Abstract
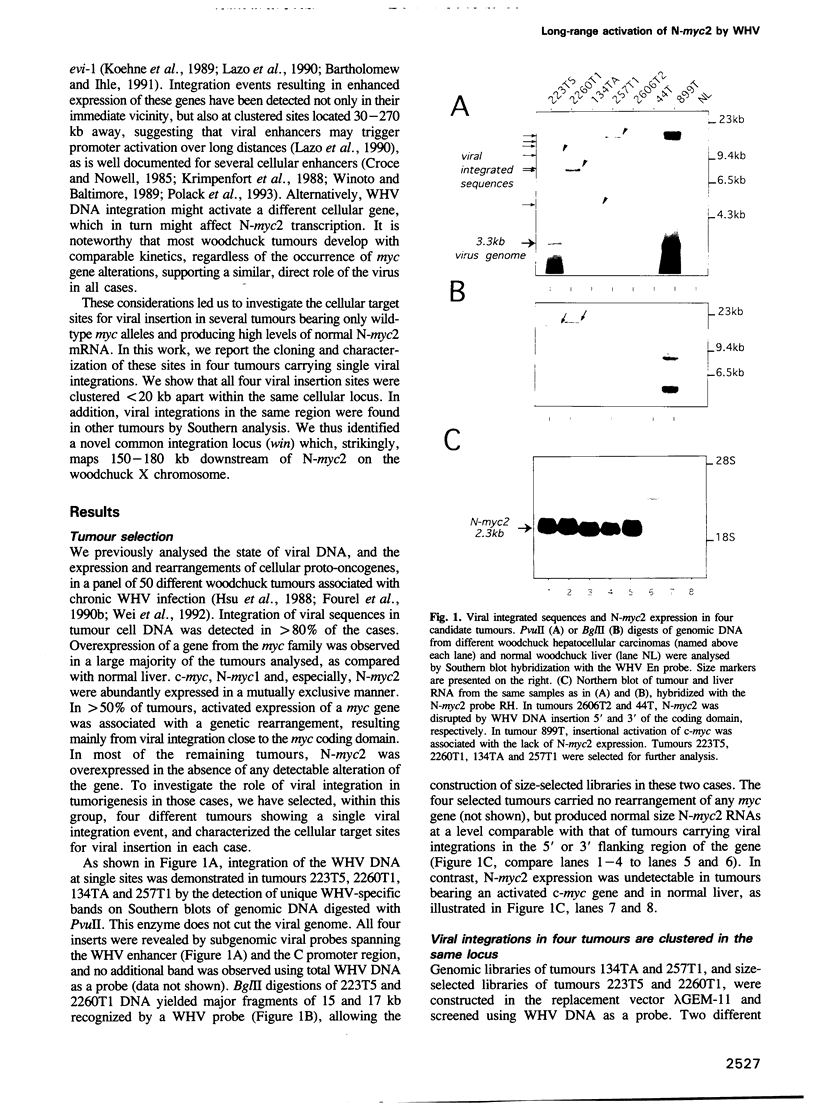
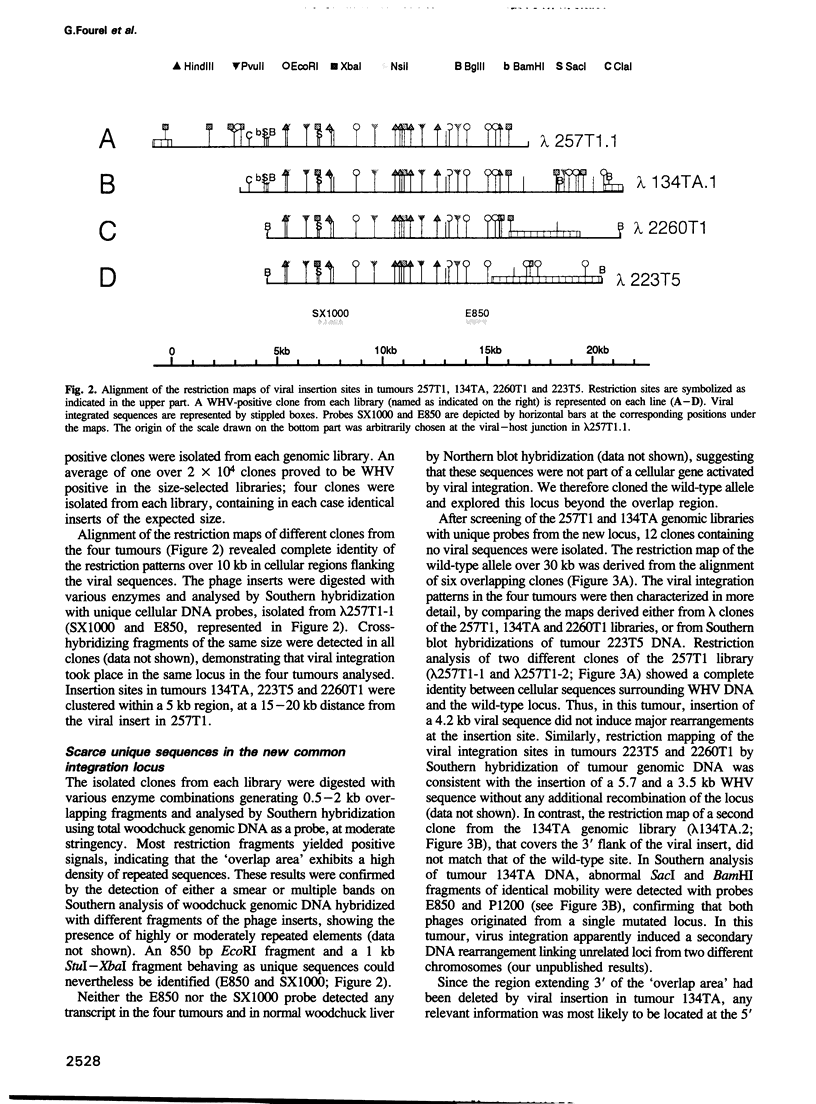
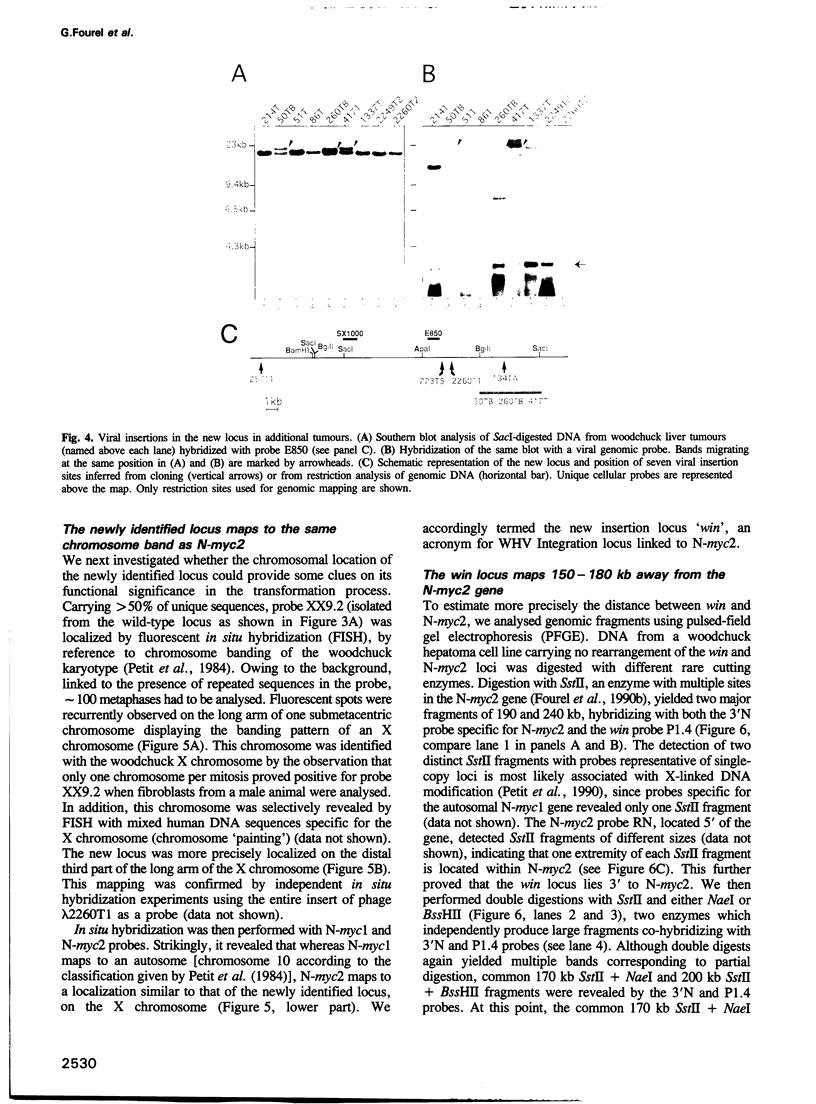
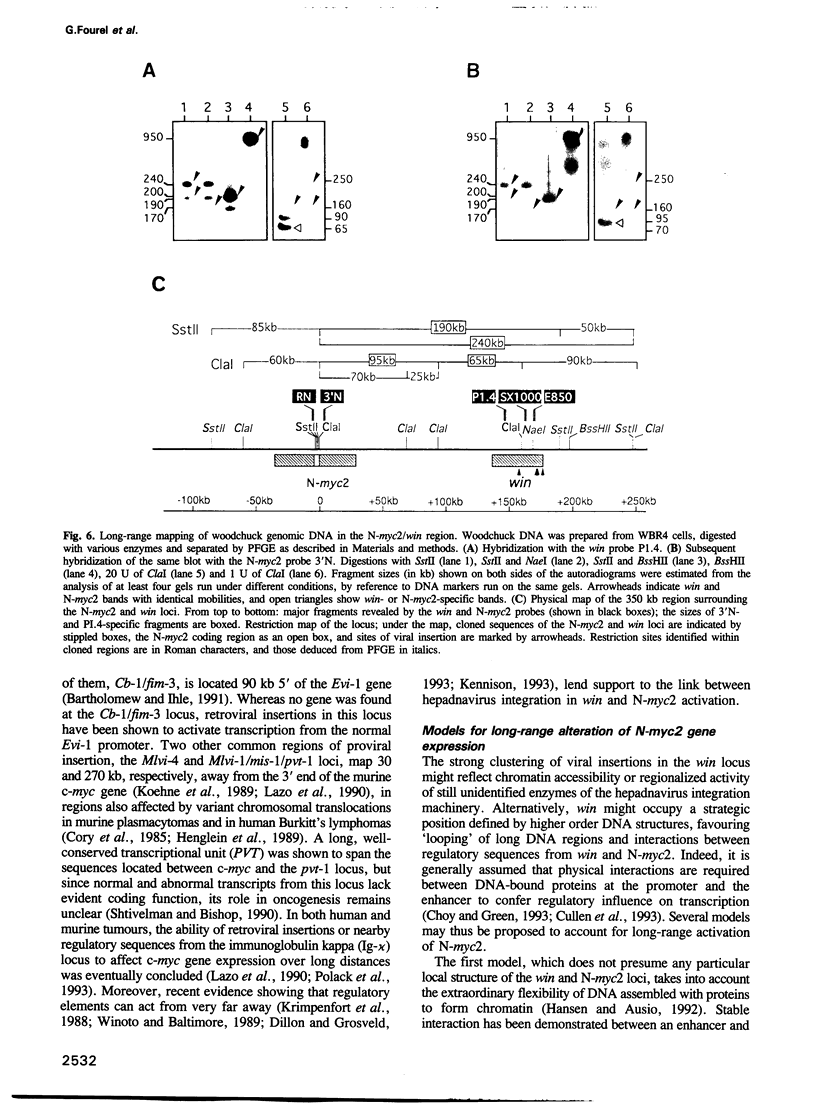
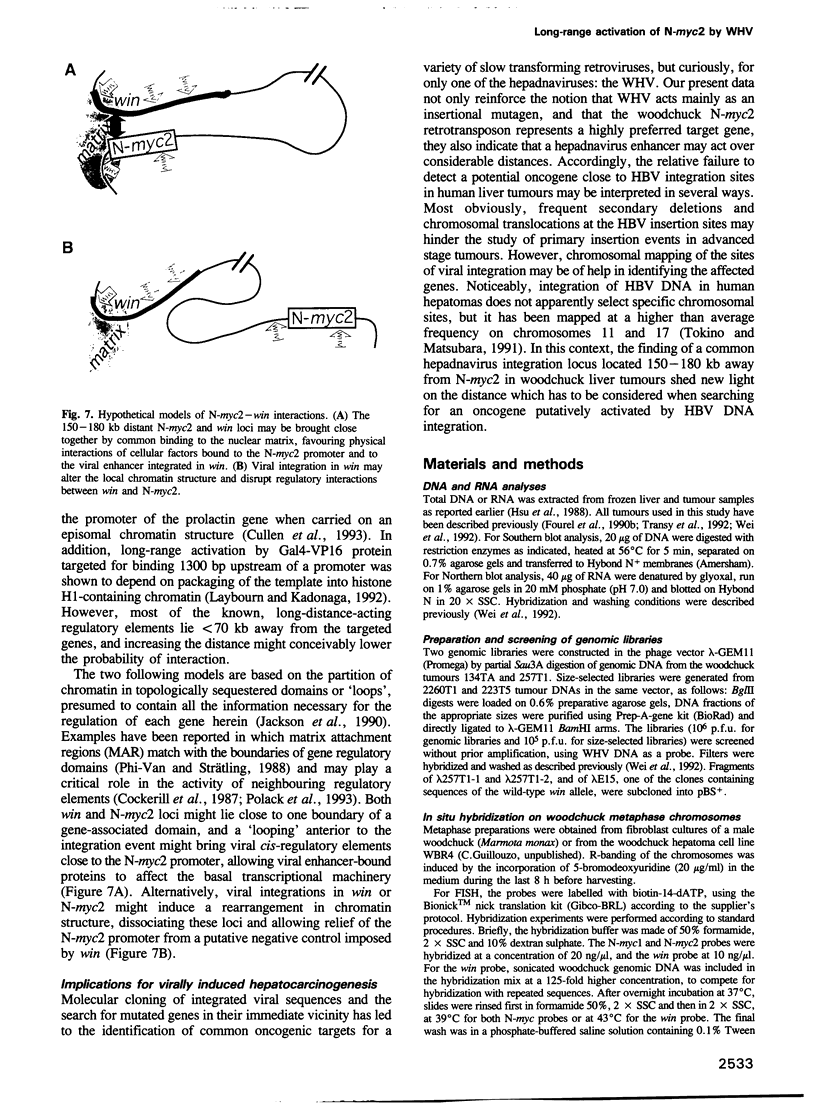
Insertional mutagenesis of host genes, a common oncogenic strategy of slow transforming retroviruses, has recently been described for a DNA virus of the hepadnavirus group: the woodchuck hepatitis virus. This virus causes insertional activation of myc genes, mainly the intronless N-myc2 oncogene, in > 50% of woodchuck liver tumours. In most remaining tumours, N-myc2 is overexpressed without any apparent genetic alteration. To elucidate the role of the virus in such cases, we have cloned and analysed single integration sites in four woodchuck tumours carrying wild-type myc alleles. All sites were clustered within < 20 kb in a single locus, in which scarce unique sequences showed no detectable transcriptional activity. By fluorescent in situ hybridization, N-myc2 and the new locus (win) were localized to the same region of the long arm of the woodchuck X chromosome, and a 150-180 kb intervening distance was deduced from pulse-field gel analysis. The detection of viral integrations in win in additional tumours that produced abundant N-myc2 transcripts further substantiates the link between these two loci in woodchuck tumorigenesis. We propose that efficient activation of the N-myc2 promoter by the hepadnavirus enhancer acting over a long distance might operate in liver cell transformation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y., Lin C. C., Chien C. S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981 Nov 21;2(8256):1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- Choy B., Green M. R. Eukaryotic activators function during multiple steps of preinitiation complex assembly. Nature. 1993 Dec 9;366(6455):531–536. doi: 10.1038/366531a0. [DOI] [PubMed] [Google Scholar]
- Cockerill P. N., Yuen M. H., Garrard W. T. The enhancer of the immunoglobulin heavy chain locus is flanked by presumptive chromosomal loop anchorage elements. J Biol Chem. 1987 Apr 15;262(11):5394–5397. [PubMed] [Google Scholar]
- Cory S., Graham M., Webb E., Corcoran L., Adams J. M. Variant (6;15) translocations in murine plasmacytomas involve a chromosome 15 locus at least 72 kb from the c-myc oncogene. EMBO J. 1985 Mar;4(3):675–681. doi: 10.1002/j.1460-2075.1985.tb03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Nowell P. C. Molecular basis of human B cell neoplasia. Blood. 1985 Jan;65(1):1–7. [PubMed] [Google Scholar]
- Cullen K. E., Kladde M. P., Seyfred M. A. Interaction between transcription regulatory regions of prolactin chromatin. Science. 1993 Jul 9;261(5118):203–206. doi: 10.1126/science.8327891. [DOI] [PubMed] [Google Scholar]
- Dejean A., Bougueleret L., Grzeschik K. H., Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to v-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1986 Jul 3;322(6074):70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- Dillon N., Grosveld F. Transcriptional regulation of multigene loci: multilevel control. Trends Genet. 1993 Apr;9(4):134–137. doi: 10.1016/0168-9525(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Fourel G., Tiollais P., Buendia M. A. Nucleotide sequence of the woodchuck N-myc gene (WN-myc1). Nucleic Acids Res. 1990 Aug 25;18(16):4918–4918. doi: 10.1093/nar/18.16.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel G., Transy C., Tennant B. C., Buendia M. A. Expression of the woodchuck N-myc2 retroposon in brain and in liver tumors is driven by a cryptic N-myc promoter. Mol Cell Biol. 1992 Dec;12(12):5336–5344. doi: 10.1128/mcb.12.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourel G., Trepo C., Bougueleret L., Henglein B., Ponzetto A., Tiollais P., Buendia M. A. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumours. Nature. 1990 Sep 20;347(6290):294–298. doi: 10.1038/347294a0. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Girones R., Cote P. J., Hornbuckle W. E., Tennant B. C., Gerin J. L., Purcell R. H., Miller R. H. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1846–1849. doi: 10.1073/pnas.86.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. C., Ausio J. Chromatin dynamics and the modulation of genetic activity. Trends Biochem Sci. 1992 May;17(5):187–191. doi: 10.1016/0968-0004(92)90264-a. [DOI] [PubMed] [Google Scholar]
- Hansen L. J., Tennant B. C., Seeger C., Ganem D. Differential activation of myc gene family members in hepatic carcinogenesis by closely related hepatitis B viruses. Mol Cell Biol. 1993 Jan;13(1):659–667. doi: 10.1128/mcb.13.1.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henglein B., Synovzik H., Groitl P., Bornkamm G. W., Hartl P., Lipp M. Three breakpoints of variant t(2;8) translocations in Burkitt's lymphoma cells fall within a region 140 kilobases distal from c-myc. Mol Cell Biol. 1989 May;9(5):2105–2113. doi: 10.1128/mcb.9.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T., Möröy T., Etiemble J., Louise A., Trépo C., Tiollais P., Buendia M. A. Activation of c-myc by woodchuck hepatitis virus insertion in hepatocellular carcinoma. Cell. 1988 Nov 18;55(4):627–635. doi: 10.1016/0092-8674(88)90221-8. [DOI] [PubMed] [Google Scholar]
- Jackson D. A., Dickinson P., Cook P. R. The size of chromatin loops in HeLa cells. EMBO J. 1990 Feb;9(2):567–571. doi: 10.1002/j.1460-2075.1990.tb08144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison J. A. Transcriptional activation of Drosophila homeotic genes from distant regulatory elements. Trends Genet. 1993 Mar;9(3):75–79. doi: 10.1016/0168-9525(93)90227-9. [DOI] [PubMed] [Google Scholar]
- Koehne C. F., Lazo P. A., Alves K., Lee J. S., Tsichlis P. N., O'Donnell P. V. The Mlvi-1 locus involved in the induction of rat T-cell lymphomas and the pvt-1/Mis-1 locus are identical. J Virol. 1989 May;63(5):2366–2369. doi: 10.1128/jvi.63.5.2366-2369.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimpenfort P., de Jong R., Uematsu Y., Dembic Z., Ryser S., von Boehmer H., Steinmetz M., Berns A. Transcription of T cell receptor beta-chain genes is controlled by a downstream regulatory element. EMBO J. 1988 Mar;7(3):745–750. doi: 10.1002/j.1460-2075.1988.tb02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Threshold phenomena and long-distance activation of transcription by RNA polymerase II. Science. 1992 Sep 18;257(5077):1682–1685. doi: 10.1126/science.1388287. [DOI] [PubMed] [Google Scholar]
- Lazo P. A., Lee J. S., Tsichlis P. N. Long-distance activation of the Myc protooncogene by provirus insertion in Mlvi-1 or Mlvi-4 in rat T-cell lymphomas. Proc Natl Acad Sci U S A. 1990 Jan;87(1):170–173. doi: 10.1073/pnas.87.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux N., Dutrillaux B., Viegas-Péquignot E. A simple method for simultaneous R- or G-banding and fluorescence in situ hybridization of small single-copy genes. Cytogenet Cell Genet. 1992;59(4):311–312. doi: 10.1159/000133277. [DOI] [PubMed] [Google Scholar]
- Loc P. V., Strätling W. H. The matrix attachment regions of the chicken lysozyme gene co-map with the boundaries of the chromatin domain. EMBO J. 1988 Mar;7(3):655–664. doi: 10.1002/j.1460-2075.1988.tb02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Van Davelaar M. J., Knight S. S., Salazar F. H., Garcia G., Popper H., Robinson W. S. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4543–4546. doi: 10.1073/pnas.83.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Robinson W. S. Common evolutionary origin of hepatitis B virus and retroviruses. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2531–2535. doi: 10.1073/pnas.83.8.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Long-range restriction map of the terminal part of the short arm of the human X chromosome. Proc Natl Acad Sci U S A. 1990 May;87(10):3680–3684. doi: 10.1073/pnas.87.10.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C., Levilliers J., Weissenbach J. Physical mapping of the human pseudo-autosomal region; comparison with genetic linkage map. EMBO J. 1988 Aug;7(8):2369–2376. doi: 10.1002/j.1460-2075.1988.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit D., Couturier J., Viegas-Péquignot E., Lombard M., Dutrillaux B. Très grande similitude entre le caryotype ancestral des écureuils (rongeurs) et celui des primates et des carnivores. Ann Genet. 1984;27(4):201–212. [PubMed] [Google Scholar]
- Polack A., Feederle R., Klobeck G., Hörtnagel K. Regulatory elements in the immunoglobulin kappa locus induce c-myc activation and the promoter shift in Burkitt's lymphoma cells. EMBO J. 1993 Oct;12(10):3913–3920. doi: 10.1002/j.1460-2075.1993.tb06069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper H., Roth L., Purcell R. H., Tennant B. C., Gerin J. L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci U S A. 1987 Feb;84(3):866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Burke K., Chou M. J., Zeldis J. B., Yang C. S., Lee C. S., Isselbacher K. J., Wands J. R., Goodman H. M. Tight clustering of human hepatitis B virus integration sites in hepatomas near a triple-stranded region. J Virol. 1987 Nov;61(11):3491–3498. doi: 10.1128/jvi.61.11.3491-3498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Bishop J. M. Effects of translocations on transcription from PVT. Mol Cell Biol. 1990 Apr;10(4):1835–1839. doi: 10.1128/mcb.10.4.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Toh H., Hayashida H., Miyata T. Sequence homology between retroviral reverse transcriptase and putative polymerases of hepatitis B virus and cauliflower mosaic virus. 1983 Oct 27-Nov 2Nature. 305(5937):827–829. doi: 10.1038/305827a0. [DOI] [PubMed] [Google Scholar]
- Tokino T., Matsubara K. Chromosomal sites for hepatitis B virus integration in human hepatocellular carcinoma. J Virol. 1991 Dec;65(12):6761–6764. doi: 10.1128/jvi.65.12.6761-6764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Transy C., Fourel G., Robinson W. S., Tiollais P., Marion P. L., Buendia M. A. Frequent amplification of c-myc in ground squirrel liver tumors associated with past or ongoing infection with a hepadnavirus. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3874–3878. doi: 10.1073/pnas.89.9.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. P., Rogler C. E. Topoisomerase I-mediated integration of hepadnavirus DNA in vitro. J Virol. 1991 May;65(5):2381–2392. doi: 10.1128/jvi.65.5.2381-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Wei Y., Fourel G., Ponzetto A., Silvestro M., Tiollais P., Buendia M. A. Hepadnavirus integration: mechanisms of activation of the N-myc2 retrotransposon in woodchuck liver tumors. J Virol. 1992 Sep;66(9):5265–5276. doi: 10.1128/jvi.66.9.5265-5276.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A., Baltimore D. A novel, inducible and T cell-specific enhancer located at the 3' end of the T cell receptor alpha locus. EMBO J. 1989 Mar;8(3):729–733. doi: 10.1002/j.1460-2075.1989.tb03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M., Berns A. Tumorigenesis by slow-transforming retroviruses--an update. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):213–235. doi: 10.1016/0304-419x(90)90005-l. [DOI] [PubMed] [Google Scholar]