Abstract
Glucoprivation is a strong signal for the initiation of gastrointestinal contractions. While this relationship between utilizable nutrient levels and gastric motility has been recognized for more than 100 years, the explanation of this phenomenon has remained incomplete. Using widely differing approaches, recent work has suggested that the hindbrain is responsible for this chemoreflex effect. Surprisingly, astrocytes may be the main glucodetector elements under hypoglycemic conditions. Our own work using in vitro live cell calcium imaging shows that astrocytes in the NST increase cytoplasmic calcium in a concentration dependent manner in reaction to reductions in glucose. This effect is reversed on restoration of normal glucose concentrations. In vivo single unit neurophysiological recordings show that brainstem neurons driving gastric motility are activated by glucoprivic stimuli. Studies in intact animals verify that both dorsal medullary and systemic glucoprivation significantly increases gastric motility. Astrocyte inactivation with fluorocitrate blocks the pro-motility effects of glucoprivation. Thus, it appears that intact astrocyte signaling may be essential to glucoregulatory control over gastric motility.
Keywords: counter-regulation, calcium imaging, nucleus of the solitary tract, dorsal motor nucleus of the vagus
Introduction
Nutrient levels, feeding behavior, and gastric motility have been linked in a correlational sense for more than 100 years. The relationship was heralded by William Beaumont’s direct observations of the digestive processes of Alexis St. Martin (Beaumont, 1833). Pavlov next observed that increases in gastric motility and secretion were related to periods of food deprivation and the anticipation of eating. This led to Pavlov’s proposal of a “cephalic phase” of digestion whereby stimuli, both internal [e.g., glucoprivation] and external [e.g., cues related to the expectation of feeding], were integrated by the brain to augment digestion in advance of feeding (Pavlov, 1910). In 1912, Cannon and Washburn connected food deprivation and the sensation of gastric contractions with the urge to eat (Cannon et al., 1912). Classic studies from the 1920’s through the 1990’s (Bulatao et al., 1924; Cato et al., 1990; Novin et al., 1973; Richter, 1941) have gone on to establish that glucoprivation is a robust signal for the initiation of feeding and perhaps the strongest known stimulant of gastric motility.
Given the crucial importance to life of glucose availability, it is not surprising that there is duplication of glucodetectors in both the periphery as well as within the central nervous system. The loci of glucodetection important to homeostasis are still a matter of controversy, though there is ample evidence for hepatic vagal, solitary nucleus, ventral medullary and hypothalamic sensors (Borg et al., 1999; Novin et al., 1973; Ritter et al., 2000). This distributed sensor/regulator arrangement is observed for other critical physiological parameters such as fluid volume and osmolality (Antunes-Rodrigues et al., 2004; Simard et al., 2004) and blood gases (Bianchi et al., 1995; Gourine et al., 2011).
The nucleus of the solitary tract [NST] is the most likely site for the convergence of glucodetection and the regulation of gastric motility. Several reports provide evidence for the NST as a gluco-sensory structure (Marty et al., 2005; Ritter et al., 2000) that maintains efferent connections necessary for regulating nutrient homeostasis and digestion (Adachi et al., 1995; Dallaporta et al., 1999; Dallaporta et al., 2000; Mizuno et al., 1984; Rogers et al., 2012; Yettefti et al., 1995). Additionally, the NST is the recipient of vagal afferent projections from the gut. The NST integrates this vagal input and controls gastric motility and secretion via short axon projections to the immediately subjacent dorsal motor nucleus of the vagus [DMN]. Most of these gastric “vago-vagal” motility control reflexes are inhibitory [for example, see FIGURE 1]; the result of the withdrawal of tonic excitatory vagal efferent inputs to the stomach. Additionally, vagal afferents from the gut can activate a parallel “non-adrenergic, non-cholinergic” [NANC] efferent path that causes the active inhibition of gastric smooth muscle. This NANC path is probably composed of vagal afferents that activate noradrenergic neurons in the NST that, in turn, activate the NANC path neurons in the DMN. These NANC-DMN neurons project to the gastric enteric plexus where they activate nitregic or adrenergic neurons to cause the relaxation of the stomach (Abrahamsson, 1986; Barbier et al., 1993; Rogers et al., 2012; Rogers et al., 1999; Takahashi et al., 1997).
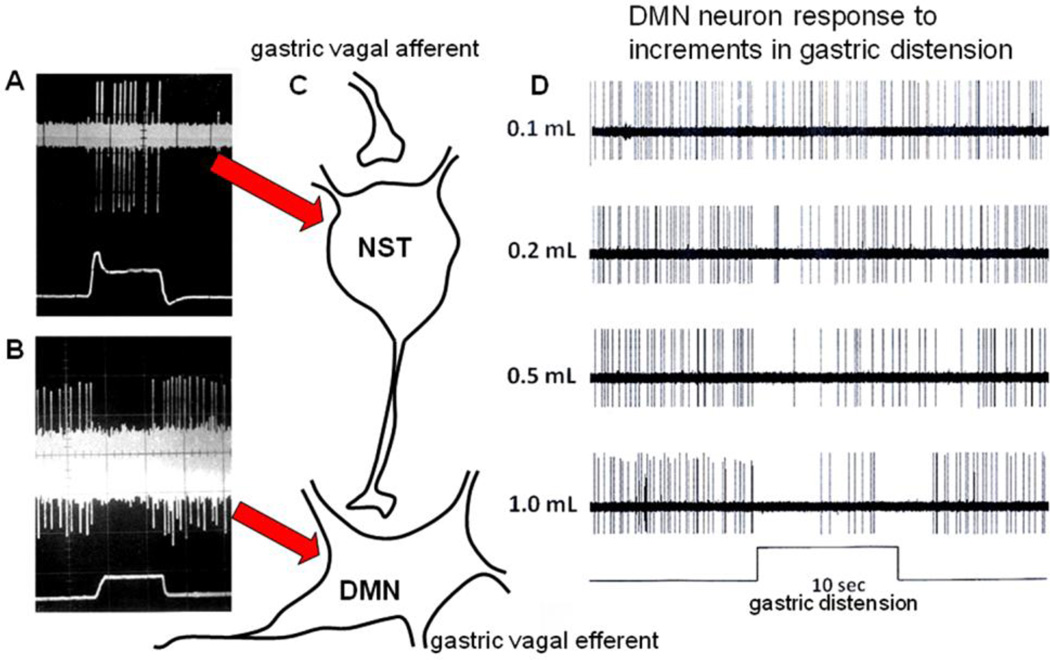
FIGURE 1.
In vivo electrophysiological recordings identifying gastric-related second order visceral sensory neurons in the nucleus of the solitary tract (NST) [A] and parasympathetic efferent neurons in the dorsal motor nucleus (DMN) [B] responding to mild distension (0.5mL) of a balloon in the gastric antrum (from McCann and Rogers, 1992). Note the characteristic, spontaneous, pacemaker activity of the DMN neuron that is interrupted by distension of the stomach (lower trace). [C] Schematic representation of the vago-vagal reflex connection responsible for the gastric accommodation reflex whereby antral distension produces relaxation of the corpus through removal of tonic vagal efferent cholinergic excitation. DMN neuronal responses to incremental changes in gastric inflation [D] show that the reflex is quite sensitive; the threshold for initiation of the reflex in the rat is approximately 0.1–0.2mL distension.
Until recently, there would have been unanimous agreement that the cells responsible for sensing, signaling, and transmitting data concerning glucose availability are neurons. However, a highly controversial and provocative paper by Nell Marty et al. (Marty et al., 2005) has thrown the primacy of neuronal glucodetection into question in favor of the astrocyte.
Astrocytes and integrated brain function
The classic neurophysiological role for the astrocyte in the control of CNS function has been seen as passive and supportive. In this view, the astrocyte controls the extracellular nutrient, metabolite, and ionic environment for the neuron, while also working to dispose of and recycle released neurotransmitters and their break-down products (Volterra et al., 2005). While astrocytes certainly perform these important functions, recent neurophysiological studies in mixed neural-glial culture suggest that astrocytes are critical regulators of neuronal excitability and synaptic efficiency (Haydon et al., 2006). Astrocytes possess a broad array of receptors for neurotransmitters, hormones, cytokines, and other agonist peptides. Agonist inputs to these astrocytic receptors can cause dramatic increases in cytoplasmic calcium (Haydon et al., 2006), which, in turn, are coupled to astrocytic release of a variety of neuroactive substances, especially glutamate and ATP.
Agonist-induced calcium signals in astrocytes can cause the delayed activation [or inhibition] of adjacent neurons (Parpura et al., 2000). Neurophysiological studies in neuronalglial co-cultures suggest, for example, that agonists acting primarily on astrocytes can generate a delayed activation signal in neurons as a result of a calcium-induced exocytosis of glutamate from the glia (Parpura et al., 2000). Depending on the circuitry under study, astrocytic glutamate release can affect neuronal excitability through action on metabotropic, NMDA, and/or AMPA receptors (Haydon et al., 2006). Again, depending on the specifics of the circuitry, these astrocyte-glutamate mediated effects can increase or decrease the post-synaptic sensitivity of neurons to presynaptic input (Fellin et al., 2006). While there is strong evidence for astrocytic glutamate release to exert significant effects on neuronal function, recent studies show that similar effects can also be produced by astrocytic release of other agonists including serine, ATP, or nitric oxide (Haydon et al., 2006).
Astrocytes are also implicated in the presynaptic regulation of afferent neuronal signal traffic. Glial release of ATP could act at a variety of pre-synaptic purinergic receptors to produce a range of circuit-specific effects (Burnstock et al., 2011; Jin et al., 2004). In particular, ATP can act at P2X3 receptors on vagal afferents within the NST to provoke significant presynaptic glutamate release [(Jin et al., 2004; Rogers et al., 2006a; Rogers et al., 2006b; Shigetomi et al., 2004); see also “Youtube” movie, search: “K5RCR”, see movie of ATP effects on vagal afferents]. In an apparent contradiction, glial release of ATP can also produce both pre- and post-synaptic inhibition following the ectoenzyme conversion of ATP to adenosine, an A1 receptor agonist (Newman, 2003). It is also possible that astrocytic release of glutamate may have significant effects at presynaptic terminals via activation of NMDA and metabotropic receptors (Czaja et al., 2006; Page et al., 2005).
Just as astrocytes can release gliotransmitters that alter neural circuit function, a plethora of neuronal afferent transmitters including norepinephrine, glutamate, GABA, acetylcholine, histamine, adenosine and ATP (Haydon et al., 2006) can regulate calcium signaling in astrocytes. It is interesting to consider the possibility that visceral afferent inputs could influence the chemosensitivity of NST astrocytes. For example, if glia serve as important chemosensory or sentinel elements for cytokines, chemokines, hormones, nutrients, etc, then neural input to NST glial cells (McDougal et al., 2011) should certainly affect their sensitivity to these physiological parameters.
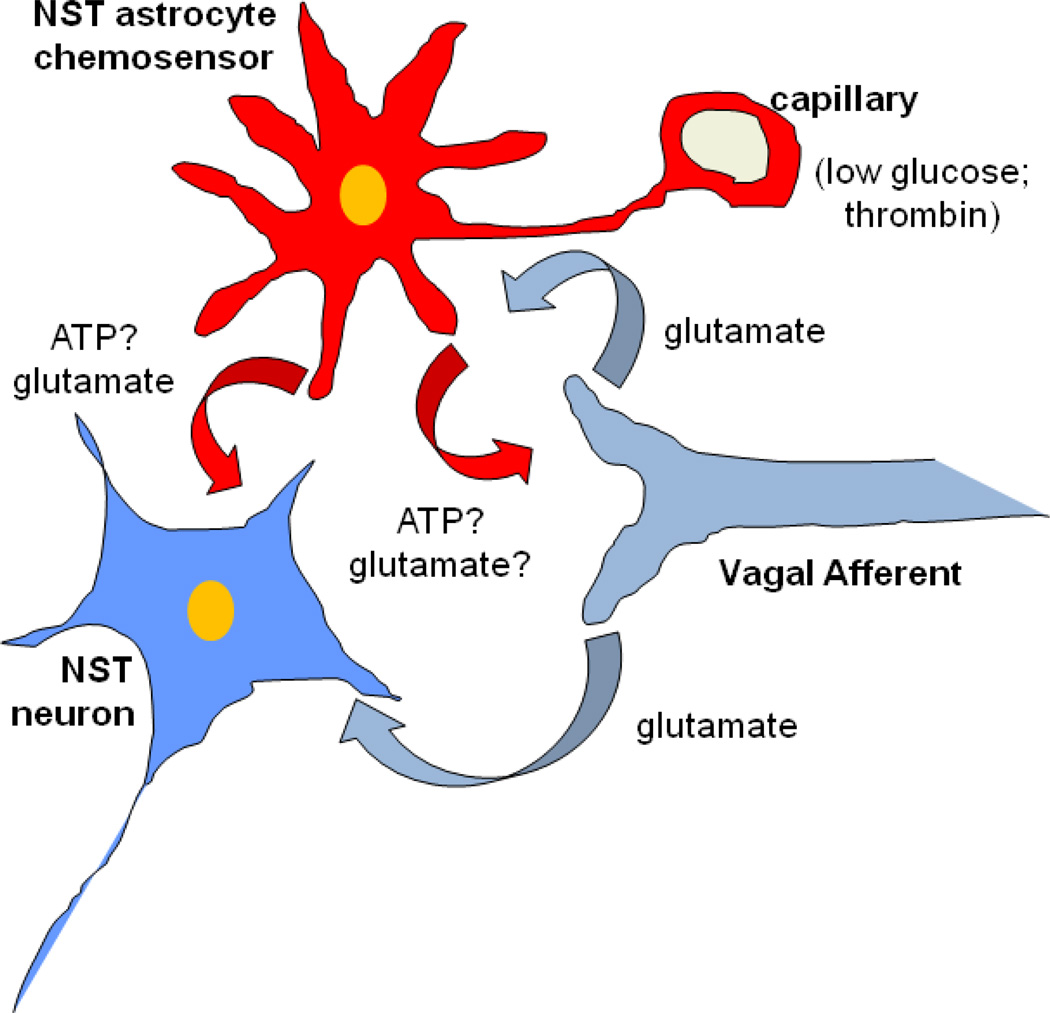
It seems likely that astrocytes, serving as local chemodetectors, can affect autonomic control through gliotransmitter release onto neuronal autonomic control circuitry (Funk, 2010; Gourine et al., 2011; Hermann et al., 2009b; McDougal et al., 2011). It is also the case, however, that NST astrocytes receive vagal afferent inputs through AMPA receptors (McDougal et al., 2011). This mutual relationship between astrocyte and neuron opens the possibility that astrocyte chemosensitivity can regulate autonomic reflexes and can, itself, be regulated by visceral afferent inputs to the NST tracking autonomic function [FIG. 2].
FIGURE 2.
Schematic representation of possible relationship between afferents, astrocytes, and NST neurons.
Data suggest that a complex relationship may exist between vagal afferents, first order neurons and astrocytes in the nucleus of the solitary tract (NST). NST-astrocytes are probably sensitive to a variety of chemical stimuli, including glucose (Marty et al., 2005), pH (Gourine et al., 2010), pO2/CO2 (Erlichman et al., 2010), and proteinases (Hermann et al., 2008b). Astrocytes probably use glutamate and ATP or adenosine to activate or inhibit NST neurons involved in regulating autonomic reflex functions like gastric motility. Recently, we have shown that NST astrocytes [in addition to neurons] receive AMPA-glutamate inputs from vagal afferents (McDougal et al., 2011). In turn, astrocytes may powerfully regulate visceral afferent input to second-order autonomic control neurons (and glia) through the release of glutamate which acts on presynaptic NMDA receptors (Ritter, 2011) and the release of ATP onto vagal afferent terminal P2X3 receptors (Jin et al., 2004). Such an arrangement could suggest the hypothesis that local and circulating agents may act directly on glia to powerfully regulate the synaptic strength of hindbrain autonomic reflexes and visceral afferent circuits such as those involved in feeding behavior. Additionally, it is hypothesized that vagal afferent input can modulate the sensitivity of glial chemosensation via glutamatergic input. Such an intertwined system would allow for maximal range of feedback and feedforward regulation of reflexes. Modified from Hermann, Van Meter, Rood, and Rogers, 2009.
ATP = adenosine triphosphate; NST = nucleus of the solitary tract.
Astrocyte interactions with gastric motility-regulating neurons in the brainstem
Our laboratory is interested in the relationship between disease and failure of autonomic control of digestion. Specifically, we have studied the connection between the activated immune system (as occurs in numerous disease processes) and digestive system failure as characterized by nearly absent gastric motility, nausea and vomiting (Hermann et al., 2008a). For example, the early, proinflammatory cytokine, tumor necrosis factor (TNF) is released by macrophages and microglia in response to infection, tumorigenesis, and radiation. Our work has established that TNF directly interacts with neural circuitry in the dorsal vagal complex of the hindbrain that regulates gastric motility. We have also shown that TNF produces a significant suppression of gastric motility and transit by increasing the sensitivity of inhibitory vago-vagal reflex circuits (Rogers et al., 2006b).
Bleeding trauma, especially closed head injury, can also produce a catastrophic failure of autonomic control, including regulation of gastric function. As seen with immune activation and TNF production, bleeding trauma causes gastrointestinal stasis (Lu et al., 1997; Young et al., 1992). A large body of clinical literature had attributed this effect to increased intracranial pressure, even though non-CNS bleeding trauma also causes gastric stasis (Berkowitz et al., 1995; Sodhi et al., 2002). With the demonstration that the gastric stasis of head injury cannot be produced by intracranial pressures typical of head injury (Garrick et al., 1988; Larson et al., 1984), it seemed likely that some function of bleeding was responsible for the reduced digestive functions following injury. One obvious suspect is thrombin, a potent protease produced by the clotting cascade. We proposed that thrombin could have direct effects on vago-vagal control circuitry by acting on an unusual class of G-protein-coupled receptor, the proteinase-activated receptors [PAR]. PARs possess an intrinsic short peptide ligand connected to the receptor with a protein “tether”. Proteinase action [e.g., thrombin] liberates the tethered ligand to act on the PAR, triggering a Gq-mediated transduction event. Earlier work (Weinstein et al., 1995) reported that PARs are constitutively expressed in the dorsal vagal complex. When we injected either thrombin or the short PAR ligand peptide into the fourth ventricle overlying the dorsal vagal complex, we observed a very significant suppression of gastric motility and transit [FIG. 3] (Hermann et al., 2009b). Given that gastric function is neurally mediated, we had assumed that PARs would be located on the neuronal components of the vago-vagal reflex; i.e., NST or DMN neurons. We were quite surprised to find that PAR type1 receptors are expressed exclusively on astrocytes and not neurons in this complex [FIG. 4] (Hermann et al., 2009b).
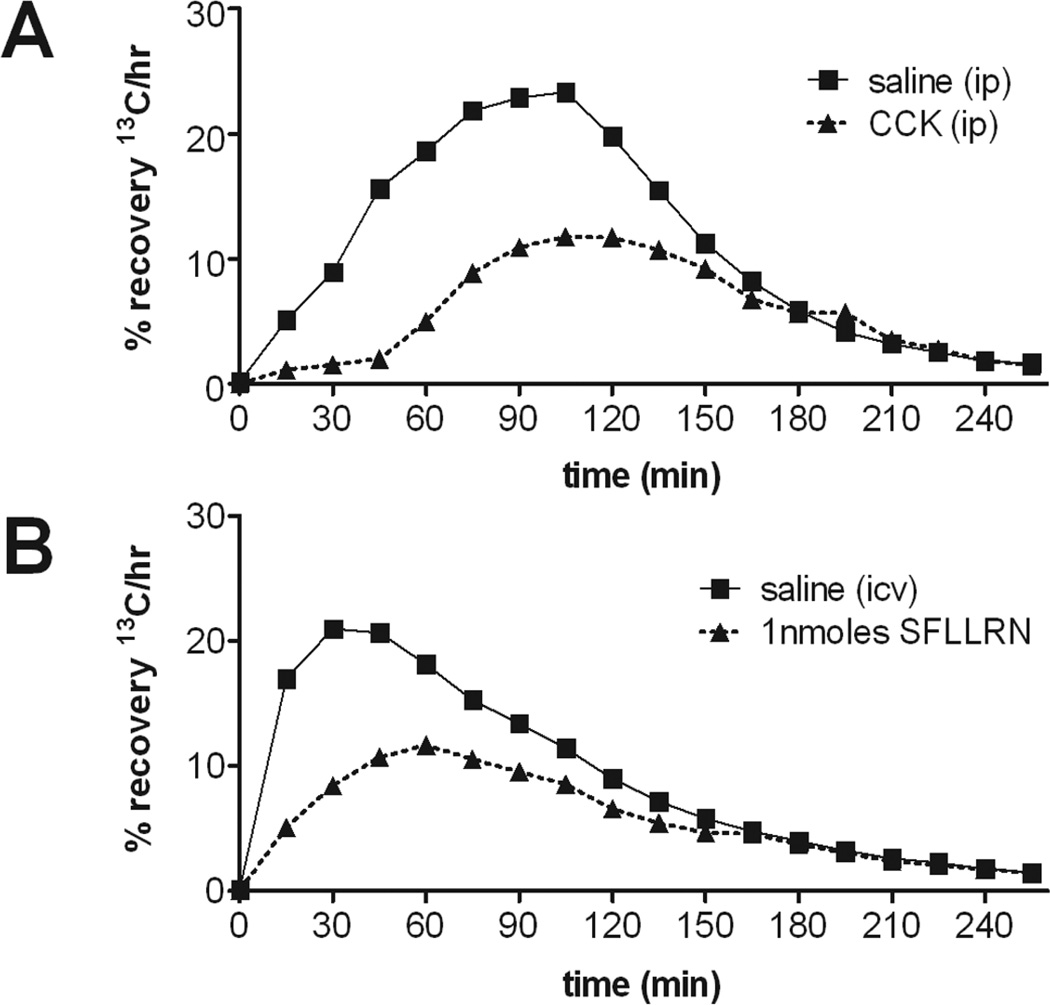
FIGURE 3.
Gastric emptying rate in an awake, freely moving animal can be monitored by determining the rate of appearance of 13C in respired CO2 that had been ingested as 13C- tagged sodium octanoate doped meal. 13C in respired CO2 is indicative of the transit of the carbohydrate meal from the stomach to the duodenum. This 13C method captures the entire time course of the transit event [samples taken at 15min intervals]. Specific parameters of this transit can be extracted for comparisons such as (i) Tlag = the time at which rate of excretion of 13CO2 is maximal, (ii) T1/2 = the gastric half-emptying time and (iii) GEC = the gastric emptying coefficient; a global index of rate of emptying [data not shown here; see original manuscript]. Each animal served as its own control; therefore, paired t-test comparisons were made between the saline control and the agonist condition for each animal. Presented here are single examples of such paired gastric transit experiments of individual animals. Microinjection of the PAR1-selective agonist SFLLRN-NH2 [1nmoles] into the fourth ventricle caused a significant reduction in gastric transit as measured by Tlag and T1/2 [B]. For comparison, the dynamics of gastric transit suppression caused by intraperitoneal injection of CCK-8 (cholecystokinin) is shown in [A]. Modified from Hermann, Van Meter, Rood, and Rogers, 2009
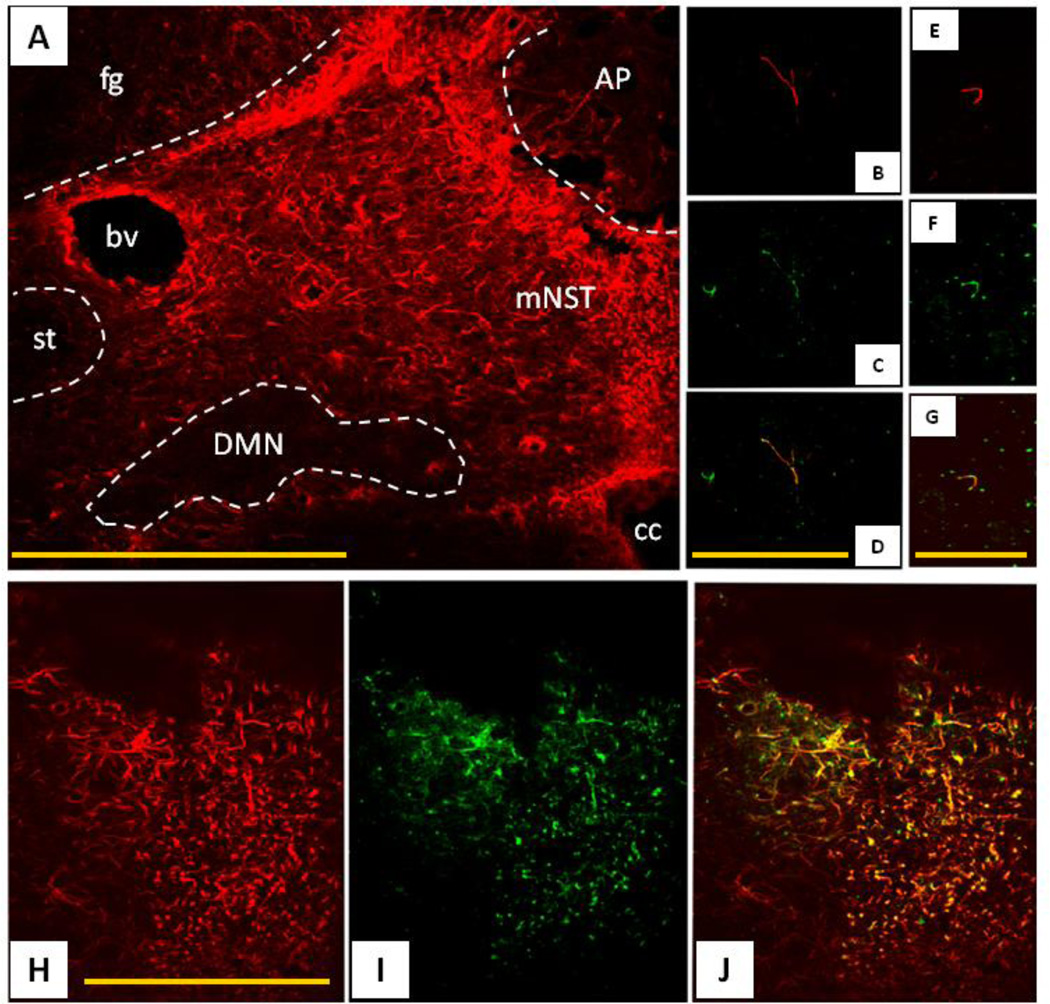
FIGURE 4.
Immunohistochemical identification of the location of proteinase-activated receptor 1 [PAR1]-positive cells in the dorsal vagal complex.
[A] Low power view of the distribution of glial fibrillary acidic protein-immunopositive [GFAP-ir = red] astrocytes in the dorsal vagal complex. Note that the medial solitary nucleus [mNST] contains substantially more astrocytes than surrounding areas, including the dorsal motor nucleus of the vagus [DMN] and the solitary tract [st].
[B–D] Single astrocytic fiber [GFAP-ir = red] in DMN possesses PAR1 receptors [PAR1-ir = green] as seen in merged image [D]. The DMN is practically devoid of astrocytic profiles, though the few that are there show co-localization with PAR1-ir.
[E–G] Single GFAP profile within the fascicles of the ST; also possesses PAR1 receptors as seen in the merged image [G]. This area shows the same pattern as seen in the DMN.
[H–J] Dense astrocytic label [GFAP-ir = red] in the mNST is extensively double labeled for PAR1-ir [green].
AP = area postrema, bv = blood vessel, cc = central canal, DMN = dorsal motor nucleus of the vagus, fg = fasciculus gracilus, mNST = medial nucleus of the solitary tract, st = solitary tract Scale bars: A = 200 microns ; B,C,D = 20microns ; E,F,G = 15microns ; H,I,J = 30microns. From Hermann, Van Meter, Rood, and Rogers 2009.
Astrocytes are electrically inexcitable and possess relatively passive electrical membrane properties dominated by strong potassium conductances (Haydon et al., 2006). This essential lack of “behavior” or responsiveness certainly lent support to the century-old theories of Cajal (Cajal, 1995) that astrocytes were important to the maintenance of neuronal function but not necessarily actively engaged in the control of neural circuits. These characteristics made the study of astrocyte signaling difficult using the classic methods available to neuroscientists. However, with the advent of modern confocal calcium imaging methods, it has been revealed that a variety of physiological stimuli provoke astrocytes to respond dynamically by producing large increases in cytoplasmic calcium (i.e., calcium waves). Using confocal live cell calcium imaging methods on in vitro brainstem slices, we have observed that NST astrocytes respond to the presence of PAR agonists with dramatic oscillatory calcium waves [FIG. 5; see also “Youtube” movie, search: “K5RCR” see movie of astrocytes]. Within a few seconds delay, NST neurons also respond with a monotonic increase in cytoplasmic calcium; an indicative of activation. The effect of PAR agonist on neuronal calcium signaling was eliminated by glutamate antagonists but not by TTX. Glial effects of PAR agonist persisted in the presence of both TTX and glutamate antagonist [FIG. 6]. These results suggest the possibility that astrocytes in the NST are capable of detecting important events [such as the presence of thrombin] and producing changes in autonomic control via direct action on vago-vagal neural control circuits in the dorsal vagal complex.
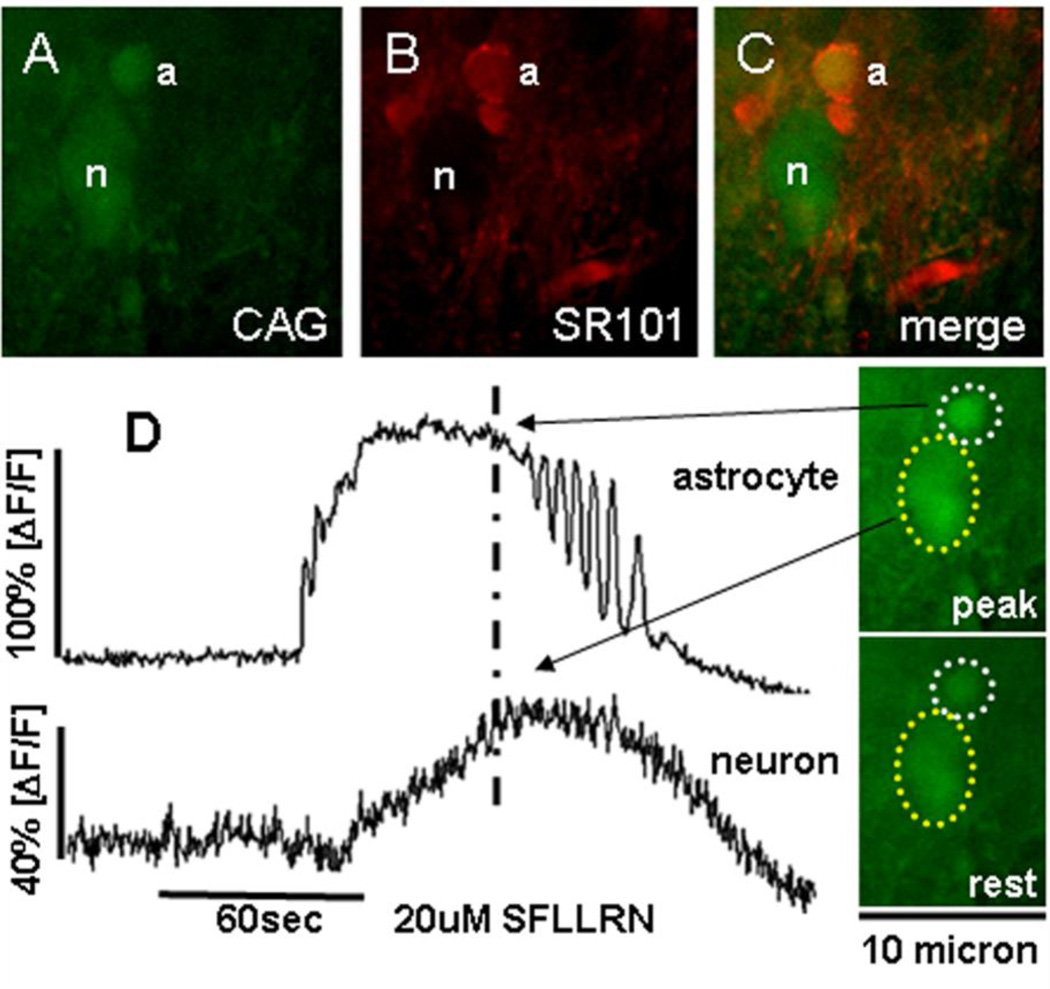
FIGURE 5.
Response patterns of astrocytes and neurons in hindbrain slice preparation to the proteinase-activated receptor ligand, SFLLRN-NH2.
[A] Calcium Green [CAG; green], is a Ca++ reporter dye that is taken up by both astrocytes (a) and neuronal cells (n) in this NST area.
[B] SR101 [red] is an astrocyte-specific vital dye used to identify astrocytes.
[C] Same field as seen in [A] and [B]; now demonstrating in vitro glial and neuronal identification. (Double fluorescence confocal images were taken through 509 and 607nm bandpass filters.)
[D] Responses of the same identified NST glial and neuron cells in A–C following 60sec bath exposure to 20uM SFLLRN-NH2. Activation of cells is seen as increased fluorescence by CAG; quantitated as percent change in fluorescence (ΔF) relative to baseline fluorescence; (ΔF/F)%. Note the delay in onset and peak of the neuronal response [bottom trace] relative to the glial response [upper trace] to SFLLRN-NH2. Raw 500nm long-pass filtered image frames on the right illustrate relative calcium levels in the astrocyte and neuron [outlined in dotted circles] at approximately the peak of the SFLLRN-NH2 response [indicated by dashed vertical line through the response curves] and at rest. Note that in the presence of glutamate antagonists [not shown here; see original manuscript Hermann, Van Meter, Rood, and Rogers 2009], the neuronal response to SFLLRN-NH2 was lost but the astrocytic response was not.
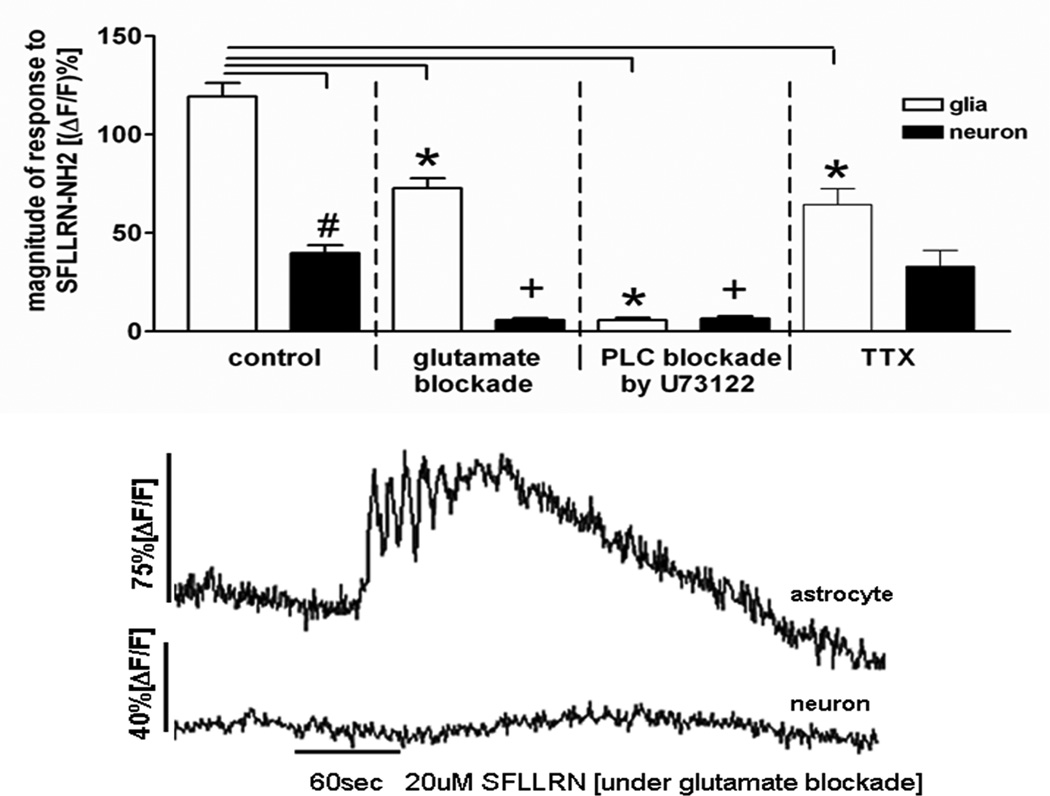
FIGURE 6.
Response patterns of astrocytes and neurons in hindbrain slice preparation to the proteinase-activated receptor ligand, SFLLRN-NH2 during blockade [i.e., glutamatergic, PLC, or TTX].
Similar to the calcium-imaging experiments described in Figure 5, laser confocal imaging of the response patterns of glia and neurons within the solitary nucleus of the in vitro slice preparation were monitored. Activation of cells is seen as increased fluorescence by CAG; quantitated as percent change in fluorescence (ΔF) relative to baseline fluorescence; (ΔF/F)%. Glia and neurons have different response patterns [e.g., time of onset and magnitude] to SFLLRN-NH2. Magnitude of response to SFLLRN-NH2 is nearly 3× greater in glia than neurons [left panel; # unpaired t-test, p<0.001].These differences are further delineated in the presence of a glutamate antagonist cocktail [kynurenate, MK 801, plus AP3] which abolished the neuronal “response” to SFLLRN-NH2, while the glial response appeared only slightly muted [bar graph and raw data trace]. When pretreated with the PLC inhibitor, U73122, for 10min prior to the SFLLRN-NH2 challenge, PLC inhibition abolished the SFLLRN-NH2 response in both glia and, in turn, neurons. Lastly, when SFLLRN-NH2 was applied in the presence of TTX, the fundamental response to SFLLRN-NH2 in both glia and neurons was still seen. Unlike the glutamate blockade condition, where the neuronal response was essentially abolished, under TTX conditions [which blocks action potential dependent transmitter release], the neuronal response was not affected. These results suggest that the glia are the primary targets of SFLLRN-NH2; that neurons are subsequently activated by glutamate release from activated glia; and that glia may be under tonic vagal afferent influence (McDougal et al., 2011). [*ANOVA glial response, Dunnett’s post test p<0.001; +ANOVA neuronal response, Dunnett’s post test p<0.001]. From Hermann, Van Meter, Rood, and Rogers 2009.
Astrocytes as potential hypoglycemic glucosensors
The NST, as a hindbrain structure, is commonly associated with the central detection of hypoglycemia. Systemic administration of the glucoprivic compound 2-deoxy-glucose (2-DG; glucose analog commonly is used to mimic conditions of low intracellular glucose availability (Ritter et al., 1978)) evokes increased c-fos expression in the NST of rats (Ritter et al., 1998). Focal administration of a similar glucoprivic agent (5-thio-D-glucose) directly into the NST of rats also drives counter regulatory responses such as increased food intake (Ritter et al., 2000) and increases in serum glucagon and stress hormone levels (Andrew et al., 2007). It is of interest to note that there is also strong evidence suggesting astrocytic involvement in the detection of low glucose availability. For example, systemic administration of the selective glial toxin (methionine sulfoximine) blocks 2-DG induced c-fos expression in NST neurons (Young et al., 2000). In studies by Marty and colleagues (Marty et al., 2005), transgenic mice with type II glucose transporter (GLUT2)-knocked out, demonstrated defects in hypoglycemic counter-regulation. Selective re-expression of GLUT2 in astrocytes, but not neurons, rescued the normal counter-regulation response in these knockout mice. These observations are not as unusual as they may first appear. As described above, we have observed other circumstances where it appears that astrocytes are functioning as sensors to, ultimately, drive neural responses with regard to digestive control (Hermann et al., 2008a; Hermann et al., 2009a; Hermann et al., 2008b). Thus, the possibility exists that NST astrocytes are directly responsive to glucoprivation.
Using in vitro live cell calcium imaging methods, we have monitored changes in intracellular calcium levels in NST astrocytes during low glucose and glucoprivic challenges. Coronal brainstem slices were imaged over the course of 30min. Changes in intracellular calcium levels were measured as time-lapse changes in the fluorescence of the calcium indicator dye, Calcium Green-1AM. Slices were exposed to a low glucose challenge [e.g. 0.5mM glucose] or glucoprivic challenge [5mM 2-DG in 5mM glucose] which was preceded and followed by periods of normal glucose conditions [5mM glucose]. Our preliminary evidence (McDougal et al., 2012) suggests that a subset of NST astrocytes respond to both low glucose and blocked glucose utilization challenges. The majority of responsive astrocytes displayed an increase in cytoplasmic calcium levels which lasted for the duration of the glucoprivic stimulus [FIG. 7]. This activation was preserved in the presence of TTX, therefore eliminating the possibility that the astrocytic response was driven secondarily to activation of glucosensitive neurons. Thus, these results suggest that NST astrocytes are intrinsically capable of signaling conditions of low glucose availability.
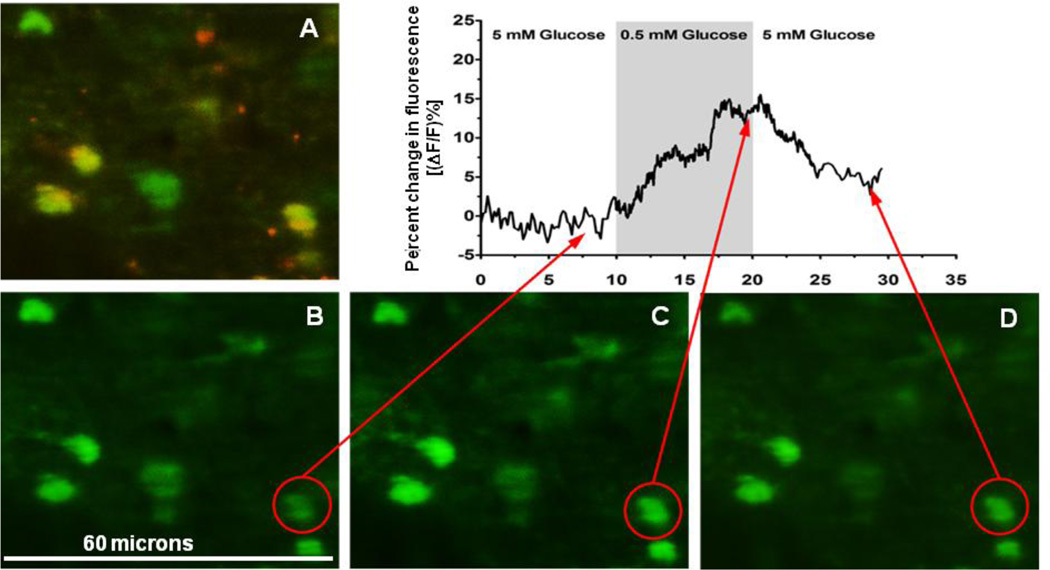
FIGURE 7.
Illustration of an in vitro slice recording of calcium signals in astrocytes in the NST.
[A] Photomicrograph showing SR101 vital staining identification of astrocytes in the NST. Calcium-green labels all CNS cells [green] while SR101 [red] is specific to astrocytes.
[B] Single frame of calcium-green filled cells responding to normal glucose levels of perfusion [control].
[C] Same cells as seen in [A] and [B]; frame captured at the peak of the response to low glucose which increases astrocyte calcium.
[D] Same cells after the restoration of normal glucose.
Dynamics of the change in fluorescence of the astrocyte circled in red in frames B–D are plotted above the photomicrographs. Notice that two other astrocytes to the left also increase cytoplasmic glucose in response to low glucose.
Astrocytes as necessary regulators of gastric function in hypoglycemia?
Data collected over almost 100 years have shown that low availability of nutrients, particularly glucose, produces a profound increase in gastric motility (Bulatao et al., 1924; Cato et al., 1990). We have already reviewed that astrocytes in the hindbrain have been implicated as necessary glucosensors (Marty et al., 2005) and the preliminary data we presented above provides physiological evidence that astrocytes in the NST are sensitive to both low glucose levels and low glucose availability. Previously, we have shown that astrocytes activated by PAR agonists have significant effects to activate NST neurons and, ultimately, cause suppression of gastric transit (Hermann et al., 2009b). Therefore, we hypothesize that astrocytic detection of hypoglycemic conditions may influence control of gastric motility.
Our preliminary studies (McDougal et al., 2012) in anesthetized rats that have been instrumented with miniature gastric strain gauges (Rogers et al., 1987), we show that application of 2-deoxyglucose [2DG; a competitive blocker of glucose utilization] to the floor of the fourth ventricle significantly elevated gastric motility [FIG 8], similar to that reported by Cato (Cato et al., 1990). The Krebs’ cycle inhibitor, fluorocitrate [FC], which is preferentially taken up by glia (Hassel et al., 1992), blocks the production of tricarboxylic acid cycle intermediates and, therefore, transiently suppresses their signaling activity (Erlichman et al., 1998; Swanson et al., 1994). FC had no effect of its own on gastric motility when applied to the floor of the fourth ventricle; however, FC blocked the effect of 2DG to increase motility. Even more impressive was the effect of insulin-induced hypoglycemia to activate gastric motility. As reported earlier by Cato (Cato et al., 1990), subcutaneous administration of insulin [10U/kg] produced a dramatic increase in gastric motility. Fourth ventricular FC substantially reduced this peripheral insulin-induced gastric hypermotility [FIG 8; (McDougal et al., 2012)].
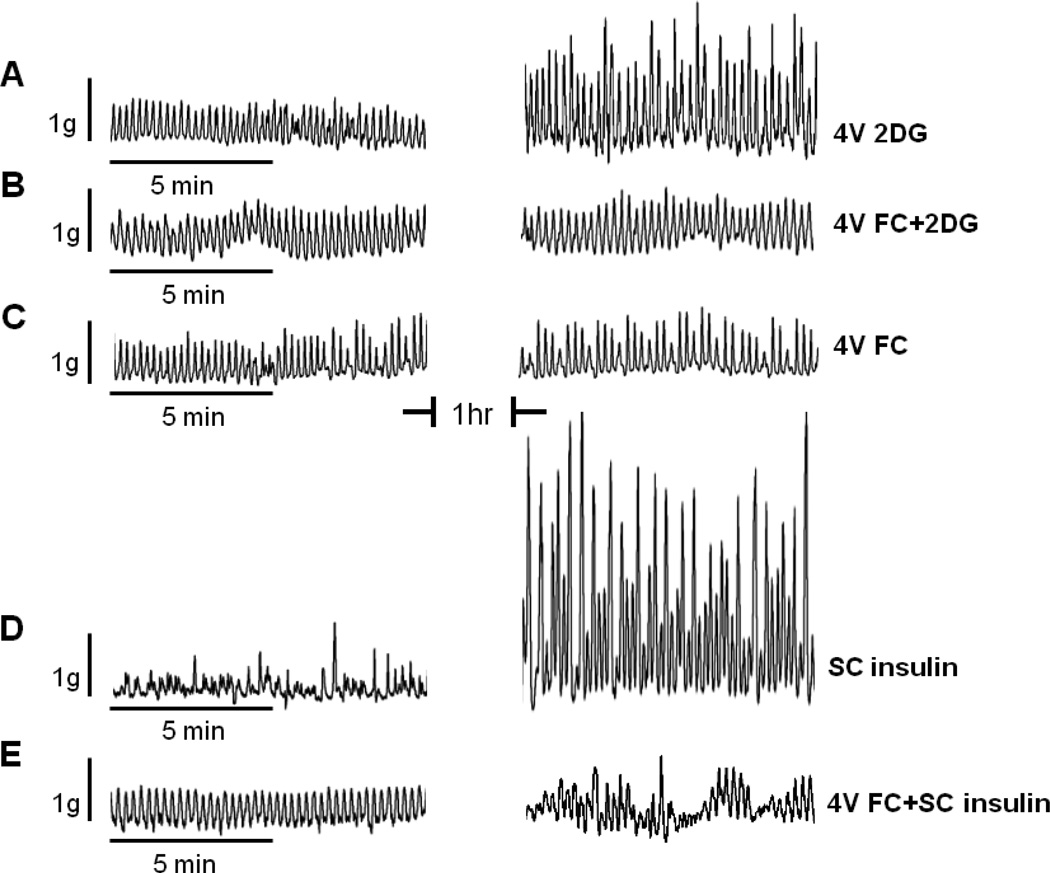
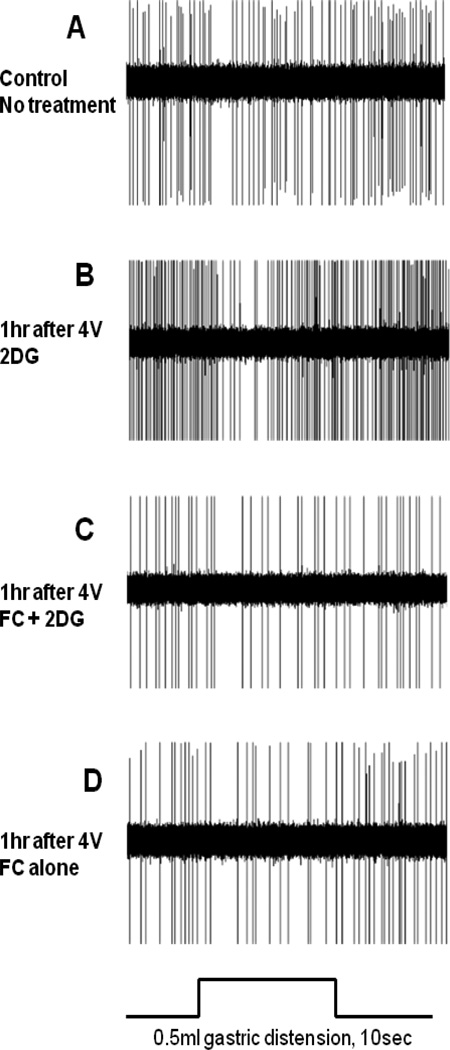
FIGURE 8.
Demonstration of the effect of fluorocitrate [FC] to block increases in gastric motility evoked by glucoprivic stimuli in the anesthetized rat. Plots on the left are gastric strain gauge recordings taken before any experimental manipulation. Plots on the right are strain gauge recordings one hour after the application of a stimulus. [A] 2DG applied to the floor of the fourth ventricle [4V 2DG] caused a substantial increase in gastric motility. [B] Fluorocitrate, an astrocyte-specific metabolic antagonist, applied to the fourth ventricle before 2DG [4V FC+2DG] blocked the 2DG effect. [C] FC applied to the fourth ventricle [4V FC] alone had no effect on basal gastric motility pattern. [D] Subcutaneous insulin produced a dramatic increase in gastric motility [SC insulin]. [E] Fourth ventricular FC largely blocked the effects of insulin to increase gastric motility. These data suggest that astrocytes in the hindbrain detect glucoprivic conditions and can activate vagal pro-motility circuits.
2DG = 2-deoxy-d-glucose; 4V = fourth ventricular; FC = fluorocitrate; SC = subcutaneous
Gastric-DMN (i.e., vagal efferent neurons) activity drives enteric cholinergic excitation of gastric smooth muscle (Grundy et al., 2002; Schemann et al., 1992). In vivo electrophysiological recordings made from physiologically identified gastric reflex DMN neurons suggest how FC might change the function of basic gastric regulatory circuits. Our preliminary results show that application of 2DG to the fourth ventricle causes a substantial increase in the basal firing rate of vagal efferent neurons. While FC, alone, has no effect to alter basal firing of DMN neurons, this gliotoxin blocks the 2DG-DMN activation effect. Interestingly, it appears that the basic reflex function of DMN neurons remains intact [FIG. 9]. That is, mild balloon distension of the antrum elicits the well recognized reduction in spontaneous DMN activity that is the result of the action of the gastric accommodation reflex; this reflex involves vagal afferent activation of NST neurons and their reflex inhibition of the DMN (Rogers et al., 2012; Viard et al., 2012). However, 2DG-mediated increases in DMN basal activity are sufficiently large to override the underlying inhibitory action of the reflex, leaving DMN-commanded increases in motility intact [FIG. 9].
FIGURE 9.
Neurophysiological recordings made from gastric-DMN neurons.
Relative to control conditions [A], the primary effect of cellular glucoprivation [4V 2DG] was an increase in the basal firing rate of DMN neurons identified as part of the gastric accommodation reflex [B]. Application of the transient gliotoxin, fluorocitrate [4V FC], prior to 4V 2DG, blocked the effects of cellular glucoprivation to increase the excitability of gastric-DMN neurons [C]. Presence of FC alone in the 4V [D] did not appear to affect the basal firing rate of gastric-DMN neurons.
Mild balloon distension [0.5ml] of the gastric antrum elicits a vagal afferent-NST-DMN neuron reflex characterized by a rapid reduction in DMN firing [see also FIG 1]. This reduction in DMN firing rate causes an immediate reduction in gastric motility referred to as gastric accommodation reflex. With the elevated basal activity of DMN neurons in response to 4V 2DG, we can explain the increased motility observed in FIG 8A. Thus, although gastric distension still elicits a drop in DMN firing rate, the reflex is dwarfed relative to the dramatic increase in basal DMN firing caused by exposure to 2DG.
Discussion
Taken together, previously published work and our preliminary data suggest that astrocytic detection of glucoprivation is necessary for the increase in gastric motility provoked by hypoglycemia (Bulatao et al., 1924; Cato et al., 1990). Evidence has been provided that astrocytes could be necessary to the initiation of hypoglycemia-induced autonomic changes organized by the hindbrain (Marty et al., 2005; Young et al., 2000). Astrocytes in the hindbrain appear capable of driving local neuronal circuits in the NST to trigger these changes in gastric motility (Hermann et al., 2009b). Our recent pilot data suggests that NST astrocytes, indeed, dramatically increase cytoplasmic calcium in response to reductions in glucose (McDougal et al., 2012). Such increases in cytoplasmic calcium are necessary triggers for gliotransmitter release (Verkhratsky et al., 2012). Work in progress suggests that FC blockade of astrocyte activity blocks the large increases in motility produced by different models of glucoprivation. Complementary in vivo electrophysiological recording studies suggest that astrocyte-mediated increases in gastric motility occur via the activation of gastric efferent DMN neurons. While our preliminary data suggest a necessary role for the NST astrocyte in autonomic adjustments to glucose availability, there are a number of issues that will need to be resolved before we have a mechanistic understanding of this connection.
For example, it is not immediately clear how the reduction in glucose availability signals an increase in cytoplasmic calcium in the NST astocyte. While astrocytic expression of GLUT2 appears necessary for proper glucose counter-regulation, the transduction mechanisms often coupled to GLUT2 produce changes in calcium that run in the opposite direction to that observed for astrocytes That is, an increase in extracellular glucose produces increases in intracellular calcium in pancreatic beta cells and neurons (Holsbeeks et al., 2004; Levin et al., 2004; Schuit et al., 2001). However, others have reported that severe glucose restriction in cultured astrocytes causes an increase in astrocytic cytoplasmic calcium signal (Arnold, 2005). Similar to our observations in the brain slice preparation, this increase in cytoplasmic calcium is reversed with the restoration of normal bath glucose. There is some evidence suggesting that astrocytes are highly dependent on glycolysis for ATP production (Kahlert et al., 2000). Removal of glucose or blocking glucose utilization may transiently starve astrocytes of glucose for ATP production. Mitochondrial-derived ATP [i.e., tricarboxcylic acid cycle] does not ameliorate the effect of the ATP loss due to the interruption in glycolysis. With impaired glycolysis, the calcium-ATPase pump in the endoplasmic reticulum [ER] of astrocytes fails and ER calcium is released to the cytoplasm (Arnold, 2005; Kahlert et al., 2000). While these results are basically consistent with our observations in the slice preparation, it is not clear if this mechanism is responsible for the cytoplasmic calcium signal seen by our laboratory in in situ NST astrocytes. For example, the response time for astrocytes in the NST slice preparation is about five-fold faster than that reported for cultured cortical astrocytes. This would argue for a much more active low-glucose detection mechanism for astrocytes located in the NST.
This raises the interesting possibility that GLUT2 may be directly mediating the astrocytic activation observed during conditions of low glucose availability. GLUT2 is the only mammalian glucose transporter known to act as a “transceptor”, i.e., a protein which functions as both a transporter and receptor. The receptor function of mammalian GLUT2 is mediated by the large intracellular loop between the 6th and 7th transmembrane domains (Guillemain et al., 2000). Blocking the receptor function of GLUT2 by overexpression of the intracellular loop domain in transgenic mice leads to altered glucose homeostasis (Stolarczyk et al., 2007) and feeding behavior (Stolarczyk et al., 2010). The transporter function of GLUT2 has been linked to the activation of hepatic transcription factor [also known as a carbohydrate response element binding protein (ChREBP), (Uyeda et al., 2006)]. However, it seems unlikely that any GLUT2-mediated transcriptional regulation in NST astrocytes could bring about the rapid changes in intracellular calcium signaling observed in response to low glucose availability. Instead, it seems more likely that there may be a direct interaction between the intracellular loop domain of GLUT2 and signaling pathways present in NST astrocytes. At present, there is no information available as to how it might function.
It is also not clear how astrocyte activation can increase vagal efferent excitability that, in turn, causes an increase in gastric motility. Recall that PAR activation of NST astrocytes causes a release of glutamate and, ultimately, suppression of gastric motility through action on NST neurons (Hermann et al., 2009b). NST neurons are the principal source of control over the vagal efferent DMN neurons that control the stomach (Rogers et al., 2012) and distension-induced inhibition of DMN neurons is mediated by glutamate-induced excitation of gastric-NST neurons (Zhang et al., 2003). Therefore, some other mechanism must connect those NST astrocytes that are activated by low glucose with the NST-DMN circuitry that, ultimately and powerfully, increase gastric motility.
Third, it is not at all clear how astrocytic glucodetectors interact with glucose sensing neurons in the NST. One possibility is that the NST neurons controlled by glucose sensing astrocytes may control gastric motility while NST neurons with intrinsic glucosensitivity may control other functions, such as the onset of feeding. Another possibility is that neurons that are apparently glucose sensitive are, in fact, functionally dependent on adjacent astrocytes in order to maintain their sensitivity. In this case, fluorocitrate may eliminate intrinsic astrocytic glucosensitivity which, in turn, also depresses the glucosensitivity of adjacent neurons.
To paraphrase Cajal’s comments at the end of his chapter on neuroglia (Cajal, 1995) “it is entirely possible that these astrocytes can regulate a variety of autonomic functions although we have absolutely no idea how this might occur”. Fortunately, the advent of in vitro cellular calcium imaging should make investigations into these questions feasible.
Acknowledgments
This work was supported by NIH grants NS52142, DK56373, HD47643, and NS60664.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamsson H. Non-adrenergic non-cholinergic nervous control of gastrointestinal motility patterns. Arch Int Pharmacodyn Ther. 1986;280:50–61. [PubMed] [Google Scholar]
- Adachi A, Kobashi M, Funahashi M. Glucose-responsive neurons in the brainstem. Obes Res. 1995;3(Suppl 5):735S–740S. doi: 10.1002/j.1550-8528.1995.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1792–R1798. doi: 10.1152/ajpregu.00777.2006. [DOI] [PubMed] [Google Scholar]
- Antunes-Rodrigues J, de Castro M, Elias LL, Valenca MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- Arnold S. Estrogen suppresses the impact of glucose deprivation on astrocytic calcium levels and signaling independently of the nuclear estrogen receptor. Neurobiol Dis. 2005;20:82–92. doi: 10.1016/j.nbd.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Barbier AJ, Lefebvre RA. Involvement of the L-arginine: nitric oxide pathway in nonadrenergic noncholinergic relaxation of the cat gastric fundus. J Pharmacol Exp Ther. 1993;266:172–178. [PubMed] [Google Scholar]
- Beaumont W. Experiments & observations on the gastric juice. Plattsburgh: F.P. Allen; 1833. [DOI] [PubMed] [Google Scholar]
- Berkowitz N, Schulman LL, McGregor C, Markowitz D. Gastroparesis after lung transplantation. Potential role in postoperative respiratory complications. Chest. 1995;108:1602–1607. doi: 10.1378/chest.108.6.1602. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Borg MA, Borg WP, Tamborlane WV, Brines ML, Shulman GI, Sherwin RS. Chronic hypoglycemia and diabetes impair counterregulation induced by localized 2-deoxyglucose perfusion of the ventromedial hypothalamus in rats. Diabetes. 1999;48:584–587. doi: 10.2337/diabetes.48.3.584. [DOI] [PubMed] [Google Scholar]
- Bulatao E, Carlson A. Contributions to the physiology of the stomach: influence of experimental changes in blood sugar level on gastric hunger contractions. Am J Physiol. 1924;69:107–115. [Google Scholar]
- Burnstock G, Fredholm BB, Verkhratsky A. Adenosine and ATP receptors in the brain. Curr Top Med Chem. 2011;11:973–1011. doi: 10.2174/156802611795347627. [DOI] [PubMed] [Google Scholar]
- Cajal SRy. Histology of the nervous system. New York: Oxford University Press; 1995. [Google Scholar]
- Cannon WB, Washburn AL. An Explanation of Hunger. Am J Physiol. 1912;29:441–454. [Google Scholar]
- Cato RK, Flanagan LM, Verbalis JG, Stricker EM. Effects of glucoprivation on gastric motility and pituitary oxytocin secretion in rats. Am J Physiol. 1990;259:R447–R452. doi: 10.1152/ajpregu.1990.259.3.R447. [DOI] [PubMed] [Google Scholar]
- Czaja K, Ritter RC, Burns GA. N-methyl-D-aspartate receptor subunit phenotypes of vagal afferent neurons in nodose ganglia of the rat. J Comp Neurol. 2006;496:877–885. doi: 10.1002/cne.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaporta M, Himmi T, Perrin J, Orsini JC. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport. 1999;10:2657–2660. doi: 10.1097/00001756-199908200-00040. [DOI] [PubMed] [Google Scholar]
- Dallaporta M, Perrin J, Orsini JC. Involvement of adenosine triphosphate-sensitive K+ channels in glucose-sensing in the rat solitary tract nucleus. Neurosci Lett. 2000;278:77–80. doi: 10.1016/s0304-3940(99)00898-8. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC. Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol. 2010;108:1803–1811. doi: 10.1152/japplphysiol.01321.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- Fellin T, Sul JY, D'Ascenzo M, Takano H, Pascual O, Haydon PG. Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP. Novartis Found Symp. 2006;276:208–217. doi: 10.1002/9780470032244.ch16. discussion 217–221, 233–237, 275–281. [DOI] [PubMed] [Google Scholar]
- Funk GD. The 'connexin' between astrocytes, ATP and central respiratory chemoreception. J Physiol. 2010;588:4335–4337. doi: 10.1113/jphysiol.2010.200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick T, Mulvihill S, Buack S, Maeda-Hagiwara M, Tache Y. Intracerebroventricular pressure inhibits gastric antral and duodenal contractility but not acid secretion in conscious rabbits. Gastroenterology. 1988;95:26–31. doi: 10.1016/0016-5085(88)90286-7. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasparov S. Astrocytes as brain interoceptors. Exp Physiol. 2011;96:411–416. doi: 10.1113/expphysiol.2010.053165. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D, Schemann M. Motor control of the stomach. In: Brookes S, Costa M, editors. Innervation of the gastrointestinal tract. Vol. 14. London, UK: Taylor and Francis; 2002. pp. 57–102. [Google Scholar]
- 27.Guillemain G, Loizeau M, Pincon-Raymond M, Girard J, Leturque A. The large intracytoplasmic loop of the glucose transporter GLUT2 is involved in glucose signaling in hepatic cells. J Cell Sci. 2000;113(Pt 5):841–847. doi: 10.1242/jcs.113.5.841. [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res. 1992;576:120–124. doi: 10.1016/0006-8993(92)90616-h. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNFalpha: a trigger of autonomic dysfunction. Neuroscientist. 2008a;14:53–67. doi: 10.1177/1073858407305725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC. TNF activates astrocytes and catecholaminergic neurons in the solitary nucleus: implications for autonomic control. Brain Res. 2009a;1273:72–82. doi: 10.1016/j.brainres.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Van Meter MJ, Rogers RC. CXCR4 receptors in the dorsal medulla: implications for autonomic dysfunction. Eur J Neurosci. 2008b;27:855–864. doi: 10.1111/j.1460-9568.2008.06058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann GE, Van Meter MJ, Rood JC, Rogers RC. Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. J Neurosci. 2009b;29:9292–9300. doi: 10.1523/JNEUROSCI.6063-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsbeeks I, Lagatie O, Van Nuland A, Van de Velde S, Thevelein JM. The eukaryotic plasma membrane as a nutrient-sensing device. Trends Biochem Sci. 2004;29:556–564. doi: 10.1016/j.tibs.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert S, Reiser G. Requirement of glycolytic and mitochondrial energy supply for loading of Ca(2+) stores and InsP(3)-mediated Ca(2+) signaling in rat hippocampus astrocytes. J Neurosci Res. 2000;61:409–420. doi: 10.1002/1097-4547(20000815)61:4<409::AID-JNR7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Larson GM, Koch S, O'Dorisio TM, Osadchey B, McGraw P, Richardson JD. Gastric response to severe head injury. Am J Surg. 1984;147:97–105. doi: 10.1016/0002-9610(84)90041-2. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- Lu WY, Rhoney DH, Boling WB, Johnson JD, Smith TC. A review of stress ulcer prophylaxis in the neurosurgical intensive care unit. Neurosurgery. 1997;41:416–425. doi: 10.1097/00006123-199708000-00017. discussion 425-416. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, Binnert C, Beermann F, Thorens B. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J.Physiol. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci. 2011;31:14037–14045. doi: 10.1523/JNEUROSCI.2855-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal DH, Rogers RC, Hermann GE. Astrocytes as glucosensors in the solitary nucleus, Society for Neuroscience. New Orleans, LA: 2012. [Google Scholar]
- Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Novin D, VanderWeele DA, Rezek M. Infusion of 2-deoxy-D-glucose into the hepatic-portal system causes eating: evidence for peripheral glucoreceptors. Science. 1973;181:858–860. doi: 10.1126/science.181.4102.858. [DOI] [PubMed] [Google Scholar]
- Page AJ, Young RL, Martin CM, Umaerus M, O'Donnell TA, Cooper NJ, Coldwell JR, Hulander M, Mattsson JP, Lehmann A, Blackshaw LA. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology. 2005;128:402–410. doi: 10.1053/j.gastro.2004.11.062. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. The work of the digestive glands, Vol. Charles Griffin. 2nd ed. Philadelphia, PA: 1910. The centrifugal (efferent) nerves to the gastric glands and of the pancreas; pp. 48–59. [Google Scholar]
- Richter CP. Increased dextrose appetite of normal rats treated with insulin. Am J Physiol. 1941;135:781–787. [Google Scholar]
- Ritter RC. A Tale of Two Endings: Modulation of Satiation by NMDA Receptors On or Near Central and Peripheral Vagal Afferent Terminals. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Roelke M, Neville M. Glucoprivic feeding behavior in absence of other signs of glucoprivation. Am J Physiol. 1978;234:E617–E621. doi: 10.1152/ajpendo.1978.234.6.E617. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- Ritter S, Llewellyn-Smith I, Dinh TT. Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res. 1998;805:41–54. doi: 10.1016/s0006-8993(98)00655-6. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Oxytocin, oxytocin antagonist, TRH, and hypothalamic paraventricular nucleus stimulation effects on gastric motility. Peptides. 1987;8:505–513. doi: 10.1016/0196-9781(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Brainstem control of gastric function. In: Johnson LR, editor. Physiology of the gastrointestinal tract. Fourth ed. Elsevier Academic Press; 2012. pp. 861–892. [Google Scholar]
- Rogers RC, Hermann GE, Travagli RA. Brainstem pathways responsible for oesophageal control of gastric motility and tone in the rat. J.Physiol. 1999;514(Pt 2):369–383. doi: 10.1111/j.1469-7793.1999.369ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, Nasse JS, Hermann GE. Live-cell imaging methods for the study of vagal afferents within the nucleus of the solitary tract. J Neurosci Methods. 2006a;150:47–58. doi: 10.1016/j.jneumeth.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Van Meter MJ, Hermann GE. Tumor necrosis factor potentiates central vagal afferent signaling by modulating ryanodine channels. J Neurosci. 2006b;26:12642–12646. doi: 10.1523/JNEUROSCI.3530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Grundy D. Electrophysiological identification of vagally innervated enteric neurons in guinea pig stomach. Am J Physiol. 1992;263:G709–G718. doi: 10.1152/ajpgi.1992.263.5.G709. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50:1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- Shigetomi E, Kato F. Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci. 2004;24:3125–3135. doi: 10.1523/JNEUROSCI.0090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Sodhi SS, Guo JP, Maurer AH, O'Brien G, Srinivasan R, Parkman HP. Gastroparesis after combined heart and lung transplantation. J.Clin.Gastroenterol. 2002;34:34–39. doi: 10.1097/00004836-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Stolarczyk E, Guissard C, Michau A, Even PC, Grosfeld A, Serradas P, Lorsignol A, Penicaud L, Brot-Laroche E, Leturque A, Le Gall M. Detection of extracellular glucose by GLUT2 contributes to hypothalamic control of food intake. Am J Physiol Endocrinol Metab. 2010;298:E1078–E1087. doi: 10.1152/ajpendo.00737.2009. [DOI] [PubMed] [Google Scholar]
- Stolarczyk E, Le Gall M, Even P, Houllier A, Serradas P, Brot-Laroche E, Leturque A. Loss of sugar detection by GLUT2 affects glucose homeostasis in mice. PLoS One. 2007;2:e1288. doi: 10.1371/journal.pone.0001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Graham SH. Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res. 1994;664:94–100. doi: 10.1016/0006-8993(94)91958-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J.Physiol. 1997;504(Pt 2):479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Parpura V. Calcium signalling in astroglia. Mol Cell Endocrinol. 2012;353:45–56. doi: 10.1016/j.mce.2011.08.039. [DOI] [PubMed] [Google Scholar]
- Viard E, Rogers RC, Hermann GE. Systemic cholecystokinin amplifies vago-vagal reflex responses recorded in vagal motor neurones. J Physiol. 2012;590:631–646. doi: 10.1113/jphysiol.2011.224477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J.Neurosci. 1995;15:2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yettefti K, Orsini JC, el Ouazzani T, Himmi T, Boyer A, Perrin J. Sensitivity of nucleus tractus solitarius neurons to induced moderate hyperglycemia, with special reference to catecholaminergic regions. J Auton Nerv Syst. 1995;51:191–197. doi: 10.1016/0165-1838(94)00130-c. [DOI] [PubMed] [Google Scholar]
- Young B, Ott L, Yingling B, McClain C. Nutrition and brain injury. J.Neurotrauma. 1992;9(Suppl 1):S375–S383. [PubMed] [Google Scholar]
- Young JK, Baker JH, Montes MI. The brain response to 2-deoxy glucose is blocked by a glial drug. Pharmacol Biochem Behav. 2000;67:233–239. doi: 10.1016/s0091-3057(00)00315-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Fogel R. Involvement of glutamate in gastrointestinal vago-vagal reflexes initiated by gastrointestinal distention in the rat. Auton Neurosci. 2003;103:19–37. doi: 10.1016/s1566-0702(02)00145-5. [DOI] [PubMed] [Google Scholar]