Abstract
Objective
Vascular remodeling occurs after endothelial injury resulting in smooth muscle cell (SMC) proliferation and vascular fibrosis. We previously demonstrated that the blood pressure-regulating hormone aldosterone enhances vascular remodeling in mice at sites of endothelial injury in a placental growth factor (PlGF)-dependent manner. We now test the hypothesis that SMC mineralocorticoid receptors (MR) directly mediate the remodeling effects of aldosterone and further explore the mechanism.
Approach and Results
A wire-induced carotid injury model was performed in wild type (WT) mice and mice with inducible SMC-specific deletion of MR (SMC-MR-KO). Aldosterone did not affect re-endothelialization after injury in WT mice. Deletion of SMC-MR prevented the 79% increase in SMC proliferation induced by aldosterone after injury in MR-Intact littermates. Moreover, both injury-induced and aldosterone-enhanced vascular fibrosis were attenuated in SMC-MR-KO mice. Further exploration of the mechanism revealed that aldosterone-induced vascular remodeling is prevented by blockade of the PlGF-specific receptor, VEGFR1, in vivo. Immunohistochemistry of carotid vessels shows that the induction of VEGFR1 expression in SMC after vascular injury is attenuated by 72% in SMC-MR-KO mice. Moreover, aldosterone induction of vascular PlGF mRNA expression and protein release are also prevented in vessels lacking SMC-MR.
Conclusions
These studies reveal that SMC-MR is necessary for aldosterone-induced vascular remodeling independent of renal effects on blood pressure. SMC-MR contributes to induction of SMC VEGFR1 in the area of vascular injury and to aldosterone-enhanced vascular PlGF expression and hence the detrimental effects of aldosterone are prevented by VEGFR1-blockade. This study supports exploring MR antagonists and VEGFR1-blockade to prevent pathological vascular remodeling induced by aldosterone.
Keywords: vascular remodeling, smooth muscle cells, aldosterone, mineralocorticoid receptor, VEGF
Introduction
Vascular remodeling occurs in response to endothelial damage and contributes to vascular pathologies including vascular stiffness due to hypertension and aging, atherosclerosis, vein graft failure, restenosis after percutaneous vascular procedures, and cardiac transplant vasculopathy (reviewed in 1). Endothelial damage can be caused by mechanical injury or by cardiovascular risk factors including dyslipidemia, hypertension, diabetes, or smoking. In the area of endothelial damage the normally quiescent smooth muscle cells (SMC) proliferate and produce extracellular matrix that contributes to vascular thickening and fibrosis. Although much has been learned about mechanisms of vascular remodeling, our current cardiovascular therapies are still limited by adverse remodeling that contributes to myocardial infarction (MI), stroke and the high failure rate of vein grafts, transplants and even stents. Thus there remains a need to identify novel contributors to vascular remodeling that might be more effective targets to prevent adverse cardiovascular events and improve the efficacy of our interventions.
Aldosterone is a steroid hormone that regulates blood pressure (BP) by acting on renal mineralocorticoid receptors (MR) to induce genes in the kidney that promote sodium retention2. MR antagonists, including spironolactone and eplerenone, are used to treat hypertension and heart failure and significantly reduce cardiovascular mortality in randomized trials3–6. Additional clinical data suggest that aldosterone promotes atherosclerotic ischemic events including MI and stroke and increases mortality7,8. In animal models of vascular injury, aldosterone enhances vascular remodeling9,10. Conversely, MR antagonists decrease vascular remodeling in animal models of hypertension11, balloon injury10, stent implantation12, vein grafting13, and hyperlipidemia-induced atherosclerosis14. Thus aldosterone plays an important role in vascular remodeling that has largely been attributed to BP elevation with secondary vascular consequences. However, it has recently become clear that aldosterone also has extra-renal actions. MR is expressed in vascular SMC and endothelial cells (EC) where it regulates genes involved in vascular inflammation, fibrosis and calcification15–19. Indeed we have recently demonstrated in a mouse model with inducible, SMC-specific MR deletion, that SMC-MR directly contributes to vascular contractile function and BP elevation with aging20. In humans, the cardiovascular protective effects of MR antagonists exceed the expected effects of modest changes in systemic BP3,4,21. These clinical and experimental findings support the possibility that aldosterone could act directly on MR in the vasculature to contribute to vascular remodeling. If so, understanding the vascular cellular target that mediates aldosterone-enhanced remodeling (EC versus SMC) and the potential molecular downstream mechanisms could provide novel therapeutic strategies.
The vascular endothelial growth factors (VEGF) are a family of secreted proteins that contribute to angiogenesis. VEGFs modulate vascular SMC and EC cell function via transmembrane VEGF type 1 and type 2 receptors (VEGFR1 and VEGFR2)22. We previously discovered that aldosterone specifically regulates vascular expression of the VEGF-family member, placental growth factor (PlGF)9,18, and that PlGF is necessary for aldosterone-enhanced vascular remodeling9. PlGF specifically binds to VEGFR1 while other VEGFs signal through both receptors22. In injured mouse vessels and in human vessels with atherosclerotic disease, aldosterone further enhances PlGF expression and specifically upregulates VEGFR19. These data identify PlGF/VEGFR1 as a potential mediator of aldosterone-enhanced vascular remodeling. In this study we test the hypothesis that SMC-MR directly mediates the remodeling effects of aldosterone on injured vessels and further explore the role of PlGF/VEGFR1 as a potential downstream mechanism and therapeutic target to prevent aldosterone-enhanced vascular remodeling in vivo.
Materials and Methods
See online methods supplement for details.
Mice and wire carotid injury model
All animals were handled in accordance with NIH standards, and the procedures were approved by the Tufts Medical Center Institutional Animal Care and Use Committee. Male MRf/f/SMA-Cre-ERT2+ (SMC-MR-KO) and MRf/f/SMA-Cre-ERT2– littermates (MR-Intact) were induced by intraperitoneal Tamoxifen daily and studies were performed 4 weeks later20.One day prior to carotid injury, vehicle or aldosterone infusion pumps (240µg/kg/d) were inserted and a Bromodeoxyuridine (BrDU) infusion pump placed at the time of injury. Two weeks after injury, BrDU positive cells, medial area and extracellular matrix quantified histologically by treatment- and genotype-blinded investigators and re-endothelialization was assessed by staining with Evans Blue dye. Mice were injected intraperitoneally with VEGFR1- or VEGFR2-blocking antibody at 35 mg/kg diluted in phosphate buffered saline (PBS) at the time of injury and every 2 days for a total of 5 injections. One group of control mice was treated with PBS alone and another group with control IgG antibody (Innovative Research, #Ir-RT-GF) and the data from these 2 control groups was pooled and referred to as “control IgG”.
Statistics
Values are reported as mean ± SEM. Within-group differences were assessed with 1-way ANOVA with Student-Newman-Keuls post-hoc test or 1-way ANOVA on ranks with Dunn’s method post-hoc test when appropriate. Carotid injury analyses were performed by 2-way ANOVA with Student-Newman-Keuls post-hoc test. P < 0.05 was considered significant.
Results
Aldosterone does not alter the rate of re-endothelialization following vascular injury
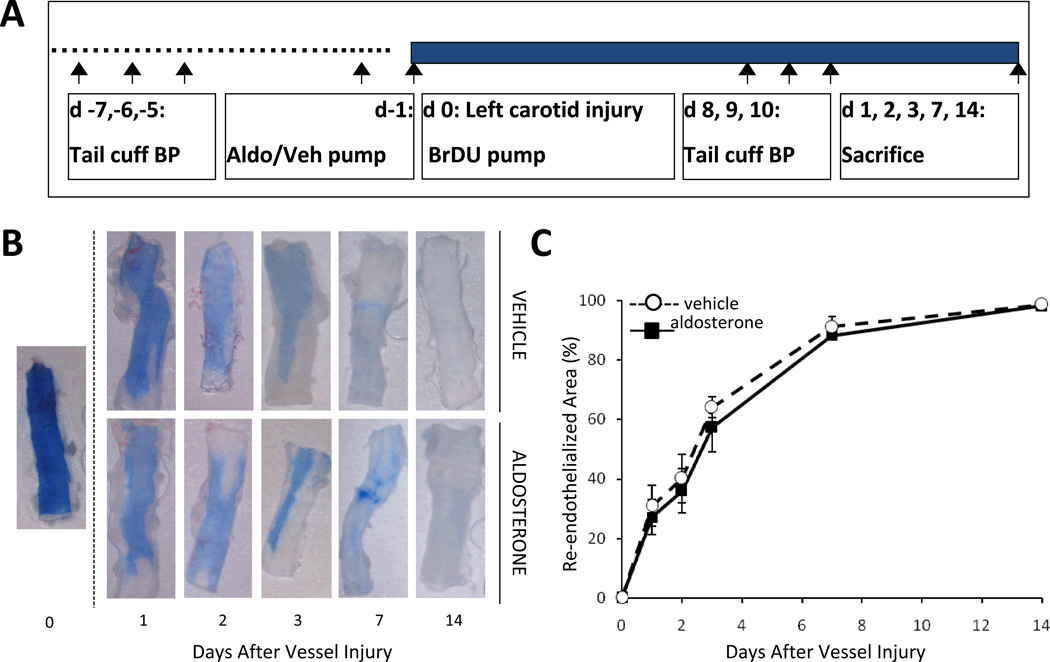
We set out to explore the mechanism by which aldosterone infusion enhances vascular remodeling specifically at sites of vascular injury without significantly changing blood pressure9. It has been suggested that the rate of endothelial re-growth after arterial injury determines the degree of vascular remodeling with accelerated re-endothelialization leading to an attenuated injury response23. Thus we first examined the effect of aldosterone on the rate of re-endothelialization in a mouse carotid wire injury model. In this model, an aldosterone or vehicle infusion pump is inserted 1 day prior to carotid endothelial denudation by wire injury (Figure 1A). After wire-induced carotid injury, Evans blue dye is infused to mark the areas of denuded carotid endothelium. Representative images of injured carotid arteries immediately after the initial injury (day 0) and 1, 2, 3, 7, and 14 days after injury are shown in Figure 1B. Evans blue staining confirms complete denudation of the endothelium on day 0. Complete re-endothelialization of the artery is confirmed14 days after injury. Quantification of the residual denuded area reveals no significant difference in the percentage of area covered with endothelium in arteries from aldosterone compared to vehicle treated mice at all time points after injury (Figure 1C). These results suggest that aldosterone is not enhancing the vascular remodeling response by altering endothelial cell proliferation or migration and may instead be acting on MR elsewhere in the vessel so we next focused on the smooth muscle cells.
Figure 1. Aldosterone-enhanced vascular injury is independent of effects on endothelial re-growth.
(A) Schematic of the mouse wire carotid injury model used for all in vivo studies. Mice were implanted with vehicle (Veh) or aldosterone (Aldo) infusion pumps one day prior to carotid denudation by wire injury. Tail cuff blood pressure (BP) measurements were conducted prior to and after injury. Mice were sacrificed at day 14 after injury unless otherwise indicated. (B) Representative carotid arteries showing Evan’s Blue dye marking in blue the denuded area at the indicated times following carotid injury in C57Bl/6 mice. (C) Quantification of re-endothelialization calculated as the percent of the carotid area without Evan’s Blue staining. N=3–5 mice/treatment/time. There is no significant difference between vehicle and aldosterone treated vessels.
Aldosterone-enhances vascular injury by direct, blood pressure-independent, effects on SMC-MR
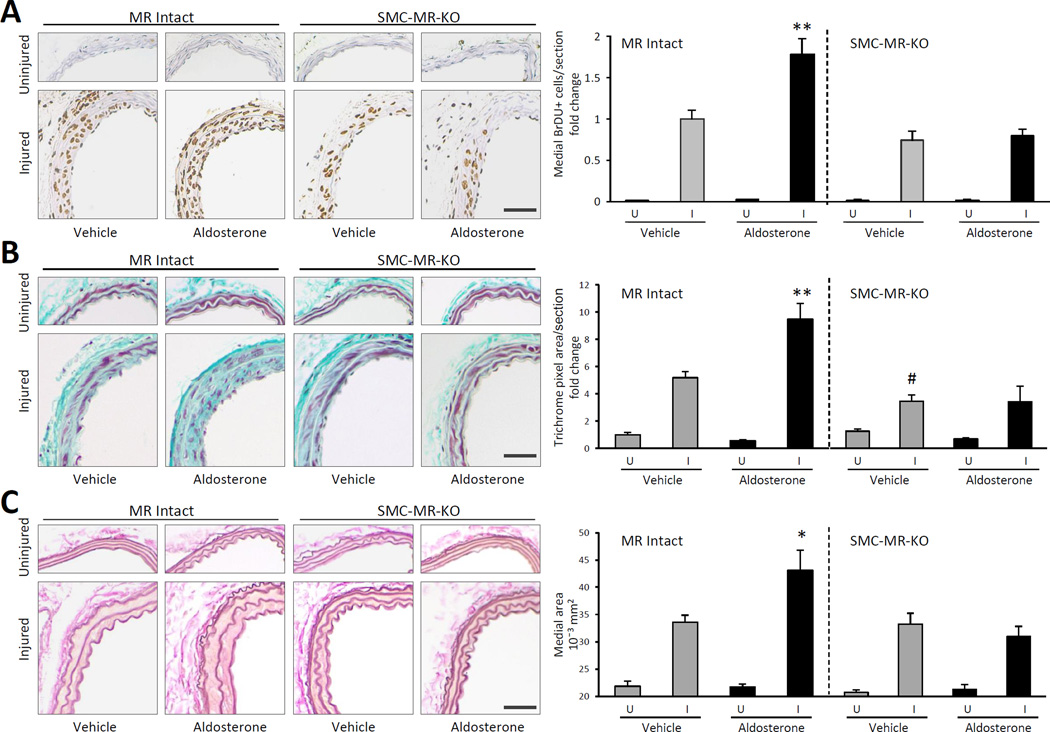
The role of SMC-MR in aldosterone-stimulated vascular injury was directly examined using a mouse model with MR genetically deleted in adulthood specifically from SMC (SMC-MR-KO) compared with MR Intact littermate controls20. Prior studies reveal that at 3-months of age, SMC-MR-KO mice have no significant difference in systemic BP with or without aldosterone infusion when compared with MR Intact controls as measured by telemetry20. This is confirmed by tail cuff plethysmography in the specific mice used for carotid injury that cannot have concurrent telemetry (Table 1). Mice underwent the carotid injury protocol (Figure 1A) with insertion of a bromodeoxyuridine (BrDU) infusion pump at the time of injury to mark proliferating cells and vascular remodeling was quantified 14 days after injury. Aldosterone was infused at a low dose that increases circulating aldosterone levels significantly and similarly in both genotypes to levels consistent with those seen in patients with cardiovascular disease with no effect on systolic BP or body weight (Table 1). In uninjured vessels there is minimal SMC proliferation, as measured by medial BrDU positive nuclei, regardless of the presence of SMC-MR or exogenous aldosterone consistent with the lack of effect of aldosterone on remodeling in the absence of endothelial damage. Vascular injury enhances SMC proliferation, even in the absence of SMC-MR (p<0.001 for injured versus uninjured), thus all further comparisons are made between the injured vessels only. In MR-intact mice, aldosterone significantly enhances SMC proliferation after injury (Figure 2A), as we previously published in wild type C57Bl/6 mice9. However, aldosterone fails to promote SMC proliferation in SMC-MR-KO mice (Figure 2A). Aldosterone infusion also significantly enhances injury-induced vascular fibrosis in MR Intact mice but not in SMC-MR-KO mice (Figure 2B). Interestingly, even in the absence of excess aldosterone, SMC-MR deficiency attenuates vascular fibrosis, supporting the concept that SMC-MR contributes to the fibrotic response to vascular injury in the presence of physiologic and pathologic levels of aldosterone (Figure 2B). Finally, vascular injury causes an increase in vessel medial area that is enhanced in the presence of excess aldosterone in MR Intact but not in SMC-MR-KO mice (Figure 2C). Taken together, these data support the new concept that aldosterone-enhances vascular remodeling by direct effects on MR in SMC and that SMC-MR contributes to basal and aldosterone-enhanced vascular fibrosis in this injury model.
Table 1.
Increased serum aldosterone to pathological levels in aldosterone-infused MR intact and SMC-MR-KO mice with no change in blood pressure or animal weight.
| Treatment | Strain | pre- treatment weight (g) |
pre- treatment SBP (mmHg) |
pre- treatment DBP (mmHg) |
pre-harvest weight (g) |
treatment SBP (mmHg) |
treatment DBP (mmHg) |
serum aldosterone (nM) |
|---|---|---|---|---|---|---|---|---|
| vehicle | MR Intact | 27.3 ± 1.2 | 109.5 ± 3.4 | 81.7 ± 3.8 | 28.0 ± 1.0 | 115.7 ± 2.5 | 85.9 ± 2.8 | 1.2 ± 0.14 |
| aldosterone | MR Intact | 26.9 ± 0.7 | 104.8 ± 3.0 | 76.2 ± 3.3 | 27.8 ± 0.7 | 107.1 ± 2.4 | 80.1 ± 2.7 | 7.6 ± 0.71* |
| vehicle | SMC-MR-KO | 27.4 ± 0.8 | 104.3 ± 3.1 | 76.7 ± 3.1 | 28.2 ± 0.7 | 109.1 ± 4.1 | 80.5 ± 3.9 | 1.3 ± 0.11 |
| aldosterone | SMC-MR-KO | 27.3 ± 0.6 | 106.4 ± 3.3 | 77.5 ± 3.3 | 28.1 ± 0.5 | 110.0 ± 3.0 | 81.0 ± 2.8 | 6.5 ± 0.74* |
SBP=systolic blood pressure, DBP=diastolic blood pressure.
P<0.001 versus vehicle.
g=grams.
Figure 2. Smooth muscle cell MR is necessary for aldosterone-enhanced vascular remodeling after injury in vivo.
Mice with MR specifically deleted from SMC (SMC-MR-KO) and MR intact littermates (MR Intact) underwent wire carotid injury along with two weeks of vehicle or aldosterone treatment. (A) Medial SMC proliferation was quantified in BrDU-stained sections of uninjured (U) and injured (I) mouse carotid arteries. Representative carotid artery sections are shown on left and the fold change in the number of medial BrDU positive SMC per section compared with MR Intact, vehicle-treated, injured vessels is indicated on right. (B) Fibrosis was quantified in trichrome-stained sections of uninjured and injured mouse carotid arteries. Representative carotid artery sections are shown on left and the fold change in medial trichrome pixel area compared with MR Intact, vehicle-treated, uninjured vessels is indicated on right. (C) Medial vessel area was quantified in Elastin-stained sections of uninjured and injured mouse carotid arteries treated with vehicle or aldosterone. Representative carotid artery sections are shown on left and the average medial area for all animals is indicated on right. Scale bar: 0.5mm. N=14–16 mice/genotype and treatment. *P < 0.01, **P < 0.001 versus all other injured vessels; # P<0.05 versus MR Intact, vehicle-treated, injured vessels.
VEGFR1 blockade prevents aldosterone-enhanced vascular remodeling in vivo
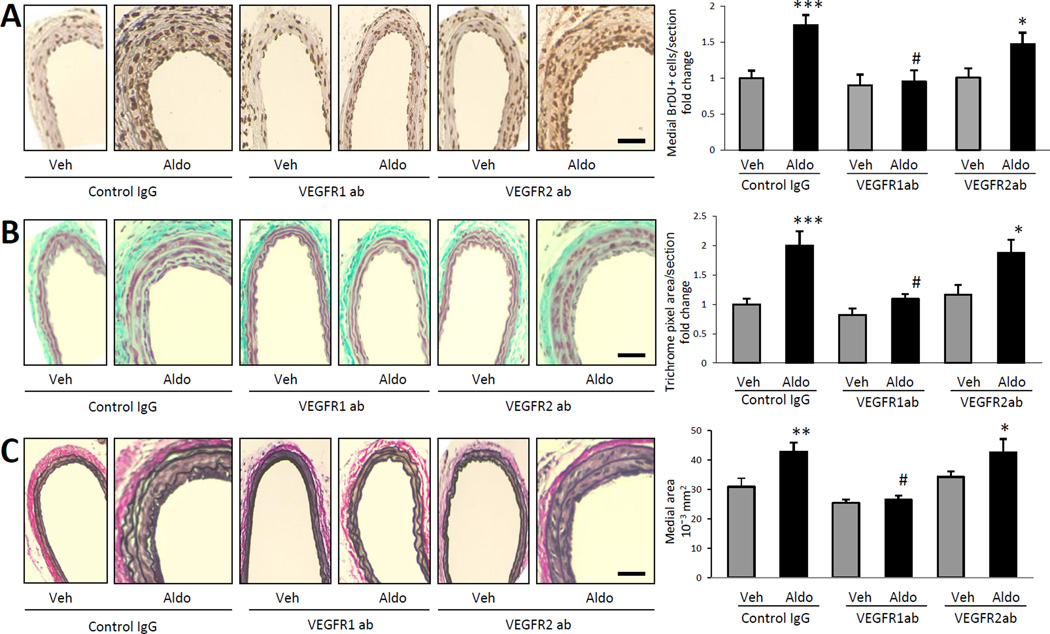
We previously demonstrated that aldosterone-induced vascular remodeling is dependent on the presence of the growth factor PlGF9 that binds specifically to VEGFR1. To explore the role of VEGF receptors in aldosterone-induced remodeling in vivo, the aldosterone-enhanced wire carotid injury model was repeated with injection of control IgG, VEGFR1- or VEGFR2-blocking antibodies. BP measurements reveal no significant effect of VEGF-blocking antibodies on BP at these doses (Supplemental Table I). As expected, aldosterone infusion significantly enhances injury-induced SMC proliferation, vascular fibrosis, and medial thickening in mice treated with control IgG (Figure 3). VEGFR1-blocking antibody, but not VEGFR2-blocking antibody, prevents aldosterone-enhanced SMC proliferation (Figure 3A), vascular fibrosis (Figure 3B) and medial thickening (Figure 3C). Taken together, these data suggest that VEGFR1 plays a significant and specific role in aldosterone-enhanced vascular remodeling in vivo.
Figure 3. VEGFR1 blockade prevents aldosterone-enhanced vascular remodeling after injury.
Mice underwent wire carotid injury along with vehicle (Veh) or aldosterone (Aldo) infusion and injection of control IgG, VEGFR1- or VEGFR2-blocking antibody (ab). (A) Medial smooth muscle proliferation (SMC) proliferation was quantified in BrDU-stained sections of injured mouse carotid arteries. Representative carotid artery sections are shown on the left. Bars on the right represent fold change in medial BrDU+ SMC relative to Veh with Control ab treatment. (B) Extra-cellular matrix deposition was quantified in trichrome-stained sections of injured mouse carotid arteries. Representative carotid artery sections are shown on the left. Bars on the right represent the fold change in medial trichrome pixel area relative to Veh with control IgG. (C) Medial vessel area was quantified in elastin-stained sections of injured mouse carotid arteries. Representative carotid artery sections are shown on left. Bars on the right represent average medial area for all animals. Scale bar: 0.5mm. N=8–12. *P<0.05, **P<0.01, ***P<0.001 versus all Veh-treated. #P<0.01 versus Aldo with Control IgG and Aldo with VEGFR2ab treatment.
Since the carotid injury studies in Figures 2 and 3 implicate direct effects of aldosterone on SMC-MR in promoting cell proliferation, we attempted to explore the mechanism using an in vitro model of mouse carotid SMC proliferation. Aldosterone enhances proliferation of primary mouse carotid SMC in a dose-dependent manner (Supplemental Figure IA) as it does for other cultured SMC24. However, at physiologically (1 nM) and pathologically (5–10 nM) relevant aldosterone concentrations, the increase in proliferation in vitro is modest (<20%) and much less than the enhanced proliferative response to the same concentration of aldosterone in vivo (70–80%). Further studies revealed that aldosterone treatment of cultured primary mouse carotid SMC results in a non-significant trend towards increased PlGF expression (much less than the 300% increase in whole vessels (9 and Figure 5A)) and only a modest but significant increase in VEGFR1 expression that is prevented by co-treatment with the MR-specific antagonist eplerenone (Supplemental Figure IB). In vitro, VEGFR1-blockade inhibits the modest aldosterone-enhanced SMC proliferation while VEGFR2-blockade prevents a significant aldosterone-induced increase in proliferation but is not significantly decreased compared to aldosterone with IgG (Supplemental Figure IC). We conclude that although the PlGF/VEGFR1 pathway is modestly activated by aldosterone and contributes to carotid SMC proliferation in vitro, this in vitro system does not completely recapitulate the effect of aldosterone on the proliferative response to injury in vivo. Therefore, further exploration of the mechanism was performed in vivo and in whole vessels.
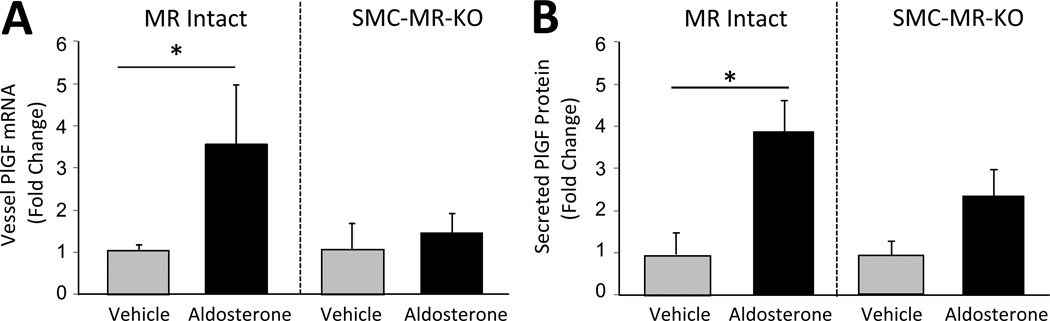
Figure 5. SMC-MR is necessary for aldosterone regulation of vascular PlGF expression and release.
(A,B) Mouse aortas from 3-month old MR Intact and SMC-MR-KO mice were treated for 8 hours ex vivo with vehicle (grey bars) or 100nM aldosterone (black bars). (A) PlGF mRNA expression was quantified by QRT-PCR of vessel RNA and (B) PlGF protein secretion was quantified by ELISA of vessel conditioned media. *P < 0.05 versus vehicle.
SMC-MR contributes to VEGFR1 induction on SMC after vascular injury
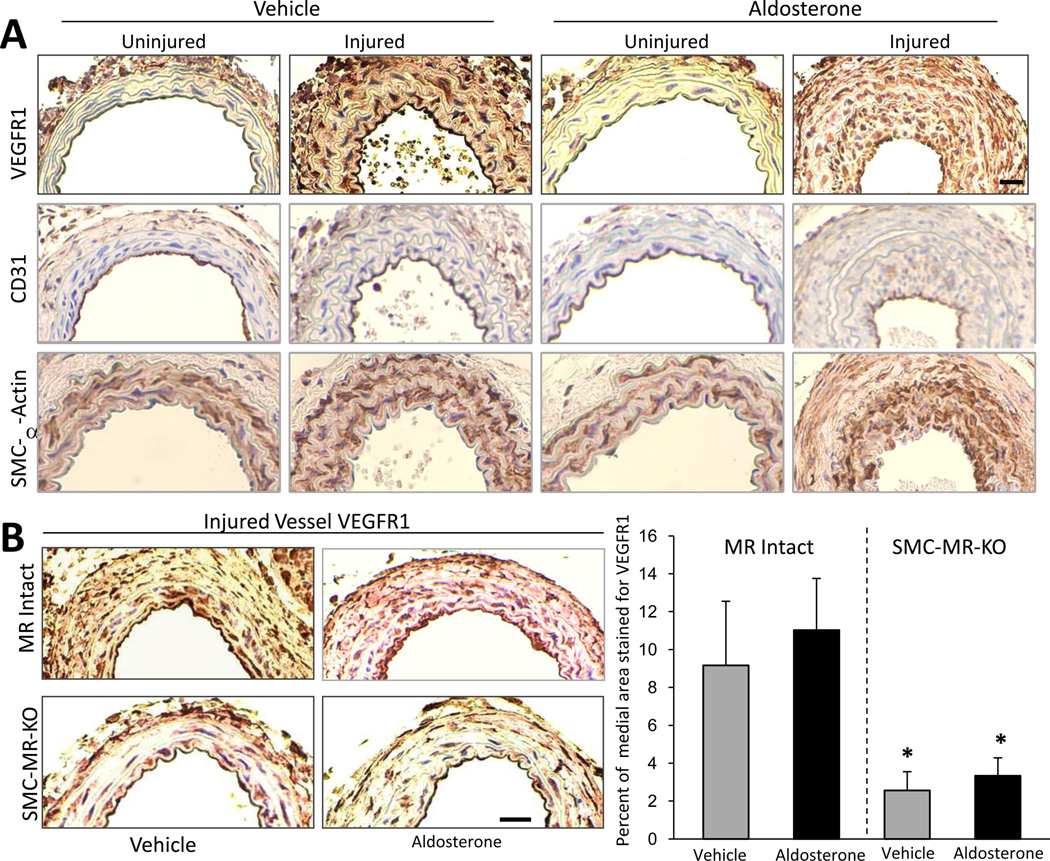
In healthy vessels, VEGFR1 is expressed exclusively in the endothelium however, upon vascular injury, VEGFR1 expression is activated on vascular SMC by unclear mechanisms25. To examine the vascular compartments in which VEGFR1 is expressed in the wire injury model, immunohistochemistry with VEGFR1-specific antibody was performed on uninjured and injured carotid arteries 14 days after unilateral endothelial injury. In this model, we confirm that uninjured vessels express VEGFR1 only in the endothelium while VEGFR1 is expressed robustly on EC and SMC in injured vessels even without exogenous aldosterone administration (Figure 4A). Serial sections incubated with CD31 (an EC marker) and SMC alpha actin (a SMC marker) are included in Figure 4A to confirm the identity of each cell type in the vessel.
Figure 4. SMC-MR contributes to injury-induced SMC VEGFR1 expression.
(A) VEGFR1 is expressed exclusively on EC in uninjured vessels and is induced on SMC after vascular injury. Immunohistochemistry of serial carotid artery sections 14 days after unilateral carotid injury of wild type mice using VEGFR1 antibody to localize expression and anti CD31 antibody to label endothelial cells and anti smooth muscle α-actin antibody to label SMC. (B) SMC-MR contributes to induction of SMC VEGFR1 expression after endothelial injury. VEGFR1 immunohistochemistry of sections of injured carotids from MR-Intact and SMC-MR-KO littermates 14 days after injury. Representative carotid artery sections are shown on the left and the percent of the medial area that stains positive for VEGFR1 is quantified on the right. Scale bar: 0.5mm. N=8. *P<0.05 versus MR Intact vehicle- and aldosterone-treated vessels.
To explore if SMC-MR is involved in the mechanism of the injury-induced SMC expression of VEGFR1, medial VEGFR1 immunoreactivity was quantified in serial sections of the injured vessels from the study in Figure 2 (Figure 4B). VEGFR1 expression is detected in the media in all the injured vessels, however in vessels from SMC-MR-KO, there is a significant and substantial reduction in the percentage of the vessel staining positive for VEGFR1 regardless of the presence of excess aldosterone. Taken together, these data support that SMC-MR contributes substantially to the induction of VEGFR1 expression on SMC after a vessel is injured.
SMC-MR is necessary for aldosterone induction of vascular PlGF
We previously demonstrated that aldosterone stimulates vascular PlGF transcription and release from intact and injured mouse vessels and from diseased human vessels9. To investigate whether PlGF production is dependent on SMC-MR, whole vessels from MR Intact and SMC-MR-KO mice were treated with aldosterone ex vivo and PlGF levels were assessed. In MR Intact vessels aldosterone increases vascular PlGF mRNA and extravascular PlGF protein more than 3 fold (Figure 5) as previously demonstrated in WT vessels9. In vessels lacking SMC-MR, the aldosterone-induced increase in PlGF message and protein secretion are prevented, demonstrating that aldosterone regulation of vascular PlGF requires SMC-MR. Thus, deletion of SMC-MR attenuates the local upregulation of VEGFR1at the site of injury (Figure 4B), prevents aldosterone-induced vascular PlGF production (Figure 5), and prevents aldosterone-enhanced vascular remodeling after injury (Figure 2).
Discussion
In summary, we have demonstrated that SMC-MR is required for aldosterone-enhanced vascular remodeling in vivo. Aldosterone induces vascular SMC proliferation and fibrosis after injury without effecting endothelial re-growth. These adverse effects of the hormone are completely lost in mice with the MR specifically deleted from SMC in adulthood. SMC-MR also contributes to injury-associated vascular fibrosis even without addition of exogenous aldosterone. Blockade of VEGFR1 signaling with receptor-specific antibodies also prevents aldosterone-induced SMC proliferation and vascular fibrosis after injury in vivo. Further mechanistic studies reveal that SMC-MR directly contributes to the induction of VEGFR1 expression on SMC at the site of vascular injury and is necessary for aldosterone-induction of the VEGFR1 ligand, PlGF.
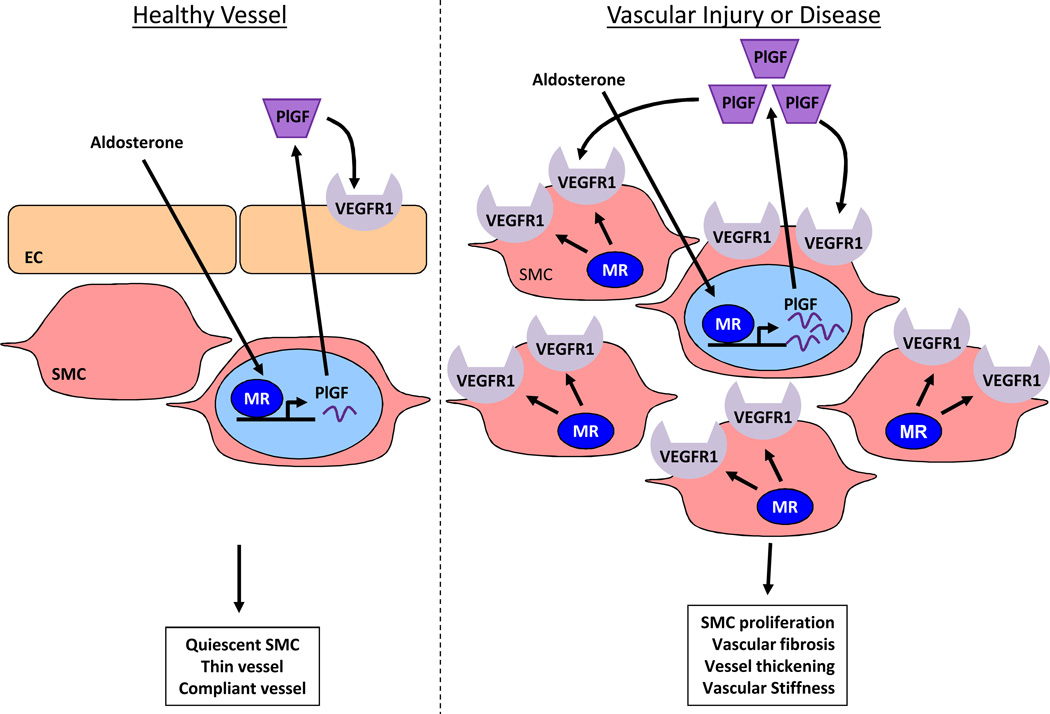
Putting these data together with previously published work9, 18, 25 provides a new model by which SMC-MR contributes directly to vascular remodeling (Figure 6). This model addresses the longstanding conundrum as to why aldosterone alone has no adverse vascular phenotype unless combined with a cause for endothelial dysfunction, including high salt intake26, congestive heart failure27, hyperlipidemia28, or localized vascular injury9. In healthy vessels with an intact endothelium, VEGFR1 is expressed only on EC and aldosterone activation of SMC-MR only modestly increases PlGF transcription and release9 with no effect on SMC proliferation and fibrosis as PlGF receptors are not expressed on SMC under these conditions (Figure 6, left). Thus in healthy vessels, aldosterone does not disrupt the quiescent SMC phenotype and vessels do not undergo adverse remodeling even when aldosterone levels are high (for example in healthy individuals (or mice) on a low sodium diet). However, when vessels are injured or diseased, SMC-MR contributes to substantial upregulation of PlGF9, 18 and to the expression of VEGFR1 on SMC resulting in SMC proliferation and vascular fibrosis25, 29. Conversely, PlGF deficiency9 or VEGFR1 blockade prevents aldosterone-enhanced remodeling after injury in mice. This data from mouse models is consistent human data demonstrating that aldosterone enhances PlGF and VEGFR1 expression in vessels from patients with severe atherosclerosis (undergoing coronary artery bypass grafting) but not in healthy human vessels (transplant donors)9. Based on these data, we propose the model in Figure 6 (right) that in diseased or injured vessel, SMC-MR locally enhances PlGF release and SMC VEGFR1 expression resulting in enhanced SMC proliferation, vascular fibrosis, and vessel thickening after injury. By this mechanism, aldosterone and SMC-MR promote adverse vascular remodeling that contributes to luminal narrowing and vascular stiffness, important contributors to cardiovascular disease. Treatment of diseased human vessels with the MR antagonist spironolactone ex vivo suppresses PlGF production9 thus inhibition of this mechanism may contribute to the beneficial effects of MR antagonists in cardiovascular patients3, 4, 6. Specific inhibition of VEGFR1 prevented aldosterone-enhanced remodeling in vivo supporting the concept that SMC-MR-regulated pathways could be targeted to prevent or treat cardiovascular diseases.
Figure 6. Model for the role of the SMC-MR/PlGF/VEGFR1 pathway in aldosterone-induced vascular remodeling after injury.
In the healthy uninjured vasculature (left), vascular endothelial growth factor type 1 receptors (VEGFR1) are expressed exclusively on endothelial cell (EC) and not on smooth muscle cells (SMC). Aldosterone activation of SMC mineralocorticoid receptors (MR) in this setting modestly enhances placental growth factor (PlGF) transcription and local release that can act only on EC expressing VEGFR1. Thus in healthy vessels, aldosterone does not disrupt the quiescent SMC phenotype and vessels do not undergo adverse remodeling even when aldosterone levels are high. In the setting of vascular injury or disease (right), SMC-MR contributes to local upregulation of VEGFR1 expression on SMC and aldosterone activation of SMC-MR contributes to substantial PlGF expression and release. PlGF mediates aldosterone-enhanced SMC proliferation and vascular fibrosis after injury by binding to VEGFR1 receptors on SMC. This model provides insight into how aldosterone acts synergistically with endothelial injury to contribute to adverse vascular remodeling and how MR antagonists prevent adverse cardiovascular events in clinical trials.
For over half a century, aldosterone and MR have been known to regulate BP by renal sodium retention. Based on this knowledge, the detrimental cardiovascular effects of aldosterone have been attributed to secondary vascular responses to elevated BP. In this study, the low dose aldosterone infusion enhanced vascular remodeling without increasing BP. Moreover, aldosterone-enhanced vascular remodeling is completely prevented by the specific deletion of MR from SMC. We previously demonstrated that SMC-MR-KO mice have intact renal MR function, normal renal sodium handling and no difference in telemetric BP at the age used for this study20 which we confirm by tail cuff BP here. The use of an inducible model of MR deletion also prevents developmental effects of MR deletion from contributing to altered vascular remodeling responses. Taken together, these results support a new paradigm in which direct MR activation in the SMC of the vasculature is responsible for enhanced vascular remodeling independent of renal MR and BP alterations.
There are several limitations and future directions to this study that should be noted. First, the wire carotid injury model in C57Bl/6 mice is a reproducible model to examine medial SMC proliferation and collagen deposition in the vessel wall. These processes are paramount in vascular remodeling induced by hypertension and aging and also occur in the setting of atherosclerosis and vascular injury in which proliferation is accompanied by SMC migration and neointima formation. Aldosterone contributes to neointimal formation in other models10 but this occurs only rarely in the wire injury model in C57Bl/6 mice (<10% of mice). Thus, the direct role of SMC-MR, PlGF and VEGFR1 in neointima formation warrants further exploration in vascular injury models with more reproducible neointimal responses. This would have important clinical implications since the aldosterone-enhanced mechanism of vascular remodeling identified here appears to dissociate SMC proliferation from endothelial re-growth, a desired situation for drugs to prevent restenosis of vascular stents in which neointima formation is an important component of the pathology. PlGF and VEGF are also vasodilators and as a result, novel cancer therapeutics that block VEGF receptors cause hypertension30 and further exploration of the role of VEGF signaling in vascular remodeling could have important implications for the growing patient populations treated with anti-VEGF therapy for malignancy and other conditions. In this study, we confirm that there is no substantial difference in tail cuff BP at the concentrations of blocking antibodies or aldosterone used. Telemetric monitoring is the gold standard for BP measurements in rodents however, since the catheter is inserted via the carotid artery, this cannot be performed concurrently with carotid injury. We previously demonstrated by telemetry that there is no BP difference in young SMC-MR-KO mice compared to MR Intact controls at baseline or with aldosterone infusion20. Here we confirm by tail cuff plethysmography that the substantial changes in arterial remodeling responses are not due to large changes in BP although small BP differences (5–10 mmHg or less) cannot be accurately distinguished by this technique. Finally, understanding how the downstream signaling of MR and VEGFR1 converge in SMC cells to coordinate the proliferative response to injury is an important area for future investigation. Indeed, both MR24 and VEGFR125 signaling have been shown to promote SMC proliferation by activation of MAP-kinase signaling in vitro. Since the SMC-MR/PlGF/VEGFR1 mechanism identified here is specifically activated in the setting of vascular injury that is not easily reproduced in vitro, future in vivo studies will be important to explore the downstream signaling events that mediate aldosterone-induced vascular remodeling.
In conclusion, these data support a novel mechanism of aldosterone-enhanced remodeling in which direct activation of SMC-MR promotes adverse vascular remodeling by regulation of VEGFR1 and PlGF in SMC in areas of vascular injury. This mechanism is independent of alterations in endothelial re-growth, renal MR activation, and systemic BP. Clinically, elevated serum aldosterone is increasingly common due to an association with resistant hypertension31, heart failure, and obesity32 and aldosterone levels correlate with increased risk of MI, stroke and death7. Furthermore, SMC-MR-activation contributes to vascular fibrosis in this model even without added aldosterone and hence might contribute to vascular remodeling even in patients with normal serum aldosterone levels. Interestingly, the basal level of vascular fibrosis after injury is not significantly altered by VEGFR1 blockade supporting an additional mechanism by which SMC-MR contributes to fibrosis that remains to be explored. Thus, the mechanisms identified in this study likely contribute to vascular remodeling in rapidly growing populations of patients at high risk for cardiovascular disease and have important implications for developing new therapeutic strategies to treat vascular diseases. Current aldosterone antagonists therapies are limited by off target (gynecomastia) and renal (hyperkalemia) side effects. Thus, understanding the mechanisms by which vascular MR activation contributes directly to vascular disease could identify novel targets, including the PlGF/VEGFR1 pathway, which could reap the vascular benefits of MR blockade without the systemic side effects.
Supplementary Material
Significance.
This manuscript describes a new mechanism for the detrimental vascular effects of the blood pressure regulating hormone aldosterone and for the beneficial effects of mineralocorticoid receptor (MR) antagonist drugs. The results demonstrate a blood-pressure independent role for aldosterone in vascular remodeling that is directly mediated by MR in the smooth muscle cells of the vasculature. It provides a mechanistic explanation by which aldosterone enhances vascular remodeling specifically in areas of endothelial damage by upregulating SMC expression of type 1 vascular endothelial growth factor receptors (VEGFR1) at sites of injury and promoting local production of the VEGFR1 ligand, PlGF. Finally, it identifies the SMC-MR/VEGFR1 pathway as a target that prevents aldosterone-enhanced vascular SMC proliferation after endothelial injury without affecting endothelial re-growth with potential implications for new therapies to prevent the detrimental sequelae of hypertension and other adverse vascular remodeling outcomes such as vein graft failure, in-stent-restenosis, or transplant allograft vasculopathy.
Acknowledgements
The authors would like to thank Pierre Chambon and Daniel Metzger for generously sharing the floxed MR mice and the SMA-Cre-ERT2 mice.
Sources of Funding: This work was supported by grants from the US National Institutes of Health (NIH HL095590 to I.Z.J.) and the American Heart Association (AHA GIA0855920D to I.Z.J., GRNT7240000 to I.Z.J., and POST7590096 to A.M.). S.A.K is an investigator of the Howard Hughes Medical Institute.
Nonstandard abbreviations
- BP
Blood pressure
- BrDU
bromodeoxyuridine
- EC
endothelial cells
- ECM
extracellular matrix
- KO
knock out mouse
- MR
Mineralocorticoid Receptor
- PBS
phosphate buffered saline
- PlGF
Placental Growth Factor
- SMC
smooth muscle cells
- SMC-MR-KO
smooth muscle cell-specific MR knockout mouse
- VEGF
vascular endothelial growth factor
- VEGFR1
type 1 vascular endothelial growth factor receptor
- VEGFR2
type 2 vascular endothelial growth factor receptor
- WT
wild type
Footnotes
Disclosures: The authors have no relevant financial relationships to disclose.
Reference List
- 1.Goel SA, Guo LW, Liu B, Kent KC. Mechanisms of post-intervention arterial remodelling. Cardiovascular Research. 2012;96:363–371. doi: 10.1093/cvr/cvs276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65(2):61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Reichek N, Willenbrock R, et al. Effects of eplerenone, enalapril, and eplerenone/enalapril in patients with essential hypertension and left ventricular hypertrophy: the 4E-left ventricular hypertrophy study. Circulation. 2003;108:1831–1838. doi: 10.1161/01.CIR.0000091405.00772.6E. [DOI] [PubMed] [Google Scholar]
- 6.Zannad F, Mcmurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 7.Ivanes F, Susen S, Mouquet F, et al. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. European Heart Journal. 2012;33:191–202. doi: 10.1093/eurheartj/ehr176. [DOI] [PubMed] [Google Scholar]
- 8.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. Journal of the American College of Cardiology. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe IZ, Newfell BG, Aronovitz M, et al. Placental growth factor mediates aldosterone-dependent vascular injury in mice. Journal of Clinical Investigation. 2010;120:3891–3900. doi: 10.1172/JCI40205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Belle E, Bauters C, Wernert N, et al. Neointimal thickening after balloon denudation is enhanced by aldosterone and inhibited by spironolactone, and aldosterone antagonist. Cardiovascular Research. 1995;29:27–32. [PubMed] [Google Scholar]
- 11.Rocha R, Martin-Berger CL, Yang P, Scherrer R, Delyani J, McMahon E. Selective aldosterone blockade prevents angiotensin II/salt-induced vascular inflammation in the rat heart. Endocrinology. 2002;143:4828–4836. doi: 10.1210/en.2002-220120. [DOI] [PubMed] [Google Scholar]
- 12.Ward MR, Kanellakis P, Ramsey D, Funder J, Bobik A. Eplerenone suppresses constrictive remodeling and collagen accumulation after angioplasty in porcine coronary arteries. Circulation. 2001;104:467–472. doi: 10.1161/hc3001.091458. [DOI] [PubMed] [Google Scholar]
- 13.Ehsan A, McGraw AP, Aronovitz M, et al. Mineralocorticoid receptor antagonism inhibits vein graft remodeling in mice. J Thorac Cardiovasc Surg. 2012;145:1642–1649. doi: 10.1016/j.jtcvs.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keidar S, Hayek T, Kaplan M, et al. Effect of eplerenone, a selective aldosterone blocker, on blood pressure, serum and macrophage oxidative stress, and atherosclerosis in apolipoprotein E-deficient mice. Journal of Cardiovascular Pharmacology. 2003;41:955–963. doi: 10.1097/00005344-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Caprio M, Newfell BG, LaSala A, et al. Functional Mineralocorticoid Receptors in Human Vascular Endothelial Cells Regulate ICAM-1 Expression and Promote Leukocyte Adhesion. Circulation Research. 2008;102:1359–1367. doi: 10.1161/CIRCRESAHA.108.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circulation Research. 2005;96:643–650. doi: 10.1161/01.RES.0000159937.05502.d1. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27:799–805. doi: 10.1161/01.ATV.0000258414.59393.89. [DOI] [PubMed] [Google Scholar]
- 18.Newfell BG, Iyer LK, Mohammad NN. Aldosterone Regulates Vascular Gene Transcription via Oxidative Stress-Dependent And -Independent Pathways. Arterioscler Thromb Vasc Biol. 2011;31:1871–1880. doi: 10.1161/ATVBAHA.111.229070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCurley A, Jaffe IZ. Mineralocorticoid Receptors in Vascular Function and Disease. Molecular and Cellular Endocrinology. 2012;350:256–265. doi: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCurley A, Pires PW, Bender SB, et al. Direct Regulation of Blood Pressure by Smooth Muscle Cell Mineralocorticoid Receptors. Nature Medicine. 2012;18:1429–1433. doi: 10.1038/nm.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy DG, Rocha R, Funder JW. Distinguishing the antihypertensive and electrolyte effects of eplerenone. Journal of Clinical Endocrinology & Metabolism. 2004;89:2736–2740. doi: 10.1210/jc.2003-032149. [DOI] [PubMed] [Google Scholar]
- 22.Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Letters. 2006;580:2879–28870. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 23.Versari D, Lerman LO, Lerman A. The importance of reendothelialization after arterial injury. Current Pharmaceutical Design. 2007;13:1811–1824. doi: 10.2174/138161207780831239. [DOI] [PubMed] [Google Scholar]
- 24.Ishizawa K, Izawa Y, Ito H, et al. Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension. 2005;46:1046–1052. doi: 10.1161/01.HYP.0000172622.51973.f5. [DOI] [PubMed] [Google Scholar]
- 25.Parenti A, Brogelli L, Filippi S, Donnini S, Ledda F. Effect of hypoxia and endothelial loss on vascular smooth muscle cell responsiveness to VEGF-A: role of flt-1/VEGF-receptor-1. Cardiovascular Research. 2002;55:201–212. doi: 10.1016/s0008-6363(02)00326-7. [DOI] [PubMed] [Google Scholar]
- 26.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. American Journal of Physiology - Heart & Circulatory Physiology. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90:765–770. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGraw AP, Bagley J, Chen W, et al. Aldosterone Increases Early Atherosclerosis and Promotes Plaque Inflammation through a Placental Growth Factor-dependent Mechanism. J Am Heart Assoc. 2013;2:e000018. doi: 10.1161/JAHA.112.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couper LL, Bryant SR, Eldrup-Jorgensen J, Bredenberg CE, Lindner V. Vascular endothelial growth factor increases the mitogenic response to fibroblast growth factor-2 in vascular smooth muscle cells in vivo via expression of fms-like tyrosine kinase-1. Circulation Research. 1997;81:932–939. doi: 10.1161/01.res.81.6.932. [DOI] [PubMed] [Google Scholar]
- 30.Patel TV, Morgan JA, Demetri GD, et al. A preeclampsia-like syndrome characterized by reversible hypertension and proteinuria induced by the multitargeted kinase inhibitors sunitinib and sorafenib. Journal of the National Cancer Institute. 2008;100:282–284. doi: 10.1093/jnci/djm311. [DOI] [PubMed] [Google Scholar]
- 31.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–e526. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 32.Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. Journal of Clinical Endocrinology & Metabolism. 2007;92:4472–4475. doi: 10.1210/jc.2007-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.