Abstract
New advances in achieving hematopoietic chimerism may facilitate immunological tolerance to kidney transplants.
In 1953, Billingham et al. reported the Nobel Prize–winning finding that neonatal inoculation of mice with allogeneic lymphoid cells could induce long-lived donor hematopoietic chimerism and that the resulting intermingling of donor and host immune cells throughout the host yielded immunological tolerance to donor-strain grafts (1). This discovery brought with it the promise of organ transplantation without the morbidity of lifelong immunosuppression and set routine attainment of immunological tolerance as the field’s seemingly unreachable Holy Grail. Now, Leventhal et al. (2) add to advances in the last few years that suggest that the 6-decade-long quest for tolerance for kidney transplant patients may finally be nearing its end.
Transplantation tolerance has been effectively induced by many different methods in experimental small-animal systems, but very few of these routes have survived more stringent translational validation in preclinical large-animal models. Billingham’s approach capitalized on the immaturity of the immune system in neonatal rodents to allow integration of foreign immune cells without rejection; yet, defining host conditioning regimens to engender chimerism development in adults has proven more challenging. Indeed, reliance on donor hematopoietic chimerism has yielded the most consistent and robust transplantation tolerance in large-animal models and adult rodents, but a major roadblock has been achieving sufficient chimerism across strong histocompatibility barriers [specifically, mismatched major histocompatibility complex (MHC) proteins] to gain tolerance without the transferred immunocompetent lymphocytes attacking recipient antigens perceived as “foreign”—graft -versus-host disease (GVHD). GVHD has posed a formidable barrier; clinically acceptable regimens must meet the high benefit-to-risk standard of conventional lifelong immunosuppression, which yields excellent long-term outcome with live-donor kidney graft survival exceeding 95 and >60% at 1 and 10 years, respectively. Although bone marrow transplant physicians routinely succeed with full replacement of recipient marrow to achieve donor chimerism in MHC-matched donor recipient pairs, success in the setting of MHC mismatch requires more intense and toxic conditioning as well as the risk of morbid and sometimes lethal GVHD—complications unacceptable in a renal allograft recipient.
Mouse studies from nearly 3 decades ago by Ildstad and Sachs (3) determined that stable allogeneic lymphoid chimerism could be achieved but relied on myeloablative conditioning. Sachs and Sharabi subsequently obtained stable mixed chimerism without ablation of the host’s own marrow (nonmyeloablative mixed-chimerism)—a more clinically admissible approach that uses a reduced dose of whole-body irradiation counterbalanced by potent T cell–targeted antibodies and local thymic irradiation (4). In preparation for clinical application, the Sachs team extended its efforts to large animals, achieving stable mixed chimerism and donor-specific transplantation tolerance in the absence of GVHD (5).
More direct evidence that donor chimerism would permit successful organ transplantation was found in the specific setting of patients who underwent myeloablative human lymphocyte antigen (HLA)–matched bone marrow transplantation for hematologic disorders and years later developed renal failure requiring transplantation. With the kidney donor being the same individual who previously donated bone marrow, kidney transplants succeeded without the need for immunosuppression, affirming the principle that donor chimerism carried with it a permit for successful transplant from the same donor (6).
It is with this firm scientific foundation that experimental protocols were initiated to attempt human kidney transplant acceptance through donor hematopoietic chimerism with only nonmyeloablative conditioning. Three groups have now reported success: one apparently only successful in HLA-matched donor-recipient pairs (7) and two succeeding even in the more challenging situation of pairs disparate for HLA antigens. The first to succeed, Kawai et al., reported four of five patients who gained stable immunosuppression-free kidney graft survival now 5.5 to 9.5 years after transplant (8). This landmark feat was accomplished by a nonmyeloablative conditioning regimen including an antibody to CD2 to deplete T cells, cyclophosphamide, and thymic irradiation in conjunction with donor bone marrow infusion. Rituximab, an antibody that depletes B cells, was added to the treatment regimen after the third patient experienced an antibody-mediated rejection.
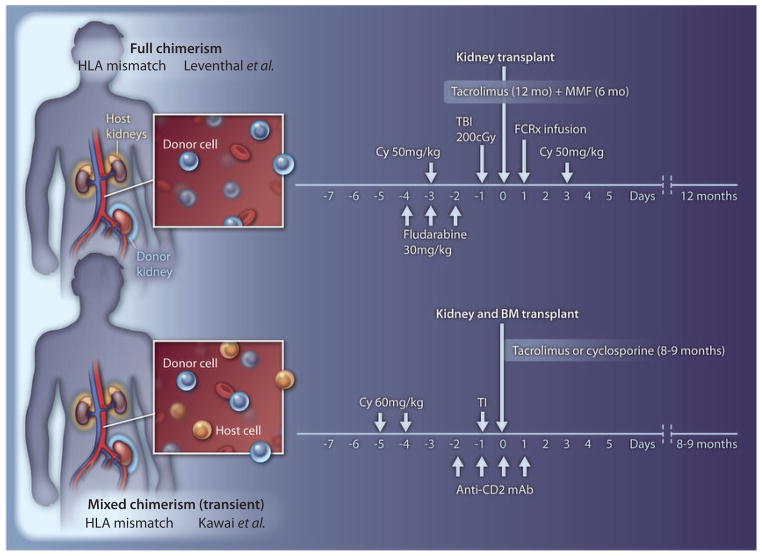
The trial has now been extended to 10 patients, with 7 achieving the goal of long-term graft survival in the absence of maintenance immunosuppression. Three recipients failed because of early humoral rejection, thrombotic microangiopathy, and acute rejection after cessation of immunosuppression. As observed in their studies in monkeys, long-lived allograft tolerance was achieved in the majority of cases despite the fact that donor chimerism in all patients was short-lived, lasting less than 3 weeks (Fig. 1). Loss of chimerism, presumably through immune-mediated processes, was also accompanied by engraftment syndrome and often transient graft dysfunction.
Fig. 1.
In the Leventhal trial (2), an intensive conditioning regimen was used, including total body irradiation (TBI), cyclophosphamide (Cy), fludarabine, and postoperative immunosuppression with mycophenolate mofetil (MMF) and tacrolimus. This regimen, together with bone marrow stem cells and FCRx, achieved full allogeneic chimerism (top), with replacement of essentially all host hematopoietic cells with those of the donor (blue). The conditioning used by Kawai et al. (8) consisted of cyclophosphamide, thymic irradiation (TI), antibody to CD2, and postoperative calcineurin inhibitor therapy (peritransplant rituximab was later added). This regimen resulted in transient mixed-chimerism, with both host (orange) and donor (blue) hematopoietic cells coexisting.
In this issue of Science Translational Medicine, Leventhal et al. report the only other instance of successful tolerance in non–HLA matched kidney transplant pairs (2). Their conditioning strategy of total body irradiation (200 grays), fludarabine and cyclophosphamide, and administration of a novel cellular therapeutic, FCRx, with donor hematopoietic stem cells consistently resulted in high percentages of donor chimerism without GVHD. Although the number of patients was relatively small and the follow-up brief, the results are striking: Multilineage full donor chimerism was achieved (Fig. 1) in all subjects at 1 month, and five of eight (including each of the last four) persisted long-term, allowing weaning from all maintenance immunosuppression by 1 year with rejection-free kidney graft function for 4 to 18 months. Although no definitive cases of GVHD were observed, in one case a skin rash developed in which the diagnosis of GVHD was entertained; however, the biopsy was nondiagnostic. The patient subsequently went on to lose the graft to unclear causes during an episode of sepsis and, because of pancytopeina, required hematologic rescue with previously recovered autologous stem cells. In the other two instances in which tolerance was not successful, weaning was halted, with development of membranous nephropathy in one case and signs of rejection in the other.
Induction protocols that rely on high doses of cyclophosphamide and fludarabine, similar to that used by Leventhal et al., have been applied previously in the setting of nonmatched bone marrow transplantation for hematological disorders but demonstrated a 13% graft failure rate and a 6% rate of severe (Grade III–IV) GVHD (9). Thus, the secret to the unprecedented level of success by Leventhal et al. may be the addition of a special population of bone marrow–derived cells termed facilitator cells (FCs), which in animal models have been found to improve engraftment and to avoid GVHD, possibly through induction of regulatory T cells. FCs are a mixed cell population: Precursor plasmacytoid DCs (pre-pDCs) are required but not sufficient for the beneficial FC effect to be observed.
Unfortunately, the lack of a direct control group makes it impossible to ascribe the remarkable results unequivocally to the administration of the FCRx product. This problem is compounded by the fact that the authors elected not to fully disclose the details of donor FC manufacturing and FCRx cell product composition for the purpose of intellectual property protection, which may impede validation of the results by other investigators.
The results of Leventhal et al. provide a fascinating foil to the approach used by Kawai et al. in that both achieved a comparable level of success; however, the dramatic difference in the extent and duration of chimerism raises the interesting question as to whether a transient or stable-donor chimerism-based strategy is preferable. Transient chimerism is attractive because it should essentially eliminate the potential for GVHD, but the absence of persistent chimerism may compromise the resilience of tolerance. Indeed, one rejection episode from Kawai et al. occurred in proximity to an infection, which hints that the tolerant state after transient chimerism may be metastable and corruptible by nonspecific inflammatory triggers.
On the other hand, although full chimerism may ensure stability, complete replacement of recipient myeloid and lymphoid lineages by donor cells could compromise immunocompetence and may incur an ongoing risk of chronic GVHD persisting even late posttransplant (10). Although it is certainly encouraging that GVHD was not evident in this pilot experience, we are not aware of other similar examples of mismatched donor chimerism that has not been accompanied by substantial GVHD risk. Thus, the debate on the superiority of transient versus persistent chimerism hinges on the longer-term results of the latter—specifically, whether the patients remain free of GVHD and opportunistic infections.
If the Leventhal et al. results are sustained and expanded in number, they may potentially have an enormous, paradigm-shifting impact on solid-organ transplantion. Future modifications would be needed, however, to expand application to deceased donor transplants in which preemptive conditioning is not feasible as well as to other more complex organs such as the liver, heart, and lung, in which intensive conditioning would not be tolerated in the perioperative period. Although only a taste of things to come, few transplant developments in the past half-century have been more enticing than these that put transplantation tolerance within our grasp.
REFERENCES AND NOTES
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R, Ildstad ST. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. doi: 10.1126/scitranslmed.3003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 4.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang CA, Fuchimoto Y, Scheier-Dolberg R, Murphy MC, Neville DM, Jr, Sachs DH. Stable mixed chimerism and tolerance using a nonmyeloablative preparative regimen in a large-animal model. J Clin Invest. 2000;105:173–181. doi: 10.1172/JCI7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114:954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 7.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 8.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolaños-Meade J, Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA, Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohty M, Apperley JF. Long-term physiological side effects after allogeneic bone marrow transplantation. Hematology. 2010;2010:229–236. doi: 10.1182/asheducation-2010.1.229. [DOI] [PubMed] [Google Scholar]