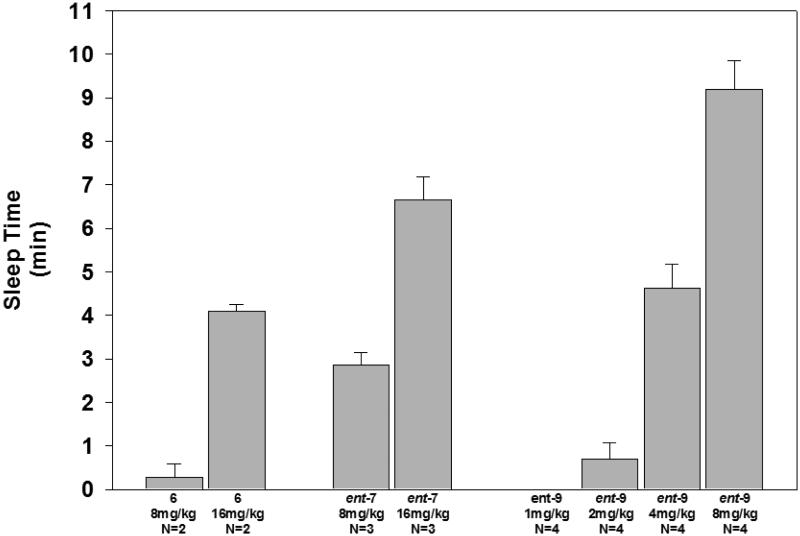
The potency, rate of onset, and rate of recovery for compounds ent-7 and ent-9 relative to these parameters for anesthetic steroid 6 were made using tail vein injections in mice. The duration of anesthesia, defined as loss of righting reflex, observed for the compounds is shown in Figure 5. We previously found that the threshold dose for mice LRR in mice by this route of administration was 8 mg/kg for steroid 6 which caused LRR of less than 1 min duration.15 A 16 mg/kg dose of steroid 6 caused LRR of about 4 min duration. At a dose of 8 mg/kg compound ent-7 caused LRR of about 3 min duration, and at a dose of 16 mg/kg duration of LRR was increased to about 7 min. The threshold dose for compound ent-7 was not determined. For compound ent-9, no LRR was observed at a dose of 1 mg/kg and LRR of about 1 min was observed at a threshold dose of 2 mg/kg. At 4 mg/kg, LRR induced by compound ent-9 lasted about 5 min, and at a dose of 8 mg/kg LRR lasted for about 9 min. For all three compounds the onset of anesthesia was immediate (< 30 sec), and recovery was characterized by a rapid progression over 1–2 min from an initial return of leg movement followed by righting and subsequent walking around the cage.
Figure 5.
Duration of anesthesia (LRR) induced by tail vein injection of alphaxalone (6), and ent-7 and ent-9. The compounds were dissolved in 22.5% aqueous 2-(hydroxypropyl)-β-cyclodextrin. For compound ent-9, serial dilutions of the 8 mg/kg stock solution with 0.9% saline were made to obtain the lower doses tested.
DISCUSSION
This is the second SAR study to follow up on our initial observation that ent-androsterone and ent-etiocholanolone are modulators of GABAA receptors.9,10 In the first follow up study, we presented a model for how the pair of androsterone enantiomers were aligned when bound to the site that causes steroid potentiation of GABAA receptors.13 In this study, we further validate and refine that model (Figure 1) and also show that it is useful for identifying structural features that could lead to development of novel clinically useful intravenous general anesthetics. We identify two candidate ent-steroids that have this potential.
Three predictions were made based on our original model and prior SAR studies of steroids and ent-steroids that potentiate GABAA receptors. The first concerned the effect of an axial 4α-OMe group on the activity of compound ent-1. It was shown about 40 years ago in the published SAR studies that led to the clinical use of anesthetic steroid 6 that axial 2β-alkoxy groups were compatible with high anesthetic activity and it was stated that various axial 4β-groups were not.14 We confirmed this SAR using steroids 2 (axial 2β-OMe substituent) and 3 (axial 4β-OMe substituent). Our model predicted that as a consequence of the inverted binding of ent-steroids relative to steroids at the steroid potentiation site, an ent-steroid with an axial 2α-OMe group would yield a compound with low activity (ent-2), whereas an ent-steroid with an axial 4α-OMe group would yield a compound (ent-3) with about the same anesthetic activity as steroid 2. This was found to be correct and consistently observed in all three of our screening bioassays ([35S]-TBPS displacement, oocyte electrophysiology, tadpole LRR and LSR).
The second prediction was that for active ent-steroids, a more favorable position for the D-ring hydrogen bond acceptor ketone group found at position C-17 in steroid potentiators, would be at position C-16 in ent-steroid potentiators. It also implies that moving the ketone group to the C-16 position in steroids would decrease the activity of steroid potentiators. The prediction for the ent-steroids was evaluated by comparing the actions of compounds ent-1 and ent-3 with those of ent-7 and ent-9, respectively. In both cases, this structural change produced compounds with much higher activity in all three bioassays. The prediction for the steroids was evaluated with the steroid pairs 1 and 7 as well as with steroid pairs 2 and 8. The lower activity expected for steroids 7 and 8 was observed in the [35S]TBPS displacement and oocyte electrophysiology screens which measure actions at GABAA receptors. However, these two compounds were modestly more potent in the tadpole LRR and LSR screen. This result was unexpected, but could be explained by steroid 7 and 8 modulation of other receptors that contribute to LRR and LSR in tadpoles.
The third prediction was that the activities of the ent-steroids would be more greatly affected by removal of the C-18 Me group than they would be by removal of the C-19 Me group. In the model shown in Figure 1, the C-18 and C-19 Me groups of both steroids and ent-steroids are in nearly the same place in three-dimensional space. This implies that the effects observed for C-18 and C-19 Me groups in steroids and ent-steroids should be the same. In steroids, their potentiation effects are little altered when the C-19 Me group is absent.24 By contrast, removal of the C-18 Me group can have a major impact on [35S]TBPS displacement potency and single channel kinetics.16,17 Hence, we expected similar effects for the C-18 and C-19 Me group substitution pattern in active ent-steroids. The effect of this Me group substitution pattern was evaluated using compounds ent-7 and ent-10–ent-12.
Compound ent-11 lacks the C-19 Me group. Relative to compound ent-7, which has both the C-18 and C-19 Me groups, ent-11 has diminished activity in all three screening bioassays. Removing the C-18 Me group, as in compound ent-10, causes a larger loss of activity relative to compound ent-7 than does removal of the C-19 Me group (ent-11) in all three screening bioassays. Compound ent-12, which lacks both the C-18 and C-19 Me groups, is not an effective potentiator in the electrophysiology screen and is inactive in the tadpole screens at or below 10 μM. This di-nor ent-steroid is a very weak displacer of [35S]TBPS. Overall the activities of compounds ent-7 and ent-11, the compounds with the C-18 Me group, are similar to each other, and compounds ent-10 and ent-12, the compounds without the C-18 Me group are similar to each other. Consistent with expectations, those with the C-18 Me group have the higher activity. We attribute the effect of the C-19 Me group to be explained in terms of its effect on logP. Removing this substituent, lowers logP (ent-7, logP 3.71; ent-11, logP 3.24) thereby decreasing a compound’s accumulation in the membrane where the steroid potentiation sites on the GABAA receptor are located.26,28 As a consequence of its lower effective concentration in the membrane bilayer, compound ent-11 has modestly decreased activity. With regard to the large loss of activity found when the C-18 Me group is not present, we propose that this is due to removal of a structural feature that is required to meet pharmacophore requirements for high activity. The calculated logP values for compounds ent-10 and ent-11 are the same (3.24) indicating that logP alone does not adequately explain why compounds without a C-18 Me group have such low activity. The very low activity of compound ent-12 (10 μM), which lacks both Me groups is likely due to the combination of the logP (2.77) and pharmacophore requirement effects. The correlation of logP with biological activity is further addressed later in the Discussion.
We carried out electrophysiologcal experiments with three different mutated forms of GABAA receptors to verify that potentiation effects of ent-16-ketosteroids (ent-7, ent-9) were affected by these mutations in the same way as those of endogeneous neurosteroid 5. The effects of all three mutations on the actions of the two ent-16-ketosteroids and steroid 5 were the same. We infer that the actions of the ent-steroids examined in this study are mediated by the previously identified steroid modulation site.26
We conducted single-channel measurements on the effects of analogues ent-7 and ent-9–ent-12 for two reasons. The first goal was to determine the kinetic components through which these ent-steroids potentiate rat α1β2γ2L receptors, for comparison with changes observed in the presence of steroid 5. The second goal was to determine whether changes in ent-steroid structure correlate with the specific kinetic effects. Previous studies employing single-channel kinetic analysis have revealed, for example, that the presence of the C-18 Me group in a steroid as well as the configuration at C-5 (α vs. β) can determine the ability of a steroid to affect the mean duration of long channel openings.16 Data presented in this study significantly extend prior knowledge and satisfy both of these goals.
We found that compound ent-9 had the identical profile of single-channel kinetic effects as the previously studied endogenous neurosteroid 5.28 Removing the axial 4α-OMe group (ent-7) removed the effect of increasing the mean lifetime of long open events. We then examined the effect of C-18, C-19 Me substituents on the profile of kinetic effects observed with compound ent-7. We found that the C-19 Me group’s effect correlated with open time properties. By contrast, the C-18 Me group’s effect correlated with decreased prevalence of the long closed state. The C-18 Me group seems to be a specific structural effect, whereas the C-19 Me group does not, since some cyclopenta[b]phenanthacene and cyclopenta[b]anthracenes analogues without a Me group placed in a position equivalent to that occupied by the steroid C-19 Me group, but containing the equivalent of a steroid C-18 Me group, produce similar effects on long open state events.29 For this reason, we postulate that the C-19 Me group in both steroids and ent-steroids having a trans A,B-ring fusion increases analogue potentiation predominately by an effect on logP. Comparison of single channel data (Table 4) with whole-cell recordings (Table 2) indicates that the kinetic component that contributes most to enhancement of the whole-cell peak response is the reduction in the fraction of long closed events. We note that this is in agreement with our previous study examining GABAA receptor potentiation by steroid 5.28,30
The correlation of logP with the biological activity of neurosteroids merits further discussion. The introduction of polar substituents at the C-11 and/or the C-21 position of neurosteroid 5 yields analogues with reduced activity at GABAA receptors. The decreased activity of the more polar analogues strongly correlates with logP.31,32 However, the correlation does not rule out the possibility that the polar substituents yield analogues which do not satisfy the pharmacophore requirements for maximal activity and that the correlation with logP is fortuitous. More generally, it implies that making structural changes that affect logP and pharmacophore requirements are difficult to interpret when both parameters change in the same direction. There are two ways to circumvent this commonly encountered interpretation difficulty in SAR studies. One is to use enantiomers which have identical logP values. Thus, changes in pharmacological activity (in bioassays where pharmacokinetic and metabolism issues are not confounding factors) can be unambiguously correlated with fit to the pharmacophore requirements. The second way is to identify analogues wherein the logP and pharmacological activity move in opposite directions. Both approaches are exemplified in this study.
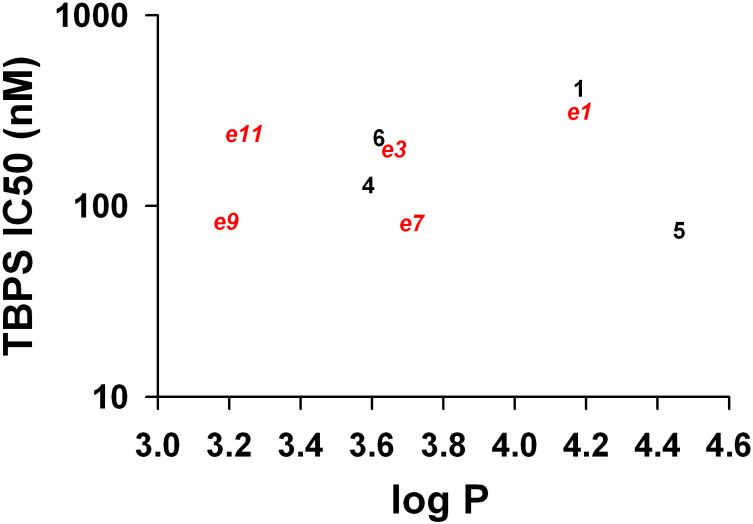
Overall, we failed to find a correlation between [35S]TBPS IC50 values and logP for those compounds which strongly displaced [35S]TBPS IC50 < 500 nM) as shown in Figure 6. As a specific example, the endogeneous neurosteroid 5 and analogue ent-9 have very different logP values (5, logP 4.46; ent-9, 3.19) but essentially the same IC50 values. In total, five enantiomeric pairs of steroids were examined in this study and in all cases the pairs of enantiomers had different activities. Since our mutagenesis data support the conclusion that the pairs of enantiomers act at the same steroid potentiation site on the GABAA receptor, this most likely reflects the ability of one enantiomer in each pair, independent of logP, to meet pharmacophore requirements better than the other enantiomer. It remains possible that the neurosteroid potentiation site has a different induced fit when bound to steroids than it does when bound to ent-steroids, but this does not invalidate our conclusion. Rather, it only indicates that receptor–ligand interactions should not be viewed as a rigid lock and key fit, but as an induced fit between both molecules as is now widely appreciated in SAR studies.
Figure 6.
Correlation between compound logP and log TBPS IC50 values for compounds with reported IC50 values below 500 nM. Red symbols represent enantiomers (“e”). Black symbols represent natural compounds. There was no correlation between logP and log TBPS IC50 (or between logP and TBPS IC50, Supplemental Material Figure S3).
Finally, we examined the in vivo anesthetic effects of compounds ent-7 and ent-9 in mice (Figure 5). At equivalent doses, both compounds induced LRR for a longer period of time than did anesthetic steroid 6. Compound ent-9 was particularly potent at causing LRR. It induced about twice the length of LRR at a dose of 8 mg/kg as did anesthetic steroid 6 at the higher dose of 16 mg/kg. Our expectation is that the lower logP value of compound ent-9 will increase its water solubility and that ent-steroids, in general, may provide a structural framework for the development of anesthetic steroid analogues with improved water solubility.
Additional efforts will be required to determine the full potential of ent-steroids as clinically useful drugs. As noted above, compound ent-9 has the potential to be a clinically useful anesthetic. Other ent-steroids could be potentially useful for treating conditions in which increasing neuronal inhibition by potentiation of GABAA receptor function has proven useful (e.g., seizures, anxiety, depression).1,33 Since enzymes and nuclear hormone receptors that bind endogeneous steroids have structurally defined chiral binding sites, there is the potential that ent-steroids will not be bound with high affinity to these proteins. If so, then ent-steroid drugs might not strongly interfere with endogenous steroid hormone biosynthesis or be agonists for steroid hormone receptors. Several examples of the failure of ent-steroid hormones to act as agonists at nuclear receptors are already reported in the literature.34-36 Results from those studies led to the suggestion that ent-steroid drugs have the potential to treat brain disorders or traumatic brain injury without acting as hormones that stimulate the development and growth of hormone-dependent cancers.36,37
It is also possible that the half-lives of ent-steroid drugs may be quite different, and potentially longer, than those of steroid drugs used as anticonvulsants, anxiolytics, and other neuroactive agents. In this regard, liver metabolism of ent-steroids has received almost no attention thus far. One reported study of the glucuronidation of three ent-steroids showed that the enantiomers are preferred substrates for different glucuronosyltransferases than those that accept the naturally occurring enantiomers as substrates.38 Other differences in liver metabolism are also likely to occur. Thus, it is likely that any effort to develop ent-steroids as drugs will require a great deal of effort with the attendant generation of a lot of new knowledge as so little is known about the in vivo actions and metabolism of ent-steroids in humans.
CONCLUSION
We conclude that a previously proposed model showing the alignment of androsterone enantiomers at the steroid potentiation site on GABAA receptors has predictive value. The model accurately predicted: 1) that the potentiating effects of compound ent-1 would be increased in an analogue with the 16-ketone group (ent-7); 2) that high activity would be maintained by adding by adding an axial 4α-OMe substituent (ent-9), but not an axial 2α-OMe group (ent-8); and 3) that the C-18 Me group in ent-16-ketosteroids is more important for enhancement of potentiation than the C-19 Me group. We compared the length of loss of righting reflex in mice induced by tail vein injection of anesthetic steroid 6 and analogues ent-7 and ent-9. We found that both ent-steroids induced a longer period of loss of righting reflex at a lower dose than did steroid 6. We conclude that the high activity of these two compounds results from their ability to satisfy pharmacophore requirements, not because of an increase in logP, since compound ent-9, has a lower logP than anesthetic steroid 6 and both of these ent-neurosteroids have a lower logP than endogeneous neurosteroid 5. We propose that ent-steroids have high potential for development as both intravenous anesthetics and drugs to treat brain disorders that are alleviated by potentiation of GABAA receptor function.
EXPERIMENTAL SECTION
General Methods
Solvents were either used as purchased or dried and purified by standard methodology. Extraction solvents were dried with anhydrous Na2SO4 and after filtration, removed on a rotary evaporator. Flash column chromatography was performed using silica gel (32–63 μm) purchased from Scientific Adsorbents (Atlanta, GA). Melting points were determined on a Kofler micro hot stage and are uncorrected. FT-IR spectra were recorded as films on a NaCl plate. NMR spectra were recorded in CDCl3 at ambient temperature at 300 or 400 MHz (1H), 74 or 100 MHz (13C). Purity of > 95% was determined for all evaluated compounds by combustion analysis for C,H performed by M-H-W Laboratories (Phoenix, AZ). Steroids 1,5,6,13,14 and 18 were purchased from Steraloids (Newport, RI). K(sec-Bu)3BH (K-Selectride) was purchased from Aldrich Chemical Co. (Milwaukee, WI). The logP values were calculated using ChemDraw from CambridgeSoft (Cambridge, MA).
(5β,8α,9β,10α,13α,14β)-Androstane-3,17-dione (ent-1)
The compound was prepared as previously described.9
(2α,3β,5β,8α,9β,10α,13α,14β)-3-Hydroxy-2-methoxyandrostan-17-one (ent-2)
The 2α,3α-epoxide ent-17 (250 mg, 0.86 mmol), containing minor amounts of the 2β,3β-epoxide, was dissolved in MeOH (10 mL), a drop of conc. H2SO4 was added and the reaction was stirred at room temperature for 3 hr. The reaction mixture was made basic with aqueous NaHCO3 and the MeOH was removed under reduced pressure. The resulting residue was diluted with water and extracted with EtOAc (3 × 80 mL). The combined organic extracts were dried and concentrated to give a solid which was purified by column chromatography (silica gel eluted with 20–35% EtOAc in hexanes) to give ent-2 as a white solid (170 mg, 62%): mp 153–155 °C; [α]D23 −98.3 (c 0.23, CHCl3); IR νmax 3438, 2920, 1739, 1595, 1453, 1406, 1372, 1255, 1214 cm−1 ; 1H NMR (CDCl3) δ 3.95 (br s, 1H), 3.32 (br s, 4H), 2.45 (dd, 1H, J = 19.2 Hz, 8.7 Hz), 1.00 (m, 1H), 0.95 (s, 3H), 0.85 (s, 3H), 0.77 (m, 1H); 13C NMR δ 221.5, 80.6, 68.1, 56.6, 55.1, 51.4, 47.8, 39.0, 36.1, 35.8, 35.5, 34.5, 32.0, 31.5, 30.8, 27.8, 21.7, 20.1, 13.8, 13.1. Anal. (C20H32O2) C, H.
(2β,3α,5α)-3-Hydroxy-2-methoxyandrostan-17-one (2)
Steroid 2 (206 mg, 73%) was prepared from the natural enantiomer of compound ent-17 using the procedure described for the preparation of compound ent-2. Flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) gave product 2: mp 151–153 °C; [α]D23 +92.4 (c 0.37, CHCl3); IR νmax 3439, 1738 cm−1; 1H NMR (CDCl3) δ 3.84–3.83 (m, 1H), 3.22 (br s, 4H), 2.54 (br s, 1H), 2.38 (dd, 1H, J = 19.2 Hz, 8.8 Hz), 0.86 (s, 3H), 0.76 (s, 3H), 0.71–0.64 (m, 1H); 13C NMR (CDCl3) δ 221.4, 80.5, 67.7, 56.4, 54.9, 51.3, 47.6, 38.7, 35.9, 35.6, 35.2, 34.3, 31.8, 31.4, 30.6, 27.6, 21.5, 19.9, 13.6, 12.9. Anal. (C20H32O3) C, H.
(3β,4α,5β,8α,9β,10α,13α,14β)-3-Hydroxy-4-methoxyandrostan-17-one (ent-3)
Compound ent-3 (200 mg, 78%) was prepared from compound ent-20 using the procedure described for the preparation of ent-2. Flash column chromatography (silica gel eluted with 35% EtOAc in hexanes) gave product ent-3 as a white solid: mp 215–218 °C; [α]D23 −99 (c 0.06, CHCl3); IR νmax 3510, 2917, 2838, 1735, 1594, 1443, 1375, 1242 cm−1 ; 1H NMR (CDCl3) δ 4.02 (br s, 1H), 3.35 (s, 3H), 3.04 (s, 1H), 2.44 (dd, 1H, J = 19.0 Hz, 9.0 Hz), 0.98 (s, 3H), 0.86 (s, 3H), 0.62 (m, 1H); 13C NMR (CDCl3) δ 221.5, 85.4, 66.1, 59.0, 55.2, 51.5, 47.8, 44.0, 36.2, 35.8, 35.0, 31.8, 31.5, 31.1, 25.2, 25.0, 21.7, 19.6, 14.0, 13.8. Anal. (C20H32O3 ) C, H.
(3α,4β,5α)-3-Hydroxy-4-methoxyandrostan-17-one (3)
Steroid 3 (270 mg, 81%) was prepared from the natural enantiomer of compound ent-20 using the procedure described for the preparation of compound ent-2. Flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) gave product ent-3 as a white solid : mp 223–225 °C; [α]D20 +95 (c 0.06, CHCl3); IR νmax 3513, 1734 cm−1; 1H NMR (CDCl3) δ 3.97 (br s, 1H), 3.30 (s, 3H), 2.95–2.85 (m, 1H), 2.44 (dd, 1H, J = 19.3 Hz, 8.8 Hz), 0.93 (s, 3H), 0.81 (s, 3H); 13C NMR (CDCl3) δ 221.7, 85.7, 66.3, 59.1, 55.4, 51.7, 47.9, 44.2, 36.3, 36.0, 35.2, 32.0, 31.7, 31.3, 25.4, 25.1, 21.9, 19.8, 14.1, 14.0. Anal. (C20H32O3) C, H.
[3α,5α,17(20)Z]-3-Hydroxy-11-oxopregn-17(20)-ene-21-nitrile (4)
The compound was prepared as previously described.15
(3β,5β,8α,9β,10α,13α,14β)-3-Hydroxyandrostan-16-one (ent-7)
Compound ent-24 (88 mg, 0.27 mmol) was dissolved in methanol (10 mL) and water (0.5 mL) and K2CO3 (5 mmol, 680 mg) were added. The reaction was refluxed for 2 h, cooled to room temperature, water (30 mL) was added and the product was extracted into EtOAc (50 mL × 3). The combined extracts were dried, filtered, and the solvent removed. The residue was purified by flash column chromatography (silica gel eluted with 25–40% EtOAc in hexanes) to give product ent-7 (72 mg, 93%) as a white solid: mp 152–153 °C; [α]D23 +156.8 (c 0.25, CHCl3); IR νmax 3435, 1737 cm−1; 1H NMR (CDCl3) δ 4.06 (m, 1H), 0.87 (s, 3H), 0.81 (s, 3H); 13C NMR (CDCl3) δ 219.0, 66.4, 55.9, 54.2, 51.7, 39.3, 39.2, 39.0, 38.2, 36.3, 35.8, 34.9, 32.2, 31.9, 28.9, 28.3, 20.3, 18.1, 11.2. Anal. (C19H30O2) C, H.
(3α,5α)-3-Hydroxyandrostan-16-one (7)
Steroid 7 (50 mg, 90%) was prepared from the natural enantiomer of compound ent-24 using the procedure described for the preparation of compound ent-7. Product 7 was obtained as a white solid: mp 152–154 °C (lit mp39 153–154 °C); [α]D23 –154.0 (c 0.20, CHCl3); IR νmax 3441, 1738 cm−1; 1H NMR (CDCl3) δ 4.05 (m, 1H), 0.87 (s, 3H), 0.81 (s, 3H); 13C NMR (CDCl3) δ 219.0, 66.2, 55.8, 54.2, 51.6, 39.2, 39.1, 38.9, 38.1, 36.2, 35.7, 34.8, 32.2, 31.8, 28.8, 28.2, 20.2, 18.0, 11.2. Anal. (C19H30O2) C, H.
(2α,3β,5β,8α,9β,10α,13α,14β)-3-Hydroxy-2-methoxyandrostan-16-one (ent-8)
Compound ent-8 (120 mg, 97%) was prepared from compound ent-27 using the procedure described for the preparation of compound ent-7. Flash column chromatography (silica gel eluted with 25–40% EtOAc in hexanes) gave product ent-8 as a white solid: mp 209–211 °C; [α]D23 +144 (c 0.25, CHCl3); IR νmax 3447, 1729 cm−1; 1H NMR (CDCl3) δ 3.95 (br s, 1H), 3.32 (s, 4H), 0.95 (s, 3H), 0.85 (s, 3H); 13C NMR δ 218.9, 80.6, 68.0, 56.6, 55.8, 54.9, 51.7, 39.2, 39.1, 38.8, 38.2, 36.1, 35.3, 34.3, 32.2, 32.0, 27.8, 20.4, 18.1, 13.0. Anal. (C20H32O3) C, H.
(2β,3α,5α)-3-Hydroxy-2-methoxyandrostan-16-one (8)
Steroid 8 (68 mg, 94%) was prepared from the natural enantiomer of ent-27 using the procedure described for the preparation of compound ent-7. Product 8 was obtained as a white solid: mp 205–207 °C; [α]D23 −143.5 (c 0.20, CHCl3); IR νmax 3445, 1730 cm−1; 1H NMR (CDCl3) δ 3.92 (br s, 1H), 3.31 (s, 4H), 0.94 (s, 3H), 0.84 (s, 3H); 13C NMR (CDCl3) δ 218.9, 80.6, 68.0, 56.6, 55.8, 54.9, 51.7, 39.2, 39.1, 38.8, 38.2, 36.1, 35.3, 34.3, 32.2, 32.0, 27.8, 20.4, 18.1, 13.0. Anal. (C20H32O3) C, H.
(3β,4α,5β,8α,9β,10α,13α,14β)-3-Hydroxy-4-methoxyandrostan-16-one (ent-9)
Compound ent-9 (50 mg, 94%) was prepared from compound ent-30 using the procedure described for the preparation of compound ent-7. Flash column chromatography (silica gel eluted with 25–40% EtOAc in hexanes) gave product ent-9 as a white solid: mp 198–200 °C; [α]D23 +152 (c 0.2, CHCl3); IR νmax 3458, 1725 cm−1; 1H NMR (CDCl3) δ 4.01 (br s, 1H), 3.34 (s, 3H), 3.02 (d, 1H, J = 1.1 Hz), 0.98 (s, 3H), 0.87 (s, 3H); 13C NMR δ 219.0, 85.4, 66.2, 59.0, 55.9, 55.1, 51.8, 43.9, 39.3, 39.3, 38.1, 36.2, 34.9, 32.6, 31.6, 25.2, 25.0, 19.9, 18.1, 14.0. Anal. (C20H32O3) C, H.
(3α,4β,5α)-3-Hydroxy-4-methoxyandrostan-16-one (9)
Steroid 9 (62 mg, 90%) was prepared from the natural enantiomer of compound ent-30 using the procedure described for the preparation of compound ent-7. Steroid 9 was obtained as a white solid: mp 196–198 °C; [α]D23 −141.8 (c 0.17, CHCl3); IR νmax 3445, 1725 cm−1; 1H NMR (CDCl3) δ 3.99 (br s, 1H), 3.33 (s, 3H), 3.00 (m, 1H), 0.97 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3) δ 219.0, 85.4, 66.2, 59.0, 55.9, 55.1, 51.8, 43.9, 39.3, 39.1, 38.1, 36.2, 34.9, 32.5, 31.6, 25.2, 25.0, 19.9, 18.1, 14.0. Anal. (C20H32O3) C, H.
(3β,5β,8α,9β,10α,13α,14β)-18-Norandrostan-16-one (ent-10)
A mixture of β-ketoester ent-46 (25 mg, 0.075 mmol), LiCl (100 mg) and DMF was heated at 160 °C for 30 min under N2. The reaction mixture was cooled, diluted with water and extracted with EtOAc. The combined organic extracts were dried and concentrated to give an off-white solid. The crude product was purified by flash column chromatography (silica gel eluted with 30–40% EtOAc in hexanes) to give product ent-10 as a white solid (18 mg, 86%): mp 174–177 °C; [α]D23 +171.9 (c 0.1, CHCl3); IR νmax 3475, 2923, 2853, 1723 cm−1; 1H NMR (CDCl3) δ 4.06 ( br s, 1H), 2.36 (m, 2H), 0.78 (s, 3H); 13C NMR (CDCl3) δ 218.5, 66.4, 53.3, 50.2, 45.9, 43.8, 43.3, 41.7, 38.8, 36.2, 35.8, 32.2, 32.0, 31.1, 29.0, 28.1, 24.8, 11.1. Anal. (C18H28O2) C, H.
(3β,5β,8α,9β,10α,13α,14β)-19-Norandrostan-16-one (ent-11)
Compound ent-11 (207 mg, 98%) was prepared from compound ent-33 using the procedure described for the preparation of compound ent-7. Flash column chromatography (silica gel eluted with 25–40% EtOAc in hexanes) gave product ent-11 as a white solid: mp 160–162 °C; [α]D23 +162.0 (c 0.22, CHCl3); IR νmax 3401, 2915, 2861, 1743 cm−1; 1H NMR (CDCl3) δ 4.08 (br s, 1H), 0.87 (s, 3H); 13C NMR (CDCl3) δ 218.8, 66.3, 56.0, 50.9, 48.0, 47.0, 40.53, 40.47, 39.2, 39.1, 38.1, 35.8, 33.4, 32.9, 31.2, 25.1, 23.4, 18.1. Anal. (C18H28O2) C, H.
(3β,5β,8α,9β,10α,13α,14β)-3-Hydroxygonan-16-one. (ent-12)
K-Selectride (1M in THF, 0.45 mL) was added dropwise under N2 to a cooled solution (−78 °C) of compound ent-58 (108 mg, 0.41 mmol) in anhydrous THF (15 mL). After 2 h stirring at −78 °C, water (2 mL) was added and the mixture was allowed to reach room temperature. Then, aqueous NaOH (2 mL, 6 M), and 30% H2O2 (2 mL) were added and the reaction was stirred for 30 min. The product was extracted with CH2Cl2 (2 × 50 mL), the combined extracts were washed with aqueous HCl (1 N), saturated aqueous NaHCO3, and brine. Solvent was dried and evaporated. Flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) gave product ent-12 (76 mg, 70%) as a white solid: mp 168–169 °C (EtOAc/hexanes); [α]D23 +218.6 (c 0.17, CHCl3). IR νmax 3466, 2917, 2846, 1724 cm−1; 1H NMR (CDCl3) δ 4.08 (1H, m), 2.30–2.41 (2H, m), 1.97−2.10 (2H, m). 13C NMR (CDCl3) δ 218.5, 66.5, 49.3, 47.7, 47.3, 47.2, 46.1, 43.8, 43.6, 40.7, 35.9, 33.5, 33.1, 31.4, 31.1, 29.6, 23.6. Anal. (C17H26O2) C, H.
(5β,8α,9β,10α,13α,14β)-Androstane-3,17-dione (ent-13)
This compound was prepared as described previously.9
(3α,5β,8α,9β,10α,13α,14β)-3-Hydroxyandrostan-17-one (ent-14)
A 1M THF solution of Li(t-OBu)3AlH (6.86 mL, 1.1 equiv) was added to a cold (−40 °C) solution of compound ent-13 (1.80 g, 6.24 mmol) in THF (15 mL), and the resulting mixture was stirred at −40 °C for 90 min. A few drops of acetone were added to consume excess hydride reagent and the mixture was stirred for a few min. Then 1M HCl was added and the reaction was warmed to room temperature. The product was extracted into EtOAc and the combined organic extracts were washed with brine, dried and the solvent removed to give an oil, which was purified by flash column chromatography (silica gel eluted with 30–40% EtOAc in hexanes) to give product ent-14 as a colorless solid (1.49 g, 82%): mp 171–173 °C; [α]D23 −95.4 (c 0.13, CHCl3); IR νmax 3470, 2928, 2853, 1732, 1450, 1374 cm−1; 1H NMR (CDCl3) δ 3.59 (m, 1H), 2.41 (dd, 1H, J = 19.2 Hz, 9.0 Hz), 2.05 (dd, 1H, J = 19.2 Hz, 9.0 Hz), 0.85 (s, 3H), 0.83 (s, 3H), 0.69 (m, 1H); 13C NMR (CDCl3) δ 221.4, 71.1, 54.4, 51.4, 47.8, 44.8, 38.0, 36.9, 35.8, 35.6, 35.0, 31.5, 31.4, 30.9, 28.4, 21.7, 20.5, 13.8, 12.3. Anal. (C20H32O3) C, H.
(3α,5β,8α,9β,10α,13α,14β)-3-[(Methylsulfonyl)oxy]-androstan-17-one (ent-15)
MeSO2Cl (0.58 mL, 7.5 mmol) was added to a cold solution (0 °C) of compound ent-14 (1.45 g, 5 mmol) and Et3N (1.4 ml, 10 mmol) in CH2Cl2 (15 mL) and the mixture was slowly warmed to room temperature (~15 min) and stirred for 30 min. An aqueous saturated NaHCO3 solution was added and the product was extracted into CH2Cl2. The organic extracts were washed with brine, dried and concentrated to give an oil, which was purified by flash column chromatography (silica gel eluted with 15–30% EtOAc in hexanes) to give mesylate ent-15 as a white solid (1.84 g, 100%): mp 150–152 °C (dec); IR νmax 2919, 1737, 1452, 1352 cm−1 ; 1H NMR (CDCl3) δ 4.43 (m, 1H), 2.86 (s, 3H), 2.27 (dd, 1H, J = 19.2 Hz, 8.8 Hz), 0.71 (s, 3H), 0.70 (s, 3H), 0.57 (m, 1H); 13C NMR (CDCl3) δ 220.2, 81.3, 53.6, 50.7, 47.2, 44.2, 38.2, 36.2, 35.3, 34.9, 34.6, 34.4, 31.0, 30.2, 28.1, 27.7, 21.2, 20.0, 13.3, 11.7.
(3β,5α)-3-[(Methylsulfonyl)oxy]-androstan-17-one (15)
The known steroid 1540 (1.62 g, 99 %) was prepared from the natural enantiomer of compound ent-14 using the procedure described for the preparation of compound ent-15.
(5β,8α,9β,10α,13α,14β)-Androst-2-en-17-one (ent-16)
Mesylate ent-15 (1.60 g, 4.34 mmol) and LiBr (3.89 g, 44.7 mmol) in DMF (15 mL) were heated at 125 °C under N2 for 2 h. The reaction mixture was cooled, made basic with aqueous NaHCO3 and extracted with EtOAc. The combined organic extracts were washed with brine, dried and concentrated to give an oil which was purified by flash column chromatography (silica gel eluted with 5–20% EtOAc in hexanes) to give the a 5:1 mixture (determined by NMR) of Δ2 and Δ3 olefins as a white solid (1.03 g, 87%): IR νmax 3021, 2933, 2886, 2842, 1744, 1655, 1467, 1377 cm−1; (major isomer) 1H NMR (CDCl3) δ 5.52 (m, 2H), 2.38 (dd, 1H, J = 19.2 Hz, 8.8 Hz), 0.81 (s, 3H), 0.72 (s, 3H); (major isomer) 13C NMR(CDCl3) δ 220.8, 125.5, 125.5, 54.0, 51.2, 47.4, 41.2, 39.5, 35.6, 34.9, 34.5, 31.4, 30.4, 30.0, 28.2, 21.6, 20.0, 13.5, 11.5.
(5α)-Androst-2-en-17-one (16)
The known steroid 1639 (1.1 g, 91%, 7:1 mixture of Δ2 and Δ3 olefins) was prepared from the natural enantiomer of compound ent-15 using the procedure described for the preparation of compound ent-16.
(2α,3α,5β,8α,9β,10α,13α,14β)-2,3-Epoxyandrostan-17-one (ent-17)
Compound ent-16 (containing the unseparated Δ3 olefin, 0.97 g, 3.56 mmol) was dissolved in CH2Cl2 (20 mL) and HCO2H (1.34 mL) and then 30% H2O2 (3.3 mL) was added and the reaction was stirred for 4 h at room temperature. MeOH (20 mL) was added and after 3 min, 10% aqueous NaOH (15mL) was added and the mixture was stirred for 5 min. 10% HCl (20 mL) was added dropwise and the resulting mixture was stirred for 3 min. The product was extracted into CH2Cl2 (3 × 150 mL) and the organic extracts were dried and concentrated to give an oil which was purified by flash column chromatography (silica gel eluted with 20–30% EtOAc in hexanes) to give the epoxide ent-17 (containing the 3,4-epoxide) as a white solid (1.06 g, 100 %): IR νmax 2918, 1740, cm−1 ; (major isomer) 1H NMR (CDCl3) δ 3.10–2.98 (m, 2H), 2.40 (dd, 1H, J = 19.6 Hz, 9.0 Hz), 0.72 (s, 3H), 0.66 (s, 3H), 0.56 (m, 1H); (major isomer) 13C NMR (CDCl3) δ 220.4, 53.5, 51.8, 50.9, 50.4, 47.2, 37.9, 35.9, 35.4, 34.8, 33.4, 31.2, 30.2, 28.7, 27.8, 21.4, 19.8, 13.3, 12.3.
(2β,3β,5α)-2,3-Epoxyandrostan-17-one (17)
The known steroid 1741 (1.05 g, 91%) was prepared from the natural enantiomer of compound ent-16 (containing the unseparated Δ3 olefin) using the procedure described for the preparation of compound ent-17.
(8α,9β,10α,13α,14β)-Androst-4-ene-3,17-dione (ent-18)
This compound was prepared as previously described.7
(5β,8α,9β,10α,13α,14β)-Androst-3-en-17-one (ent-19)
To a boiling solution of compound ent-18 (750 mg, 2.63 mmol) in glacial AcOH (40 mL), Zn dust (4.5g) was added in several portions during a period of 15 min and then heating was continued an additional 15 min. The reaction was cooled, and the Zn dust was filtered, and the filtrate was collected. The filter-cake was washed with AcOH and EtOAc. Solvents were removed from the combined filtrates and washings, water was added to the residue and the product was extracted into EtOAc. The combined extracts were washed with aqueous NaHCO3, brine, dried and solvents were evaporated to give a white solid which is a mixture (2.3:1 by NMR) of product ent-19 and the epimeric (5α)-3-ene product. The product mixture was crystallized from hexanes to give pure product ent-19 (310 mg, 43%): mp 122–125 °C; IR νmax 3016, 2968, 2940, 2837, 2807, 1742, 1470, 1443, 1376, 1251 cm−1 ; 1H NMR (CDCl3) δ 5.60 (m, 1H), 5.29 (m, 1H), 2.44 (dd, 1H, J = 19.0 Hz, 9.0 Hz), 0.88 (s, 3H), 0.80 (s, 3H); 13C NMR (CDCl3) δ 221.3, 131.0 125.6, 53.5, 51.5, 47.9, 45.9, 35.8, 35.1, 35.0, 34.0, 31.6, 30.9, 27.1, 23.4, 21.8, 20.3, 13.9, 11.8.
(5α)-Androst-3-en-17-one (19)
The known steroid 1942 (350 mg, 37%) was prepared from the natural enantiomer of compound ent-18 using the procedure described for the preparation of compound ent-19.
(3β,4β,5β,8α,9β,10α,13α,14β)-2,3-Epoxyandrostan-17-one (ent-20)
Compound ent-20 (300 mg, 84%) was prepared from compound ent-19 using the procedure described for the preparation of compound ent-17. The crude product was obtained as an oil and after flash column chromatography (silica gel eluted with 20–30% EtOAc in hexanes) yielded a white solid: mp 148–150 °C; IR νmax 2928, 2882, 2859, 1740, 1472, 1446, 1405, 1373, 1251 cm−1 ; 1H NMR (CDCl3) δ 3.09 (s, 1H), 2.63 (d, 1H, J = 3.9 Hz), 2.36 (dd, 1H, J = 19.6 Hz, 9.0 Hz), 0.79 (s, 3H), 0.73 (s, 3H); 13C NMR (CDCl3) δ 220.7, 55.4, 52.5, 51.9, 51.0, 47.6, 46.6, 35.6, 34.8, 34.0, 31.3, 30.5, 30.2, 26.3, 21.5, 21.1, 20.4, 13.7, 13.3.
(2α,3α)-2,3-Epoxyandrostan-17-one (20)
The known steroid 2043 (300 mg, 95%) was prepared from the natural enantiomer of compound ent-19 using the procedure described for the preparation of compound ent-16. Steroid 20 had: IR νmax 2926, 1738, 1403, 1250 cm−1; 1H NMR (CDCl3) δ 3.05–3.04 (m, 1H), 2.61 (d, 1H, J = 3.9), 2.35 (dd, 1H, J = 18.7, 10.2), 0.76 (s, 3H), 0.70 (s, 3H); 13C NMR (CDCl3) δ 220.5, 55.4, 52.5, 51.8, 51.0, 47.5, 46.6, 35.5, 34.7, 34.0, 31.3, 30.5, 30.1, 26.3, 21.5, 21.0, 20.3, 13.7, 13.2.
(3β,5β,8α,9β,10α,13α,14β)-3-Hydroxyestran-17-one (ent-21)
Compound ent-21 was prepared from (8α,9β,10α,13α,14β,17α)-17-hydroxyestr-4-en-3-one37 using the methods reported previously for the preparation of compound ent-1 from ent-testosterone.9 Compound ent-21 had: mp 158–160 °C; [α]D = −108.5 (c 0.24, CHCl3); IR νmax 3487, 2859, 1739, 1444, 1374, 1261 cm−1; 1H NMR (CHCl3) δ 4.01 (s, 1H), 2.42 (dd, 1H, J = 19.3 Hz, 9.8 Hz), 0.86 (s, 3H); 13C NMR (CHCl3) δ 221.6, 66.3, 50.6, 48.2, 47.9, 47.0, 40.7, 40.5, 35.9, 35.8, 33.4, 32.9, 31.4, 29.8, 24.9, 23.7, 21.6, 13.8.
(3α,5α)-3-Hydroxyestran-17-one (21)
– The compound was prepared as previously described.44
(3β,5β,8α,9β,10α,13α,14β,17α)-16-(Phenylmethylene)-androstane-3,17-diol diacetate (ent-22)
Compound ent-1 (1.25 g, 4.5 mmol) and benzaldehyde (1.4 mL, 14 mmol) were added to KOH (700 mg) dissolved in EtOH (60 mL) and the reaction was stirred at room temperature for 16 h. The reaction mixture was cooled to 0 °C and CeCl3·7 H2O (7.45 g, 20 mmol) and NaBH4 (756 mg, 20 mmol) were added, the reaction was allowed to warm to room temperature and stirring continued for 3 h. Glacial AcOH (7 mL) was added and the product extracted into EtOAc (3 × 150 mL). The combined extracts were dried and the solvents removed to give an oil. The oil was dissolved in CH2Cl2 (30 mL) and AcOAc (1.4 mL, 15 mmol), NEt3 (4.2 ml, 30 mmol) and DMAP (200 mg) were added and stirring was continued at room temperature for 4 h. Aqueous saturated NaHCO3 was then added. After 1 h, the product was extracted into CH2Cl2 (3 × 125 mL). The combined extracts were dried and solvents removed to give an oil. The crude product was purified by flash column chromatography (silica gel, eluted with 25–35 % EtOAc in hexanes) to give product ent-22 as a colorless liquid containing benzyl acetate. The benzyl acetate was removed by applying high vacuum at 60 °C for 10 h, to give product ent-22 as a white solid (1.9 g, 91%): mp 162–164 °C; IR νmax 2932, 2855, 1735, 1492, 1447, 1371, 1237 cm−1; 1H NMR (CHCl3) δ 7.37– 7.17 (m, 5H), 6.21 (d, 1H, J = 2.4 Hz), 5.37 (s, 1H), 5.03 (s, 1H), 2.68 (dd, 1H, J = 16.8 Hz, 6.6 Hz), 2.21 (s, 3H), 2.07 (s, 3H), 0.83 (s, 3H), 0.78 (s, 3H); 13C NMR (CHCl3) δ 171.2, 170.7, 141.0, 137.6, 128. 3 (2 × C), 128.2 (2 × C), 126.4, 123.5, 84.6, 70.0, 54.1, 48.9, 42.9, 40.0, 36.5, 35.9, 34.8, 32.8, 32.7, 31.5, 30.9, 28.1, 26.0, 21.5, 21.2, 20.3, 12.3, 11.3.
(3α,17β)-16-(Phenylmethylene)-androstane-3,17-diol diacetate (22)
Steroid 22 (144 mg, 90%) was prepared from the natural enantiomer of compound ent-1 using the procedure described for the preparation of compound ent-21. Steroid 22 had: 1H NMR (CDCl3) δ 7.29–7.10 (m, 5H), 6.13 (d, 1H, J = 2.4 Hz), 5.29 (s, 1H), 4.94 (s, 1H), 2.62 (dd, 1H, J = 16.8 Hz, 6.6 Hz), 2.12 (s, 3H), 1.98 (s, 3H), 0.74 (s, 3H), 0.69 (s, 3H); 13C NMR (CDCl3) δ 171.1, 170.6, 140.9, 137.5, 128.2, 128.1, 126.4, 123.5, 84.5, 69.9, 54.0, 48.8, 42.9, 39.9, 36.4, 35.8, 34.8, 32.8, 32.7, 31.5, 30.9, 28.1, 26.0, 21.5, 21.1, 20.2, 12.2, 11.3.
(3β,5β,8α,9β,10α,13α,14β,17α)-3,17-Dihydroxyandrostan-16-one diacetate (ent-23)
Compound ent-23 (110 mg, 82%, starting from compound ent-1) was prepared from compound ent-22 using the procedure described for the preparation of compound ent-26. Compound ent-23 was a white solid and had: 1H NMR (CDCl3) δ 4.96 (m, 2H), 2.11 (s, 3H), 2.01 (s, 3H), 0.77 (s, 6H); 13C NMR (CDCl3) δ 211.0, 170.5, 170.2, 85.5, 69.7, 53.8, 45.2, 41.6, 39.7, 36.2, 35.9, 35.8, 34.2, 32.6, 32.4, 31.4, 27.8, 25.8, 21.4, 20.5, 19.7, 12.3, 11.2.
(3α,5α,17β)-3,17-Dihydroxyandrostan-16-one diacetate (23)
Steroid 23 (109 mg, 91%) was prepared from the natural enantiomer of compound ent-22 using the procedure described for the preparation of compound ent-26. Steroid 23 was a white solid and had: 1H NMR (CDCl3) δ 4.98–4.95 (m, 2H), 2.14 (s, 3H), 2.03 (s, 3H), 0.78 (s, 6H); 13C NMR (CDCl3) δ 211.1, 170.6, 170.3, 85.6, 69.8, 53.9, 45.3, 41.7, 39.8, 36.2, 36.0, 35.9, 34.2, 32.6, 32.5, 31.5, 27.9, 25.9, 21.4, 20.6, 19.8, 12.4, 11.2.
(3β,5β,8α,9β,10α,13α,14β)-3-Hydroxyandrostan-16-one acetate (ent-24)
Freshly prepared Sm filings (1.5 mmol, 225 mg) were added to THF (10 mL) and I2 (1.0 mmol, 254 mg) in THF (5 mL) was added at room temperature. The suspension was stirred under N2. After 30 min, the mixture became deep blue indicating SmI2 formation and stirring was continued for another 30 min. Compound ent-23 (0.28 mmol, 110 mg) in THF/methanol (10/1, 11 mL) was added. After 2 h, 10% aqueous Na2CO3 (60 mL) was added and the product was extracted into EtOAc (3 × 50 mL). The combined extracts were washed with brine (2 × 20 mL) and dried. Solvents were removed and the residue was purified by flash column chromatography (silica gel, eluted with 20% EtOAc in hexanes) to give product ent-24 (48 mg, 51%) and a compound in which the 16-ketone group had been reduced to a 16-hydroxyl group of undetermined configuration (48 mg). Jones oxidation of the 16-hydroxyl group gave additional compound ent-24 (40 mg, 43%): 1H NMR (CDCl3) δ 5.00 (br s, 1H), 2.03 (s, 3H), 0.86 (s, 3H), 0.81 (s, 3H); 13C NMR (CDCl3) δ 218.7, 170.6, 69.9, 55.8, 54.1, 51.7, 39.9, 39.1, 38.1, 35.9, 34.8, 32.7, 32.5, 32.1, 28.0, 26.0, 21.5, 20.3, 18.0, 11.3.
(3α,5α)-3-Hydroxyandrostan-16-one acetate (24)
Steroid 24 (60 mg, 91%) was prepared from the natural enantiomer of compound ent-23 using the procedure described for the preparation of compound ent-23 and had: 1H NMR (CDCl3) δ 5.00 (br s, 1H), 2.03 (s, 3H), 0.85 (s, 3H), 0.80 (s, 3H); 13C NMR (CDCl3) δ 218.7, 170.6, 69.9, 55.8, 54.1, 51.7, 39.9, 39.2, 38.1, 35.9, 34.8, 32.7, 32.5, 32.1, 28.0, 26.0, 21.5, 20.3, 18.0, 11.3.
(2α,3β,5β,8α,9β,10α,13α,14β,17α)-2-Methoxy-16-(phenylmethylene)-androstane-3,17-diol diacetate (ent-25)
Compound ent-25 (441mg, 95%) was prepared as a white solid from compound ent-2 using the procedure described for the preparation of compound ent-22 and had: mp 167–169 °C; IR νmax 2932, 1737, 1599, 1492, 1448, 1369, 1241 cm−1 ; 1H NMR (CDCl3) δ 7.36–7.06 (m, 5H), 6.21 (d, 1H, J = 2.4 Hz), 5.37 (s, 1H), 5.02 (br s, 1H), 3.36 (br s, 4H), 2.67 (dd, 1H, J = 16.8 Hz, 7.0 Hz), 2.21 (s, 3H), 2.08 (s, 3H), 0.96 (s, 3H), 0.78 (s, 3H); 13C NMR (CDCl3) δ 171.2, 170.3, 141.0, 137.6, 128.3 (2 × C), 128.2 (2 × C), 126.4, 123.6, 84.6, 77.7, 69.9, 57.0, 54.8, 48.9, 43.0, 39.9, 37.0, 36.5, 35.7, 34.3, 31.5, 30.9, 29.0, 27.7, 21.4, 21.2, 20.3, 13.1, 12.3.
(2β,3α,17β)-2-Methoxy-16-(phenylmethylene)-androstane-3,17-diol diacetate (25)
Steroid 25 (150 mg, 95%) was prepared from the natural enantiomer of compound ent-2 using the procedure described for the preparation of compound ent-22 and had: IR νmax 1737, 1240 cm−1; 1H NMR (CDCl3) δ 7.28–7.11 (m, 5H), 6.13 (d, 1H, J = 2.3 Hz), 5.28–5.27 (m, 1H), 4.94 (br s, 1H), 3.27 (br s, 4H), 2.58–2.54 (m, 1H), 2.12 (s, 3H), 1.99 (s, 3H), 0.88 (s, 3H), 0.69 (s, 3H); 13C NMR (CDCl3) δ 171.1, 170.2, 140.9, 137.5, 128.2 (2 × C), 128.1 (2 × C), 126.4, 123.5, 84.5, 77.6, 69.8, 56.9, 54.8, 48.8, 42.9, 39.9, 36.9, 36.5, 35.6, 34.2, 31.4, 30.9, 29.0, 27.6, 21.4, 21.4, 21.1, 20.3, 13.0, 12.2.
(2α,3β,5β,8α,9β,10α,13α,14β,17α)-3,17-Dihydroxy-2-methoxyandrostan-16-one diacetate (ent-26)
A stream of O3 was bubbled through a solution of compound ent-25 (345 mg, 0.70 mmol) dissolved in MeOH/EtOAc (2:1; 20 mL) at −78 °C until a blue color persisted for 10 min. The excess O3 was purged from the reaction using an O2 stream as evidenced by the solution becoming colorless. Me2S was then added (2 mL) and the reaction was allowed to warm to room temperature and then allowed to stir for 14 h. Solvents were removed and the residue was purified by flash column chromatography (silica gel, eluted with 30–40% EtOAc in hexanes) to yield compound ent-26 as a foamy white solid (265 mg, 90%): IR νmax 2934, 1762, 1742, 1451, 1370, 1240 cm−1; 1H NMR (CDCl3) δ 4.98 (d, 1H, J = 2.0 Hz), 4.97 (s, 1H), 3.36 (br s, 4H), 2.13 (s, 3H), 2.04 (s, 3H), 0.93 (s, 3H), 0.79 (s, 3H); 13C NMR (CDCl3) δ 210.9, 170.2, 170.1, 85.6, 77.5, 69.7, 56.9, 54.6, 45.2, 41.7, 39.8, 36.7, 36.2, 36.0, 35.7, 33.7, 31.5, 28.8, 27.4, 21.3, 20.6, 19.9, 13.0, 12.4.
(2β,3α,17β)-3,17-Dihydroxy-2-methoxyandrostan-16-one diacetate (26)
Steroid 26 (120 mg, 94%) was prepared as a foamy solid from the natural enantiomer of compound ent-25 using the procedure described for the preparation of compound ent-26. Steroid 26 had: IR νmax 1762, 1741, 1239 cm−1; 1H NMR (CDCl3) δ 4.94–4.93 (m, 2H), 3.28 (br s, 4H), 2.09 (s, 3H), 2.00 (s, 3H), 0.89 (s, 3H), 0.75 (s, 3H); 13C NMR (CDCl3) δ 211.0, 170.3, 170.1, 85.6, 77.5, 69.7, 56.9, 54.6, 45.2, 41.7, 39.8, 36.7, 36.3, 36.0, 35.7, 33.7, 31.5, 28.9, 27.4, 21.3, 20.6, 19.9, 13.0, 12.4.
(2α,3β,5β,8α,9β,10α,13α,14β)-3-Hydroxy-2-Methoxyandrostan-16-one acetate (ent-27)
Compound ent-27 (170 mg, 94%) was prepared as a foamy solid from compound ent-26 using the procedure described for the preparation of compound ent-24. Compound ent-27 had: IR νmax 2932, 2850, 1742, 1453, 1408, 1369, 1243, 1214 cm−1 ; 1H NMR (CDCl3) δ 4.98 (s, 1H), 3.32 (s, 4H), 2.04 (s, 3H), 0.94 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3) δ 218.5, 170.1, 77.6, 69.8, 56.9, 55.8, 54.8, 51.6, 39.8, 39.2, 39.1, 38.1, 36.7, 35.7, 34.3, 32.1, 28.9, 27.6, 21.3, 20.3, 18.0, 13.0.
(2β,3α,5α)-3-Hydroxy-2-methoxyandrostan-16-one (27)
Steroid 27 (85 mg, 83%) was prepared from the natural enantiomer of compound ent-26 using the procedure reported for the preparation of compound ent-24. Steroid 27 had: IR νmax 1741, 1243 cm−1; 1H NMR (CDCl3) δ 4.98–4.97 (m, 1H), 3.32 (s, 4H), 2.04 (s, 3H), 0.94 (s, 3H), 0.85 (s, 3H); 13C NMR (CDCl3) δ 218.6, 170.1, 77.5, 69.8, 56.9, 55.8, 54.8, 51.7, 39.8, 39.2, 39.1, 38.2, 36.7, 35.7, 34.3, 32.1, 28.9, 27.6, 21.3, 20.3, 18.1, 13.1.
(3β,4α,5β,8α,9β,10α,13α,14β)-4-Methoxy-16-(phenylmethylene)-androstane-3,17-diol diacetate (ent-28)
Compound ent-28 (300 mg, 97%) was prepared as a white solid from compound ent-3 using the procedure described for the preparation of compound ent-22. Compound ent-28 had: mp 150–152 °C; IR νmax 2935, 2859, 1738, 1599, 1492, 1448, 1371, 1241 cm−1 ; 1H NMR (CDCl3) δ 7.30–7.08 (m, 5H), 6.13 (d, 1H, J = 2.2 Hz), 5.28 (s, 1H), 4.97 (d, 1H, J = 2.3 Hz), 3.30 (s, 3H), 3.22 (s, 1H), 2.60 (m, 1H), 2.12 (s, 3H), 2.00 (s, 3H), 0.90 (s, 3H), 0.69 (s, 3H); 13C NMR (CDCl3) δ 171.2, 170.4, 141.0, 137.6, 128.3 (2 × C), 128.2 (2 × C), 126.4, 123.5, 84.6, 82.5, 68.6, 58.9, 55.0, 49.0, 45.0, 42.9, 36.4, 35.9, 34.8, 32.5, 31.8, 30.9, 25.0, 22.0, 21.4, 21.2, 19.9, 14.0, 12.3.
(3α,4β,17β)-4-Methoxy-16-(phenylmethylene)-androstane-3,17-diol diacetate (28)
Steroid 28 (126 mg, 100%) was prepared as a white semisolid from the natural enantiomer of compound ent-3 using the procedure described for the preparation of compound ent-22. Steroid 28 had: IR νmax 1733, 1241 cm−1; 1H NMR (CDCl3) δ 7.28–7.11 (m, 5H), 6.13 (d, 1H, J = 1.9 Hz), 5.28–5.26 (m, 1H), 4.97–4.96 (m, 1H), 3.29 (s, 3H), 2.62–2.57 (m, 1H), 2.11 (s, 3H), 2.00 (s, 3H), 0.90 (s, 3H), 0.69 (s, 3H); 13C NMR (CDCl3) δ 171.1, 170.4, 141.0, 137.6, 128.3, 128.2, 126.4, 123.5, 84.6, 82.5, 68.6, 58.9, 55.0, 49.0, 45.0, 42.9, 36.4, 35.9, 34.8, 32.6, 31.8, 30.9, 25.0, 22.1, 21.4, 21.1, 19.9, 14.0, 12.3.
(3β,4α,5β,8α,9β,10α,13α,14β,17α)-3,17-Dihydroxy-4-methoxyandrostan-16-one diacetate (ent-29)
Compound ent-29 (205 mg, 87%) was prepared as a white solid from compound ent-28 using the procedure described for the preparation of compound ent-23. Compound ent-29 had: mp 158–160 °C; IR νmax 2928, 1762, 1742, 1449, 1372, 1240 cm−1; 1H NMR (CDCl3) δ 5.02 (d, 1H, J = 2.0 Hz), 4.97 (s, 1H), 3.34 (s, 3H), 3.01 (br s, 1H), 2.13 (s, 3H), 2.05 (s, 3H), 0.96 (s, 3H), 0.79 (s, 3H); 13C NMR (CDCl3) δ 211.0, 170.3 (2 × C), 85.6, 82.2, 68.5, 58.8, 54.8, 45.3, 44.8, 41.6, 36.2, 36.0, 35.8, 34.3, 32.3, 31.8, 24.8, 21.9, 21.3, 20.6, 19.4, 13.9, 12.4.
(3α,4β,17β)-3,17-Dihydroxy-4-Methoxyandrostan-16-one diacetate (29)
Steroid 29 (96 mg, 96%) was prepared as a foamy solid from the natural enantiomer of compound ent-28 using the procedure described for the preparation of compound ent-26. Steroid 29 had: 1H NMR (CDCl3) δ 4.97–4.92 (m, 2H), 3.29 (s, 3H), 2.97 (br s, 1H), 2.08 (s, 3H), 2.00 (s, 3H), 0.90 (s, 3H), 0.74 (s, 3H); 13C NMR (CDCl3) δ 221.0, 170.3, 85.6, 82.2, 68.5, 58.8, 54.8, 45.3, 44.8, 41.6, 36.2, 36.1, 35.8, 34.3, 32.3, 31.9, 24.8, 21.9, 21.4, 20.6, 19.4, 14.0, 12.4.
(3β,4α,5β,8α,9β,10α,13α,14β)-3-Hydroxy-4-Methoxyandrostan-16-one acetate (ent-30)
Compound ent-30 (72 mg, 84%) was prepared as a white solid from compound ent-29 using the procedure described for the preparation of compound ent-24. Compound ent-30 had: mp 159–161 °C; IR νmax 2944, 2861, 1733, 1449, 1407, 1380, 1244, 1216 cm−1 ; 1H NMR (CDCl3) δ 5.03 (d, 1H, J = 1.6 Hz), 3.36 (s, 3H), 3.03 (br s, 1H), 2.07 (s, 3H), 0.98 (s, 3H), 0.87 (s, 3H); 13C NMR (CDCl3) δ 218.8, 170.4, 82.4, 68.6, 58.9, 55.8, 55.0, 51.8, 44.9, 39.3, 39.1, 38.1, 35.9, 34.9, 32.4, 32.4, 24.9, 22.0, 21.4, 19.9, 18.1, 14.0.
(3α,4β,5α)-3-Hydroxy-4-methoxyandrostan-16-one acetate (30)
Steroid 30 (64 mg, 78%) was prepared as a foamy solid from the natural enantiomer of compound ent-29 using the procedure described for the preparation of compound ent-24. Steroid 30 had: 1H NMR (CDCl3) δ 5.04–5.03 (m, 1H), 3.35 (s, 3H), 3.02 (br s, 1H), 2.06 (s, 3H), 0.98 (s, 3H), 0.86 (s, 3H); 13C NMR (CDCl3) δ 218.8, 170.4, 82.4, 68.6, 58.9, 55.8, 55.0, 51.8, 44.9, 39.3, 39.1, 38.1, 35.9, 34.9, 32.5, 32.4, 25.0, 22.0, 21.4, 19.9, 18.1, 14.0.
(3β,5β,8α,9β,10α,13α,14β,17α)-19-Nor-16-(phenylmethylene)-androstane-3,17-diol diacetate (ent-31)
Compound ent-31 (670 mg, 91%) was prepared as an oil from compound ent-21 using the procedure described for the preparation of compound ent-22. Compound ent-31 had: IR νmax 2919, 2862, 1738, 1600, 1492, 1446, 1370, 1240 cm−1 ; 1H NMR (CDCl3) δ 7.40–7.10 (m, 5H), 6.24 (b s, 1H), 5.40 (b s 1H), 5.07 (b s, 1H), 2.69 (dd, 1H, J = 16.7 Hz, 6.3 Hz), 2.23 (s, 3H), 2.07 (s, 3H), 0.81 (s, 3H); 13C (CDCl3) NMR δ 171.0, 170.6, 140.9, 137.5, 128.2 (4 × C), 126.4, 123.5, 84.5, 69.7, 48.0, 47.8, 46.5, 42.9, 40.4, 37.4, 36.6, 36.4, 33.2, 30.7, 30.4, 29.9, 25.0, 24.2, 21.4, 21.1, 12.1. Anal. (C29H38O4) C, H.
(3β,5β,8α,9β,10α,13α,14β,17α)-19-Nor-3,17-dihydroxyandrostan-16-one diacetate (ent-32)
Compound ent-32 (530 mg, 98%) was prepared as a white solid from compound ent-31 using the procedure described for the preparation of compound ent-26. Compound ent-32 had: mp 116–118 °C; IR νmax 2919, 2861, 1763, 1736, 1445, 1371, 1239, 1216 cm−1 ; 1H NMR (CDCl3) δ 4.91 (br s, 1H), 4.89 (s, 1H), 2.19 (dd, 1H, J = 18.7 Hz, 7.7 Hz), 2.03 (s, 3H), 1.92 (s, 3H), 0.71 (s, 3H); 13C NMR (CDCl3) δ 210.6, 170.1, 169.9, 85.4, 69.3, 47.4, 46.1, 44.1, 41.5, 39.5, 37.1, 36.2, 35.9, 35.6, 32.8, 30.3, 29.6, 24.3, 23.7, 21.1, 20.3, 12.0. Anal. (C22H32O5) C, H.
(3β,5β,8α,9β,10α,13α,14β)-19-Nor-3-hydroxyandrostan-16-one acetate (ent-33)
Compound ent-33 (290 mg, 70%) was prepared as a colorless oil from compound ent-32 using the procedure described for the preparation of compound ent-24. Compound ent-33 had: IR νmax 2918, 2862, 1737, 1444, 1408, 1378, 1244, 1214 cm−1 ; 1H NMR (CDCl3) δ 4.97 (b s, 1H), 1.97 (s, 3H), 0.82 (s, 3H); 13C NMR (CDCl3) δ 218.1, 170.3, 69.6, 55.8, 50.8, 47.8, 46.5, 40.4, 39.0, 38.9, 38.0, 37.3, 36.5, 36.5, 33.1, 31.0, 29.8, 25.0, 23.3, 21.2, 17.9. Anal. (C20H30O3) C,H.
(5β,8α,9β,10α,13α,14β,17α)-17-androstane-3-one (ent-34)
This compound was prepared as previously described.45
(5β,8α,9β,10α,13α,14β,17α)-17-[[(4-Phenylmethyl)sulfonyl]oxy]-androstan-3-one (ent-35)
Compound ent-34 (1.8 g, 6.54 mmol), p-TsCl (2.5g, 13.08 mmol) and pyridine (60 mL) were heated at 40 °C for 18 h. The reaction mixture was cooled and poured into a beaker containing crushed ice. The mixture was extracted with CH2Cl2 (3×100 mL) and the combined extracts were washed with 1N HCl to remove the pyridine, washed with brine, dried and solvent removed. The crude product was purified by flash column chromatography (silica gel eluted with10% EtOAc in CH2Cl2) to give ent-35 as a white crystalline solid (2.65 g, 91%). mp 180–182 °C; ; IR νmax 2917, 2851, 1710, 1598, 1449, 1415, 1361, 1253, 1217 cm−1; 1H NMR (CDCl3) δ 7.77 (d, 2H, J = 7.8 Hz), 7.32 (d, 2H, J = 7.8 Hz), 4.24 (t, 1H, J = 8.2 Hz), 2.44 (s, 3H), 0.98 (s, 3H), 0.80 (s, 3H); 13C NMR (CDCl3) δ 211.7, 144.4, 134.1, 129.6 (2 × C), 127.76 (2 × C), 89.8, 53.5, 49.7, 46.5, 44.5, 43.0, 38.4, 38.0, 36.0, 35.6, 35.0, 31.0, 28.6, 27.6, 23.2, 21.6, 21.6, 20.7, 11.8. 11.4.
(3β,5β,8α,9β,10α,13α,14β,17α)-17-[[(4-Phenylmethyl)sulfonyl]oxy]-androstan-3-ol (ent-36)
Compound ent-35 ( 2.8 g, 6.3 mmol) was dissolved in THF (50 mL), cooled to −78 °C and K-selectride in THF (1 M, 7.2 mL, 7.2 mmol, 1.2 eq) was added dropwise. Stirring at −78 °C was continued for 2 h. Aqueous NaOH (3 M, 20 mL) and then 30% H2O2 (20 mL) were added and the reaction was warmed to room temperature and stirred for 2 h. Water (150 mL) was added and the product was extracted into EtOAc. The combined EtOAc extracts were dried and the solvents removed to give an oil which was purified by flash column chromatography (silica gel eluted with 10% EtOAc in CH2Cl2) to give product ent-36 (2 g, 71%): mp 186–188 °C; IR νmax 3400, 2918, 2850, 1599, 1446, 1355 cm−1; 1H NMR (CDCl3) δ 7.77 (d, 2H, J = 7.1 Hz), 7.32 (d, 2H, J = 7.1 Hz), 4.24 (t, 1H, J = 7.8 Hz), 4.03 (d, 1H, J = 2.3 Hz) 2.44 (s, 3H), 0.78 (s, 3H), 0.75 (s, 3H); 13C NMR (CDCl3) δ 144.1, 134.2, 129.6 (2 × C), 127.8 (2 × C), 90.1, 66.4, 66.3, 54.1, 50.0, 43.0, 39.0, 36.1, 35.7, 35.1, 32.1, 31.3, 28.9, 28.2, 27.6, 23.2, 21.6, 20.0, 11.8, 11.1.
(3β,5β,8α,9β,10α,14β)-17-Methylandrost-14(17)-en-3-ol (ent-37)
MeMgBr (3 M in Et2O, 5 mL) was added to refluxing toluene (80 mL) containing dissolved compound ent-36 (1.34 g, 3 mmol) and refluxing was continued for 1 h under N2. The reaction was cooled, acidified with 1N HCl and extracted with CH2Cl2. The combined extracts were washed with brine, dried and concentrated to give an oil. The oil was dissolved in MeOH (20 mL) and 5 M HCl (prepared from concentrated HCl diluted with MeOH) was added and the reaction was stirred at room temperature for 15 h. The reaction was made basic by the addition of aqueous NaHCO3 and the MeOH was removed. The aqueous residue was diluted further with water and the product extracted into EtOAc. The combined extracts were dried and concentrated to give an oil, which was purified by flash column chromatography (silica gel eluted with 15–35% EtOAc in hexanes) to give product ent-37 as a colorless oil (750 mg, 91%): IR νmax 3337, 2920, 2856, 1444, 1359, 1255 cm−1; 1H NMR (CDCl3) δ 4.02 (br s, 1H), 1.58 (s, 3H), 0.68 (s, 3H); 13C NMR (CDCl3) δ 136.5, 127.4, 66.4, 53.9, 52.3, 45.3, 38.7, 37.1, 36.1, 35.8, 32.3, 32.2, 28.9, 28.3, 28.1, 25.6, 25.2, 13.4, 11.1.
(3β,5β,8α,9β,10α,14β)-3-(Methoxymethoxy)-17-methylandrost-14(17)-ene (ent-38)
Compound ent-37 (823 mg, 3 mmol) was dissolved CH2Cl2 (80 mL) and cooled to 0 °C. (i-Pr)2EtN (2.6 mL, 15 mmol) and ClCH2OMe (0.76 ml, 10 mmol) were added and the reaction was stirred at room temperature for 6 h. The reaction mixture was made basic by adding aqueous NaHCO3 solution and the product extracted into CH2Cl2. The combined extracts were washed with brine, dried and solvent removed to give a viscous liquid. The crude product was purified by flash column chromatography (silica gel eluted with 10–15% EtOAc in hexanes) to give product ent-38 as a colorless liquid (960 mg, 90%): IR νmax 2924, 2857, 1445, 1368, 1302, 1213 cm−1; 1H NMR (CDCl3) δ 4.57 (s, 2H), 3.75 (s, 1H), 3.28 (s, 3H), 1.51 (s, 3H), 0.64 (s, 3H); 13C NMR (CDCl3) δ 136.4, 127.1, 94.3, 71.4, 54.8, 53.8, 52.2, 45.3, 39.2, 37.0, 35.8, 33.5, 32.8, 32.2, 28.3, 28.0, 26.2, 25.6, 25.2, 13.2, 11.2.
(1R,4aR,4bR,7S,8aR,10aS)-7-(Methoxymethoxy)docecahydro-4b-methyl-1(3-oxobutyl-2(1H)-phenanthrenone (ent-39)
Compound ent-38 (2.38 g, 7.5 mmol) was dissolved in CH2Cl2 (100 mL) and cooled to − 78 °C. O3 was passed through the solution until it remained blue for 20 min and then O2 was passed through the solution until it became colorless. The reaction was warmed to 0 °C, AcOH (30 mL) was added and the CH2Cl2 removed on a rotary evaporator. Additional AcOH (15 mL) and Zn dust (6 g) were added and the reaction was stirred at room temperature for 3 h to decompose the ozonide intermediate. The Zn was removed by filtration through Celite and the Celite was washed with EtOAc. The combined filtrate and washings were combined and the volatile solvents removed to leave the product in AcOH. The AcOH was carefully neutralized with aqueous NaHCO3 and the product extracted into EtOAc. The combined extracts were dried and solvent removed to leave the crude product as an oil which was purified by flash column chromatography (silica gel eluted with 20–35% EtOAc in hexanes) to give product ent-39 (1.7 g, 65%) as a liquid: IR νmax 2928, 1712, 1447, 1367, 1213 cm−1; 1H NMR (CDCl3) δ 4.57 (s, 2H), 3.75 (s, 1H), 3.28 (s, 3H), 2.03 (s, 3H), 0.65 (s, 3H); 13C NMR (CDCl3) δ 212.2, 208.7, 94.3, 71.2, 54.9, 54.6, 52.1, 42.3, 41.7, 40.8, 38.7, 36.0, 33.0, 32.6, 32.0, 29.6, 28.0, 26.2, 26.1, 19.2, 10.9.
(3β,5β,8α,9β,10α,14β)-3-(Methoxymethoxy)-D-Homo-18-norandrost-13(17a)-en-17-one acetate (ent-40)
Compound ent-39 (1.55g, 4.43 mmol) was dissolved in MeOH (15 mL) and water (1.5 mL), 10% methanolic NaOH (12 mL) was added and the reaction was stirred at room temperature for 1 h. The reaction was then acidified with 3N HCl and the product extracted into CH2Cl2. The combined extracts were washed with aq. NaHCO3, brine, dried and the solvents were removed. The crude product was purified by flash column chromatography (silica gel eluted with 30–40% ethyl acetate in hexanes) to give product ent-40 as a white solid. (1.10 g, 75%): mp 98–100 °C; IR νmax 2926, 1674, 1622, 1449, 1364, 1259, 1209 cm−1 ; 1H NMR (CDCl3) δ 5.68 (s, 1H), 4.55 (s, 2H), 3.74 (s, 1H), 3.27 (s, 3H), 0.63 (s, 3H); 13C NMR (CDCl3) δ 199.5, 166.4, 123.7, 94.3, 71.1, 54.9, 52.0, 43.6, 43.0, 38.6, 36.2, 35.8, 35.8, 35.7, 33.1, 32.5, 31.1, 27.9, 26.1, 25.9, 25.3, 10.9.
(3β,5β,8α,9β,10α,13α,14β)-3-(Methoxymethoxy)-D-Homo-18-norandrostan-17-one (ent-41)
Liquid NH3 (250 mL) was condensed in a 3–necked 500 mL round bottom flask fitted with a Dewar condenser containing Dry Ice/acetone and an overhead stirrer and then placed in a cold bath at −78 °C. Li (140 mg, 20 mmol) was added and stirring continued for 30 min during which time the solution became deep blue. THF (80 mL) was added and after 10 min, compound ent-40 (942 mg, 2.7 mmol) dissolved in THF (40 mL) was added and stirring continued for 1 h. Solid NH4Cl (5 g) was added and the solution became colorless. The reaction was allowed to warm to room temperature and the liq. NH3 was allowed to evaporate. Water was added and the product extracted into EtOAc. The combined extracts were washed with brine, dried and solvent removed to give a yellow solid which was purified by flash column chromatography (silica gel eluted with 30% EtOAc in hexanes) to give product ent-41 as a white solid (650 mg, 72%): mp 85–87 °C; IR νmax 2919, 2859, 1717, 1445, 1366, 1322, 1206, 1209 cm−1 ; 1H NMR (CDCl3) δ 4.65 (s, 2H), 3.82 (s, 1H), 3.36 (s, 3H), 0.72 (s, 3H); 13C NMR (CDCl3) δ 211.9, 94.5, 71.4, 55.1, 52.9, 48.6, 47.4, 43.3, 41.3, 40.8, 39.1, 35.9, 34.3, 33.5. 32.6, 30.9, 30.3, 28.3, 26.2, 24.6, 11.2.
(3β,5β,8α,9β,10α,13α,14β)-3-Hydroxy-D-Homo-18-norandrostan-17-one (ent-42)
Compound ent-41 (500 mg, 1.50 mmol) dissolved in MeOH (20 mL) was stirred with 6 N HCl (6 mL) at room temperature for 24 h. The HCl was neutralized by careful addition of aqueous NaHCO3 solution and the MeOH removed. Water was added and the product was extracted into EtOAc. The combined extracts were washed with brine, dried and the solvents removed to give the crude product as an off-white solid which was purified by flash column chromatography (silica gel eluted with 40–50% EtOAc in hexanes). Compound ent-42 was obtained as a white solid (400 mg, 92%): mp 227–229 °C; IR νmax 3395, 2939, 2922, 2850, 1699, 1594, 1419, 1360, 1320, 1264, 1208 cm−1 ; 1H NMR (CDCl3) δ 4.04 (s, 1H), 0.71 (s, 3H); 13C NMR (CDCl3) δ 211.9, 66.2, 52.9, 48.6, 47.4, 43.2, 41.2, 40.8, 38.4, 36.1, 35.7, 34.3, 32.0, 31.0, 30.3, 28.9, 28.2, 24.6, 11.0.
(3β,5β,8α,9β,10α,13α,14β)-3-Hydroxy-D-Homo-18-norandrostan-17-one acetate (ent-43)
Compound ent-42 (370 mg, 1.27 mmol), AcOAc (0.5 mL) and Et3N (1 mL) in CH2Cl2 (8 mL) were stirred at room temperature for 24 h. Aqueous sat. NaHCO3 (5 mL) was carefully added and the mixture was stirred for 1 h. The product was extracted into CH2Cl2 and the combined extracts were washed with brine, dried and the solvent removed to give an off-white solid. The crude product was purified by flash column chromatography (silica gel eluted with 20–30% EtOAc in hexanes) to give product ent-43 as a white solid (400 mg, 95%): mp 141–143 °C; IR νmax 2920, 2860, 2733, 1717, 1446, 1361, 1246 cm−1 ; 1H NMR (CDCl3) δ 4.96(s, 1H), 2.00 (s, 3H), 0.69 (s, 3H); 13C NMR (CDCl3) δ 211.6, 170.4, 69.8, 69.6, 52.7, 48.5, 47.3, 43.1, 41.1, 40.7, 39.2, 35.7, 34.2, 32.6, 32.5, 30.8, 30.2, 27.9, 25.9, 24.5, 21.4, 21.3, 11.1, 11.0.
(3β,5α)-3-(Acetyloxy)-16,17-seco-D-homo-18-norandrostane-16,17-dioic acid (ent-44) and (3β,5α)-3-(Hydroxy)-16,17-seco-D-homo-18-norandrostane-16,17-dioic acid dimethyl ester (ent-45)
A mixture of compound ent-43 (96 mg) and a CrO3 solution (0.7 mL) [prepared by mixing CrO3 (500 mg), water (0.7mL) and acetic acid (0.8 mL)] and a solution of aqueous methanolic acetic acid (1 mL) [prepared by mixing acetic acid (30 mL), water (1 mL) and methanol (0.05 mL)] was stirred at 60 °C for 4.5 h. The reaction was cooled to room temperature, water (2.5 mL) was added and the reaction was stirred at room temperature for 13 h. Additional water (15 mL) was added and the product was extracted into CH2Cl2. The combined extracts were dried and the solvent removed to give dicarboxylic acid ent-44 as a white solid which was immediately converted without characterization to diester ent-45 upon stirring with dry HCl in MeOH (10 mL) [prepared by adding AcCl (3 mL) to MeOH (8 mL)] at room temperature for 15 h. The HCl was cautiously neutralized with aqueous NaHCO3 and the MeOH was removed. Water was added and the product was extracted into EtOAc. The combined extracts were washed with brine, dried and solvent removed to give an oil. Purification by flash column chromatography (silica gel eluted with 40% EtOAc in hexanes) yielded compound ent-45 as a colorless oil (54 mg, 51%): IR νmax 3445.55, 2924, 2858, 1738, 1436, 1361, 1258 cm−1 ; 1H NMR (CDCl3) δ 4.03 (s, 1H), 3.65 (s, 6H), 0.71 (s, 3H); 13C NMR (CDCl3) δ 173.7 (2 × C), 66.3, 53.0, 51.5, 51.4, 45.0, 40.8, 39.3, 38.8, 38.3, 36.1, 35.7, 35.3, 32.7, 31.9, 31.3, 28.9, 28.4, 24.4, 11.0.
(3β,5β,8α,9β,10α,13α,14β,17α)-3-Hydroxy-16-oxo-18-norandrostane-17-carboxylic acid methy ester (ent-46)
A mixture of compound ent-45 (53 mg, 0.14 mmol), NaOMe (27 mg, 0.5 mmol) and THF (8 mL) was heated at reflux for 0.5 h. The reaction was cooled, acidified with 1 N HCl to pH 3 and the product extracted into CH2Cl2 (3 × 75 mL). The combined extracts were washed with brine, dried and solvents removed to give a white solid. The crude product was purified by flash column chromatography (silica gel eluted with 40% EtOAc in hexanes) gave pure product ent-46 as a white solid (34 mg, 71%): mp 136–138 °C; IR νmax 3435, 2920, 2855, 1756, 1727, 1435, 1410, 1384, 1339, 1261 cm−1 ; 1H NMR (CDCl3) δ 4.06 (t, 1H, J = 2.5 Hz), 3.75 (s, 3H), 2.86 (d, 1H, J = 2.5 Hz), 2.50 (dd, 1H, J = 18.0 Hz, 5.8 Hz), 0.78 (s, 3H); 13C NMR (CDCl3) δ 210.2, 169.5, 66.3, 61.9, 53.1, 52.3, 47.1, 46.6, 43.2, 41.6, 38.7, 36.2, 35.7, 32.2, 31.9, 30.0, 28.9, 28.0, 24.5, 11.0.
(5α)-Estrane-3,17-dione (47)
This compound was prepared as previously described.20
(5α)-17-Oxo-3,4-secoestrane-2,3-dicarboxylic acid (48) and (5α)-17-Oxo-3,4-secoestrane-2,3-dicarboxylic acid dimethyl ester (49)
A solution of CrO3 (10.9 g), H2SO4 (98%, 16 mL), and water (76 mL) was added dropwise to a stirred solution of compound 47 (7.9 g, 28.5 mmol) in AcOH (80 mL) at 65 °C. The reaction was heated at 70 °C for 1 h. Then, crushed ice and water (500 mL) were added and the mixture was stirred at room temperature for 1 h. Steroid 48 precipitated and was filtered, washed with water, and dried overnight at room temperature to give pure dicarboxylic acid (5.53 g, 60%) as a white solid. The aqueous phase was extracted with CH2Cl2 (2 × 150 mL), and the combined extracts were washed with brine (2 × 100 mL), dried and evaporated to afford impure dicarboxylic acid 48 (2 g, 21%) as an oily residue. Pure steroid 48 had: 1H NMR (300 MHz, CDCl3) δ 0.88 (3H, s); 13C NMR (75 MHz, CDCl3) δ 221.5, 179.43, 179.25, 50.6, 47.9, 47.7, 43.2, 40.6, 39.4, 38.8, 36.0, 35.3, 32.3, 31.6, 29.4, 26.0, 21.7, 13.9.
AcCl (6 mL, 23 mmol) was added to steroid 48 (7.53 g) dissolved in MeOH (100 mL) and the reaction was stirred at room temperature for 2 h. Then, water (50 mL) was added and the product was extracted into CH2Cl2 (2 × 100 mL). The combined extracts were washed with water, dried and the solvent removed to yield an oily residue which was purified by flash column chromatography (silica gel eluted with 15% EtOAc in hexanes) to give steroid 49 (4.43 g, 52%) as an oil. Unreacted steroid 48 was reclaimed by washing the silica gel with MeOH and the esterification procedure was repeated to give additional product 49 (780 mg, 9%) as an oil: IR νmax 2925, 2858, 1737 cm−1; 1H NMR (CDCl3) δ 3.65 (6H, s), 0.86 (3H, s); 13C NMR (CDCl3) δ 220.9, 173.6 (2 × C), 51.7, 51.6, 50.6, 47.99, 47.90, 43.3, 40.6, 39.3, 39.1, 36.0, 35.3, 32.3, 31.7, 29.4, 25.9, 21.7, 13.9.
(5α,17β)-17-Hydroxy-3,4-secoestrane-2,3-dicarboxylic acid dimethyl ester (50)
Steroid 49 (1.55 g, 31.4 mmol) was dissolved in stirred EtOH (20 mL) and cooled in an ice-bath. NaBH4 (218 g, 5.8 mmol) was added in small portions. After 1.5 h, a solution of water (50 mL) and AcOH (2 mL) was added and the solution was stirred for 1h. The product was extracted into CH2Cl2 (2 × 125 mL), washed with an aqueous 1 N HCl, sat. aqueous NaHCO3, brine, dried and the solvent removed. Flash column chromatography (silica gel eluted with15% EtOAc in hexanes) gave secosteroid 50 (1.16 g, 74%) as an oily product: IR νmax 3452, 2950, 2921, 2867, 1733 cm−1; 1H NMR (CDCl3) δ 3.60–3.65 (7H, m), 0.73 (3H, s); 13C NMR (CDCl3) δ 173.7 (2 × C), 82.0, 51.68, 51.62, 50.2, 47.9, 43.5, 43.1, 41.2, 39.5, 39.2, 36.9, 35.4, 32.5, 30.7, 30.1, 26.3, 23.3, 11.2.
(3α,5α,17β)-17-Hydroxy-2-oxo-A-norestrane-3-carboxylic acid methyl ester (51)
A NaOMe solution (5 mL) was prepared by dissolving Na (1.14 g, 49 mmol) in MeOH (25 mL) followed by MeOH evaporation on a rotary evaporator. Dry THF (30 mL) was added and the flask was filled with N2. Steroid 50 (5 g, 14.1 mmol) dissolved in dry THF (50 mL) was slowly added. The reaction mixture was heated at 100 °C for 1 h under N2 and then allowed to attain room temperature. Then 1 N HCl (15 mL) was slowly added and the product was extracted into CH2Cl2 (2 × 100 mL). The combined extracts were washed with brine, dried and the solvent removed. Flash column chromatography (silica gel eluted with 15% EtOAc in hexanes) gave product 51 (3.7 g, 81%) as a white solid: mp 138–142 °C (EtOAc/hexanes); IR νmax 3440, 2918, 2866, 1756, 1727 cm−1; 1H NMR (CDCl3) δ 3.75 (3H, s), 3.67 (t, 1H, J = 8 Hz), 2.88 (d, 1H, J = 12.6 Hz), 2.48 (dd, 1H, J = 6.6 Hz, 18 Hz), 0.77 (3H, s). 13C NMR (CDCl3) δ 210.1, 169.7, 81.9, 62.1, 52.4, 49.7, 48.7, 47.5, 45.7, 43.6, 43.3, 41.6, 36.5, 30.5, 30.2, 29.9, 27.1, 23.3, 11.4. Anal. (C19H28O4) C, H.
(5α,17β)-17-Hydroxy-A-norestran-2-one (52)
LiCl (1.95 g, 46.1 mmol) was added to a solution of steroid 51 (3.7 g, 11.5 mmol) dissolved in DMF (50 mL) and water (0.5 mL) and the reaction was heated at 160 °C for 35 min. Then, crushed ice and water were added and the product was extracted into CH2Cl2 (2 × 120 mL). The combined extracts were washed with water and dried. After solvent evaporation, the residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give product 52 (2.6 g, 85%) as a white solid: mp 177–179 °C (EtOAc/hexanes); IR νmax 3450, 2915, 2861, 1732 cm−1; 1H NMR (CDCl3) δ 0.77 (3H, s), 2.41–2.31 (2H, m), 3.66 (t, 1H, J = 8 Hz). 13C NMR (CDCl3) δ 218.3, 82.0, 49.9, 48.9, 48.6, 46.1, 44.2, 43.9, 43.6, 41.7, 36.6, 30.9, 30.64, 30.61, 27.1, 23.4, 11.4. Anal. (C17H26O2) C, H.
(5α,17β)-17-[[(4-Methylphenyl)sulfonyl]oxy]-A-norestran-2-one (53)
A solution of steroid 52 (2.46 g, 9.37 mmol), DMAP (57 mg, 0.49 mmol), and p-TsCl (6.25 g, 32.8 mmol) in anhydrous pyridine (50 mL) was heated at 65 °C overnight. The reaction mixture was poured into ice-water and the product extracted into CH2Cl2 (2 × 75 mL). The combined extracts were washed with aqueous HCl, aqueous NaHCO3, brine, and dried. After solvent evaporation, the oily residue was purified by flash column chromatography (silica gel eluted with 10% EtOAc in hexanes) to give steroid 53 (3.56 g, 91%) as a white solid: mp 138–139 °C (ether/hexanes); IR νmax 2920, 2858, 1741, 1598, 1357, 1188, 1176 cm−1; 1H NMR (CDCl3) δ 7.80–7.77 (2H, m), 7.34–7.31 (2H, m), 4.28 (t, 1H, J = 8 Hz), 2.45 (3H, s), 2.33 (dd, 2H, J = 6.6 Hz, 18 Hz), 0.82 (3H, s). 13C NMR (CDCl3) δ 217.9, 144.6, 134.6, 129.8 (2 × C), 128.0 (2 × C), 90.0, 48.9, 48.6, 48.4, 45.9, 44.1, 43.8, 43.6, 41.2, 36.0, 30.8, 30.4, 27.7, 26.8, 23.3, 21.8, 12.0. Anal. (C24H32O4S) C, H.
(5α,17β)-2-Methylene-17-[[4-(methylphenyl)sulfonyl]oxy]-A-norestrane(54)
A solution of n-BuLi (2.5 M, 24.1 mmol, 9.6 mL) was added dropwise to a solution of MeP(Ph)3Br (8.85 g, 24.7 mmol) in dry benzene (70 mL) and dry THF (12 mL) under N2 and the mixture was stirred for 30 min. A solution of steroid 53 (2.58 g, 6.19 mmol) in dry benzene (35 mL) was added and stirred at room temperature. After 6 h, the reaction was diluted with water (50 mL) and extracted with EtOAc (2 × 75 mL). The combined extracts were washed with brine, dried and the solvents evaporated. Flash column chromatography on (silica gel eluted with 5% EtOAc in hexanes) gave steroid 54 (2.05 g, 80%) as a white solid: mp 136–137 °C (EtOAc/hexanes); IR νmax 2920, 2855, 1657, 1598, 1359 cm−1; 1H NMR (CDCl3) δ 7.80–7.77 (2H, m), 7.34–7.31 (2H, m), 4.83 (2H, m), 4.27 (t, 1H, J = 8 Hz), 2.45–2.36 (5H, m), 0.81 (3H, s); 13C NMR (CDCl3) δ 151.5, 144.5, 134.6, 129.8 (2 × C), 128.0 (2 × C), 105.9, 90.3, 51.0, 49.0, 48.9, 46.7, 43.7, 41.3, 39.6, 37.6, 36.2, 31.0, 30.8, 27.8, 26.7, 23.5, 21.8, 12.1. Anal. (C25H31O3S) C, H.
(5α)- 17-Methyl-2-Methylene-A-norgon-13(17)-ene (55)
Steroid 54 (2 g, 4.8 mmol) was dissolved in anhydrous toluene (50 mL) and heated to 100 °C. MeMgBr (3.0 M in Et2O, 8 mL, 24 mmol) was added dropwise to the stirring hot solution under an N2 atmosphere and a white precipitate appeared. The reaction was heated for 1 h at 115 °C. The flask was cooled, a few pieces of crushed ice were added, and the pH of the solution was adjusted to pH 2 by dropwise addition of 2 N aqueous H2SO4. The toluene layer was separated and the aqueous layer was extracted with EtOAc (2 × 100 mL). The combined extracts were washed with brine, dried and the solvents evaporated. Flash column chromatography on (silica gel eluted with 1% EtOAc in hexanes) gave steroid 55 (1.04 g, 90%) as an oily product: IR νmax 3067, 2921, 2851, 1657, 1441 cm−1; 1H NMR (CDCl3) δ 4.86 (2H, m), 1.62 (3H, s). 13C NMR (CDCl3) δ 151.9, 136.7, 128.3, 105.7, 100.2, 52.6, 51.5, 51.3, 47.1, 46.4, 39.7, 37.8, 37.4, 31.5, 31.2, 28.4, 25.6, 13.6.
(3aS,5aR,6S,9aR,9bS)-Decahydro-6-(3-oxobutyl)-1H-benz[e]indene-2,7-dione (56)
A solution of steroid 55 (760 mg, 3.13 mmol) in CH2Cl2 (55 mL) was treated with O3 at −78 °C until a blue color persisted (ca. 1 hr). O2 was passed through the solution for 30 min until the blue color disappeared. AcOH (50 mL) was added and the CH2Cl2 was evaporated under vacuum without heating. Then, AcOH (10 mL) and Zn dust (2.03 g, 31 mmol) were added and the reaction mixture was stirred at room temperature for 1.5 h. Zn dust was filtered off through cotton, washing with CH2Cl2 (100 mL). The Zn dust was stirred with EtOAc (50 mL) for 1 h. The solids were filtered off, the combined solvents were evaporated and the product was purified by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) to give compound 56 (390 mg, 51%) as an oil: IR νmax 3406, 2919, 2859, 1742, 1709 cm−1; 1H NMR (CDCl3) δ 2.09 (3H, s); 13C NMR (CDCl3) δ 217.0, 211.6, 209.1, 53.9, 48.4, 47.4, 46.5, 45.6, 43.6, 43.2, 41.6, 40.9, 32.0, 31.1, 30.5, 30.0, 19.5.
(8α,9β,10α,13α,14β)-Gon-4-ene-3,16-dione (ent-57)
A solution of NaOH [10% w/v in MeOH/water (9:1), 2 mL] was added to a solution of compound 56 (392 g, 1.41 mmol) in MeOH (20 mL) and the mixture was stirred at room temperature. After 3 h, a few pieces of crushed ice were added, the pH was adjusted to pH 2 by adding aqueous 1 N HCl, and the product was extracted into CH2Cl2 (2 × 70 mL). The combined extracts were washed with brine, dried and the solvent evaporated. Purification by flash column chromatography (silica gel eluted with 20% EtOAc in hexanes) gave compound ent-57 (302 mg, 82%) as a white solid: mp 147–148 °C (EtOAc/hexanes); IR νmax 2932, 2854, 1740, 1662, 1612 cm−1; 1H NMR (CDCl3) δ 5.84 (1H, s); 13C NMR (CDCl3) δ 217.4, 199.8, 165.8, 124.9, 48.7, 48.6, 46.5, 45.8, 43.6, 43.2, 42.6, 36.6, 35.3, 31.4, 30.7, 30.3, 26.5. Anal. (C17H22O2) C, H.
(5β,8α,9β,10α,13α,14β)-Gonane-3,16-dione (ent-58)
Anhydrous NH3 (10 mL) was condensed using a Dewar condenser into a three-neck flask containing Li metal (20 mg, 2.9 mmol) at −78 °C. Then, anhydrous THF (12 mL) was added and the resulting blue solution was stirred for 0.5 h. A solution of compound ent-57 (150 mg, 0.58 mmol) in dry THF (6 mL) was added dropwise to the vigorously stirred solution. After 2 h of stirring, the reaction color was discharged by careful addition of solid NH4Cl in portions and left overnight while the NH3 evaporated. The reaction was then acidified with aqueous 1 N HCl and the product was extracted into EtOAc (2 × 50 mL). The combined organic phases were washed with brine, dried and the solvent evaporated. The residue was dissolved in acetone (50 mL) and Jones reagent was added dropwise until an orange color persisted. The course of the reaction was checked by TLC. Then, 2-propanol was added dropwise until the reaction mixture turned green. After 30 min, the reaction mixture was poured into water-ice. The product was extracted into CH2Cl2 (2 × 30 mL). The combined extracts were washed with brine, dried and the solvent was evaporated. Flash column chromatography (silica gel eluted with 15% EtOAc in hexanes) gave compound ent-58 (95 mg, 63%) as a white solid: mp 188–189 °C (EtOAc/hexanes); IR νmax 2914, 2867, 1740, 1705 cm−1; 1H NMR (CDCl3) δ 0.90–2.42 (24H, m); 13C NMR (CDCl3) δ 218.1, 211.6, 49.1, 48.7, 47.3, 46.8, 46.0, 45.9, 43.7, 43.5, 43.4, 41.4, 33.8, 31.1, 31.0, 30.4, 30.0.
[35S]-TBPS Binding Methods
The methods used were as described previously.46
Xenopus Oocyte and HEK 293 Cell Electrophysiological Methods
Receptor expression and whole-cell and single-channel recordings were carried out as described previously.27, 46, 47
Tadpole Behavioral Methods
The methods used were as described previously.46
Mouse Behavioral Methods
The methods used were as described previously.48
Supplementary Material
ACKNOWLEDGMENT
This research was supported in part by NIH Grant GM47969 (D.F.C., A.S.E., C.F.Z.) and the Bantly Foundation (C.F.Z.). Washington University receives income and equity based on a license of this and related technology to Sage Therapeutics, Inc. D.F.C, A.S.E. and C.F.Z have equity holdings in Sage Therapeutics, Inc. C.F.Z serves on the Scientific Advisory Board of Sage Therapeutics, Inc. Sage Therapeutics, Inc. did not support this research or have any other role in the research or its conclusions.
aAbbreviations
- CNS
central nervous system
- GABA
γ-aminobutyric acid
- GABAA
γ-aminobutyric acid type A
- [35S]TBPS
[35S]-tert-butylbicyclophosphorothionate
- SAR
structure–activity relationships
- LRR
loss of righting reflex
- LSR
loss of swimming reflex
Footnotes
Current address: Department of Neuroprotectives; Institute of Organic Chemistry and Biochemistry; Academy of Sciences of Czech Republic, v.v.i.; Flemingovo nam. 2; 166 10 Prague 6 – Dejvice; Czech Republic
Supporting Information Available: Table of elemental analysis results, additional views of superimposed analogues (Figure S1), and structures in Table 1 with [35S]TBPS IC50 values (Figure S2). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Zorumski CF, Mennerick S. Neurosteroids as therapeutic leads in psychiatry. JAMA Psychiatry. 2013;70:659–660. doi: 10.1001/jamapsychiatry.2013.245. [DOI] [PubMed] [Google Scholar]
- 2.Brinton RD. Neurosteroids as regenerative agents in the brain: therapeutic implications. Nat. Rev. Endocrinol. 2013;9:241–250. doi: 10.1038/nrendo.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 5.Veleiro AS, Burton G. Structure-activity relationships of neuroactive steroids acting on the GABAA receptor. Curr. Med. Chem. 2009;16:455–472. doi: 10.2174/092986709787315522. [DOI] [PubMed] [Google Scholar]
- 6.Wittmer LL, Hu Y, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol. Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- 7.Nilsson KR, Zorumski CF, Covey DF. Neurosteroid analogues. 6. The synthesis and GABAA receptor pharmacology of enantiomers of dehydroepiandrosterone sulfate, pregnenolone sulfate, and (3α,5β)-3-hydroxypregnan-20-one sulfate. J. Med. Chem. 1998;41:2604–2613. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- 8.Covey DF, Nathan D, Kalkbrenner M, Nilsson KR, Hu Y, Zorumski CF, Evers AS. Enantioselectivity of pregnanolone-induced γ-aminobutyric acidA receptor modulation and anesthesia. J. Pharmacol. Exp. Ther. 2000;293:1009–1016. [PubMed] [Google Scholar]
- 9.Katona BW, Krishnan K, Cai ZY, Manion BD, Benz A, Taylor A, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABAA receptors by ent-androgens. Eur. J. Med. Chem. 2008;43:107–113. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Bracamontes J, Katona BW, Covey DF, Steinbach JH, Akk G. Natural and enantiomeric etiocholanolone interact with distinct sites on the rat α1β2γ2L GABAA receptor. Mol. Pharmacol. 2007;71:1582–1590. doi: 10.1124/mol.106.033407. [DOI] [PubMed] [Google Scholar]
- 11.Turner DM, Ransom RW, Yang JS, Olsen RW. Steroid anesthetics and naturally occurring analogs modulate the γ-aminobutyric acid receptor complex at a site distinct from barbiturates. J. Pharmacol. Exp. Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- 12.Hawkinson JE, Kimbrough CL, Belelli D, Lambert JJ, Purdy RH, Lan NC. Correlation of neuroactive steroid modulation of [35S]t-butylbicyclophosphorothionate and [3H]flunitrazepam binding and γ-aminobutyric acidA receptor function. Mol. Pharmacol. 1994;46:977–985. [PubMed] [Google Scholar]
- 13.Krishnan K, Manion BD, Taylor A, Bracamontes J, Steinbach JH, Reichert DE, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 17. Inverted binding orientations of androsterone enantiomers at the steroid potentiation site on γ-aminobutyric acid type A receptors. J. Med. Chem. 2012;55:1334–1345. doi: 10.1021/jm2014925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillipps GH. Structure–activity relationships in steroidal anaesthetics. Churchill Livingstone; New York: 1974. pp. 32–47. [DOI] [PubMed] [Google Scholar]
- 15.Stastna E, Krishnan K, Manion BD, Taylor A, Rath NP, Chen ZW, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 16. A new explanation for the lack of anesthetic effects of Δ16-alphaxalone and identification of a Δ17(20) analogue with potent anesthetic activity. J. Med. Chem. 2011;54:3926–3934. doi: 10.1021/jm2002487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J. Physiol. 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evers AS, Chen ZW, Manion BD, Han M, Jiang X, Darbandi-Tonkabon R, Kable T, Bracamontes J, Zorumski CF, Mennerick S, Steinbach JH, Covey DF. A synthetic 18-norsteroid distinguishes between two neuroactive steroid binding sites on GABAA receptors. J. Pharmacol. Exp. Ther. 2010;333:404–413. doi: 10.1124/jpet.109.164079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Rath NP, Covey DF. Neurosteroid Analogues. 13. Synthetic methods for the preparation of 2β-hydroxygonane derivatives as structural mimics of ent-3alpha-hydroxysteroid modulators of GABA(A) receptors. Tetrahedron. 2007;63:7977–7984. doi: 10.1016/j.tet.2007.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teerhuis MH, Huisman IAM, Groen MB. Synthesis of ent-nortestosterone from its naturally occurring antipode. Tetrahedron Lett. 2001;42:2869–2871. [Google Scholar]
- 20.Stastna E, Rath NP, Covey DF. The use of symmetry in enantioselective synthesis: Four pairs of chrysene enantiomers prepared from 19-nortestosterone. Org. Biomol. Chem. 2011;9:4685–4694. doi: 10.1039/c1ob05385j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nace HR, Pyle JL. Nor steroids. IX. Synthesis of A-norandrostanes via the Dieckmann cyclization. J. Org. Chem. 1971;36:81–83. doi: 10.1021/jo00800a018. [DOI] [PubMed] [Google Scholar]
- 22.Nace HR, Smith AH. Nor steroids. X. Synthesis of A-nor steroids via the Dieckmann condensation. J. Org. Chem. 1973;38:1941–1944. doi: 10.1021/jo00950a034. [DOI] [PubMed] [Google Scholar]
- 23.Kontonassios D, Sandris C. A-nor steroids. 1. The stereochemistry of a 2-carbomethoxy-A-nor-cholestan-3-one obtained via the Dieckmann condensation. Steroids. 1982;39:411–417. doi: 10.1016/0039-128x(82)90065-4. [DOI] [PubMed] [Google Scholar]
- 24.Han M, Zorumski CF, Covey DF. Neurosteroid analogues. 4. The effect of methyl substitution at the C-5 and C-10 positions of neurosteroids on electrophysiological activity at GABAA receptors. J. Med. Chem. 1996;39:4218–32. doi: 10.1021/jm960304p. [DOI] [PubMed] [Google Scholar]
- 25.Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor α1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol. Pharmacol. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 27.Steinbach JH, Akk G. Modulation of GABAA receptor channel gating by pentobarbital. J. Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akk G, Shu HJ, Wang C, Steinbach JH, Zorumski CF, Covey DF, Mennerick S. Neurosteroid access to the GABAA receptor. J. Neurosci. 2005;25:11605–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scaglione JB, Jastrzebska I, Krishnan K, Li P, Akk G, Manion BD, Benz A, Taylor A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 14. Alternative ring system scaffolds: GABA modulatory and anesthetic actions of cyclopenta[b]phenanthrenes and cyclopenta[b]anthracenes. J. Med. Chem. 2008;51:1309–1318. doi: 10.1021/jm701128r. [DOI] [PubMed] [Google Scholar]
- 30.Akk G, Covey DF, Evers AS, Mennerick S, Zorumski CF, Steinbach JH. Kinetic and structural determinants for GABA-A receptor potentiation by neuroactive steroids. Curr. Neuropharmacol. 2010;8:18–25. doi: 10.2174/157015910790909458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABAA receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The Influence of Neuroactive Steroid Lipophilicity on GABAA Receptor Modulation: Evidence for a Low-Affinity Interaction. J. Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci. Biobehav. Rev. 2013;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biellmann J-F. Enantiomeric steroids: synthesis, physical, and biological properties. Chem. Rev. 2003;103:2019–2033. doi: 10.1021/cr020071b. [DOI] [PubMed] [Google Scholar]
- 35.Katona BW, Cummins CL, Ferguson AD, Li T, Schmidt DR, Mangelsdorf DJ, Covey DF. Synthesis, characterization, and receptor interaction profiles of enantiomeric bile acids. J. Med. Chem. 2007;50:6048–58. doi: 10.1021/jm0707931. [DOI] [PubMed] [Google Scholar]
- 36.Vanlandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, Hammes SR, Jamnongjit M, Stein DG. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Green PS, Yang SH, Nilsson KR, Kumar AS, Covey DF, Simpkins JW. The nonfeminizing enantiomer of 17β-estradiol exerts protective effects in neuronal cultures and a rat model of cerebral ischemia. Endocrinology. 2001;142:400–406. doi: 10.1210/endo.142.1.7888. [DOI] [PubMed] [Google Scholar]
- 38.Sneitz N, Krishnan K, Covey DF, Finel M. Glucuronidation of the steroid enantiomers ent-17β-estradiol, ent-androsterone and ent-etiocholanolone by the human UDP-glucuronosyltransferases. J. Steroid Biochem. Mol. Biol. 2011;127:282–288. doi: 10.1016/j.jsbmb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fajkos J, Sorm F. Steroids. IX. Synthesis of 3α-hydroxy-16-oxoandrostane and 3,16-dioxo-4-androstane. Colectl. Czech. Chem. Commun. 1954;19:349–356. [Google Scholar]
- 40.Kamijo S, Matsumura S, Inoue M. CCl3CN: a crucial promoter of mCPBA-mediated direct ether oxidation. Org. Lett. 2010;12:4195–4197. doi: 10.1021/ol1018079. [DOI] [PubMed] [Google Scholar]
- 41.Gardi R, Vitali R, Ercoli A. Ethers of 3α-hydroxy-5α-androstane derivatives. Gazz. Chim. Ital. 1962;92:632–646. 1. [Google Scholar]
- 42.McKenna J, Norymberski JK, Stubbe RD. Partial reduction of steroid hormones and related substances. Part III. The reaction of αβ-unsaturated ketones with zinc in acetic acid. J. Chem. Soc. 1959:2502–2509. [Google Scholar]
- 43.da Silva EJ, Roleira FM, Sa e Melo ML, Neves AS, Paixao JA, de Almeida MJ, Silva MR, Andrade LC. X-ray and deuterium labeling studies on the abnormal ring cleavages of a 5β-epoxide precursor of formestane. Steroids. 2002;67:311–319. doi: 10.1016/s0039-128x(01)00164-7. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Zorumski CF, Covey DF. Neurosteroid analogues: structure-activity studies of benz[e]indene modulators of GABAA receptor function. 1. The effect of 6-methyl substitution on the electrophysiological activity of 7-substituted benz[e]indene-3-carbonitriles. J. Med. Chem. 1993;36:3956–3967. doi: 10.1021/jm00076a025. [DOI] [PubMed] [Google Scholar]
- 45.Hu YF, Wittmer LL, Kalkbrenner M, Evers AS, Zorumski CF, Covey DF. Neurosteroid analogues. Part 5. Enantiomers of neuroactive steroids and benz[e]indenes: total synthesis, electrophysiological effects on GABAA receptor function and anesthetic actions in tadpoles. J. Chem. Soc. Perkin Trans. 1997;1:3665–3671. [Google Scholar]
- 46.Jiang X, Manion BD, Benz A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 9. Conformationally constrained pregnanes: structure–activity studies of 13,24-cyclo-18,21-dinorcholane analogues of the GABA modulatory and anesthetic steroids (3α,5α)- and (3α,5β)-3-hydroxypregnan-20-one. J. Med. Chem. 2003;46:5334–5348. doi: 10.1021/jm030302m. [DOI] [PubMed] [Google Scholar]
- 47.Li P, Covey DF, Steinbach JH, Akk G. Dual potentiating and inhibitory actions of a benz[e]indene neurosteroid analog on recombinant alpha1beta2gamma2 GABAA receptors. Mol. Pharmacol. 2006;69:2015–2026. doi: 10.1124/mol.106.022590. [DOI] [PubMed] [Google Scholar]
- 48.Bandyopadhyaya AK, Manion BD, Benz A, Taylor A, Rath NP, Evers AS, Zorumski CF, Mennerick S, Covey DF. Neurosteroid analogues. 15. A comparative study of the anesthetic and GABAergic actions of alphaxalone, Δ16-alphaxalone and their corresponding 17-carbonitrile analogues. Bioorg. Med. Chem. Lett. 2010;20:6680–6684. doi: 10.1016/j.bmcl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bracamontes JR, Li P, Akk G, Steinbach JH. A neurosteroid potentiation site can be moved among GABAA receptor subunits. J. Physiol. 2012;590:5739–47. doi: 10.1113/jphysiol.2012.237255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.