Abstract
Background
Vincristine-induced peripheral neuropathy (VIPN) is difficult to quantify in children.
Objective
The study objective was to examine the reliability, validity, and clinical feasibility of several VIPN measures for use in children with acute lymphoblastic leukemia.
Interventions/Methods
Children (N = 65) aged 1–18 years receiving vincristine at four academic centers participated in the study. Baseline and pre-vincristine VIPN assessments were obtained using the Total Neuropathy Score-Pediatric Vincristine (TNS-PV), the National Cancer Institute Common Terminology Criteria for Adverse Events, the Balis grading scale, and the FACES pain scale. TNS-PV scores (n = 806) were obtained over 15 weeks. Blood was obtained at several time-points to quantify pharmacokinetic parameters.
Results
Cronbach’s alpha for a reduced TNS-PV scale was 0.84. TNS-PV scores correlated with cumulative vincristine dosage (r = 0.53, p = 0.01), pharmacokinetic parameters (r = 0.41, p = 0.05), and grading scale scores (r = 0.46 – 0.52; p = 0.01). FACES scores correlated with the TNS-PV neuropathic pain item (r = 0.48; p = 0.01), and were attainable in all ages. A 2-item V-Rex score (vibration and reflex items) was the most responsive to change (es 0.65, p < 0.001). TNS-PV scores were attainable in 95% of children ≥ 6 years.
Conclusions
The TNS-PV is reliable and valid for measuring VIPN. It is sensitive to change over time (15 weeks) and feasible for use in children ≥ 6 years of age.
Implications for Practice
The TNS-PV may be a useful tool for assessing vincristine toxicity in children with acute lymphoblastic leukemia.
Introduction
Vincristine-Induced Peripheral Neuropathy
Vincristine-induced peripheral neuropathy (VIPN) occurs in nearly all children receiving vincristine for acute lymphoblastic leukemia.1,2 It is characterized by progressive motor, sensory, and autonomic nerve damage due to dysfunction of Aβ, Aδ, and C-fibers.3 Microtubule structure disruption and subsequent neuronal axon dysfunction, as well as inflammatory processes, have been suggested as possible pathophysiologic mechanisms.4–6 Common VIPN signs and symptoms include numbness and tingling in the hands and feet with associated neuropathic pain, asymptomatic hyporeflexia, constipation, and muscle weakness, and less commonly, patient experience impaired balance, jaw pain, and orthostatic hypotension.1,2,5,7,8 Refractory peripheral neuropathy and associated neuropathic pain are significant health problems due to their undeniable negative influence on functional status, patient safety, quality of life, and cost of care.9–12 In addition, VIPN often necessitates chemotherapy dose reductions, possibly compromising the efficacy of a potentially life-saving treatment. Therefore, efforts to prevent or minimize VIPN are critically important.
Measuring VIPN and Pain in Children
VIPN symptoms such as numbness, tingling, and neuropathic pain are difficult concepts for young children to describe, and few studies have focused on developing and testing ways to quantify these symptoms. Peripheral nerve damage can be partially quantified based on changes in nerve conduction velocity and amplitude,1 but nerve conduction studies are not feasible for use as a standard VIPN measurement approach due to the cost, inconvenience, and discomfort associated with the testing. The current standard tool for assessment of toxicities from cancer treatment-related neurotoxicity is the National Cancer Institute Common Terminology Criteria for Adverse Events grading scale however, there is conflicting evidence supporting the scale’s reliability and validity.13–17 Other chemotherapy-induced peripheral neuropathy measures have been evaluated for use in adult cancer populations,18 but only two small pilot studies published by the same lead author were found reporting results of clinimetric testing of neuropathy measures for use in children.19,20 In the most recent study, a pediatric variant of the Total Neuropathy Score (Ped-mTNS) was tested in 41 children aged 5 to18 years who were receiving vincristine or cisplatin as treatment for various cancers, and in 41 gender-matched controls.20 Using the Ped-mTNS, children were evaluated for the presence of neuropathy-specific subjective sensory, motor, and autonomic symptoms. Ped-mTNS wording of the subjective symptom questions was simplified to be appropriate for school-aged children. Physical examination approaches were used to assess light touch, pin and vibration sensitivity, strength, and deep tendon reflexes. An important finding from the earlier (2009) study was that the adapted measure was feasible for use in children based on ease of use and adequate child comprehension of subjective symptom assessment questions.19 Gilchrist and Tanner (2013) reported data supporting satisfactory internal consistency, intra- and inter-rater reliability.20 Ped-mTNS validity was supported based on predictable associations with scores from balance and manual dexterity tests, and due to the measure’s ability to distinguish between contrasting groups. Sensitivity and responsiveness to change over time were not evaluated in either study. In addition, children less than five years of age did not participate. Very young children may be especially vulnerable to cumulative vincristine neurotoxicity given their inability to communicate symptoms that would typically lead to toxicity-averting dose reductions. Neuropathy-related neuropathic pain is particularly difficult to assess because children at any age may be unable to acknowledge or describe the specific pain characteristics that distinguish neuropathic from nociceptive pain. There are numerous behavioral and self-report pediatric pain assessment instruments with strong clinimetric properties. However, based on the findings of two systematic reviews, and the national consensus guideline for pain assessment in children and adolescents arising from the Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPaCT),21–23 reliable and valid neuropathic pain measures for use in children have not been developed.
Due to the dearth of literature regarding pediatric VIPN and associated pain measurement to date, it is important that future research be focused in this area. Moreover, special emphasis should be placed on identifying simple approaches to identifying VIPN signs and symptoms in very young children, especially since most acute lymphoblastic leukemia diagnoses and subsequent treatment with vincristine occur before the age of five. The development, clinimetric testing, and utilization of VIPN and associated neuropathic pain assessment tools address a major gap in the science to date, and is consistent with one of the priority goals defined at the National Cancer Institute State-of-the-Science Meeting on Peripheral Neuropathy (March 2009). To address the current gap in pediatric VIPN measurement science, this paper reports the results of a study evaluating the clinimetric properties of four measures; the revised Total Neuropathy Score-Pediatric Vincristine (TNS-PV), the National Cancer Institute Common Terminology Criteria grading scale version 4.0, the Balis Pediatric Scale of Peripheral Neuropathies, and the FACES pain scale when used to assess VIPN in children with precursor B acute lymphoblastic leukemia receiving vincristine. The specific aims of the study were to examine the; 1) sensitivity of all four measures, 2) internal consistency and inter-rater reliability of the revised Total Neuropathy Score-Pediatric Vincristine (TNS-PV), 3) responsiveness to change of all four measures, 4) construct and convergent validity of all measures, and 5) clinical feasibility of the TNS-PV. The primary hypothesis was that there would be a statistically significant change in TNS-PV scores when comparing baseline to week 15 VIPN assessment data.
Methods
Sample and Setting
This multi-site trial was conducted at four academic medical centers; Indiana University School of Medicine/Riley Hospital for Children, the University of Michigan Comprehensive Cancer Center/Mott Children’s Hospital, Vanderbilt University/Monroe Carell Jr. Children’s Hospital, and George Washington University/Children’s National Medical Center. Sixty-five children with newly diagnosed precursor B acute lymphoblastic leukemia aged 1 to 18 years who were receiving vincristine according to a Children’s Oncology Group (COG) treatment trial (including: AALL0232, AALL 0331, AALL08P1, or AALL0932) participated in the study. Exclusion criteria were: 1) baseline (prior to first vincristine dosage) peripheral neuropathy score greater than grade 1 (per the National Cancer Institute Common Terminology Criteria version 4.0); 2) currently receiving erythropoietin, itraconazole, or vitamin supplements above 100% of the recommended daily allowance; 3) Down’s syndrome; 4) pregnant; or 5) history of a co-existing serious illness that would limit the ability to obtain neurologic assessments. All children received vincristine 1.5 mg/M2 (capped at 2 mg maximum dose) as defined by the specific COG treatment protocols. Toxicity-based dose modifications were defined according to the specific COG protocol guiding the individual child’s leukemia treatment.
Measures
Four measures were evaluated in this study, the TNS-PV, National Cancer Institute Common Terminology Criteria version 4.0 and Balis grading scales, and the FACES Pain Scale. The TNS-PV is a variant of the Total Neuropathy Score (TNS). The TNS is a neuropathy measurement composite tool that has been utilized by neurologists to more accurately quantify peripheral neuropathy. It is the most comprehensive composite tool to have been tested in patients receiving neurotoxic chemotherapy.16,24–30 This multidimensional instrument can be used to assess the characteristics and location (distally versus proximally) of neuropathy symptoms, as well as the severity and location of several physical examination findings (signs).26–29 Specifically, the TNS quantifies subjective sensory, motor, and autonomic symptoms, pin and vibration sensation, muscle strength, reflexes, sensory and motor nerve conduction, and computerized quantitative sensory testing findings.24 Evidence exists supporting the reliability and validity of shorter four- and five-item TNS variants for use in adults receiving taxanes and platinum.16,25 These abbreviated TNS variants exclude the nerve conduction and quantitative sensory testing items and retain items quantifying neuropathy signs and symptoms based on interview and physical examination findings. For the current study, a TNS variant previously tested in adults was further revised for use in children receiving vincristine (TNS-PV) (Table 1). This instrument quantifies subjective numbness, tingling, and neuropathic pain proximal extension, vibration and temperature sensation, muscle strength, deep tendon reflexes, constipation (a common sign of autonomic neuropathy), and hoarseness/vocal cord function (an indicator of laryngeal nerve neuropathy). Given that the sample was comprised of children, the pinprick sensation item was replaced with a temperature sensation item (both items assess small nerve fiber function) to eliminate pin-prick testing-related discomfort. Raters used a 128 Hz weighted tuning fork to assess vibratory sensibility and temperature sensibility (the patient’s ability to sense cold when the tuning fork is placed on the skin). Each TNS-PV item is scored using a 0–4 scale; higher scores equate to more severe neuropathy. With the original TNS scoring criteria (Form A), signs and symptoms experienced in the hands versus the feet are not differentiated. A patient with only feet symptoms could receive the same score as a patient with symptoms in the feet and hands. Since VIPN experienced in both the hands and feet represents worse neuropathy than that experienced only feet symptoms,5 the original TNS scoring approach may be less sensitive to detecting more severe VIPN based on the proximal extension of signs and symptoms as neuropathy worsens. Therefore, an alternative scoring approach (Form B) was tested whereby the presence of both lower and upper extremity symptoms reflected more severe neuropathy than the presence of lower extremity symptoms alone.
Table 1.
TNS-PV A & B Scoring
| 0 | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| Worst Subjective Symptoma,b (A) (Tingling, Numbness, Neuropathic Pain) | None | Limited to fingers or toes | Extension to ankle/wrist | Extension to knee/elbow | Above knees/elbows or functionally disabling |
| Worst Subjective Symptom a,b (B) (Tingling, Numbness, Neuropathic Pain) | None | Toes to mid-foot (not including the heel) | Mid-foot to ankle | Extend above ankle to the knee without upper extremity symptoms | Above the knee or concurrent lower and upper extremity symptoms |
| Temperature Sensibility a (A) | Normal | Reduced in fingers/toes | Reduced to wrist/ankle | Reduced to elbow/knee | Reduced above elbow/knee |
| Temperature Sensibility (B) | Normal | Absent/decreased from toes to mid-foot (not including the heel) | Absent/decreased from mid-foot to ankle | Absent/decreased above ankle to the knee | Absent/decreased above the knee or in lower and upper extremities concurrently |
| Vibration Sensibility a (A) | Normal | Reduced in fingers/toes | Reduced to wrist/ankle | Reduced to elbow/knee | Reduced above elbow/knee |
| Vibration Sensibility (B) | Normal | Absent/decreased from toes to mid-foot (not including the heel) | Absent/decreased from mid-foot to ankle | Absent/decreased above ankle to the knee | Absent/decreased above the knee or in lower and upper extremities concurrently |
| Strength a,c | Normal | Mild weakness, but can overcome resistance | Moderate weakness, can overcome gravity but not resistance | Severe weakness, cannot overcome gravity | Paralysis |
| Tendon Reflexes a | Normal | Ankle reflex reduced | Ankle reflex absent | Ankle reflex absent/others reduced | All reflexes absent |
| Autonomic/Constipation | Normal | Requiring stool softeners or dietary modification | Requiring laxatives | Obstipation requiring enemas or manual evacuation | Life threatening consequences (e.g. toxic megacolon; obstruction) including death |
| Laryngeal/Hoarseness | Normal voice/cry | Mild or intermittent hoarseness | Persistent hoarseness, but able to vocalize; may have mild to moderate edema | Whispered speech, not able to vocalize; may have marked edema | Marked dyspnea/stridor requiring tracheostomy or intubation |
Abbreviations: TNS-PV = Total Neuropathy Score-Pediatric Vincristine.
Score is based on the worst of the three symptoms.
Toe extension/flexion, ankle dorsiflexion, hip flexion, hand grip, thumb abduction, wrist extension, arm abduction; score is based on the weakest muscle group.
Two grading scales were used to rate sensory and motor neuropathy; the Balis Pediatric Scale of Peripheral Neuropathy and the National Cancer Institute Common Terminology Criteria. The Balis instrument has been commonly used to grade neurotoxicity occurring in children, and is based on a “0–4” scale. The National Cancer Institute Common Terminology Criteria has been used extensively to quantify sensory and motor neurotoxicity occurring in adults participating in cancer treatment trials, and is based on a “0–5” scale.31 For both scales, higher scores reflect worse neuropathy.
VIPN-associated neuropathic-specific pain was assessed using the FACES Pain Scale. The FACES Scale uses face drawings depicting increasing degrees of distress to correlate with increasing pain severity.32 The faces are linked with a 0–5 numerical response criterion: “0” = no hurt, “1” = hurts a little, “2” = hurts a little more, “3” = hurts even more, “4” = hurts a whole lot, and “5” = hurts the worst.
Approaches Used to Evaluate Clinimetric Properties
Neuropathy and pain measure sensitivity were judged based on whether the various measures detected a wide range of scores as opposed to only revealing scores at the low (floor effects) or high (ceiling effects) end of the scale range. Two type of TNS-PV reliability were evaluated. Internal consistency reliability was evaluated as a gauge of whether the individual TNS-PV items were measuring a similar construct. High item-item correlations would suggest that the instrument items were each measuring VIPN.33 Inter-rater reliability was assessed to determine whether two different raters would obtain the same TNS-PV score (a measure of equivalence reliability).34 Responsiveness was evaluated based on the instruments’ ability to detect changes in neuropathy and pain over time as the children received increasingly higher cumulative vincristine dosages. The extent to which the TNS-PV would predictably quantify neuropathy and pain in relationship to other related constructs (vincristine dosage) or other measures of neuropathy and pain (the National Cancer Institute and Balis grading scales, and the FACES pain scale) was assessed (construct and convergent validity). Given that VIPN worsens as children receive higher and higher cumulative vincristine doses over time, construct validity would be supported if the neuropathy and pain scores obtained using the TNS-PV, grading scales, and FACES scale were positively correlated with vincristine cumulative dosage and a pharmacokinetic parameter of vincristine metabolism (area-under-the-concentration-time-curve). Evidence of convergent validity would be based on positive correlations between the TNS-PV and other neuropathy and pain measures. Lastly, since it is often difficult to quantify neuropathy in young children, the feasibility of using the TNS-PV within a pediatric setting was evaluated based on the proportion of unattainable neuropathy assessments due to a child’s inability to appropriately cooperate with the assessment examination or to understand the concepts of vincristine-related numbness, tingling, and pain.
Procedure
The study was reviewed and approved by each site’s Institutional Review Board. Upon meeting eligibility requirements, parental/guardian consent and the child’s assent (for children 7–17 years of age) were obtained by the child’s treating physician or by a study investigator.
VIPN and pain assessments were performed by trained evaluators at each site. Non-neurologist clinician evaluators (e.g. nurses, mid-level providers, and physicians) and a pediatric neurologist from each study site were trained to perform the VIPN and pain assessments. Neurologic examination, pain assessment, and instrument scoring procedures were reviewed via face-to-face training sessions. All evaluators viewed a training video created specifically for use in this study. The training video was posted on a study-specific web site to facilitate unlimited future access. After viewing the video and completing the face-to-face training, evaluators engaged in hands-on practice supervised by a study investigator (Smith) and the site neurologist, first on individuals without VIPN, and then on children with varying degrees of neuropathy. After the initial training session, site neurologists supervised any subsequent training of clinician evaluators at their respective institutions. Before conducting examinations for the study, each clinician evaluator conducted practice exams on approximately 10 children with established peripheral neuropathy. Verbal permission to conduct the practice exam was obtained from the child and/or parent. After completing the 10 practice examinations, the site neurologist judged each evaluator’s neurologic examination and TNS-PV scoring proficiency using a skills competency checklist. All evaluators had to score 100% in physical examination and instrument scoring competency before conducting formal study assessments.
National Cancer Institute Common Terminology Criteria and Balis peripheral neuropathy grades were assigned by the patient’s primary pediatric oncology provider (someone different than study staff) on the same day that the TNS-PV score was obtained. Those grading neuropathy via a grading scale were blinded to TNS-PV scores. Likewise, trained neuropathy evaluators performing neuropathy assessments using the TNS-PV were blinded from grading scale scores.
Neuropathy and pain were evaluated at baseline (prior to Day 8 vincristine) and on the day of each subsequent vincristine treatment. Assessments were conducted prior to vincristine administration. Each child was asked to rate pain in their hands, feet, or jaw, using the FACES Pain Scale. Additional data was collected regarding the vincristine M2 and cumulative dosage received each week.
Population plasma pharmacokinetic (PK) sampling was performed in all weighing more than 10 kilograms. Four PK samples (20mls) were collected from each patient. Samples from outpatients were collected pre- and at 5, 15, and 30 minutes post vincristine administration on Day 8 of induction therapy. Four samples were also collected from inpatients 12–72 hours post vincristine. The vincristine pharmacokinetic (PK) parameters were initially estimated with a population pharmacokinetics model using the Nonlinear Mixed Effect Model statistical software program. Then the areas-under-the-concentration-time-curve (AUC) for individual subjects were calculated based on the estimated PK parameters and vincristine dose
Neurologist–non-neurologist evaluator inter-rater reliability was assessed in 19 children who had received a minimum of six vincristine doses. Participants were assessed twice on the same day using the TNS-PV, once by the trained clinician evaluator, and once by a neurologist. Raters were blinded to each other’s findings.
Analyses
The primary hypothesis was that there would be a statistically significant change in the seven TNS-PV individual item scores, and the TNS-PV (A) total score, when comparing baseline to week 15 VIPN assessment data. The week 15 time point was selected because the greatest change in VIPN was expected to occur over this time period given that vincristine dose intensity (frequency of treatment) is the highest in the first four months of acute lymphoblastic leukemia therapy. The seven TNS-PV (A) individual items were worst subjective symptom (A), temperature (A), vibration (A), reflexes, strength, autonomic/constipation, and laryngeal/hoarseness. With 50 participants, there was 82% power to detect a 0.75 effect size, if the type one error was controlled at 0.6% per test, or 5% total divided by 8 (7 items plus the TNS-PV [A] total score). The effect size is defined as the difference between the baseline and week 15 TNS-PV (A) item scores divided by the standard deviation. Analyses were completed using SPSS® for Windows (version 19.0) (2011). Descriptive statistics were used to assess demographic variables. Tests of normalcy revealed that scores from the TNS-PV, and the Balis and National Cancer Institute Common Terminology Criteria grading scales were all positively skewed, meaning that scores were low on average. Therefore in most cases, nonparametric Mann-Whitney tests and Spearman’s rho correlations were used to analyze the data. However, given the large sample size used for some analyses, parametric (Pearson) correlations were also calculated and, when similar, the parametric results have been presented.
A TNS-PV item analysis was conducted by calculating individual item and total score means, ranges, standard deviations, and inter-item correlations (Aims 1 and 2). Internal consistency reliability was assessed using Cronbach’s alpha coefficients (Aim 2). A value above 0.70 is considered adequate for a new measure.33,35 TNS-PV inter-rater reliability was assessed using weighted kappa coefficients (using the Fleiss-Cohen quadratic weights) for the component scores (Aim 2).36
Mann-Whitney tests were used to evaluate each instrument’s responsiveness to change over time, and effect sizes were calculated based on changes in VIPN and pain measure scores over time (from baseline to week 15) (Aim 3). Correlation coefficients (Spearman’s rho) were calculated to determine the associations between TNS-PV, Balis and National Cancer Institute Common Terminology Criteria, and the FACES scores, and cumulative vincristine dose and AUC (construct validity) (Aim 4). Convergent validity was determined by assessing the correlations (Pearson) between TNS-PV and grading scale scores (Balis and National Cancer Institute Common Terminology Criteria version 4.0), and the correlation between the FACES pain scale scores and the TNS-PV neuropathic pain item scores (Aim 4). Lastly, clinical feasibility was determined based on the percentage of neuropathy and pain assessments that were unattainable by age (Aim 5).
Results
Demographics
Data from 65 children were included in the analysis. Demographic data are presented in Table 2. Thirty-four males (52.3%) and 31 females (47.7%) ranging in age from 2–18 years of age participated in the study. The mean age was 6.38 years (SD 4.4), and 46.2% were less than 5 years of age. Most were Caucasian and non-Hispanic (87.5%), and were receiving vincristine treatment according to COG protocol AALL0932 (52.3%). The mean cumulative dosage received by week 15 was 12.6 mg (SD 8.0).
Table 2.
Demographics (N =65)
| Characteristic, No. (%) | Total |
|---|---|
| Age (years): | |
| Mean (SD) | 6.38 (4.35) |
| Range | 2–19 |
| Sex: | |
| Male | 34 (52.3) |
| Female | 31 (47.7) |
| Race: | |
| Caucasian | 57 (87.7) |
| African American | 5 (7.7) |
| Not reported | 3 (4.6) |
| Ethnicity: | |
| Hispanic | 4 (6.2) |
| Non-Hispanic | 57 (87.7) |
| Not reported | 4 (6.2) |
| Acute Lymphoblastic Leukemia Therapy: | |
| AALL0331 | 1 (1.5) |
| AALL0232 | 8 (12.3) |
| AALL07P4 | 6 (9.2) |
| AALL0932 | 34 (52.3) |
| No protocol | 6 (9.2) |
| Other | 10 (15.4) |
| Vincristine Cumulative Dosage Received | |
| Mean (SD) | 12.6 mg (4.9) |
| Vincristine Dosages Received | |
| Mean (SD) | 9.3 mg (8.0) |
Item Analysis – Aims 1 and 2
TNS-PV (Forms A and B) total and individual mean item scores, ranges, and standard deviations (SD) are presented in Table 3. Mean individual TNS-PV (A and B) item scores ranged from 0.10 to 1.28 (SD range 0.35 – 1.30) (N range 787–1064 assessments). The highest mean score was associated with the reflex item. All TNS-PV items encompassed the full score range (1–4). When comparing Form A to Form B scores, means and standard deviations were slightly higher when using the Form B scoring criteria. The TNS-PV (A) mean score was 3.18 (SD 3.26) and total scores ranged from 0–21. The TNS-PV (B) mean score was 3.41 (SD 3.58), and total scores ranged from 0–22. There was no statistically significant difference between Forms A and B total scores.
Table 3.
TNS-PV, Grading Scale, and FACES Means, Ranges, and Standard Deviations
| n a | Possible Range | Actual Range | % 0 Scores b | Mean | SD | |
|---|---|---|---|---|---|---|
| TNS-PV Total Score A | 800 | 0–28 | 0–21 | 21.8 | 3.18 | 3.26 |
| TNS-PV Total Score B | 790 | 0–28 | 0–22 | 21.8 | 3.41 | 3.58 |
| Worst Subjective Symptom A | 909 | 0–4 | 0–4 | 83.1 | 0.32 | 0.82 |
| Worst Subjective Symptom B | 911 | 0–4 | 0–4 | 84.0 | 0.41 | 1.08 |
| Paresthesias A | 914 | 0–4 | 0–4 | 93.4 | 0.10 | 0.43 |
| Paresthesias B | 914 | 0–4 | 0–4 | 93.1 | 0.18 | 0.72 |
| Numbness A | 914 | 0–4 | 0–4 | 94.2 | 0.10 | 0.45 |
| Numbness B | 914 | 0–4 | 0–4 | 94.2 | 0.15 | 0.67 |
| Neuropathic Pain A | 918 | 0–4 | 0–4 | 90.8 | 0.22 | 0.74 |
| Neuropathic Pain B | 915 | 0–4 | 0–4 | 91.2 | 0.23 | 0.81 |
| Temperature A | 832 | 0–4 | 0–4 | 82.1 | 0.26 | 0.65 |
| Temperature B | 800 | 0–4 | 0–4 | 82.0 | 0.30 | 0.78 |
| Vibration A | 834 | 0–4 | 0–4 | 70.5 | 0.43 | 0.83 |
| Vibration B | 787 | 0–4 | 0–4 | 68.5 | 0.52 | 1.01 |
| Strength | 932 | 0–4 | 0–4 | 68.7 | 0.44 | 0.78 |
| Tendon Reflex | 928 | 0–4 | 0–4 | 33.1 | 1.28 | 1.30 |
| Autonomic (Constipation) | 1063 | 0–4 | 0–4 | 64.5 | 0.55 | 0.80 |
| Laryngeal Neuropathy | 1064 | 0–4 | 0–4 | 92.8 | 0.08 | 0.35 |
| CTCc Grading Scale | ||||||
| Sensory | 1016 | 0–5 | 0–3 | 68.2 | 0.30 | 0.65 |
| Motor | 1016 | 0–5 | 0–3 | 77.8 | 0.38 | 0.64 |
| Balis Grading Scale | ||||||
| Sensory | 1012 | 0–4 | 0–3 | 81.9 | 0.30 | 0.63 |
| Motor | 1012 | 0–4 | 0–4 | 76.3 | 0.27 | 0.65 |
| FACES | 1037 | 0–5 | 0–5 | 91.4 | 0.19 | 0.70 |
n reflects the number of neuropathy or pain assessments.
excludes baseline scores.
Common Terminology Criteria for Adverse Events
The National Cancer Institute Common Terminology Criteria and Balis sensory and motor grading scale score means ranged from 0.27 to 0.38 (SD range 0.63 – 0.65). Only the Balis motor scale scores encompassed the full score range of 0–4. Common Terminology Criteria and Balis motor and sensory scores were low (0–2) for approximately 98% of the sample, revealing a floor effect (most scores clustered at the lowest/floor end of the scale range). Common Terminology Criteria and Balis grade 3 neuropathy occurred in approximately 2%. The highest grading scale score (grade 4) was reported in only one patient using the Balis scale. FACES Pain Scale scores ranged from 0 to 5. The mean score was low at 0.19 (SD 0.70) (N = 1037 assessments). Scores were attainable from all participating children, regardless of age.
Item-to-item correlations ranged from 0.003 (laryngeal/hoarseness and numbness) to 0.44 (strength and vibration) (Table 4). Moderately strong item-item correlations (r range 0.51 – 0.87) were found between the paresthesias, numbness, and neuropathic pain items and the worst symptom item, but this was expected because the worst symptom item score is in fact the score of the three subjective symptoms (i.e. paresthesias, numbness, and neuropathic pain) that is the worst. Statistically significant correlations (p range .01– .05) were found between almost all items; however the strength of the correlations were often low. For example, although the item-item correlations between the laryngeal (hoarseness) and autonomic (constipation) items and most other items were statistically significant, the correlations were all less than 0.13. Low item-item correlations suggest that the items in an instrument are not internally consistent/reliable (Aim 2).
Table 4.
Item-Item Correlations (Form A) (nc = 800)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. TNS-PV Total | 1.00 | ||||||||||
| 2. Paresthesias | 0.31a | 1.00 | |||||||||
| 3. Numbness | 0.44a | 0.41a | 1.00 | ||||||||
| 4. Neuropathic Pain | 0.50a | 0.36a | 0.28a | 1.00 | |||||||
| 5. Worst Symptom | 0.58a | 0.53a | 0.51a | 0.87a | 1.00 | ||||||
| 6. Temperature | 0.64a | 0.22a | 0.31a | 0.20a | 0.31a | 1.00 | |||||
| 7. Vibration | 0.64a | 0.14a | 0.29a | 0.19a | 0.23a | 0.38a | 1.00 | ||||
| 8. Strength | 0.69a | 0.19a | 0.35a | 0.35a | 0.39a | 0.38a | 0.44a | 1.00 | |||
| 9. Reflex | 0.71a | 0.11a | 0.25a | 0.14a | 0.21a | 0.37a | 0.29a | 0.31a | 1.00 | ||
| 10. Constipation | 0.33a | 0.10a | 0.09a | 0.04 | 0.08b | 0.07b | 0.05 | 0.12a | 0.03 | 1.00 | |
| 11. Hoarseness | 0.26a | 0.02 | 0.003 | 0.07b | 0.07b | 0.09a | 0.13a | 0.13a | 0.10a | 0.09a | 1.00 |
Abbreviations: TNS-PV = Total Neuropathy Score- Pediatric Vincristine.
Pearson correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
nc reflects the number of neuropathy assessments.
Internal Consistency Reliability - Aim 2
Alpha coefficients were calculated for the 7-item TNS-PV variant, and for a revised 5-item variant. The 5-item scale included worst subjective symptom, temperature, vibration, strength, and reflex items. The laryngeal and autonomic items were dropped from the 7-item TNS-PV variant due to suboptimal item-item correlations. In addition, the alpha coefficient dropped significantly when these two items were included in the scale (α = 0.68). The alpha coefficient for the 5-item TNS-PV (A) scale was 0.84 and the Form B alpha was nearly identical. Despite the improved alpha coefficient for the 5-item TNS-PV, all validity and responsiveness analyses were conducted using the original 7-item TNS-PV scores.
Inter-rater Reliability – Aim 2
Acceptable TNS-PV inter-rater reliability (IRR) was demonstrated for nearly all TNS-PV (A & B) items. TNS-PV scores obtained by trained raters correlated moderately to strongly with neurologist TNS-PV scores (Kw range 0.54 – 0.99) (n = 13–19) with one exception; the paresthesias item IRR was poor (Kw = 0.15). In nearly all cases, IRR was better when Form B was used.
Responsiveness to Change over Time – Aim 3
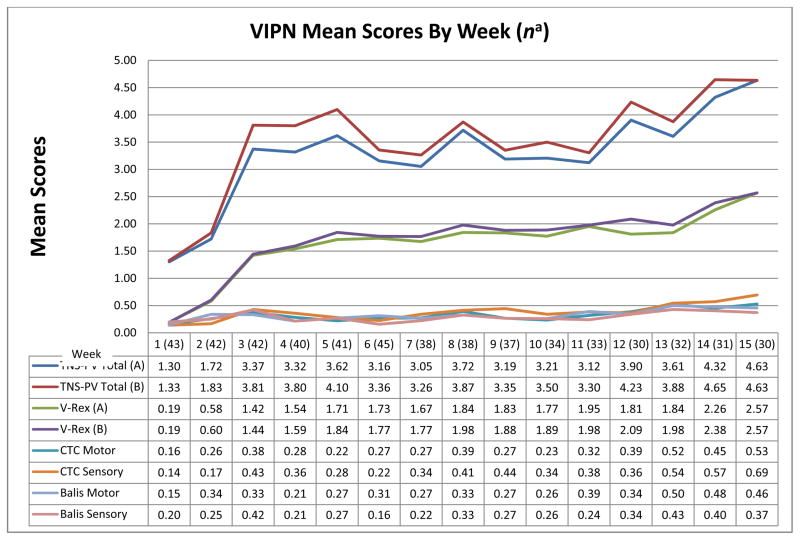
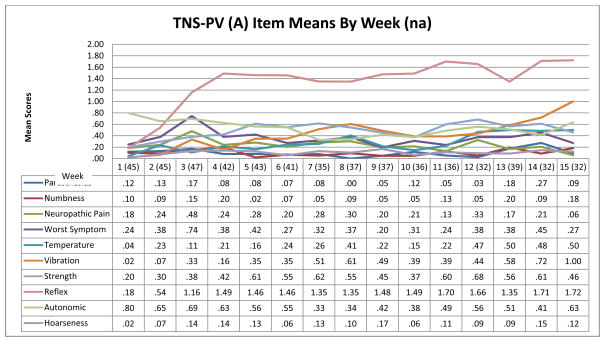
Figure 1 illustrates VIPN progression over time based on weekly mean 7-item TNS-PV (A & B) and grading scales scores from baseline to week 15. Figure 2 shows the change in mean scores of the individual TNS-PV (A) items over time. Only Form A scores are illustrated because Form A and B score patterns were similar. Figure 1 shows little change in grading scale scores, but a more obvious change in TNS-PV total scores (worsening neuropathy) over time. Both Figures 1 and 2 illustrate that VIPN develops quickly (after the first vincristine treatment) and worsens significantly by weeks 3–4. Scores temporarily decrease during weeks 6 and 7, and 9 and 10. These time points correspond with acute lymphoblastic leukemia treatment protocol-based breaks in vincristine treatment. However, despite vincristine treatment breaks, scores trend upward (VIPN worsens) over the 15 weeks. Based on moderately large and statistically significant effect sizes (es range 0.20 – 0.49, p range .04 - < .0001) (Table 5), the TNS-PV (A and B) and the CTC sensory scale were the most responsive to change when compared to other grading scales and the FACES scale. The effect size for the FACES pain scale was small (es = 0.20; p = .04), likely due to the lack of significant pain reported by participants. The reflex, temperature, and vibration items were the most responsive TNS-PV items (es range 0.31 – 0.66, p range .006 - < .0001). Because the vibration and reflex items were the most responsive of all, a 2-item TNS-PV total score was computed (V-Rex). The simpler 2-item V-Rex (A and B) was the best/most responsive measure when compared to the 7-item TNS-PV (es = 0.65; p < .0001).
Figure 1.
VIPN Measure Responsiveness to Change over Time—Weeks 1–15
n a = smallest sample from which all VIPN scores were obtained.
CTC = Common Toxicity Criteria for Adverse Events
Figure 2.
TNS-PV (A) Item Responsiveness to Change over Time—Weeks 1–15
n a = smallest sample from which all VIPN scores were obtained
Table 5.
Effect Sizes for VIPN Measures
| Summary Statistics | Mann Whitney | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Variable | n (Wk 1) | Mean (Wk 1) | SD (Wk 1) | n (Wk 15) | Mean (Wk 15) | SD (Wk 15) | Mean Rank (Wk1) | Mean Rank (Wk15) | pa | es |
| TNS-PV (A) | 43 | 1.30 | 1.49 | 30 | 4.63 | 4.28 | 28.42 | 49.30 | <.0001 | 0.49 |
| Worst Symptom A | 49 | 0.24 | 0.75 | 33 | 0.27 | 0.72 | 41.30 | 41.80 | .878 | |
| Paresthesias A | 50 | 0.12 | 0.48 | 33 | 0.09 | 0.38 | 42.30 | 41.53 | .748 | |
| Neuropathic Pain A | 49 | 0.18 | 0.73 | 34 | 0.06 | 0.34 | 42.89 | 40.72 | .329 | |
| Numbness A | 49 | 0.10 | 0.47 | 33 | 0.18 | 0.64 | 40.99 | 42.26 | .601 | |
| Temperature A | 45 | 0.04 | 0.21 | 32 | 0.50 | 0.92 | 35.56 | 43.84 | .006 | 0.31 |
| Vibration A | 48 | 0.02 | 0.14 | 33 | 1.00 | 1.20 | 32.74 | 53.02 | <.0001 | 0.58 |
| Reflex | 55 | 0.18 | 0.43 | 36 | 1.72 | 1.39 | 33.57 | 64.99 | <.0001 | 0.66 |
| Strength | 51 | 0.20 | 0.45 | 37 | 0.46 | 0.80 | 41.21 | 49.04 | .06 | |
| Autonomic/Constipation | 59 | 0.80 | 0.83 | 41 | 0.63 | 0.83 | 52.84 | 47.13 | .291 | |
| Laryngeal/Hoarseness | 60 | 0.02 | 0.13 | 41 | 0.12 | 0.40 | 49.33 | 53.44 | .066 | |
| TNS-PV (B) | 43 | 1.33 | 1.46 | 30 | 4.63 | 4.32 | 28.71 | 48.88 | <.0001 | 0.48 |
| Worst Symptom B | 49 | 0.29 | 0.87 | 33 | 0.27 | 0.72 | 41.35 | 41.73 | .908 | |
| Paresthesias B | 49 | 0.24 | 0.86 | 33 | 0.09 | 0.38 | 42.21 | 40.44 | .495 | |
| Neuropathic Pain B | 49 | 0.16 | 0.66 | 34 | 0.06 | 0.34 | 42.88 | 40.74 | .334 | |
| Numbness B | 49 | 0.08 | 0.34 | 33 | 0.18 | 0.64 | 40.97 | 42.29 | .586 | |
| Temperature B | 43 | 0.02 | 0.15 | 32 | 0.50 | 0.95 | 34.30 | 42.97 | .003 | 0.35 |
| Vibration B | 43 | 0.02 | 0.15 | 32 | 1.03 | 1.282 | 30.26 | 48.41 | <.0001 | 0.56 |
| V-Rex A | 57 | 0.19 | 0.44 | 37 | 2.57 | 2.19 | 34.82 | 67.03 | <.0001 | 0.65 |
| V-Rex B | 57 | 0.19 | 0.44 | 37 | 2.57 | 2.20 | 34.91 | 66.89 | <.0001 | 0.65 |
| CTCa Motor | 57 | 0.16 | 0.53 | 36 | 0.53 | 0.77 | 41.95 | 55.00 | .002 | 0.33 |
| CTCa Sensory | 57 | 0.14 | 0.40 | 36 | 0.69 | 0.71 | 38.70 | 60.14 | <.0001 | 0.48 |
| Balis Motor | 55 | 0.15 | 0.49 | 35 | 0.46 | 0.78 | 41.82 | 51.29 | .014 | 0.26 |
| Balis Sensory | 55 | 0.20 | 0.56 | 35 | 0.37 | 0.77 | 43.92 | 47.99 | .279 | |
| FACES | 60 | 0.25 | 0.70 | 39 | 0.03 | 0.16 | 52.46 | 46.22 | .043 | 0.20 |
based on two-tailed test; Wk = Week; Gray text highlights TNS-PV (A) data.
Common Terminology Criteria for Adverse Events.
Construct Validity – Aim 4
TNS-PV (A) (7 item) construct validity was supported based on a moderately strong correlation with vincristine cumulative dosage (r = 0.53; p = .01) (Table 6). Results were similar for other TNS-PV versions and therefore are not presented. Construct validity also is evidenced by a statistically significant correlation between TNS-PV (A) and vincristine AUC (r = 0.41; p = .05). The 2-item V-Rex was more strongly correlated with cumulative vincristine dose (r = 0.66; p = .01) and AUC (r = 0.50; p = .01) than the 7-item TNS-PV (A). Grading scale correlations with cumulative vincristine dosage were low (r range 0.18 – 0.35; p range NS - .05). There were no statistically significant associations between grading scale scores and AUC.
Table 6.
Construct Validity – VIPN Measure Correlations with Vincristine Cumulative Dose and Area under the Curve (AUC)
| Neuropathy Measure | Cumulative Dose (n) | AUC mg/min/liter (n) |
|---|---|---|
| TNS-PV (A) | 0.53a (33) | 0.41b (26) |
| V-Rex | 0.66a (34) | 0.50a (29) |
| FACES | 0.23 (45) | −0.05 (38) |
| CTCc Motor | 0.26 (45) | 0.20 (38) |
| CTCc Sensory | 0.31b (45) | 0.20 (38) |
| Balis Motor | 0.35b (45) | 0.26 (38) |
| Balis Sensory | 0.18 (45) | 0.06 (38) |
Spearman’s Rho correlation is significant at the 0.01 level (2-tailed).
Spearman’s Rho correlation is significant at the 0.05 level (2-tailed).
Common Terminology Criteria for Adverse Events.
Convergent Validity – Aim 4
Convergent validity is supported by moderately strong correlations between the TNS-PV (A) scores and the Common Terminology Criteria and Balis grading scale scores (r range 0.48 – 0.52; p = .01) (Table 7). Results were similar for other TNS-PV versions and therefore are not presented. The Common Terminology Criteria and Balis motor and sensory scales also were moderately to strongly correlated with each other (r range 0.68–0.90; p = .01). As expected, the FACES pain score was not highly correlated with TNS-PV (A), V-Rex, or grading scale scores (r range 0.20 – 0.38; p = .01), but it was moderately correlated with the neuropathic pain TNS-PV (A) sub-item (r = 0.48; p = .01).
Table 7.
Convergent Validity - VIPN Measure Correlations
| TNS-PV (A) | V-Rex | FACES | CTCa Motor | CTCa Sensory | Balis Motor | Balis Sensory | NP | |
|---|---|---|---|---|---|---|---|---|
| TNS-PV (A) | 1.00 | |||||||
| TNS-PV (A) 5-Item | 0.97 b | |||||||
| V-Rex | 0.84 b | 1.00 | ||||||
| FACES | 0.38 b | 0.20 b | 1.00 | |||||
| CTCb Motor | 0.48 b | 0.32 b | 0.28 b | 1.00 | ||||
| CTCb Sensory | 0.52 b | 0.46 b | 0.30 b | 0.70 b | 1.00 | |||
| Balis Motor | 0.46 b | 0.31 b | 0.28 b | 0.89 b | 0.70 b | 1.00 | ||
| Balis Sensory | 0.49 b | 0.30 b | 0.34 b | 0.72 b | 0.72 b | 0.67 b | 1.00 | |
| NP | 0.50 b | 0.20 b | 0.48 b | 0.39 b | 0.36 b | 0.36 b | 0.45 b | 1.00 |
Common Toxicity Criteria for Adverse Events.
Pearson correlation is significant at the 0.01 level (2-tailed).
NP = Neuropathic Pain.
n = 654–1037; n reflects the number of neuropathy assessments.
Feasibility of VIPN Assessment in Young Children – Aim 5
Vibration and temperature sensibility assessments could not be performed in 84 to 87% of children three years of age or younger (Figure 3). In addition, approximately 48% of three year olds could not provide subjective symptom information regarding numbness, paresthesias, and neuropathic pain. However, reflex and strength scores were more easily attainable in this age group. Approximately 91% of reflex and 78% of strength scores, respectively, could be assessed in very young children, including strength of specific muscles such as big toe dorsiflexion and extension of abductor pollicus brevis. Laryngeal and autonomic neuropathy assessments could be attained in nearly all children because changes in voice or bowel patterns could be observed by caregivers and/or clinical providers. TNS-PV individual item assessments were attainable in nearly all children six years of age or older. Lastly, the FACES pain scale was answered by 95% of all aged children without parental assistance. However, some children required additional coaching so that they understood that their selected “face” should reflect pain severity in their hands, feet, or jaw versus general feelings of distress.
Figure 3.
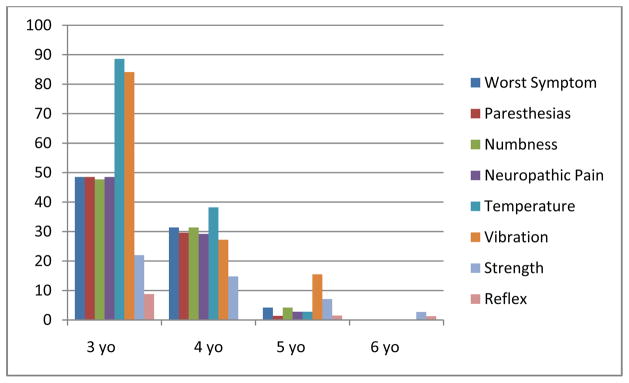
TNS-PV (A) Assessment Feasibility by Age – Percent Unattainable (na = 803)
na reflects the number of neuropathy assessments.
Discussion
Vincristine is a mainstay treatment for a number of curable pediatric malignancies, including acute lymphoblastic leukemia. While adequate vincristine dosing is correlated with improved survival, VIPN is a major cause of dose reductions. However, some children experience only mild VIPN, and thus, it is important that our assessment approaches can detect a broad range of VIPN severity. One could speculate that children with particularly high-risk disease who tolerate vincristine dose intensification (experience less severe VIPN) might benefit from better disease control by receiving higher doses of this important drug. Conversely, early or pre-emptive dose modification or even elimination of vincristine could be considered in children who are at high risk for severe VIPN. However, to effectively develop improved dosing algorithms, it is critical that we understand how VIPN signs and symptoms evolve over time. Based on an enhanced knowledge of VIPN severity and patterns, the next step should be to develop a system facilitating prediction of an individual child’s risk and expected VIPN severity very early in (if not before initiating) therapy. A crucial step in this process of treatment optimization aimed at maximizing efficacy while minimizing the potential for severe side effects is to improve the ability to measure the clinical manifestations of toxicity (the phenotype).
Williams et al. (2012) recently developed and reported the validity, reliability and other psychometric properties of the Therapy-Related Symptom Checklist for Children (TRSC-C), a 30-item instrument that measures symptom occurrence and severity during pediatric cancer treatments; the sample included 385 children, 5–17 years old, 45% of whom had acute lymphoblastic leukemia.37 Numbness in the fingers/toes and jaw pain (side effects associated with vincristine treatment) were reported by 32% and 27% of the study participants, respectively.37 These data illustrate that neuropathy is a commonly occurring problem in children with malignancy. Pain (49%) due to any cause and constipation (45%) occurred in a high percentage of the sample,37 but it is not known whether these symptoms were directly related to vincristine therapy. Although this study provided important information regarding the clinimetric properties of a patient-reported symptom assessment measure, the TRSC-C should not be used to guide vincristine dosing. The TRSC-C does not quantify subclinical evidence of VIPN (detectable signs that emerge before a child will report symptoms) via assessment of vibration and reflexes and thereby will not assist the clinician to adjust vincristine dosages early enough in the treatment course. Therefore, when toxicity will be used to guide drug dosing, VIPN measures which are sensitive to early, subtle changes in the VIPN phenotype should be used.
Capturing the neuropathy phenotype is challenging in patients of all ages; however, it is particularly difficult in children. Yet, given the great importance of vincristine in treatment of curable childhood cancers, as well as the significant potential to improve on our current use of this medication, we took on the challenge of modifying an assessment tool (TNS) to allow careful, non-invasive monitoring of children for VIPN. The current study is one of few to test the clinimetric properties of several VIPN objective and subjective assessment approaches for use in children. Our findings confirm results recently reported by Gilchrist and Tanner (2013) in a study conducted in children receiving mainly vincristine (96%) where the majority of participants (56%) were receiving treatment for acute lymphoblastic leukemia.20 The researchers demonstrated that a slightly different version of the TNS was reliable and valid when used in children older than five years of age.20 Although our findings support the reliability and validity of a TNS variant (TNS-PV), study findings also suggest that several assessment approaches were suboptimal based on poor sensitivity to detect subtle differences in signs and symptoms and suboptimal construct validity (grading scales) or an inability to pick up VIPN changes over time (several TNS-PV items). The temperature, vibration, and reflex items were the most responsive to change over time. Some of the individual TNS-PV items did not correlate with other TNS-PV items, suggesting that these poorly correlating items are not solely measuring VIPN. For example, autonomic/constipation scores did not correlate with other TNS-PV items. A plausible explanation for this low correlation is that although constipation is a common vincristine side effect, constipation results from other causes such as opioid use, as well as dietary and activity-related factors. Assessing constipation is also complicated because oncologists and families often utilize prophylactic strategies, and although the TNS-PV asks about “required” management strategies, the difference between “required” versus “prophylactic” strategies is difficult to differentiate.
Most TNS-PV items were difficult to assess in very young children, and therefore are not feasible for use in every child. Nearly all TNS-PV items were attainable in children ≥ 6 years of age. These findings suggest that clinicians could tailor their VIPN assessment approach based on the child’s age. Children ≤ 5 may not be appropriate for studies requiring a clear VIPN phenotype. Additionally, vincristine dose reductions for very young patients with discomfort or gait abnormality should be determined following a thorough clinical and neurologic evaluation. The most useful information gleaned from this research relates to how pediatric oncology clinicians can streamline their assessments in older children by using the V-Rex, a simple approach to VIPN assessment that provides far better information than the standard grading scale-based methods. Disadvantages of using the V-Rex are that a two-item scale will generally demonstrate inferior internal consistency reliability when compared to scales with more items. Also, changes in weekly scores may be less obvious as evidenced by the more subtle V-Rex peaks and valleys when compared to the 7-item TNS-PV (A) score patterns (Figure 1). Lastly, the FACES pain scale also was shown to be valid, sensitive, responsive, and feasible for use when quantifying VIPN-related pain severity in children of all ages. However, further research is needed to determine if the FACES scale specifically quantifies neuropathic as opposed to nociceptive pain, or if it could be assumed to characterize neuropathic pain once nociceptive sources have been ruled out in this population.
Although the TNS-PV forms A and B seem equivalent in terms of internal consistency reliability and validity, Form B may be better because it more accurately reflects the distal to proximal progression and, thus will elucidate more severe neuropathy when compared to Form A. As illustrated in Figure 1, Form A may underestimate neuropathy severity. This has important implications for minimizing neurotoxicity because vincristine dosing may be modified sooner when scoring VIPN using Form B versus Form A. In addition, inter-rater reliability was better when using Form B.
There are several study limitations that should be mentioned. First, caution should be taken in generalizing study findings to neuropathy caused by other neurotoxic agents, or to non-leukemia populations, since these conditions were not tested. The small sample sizes used to conduct some of the analyses may have resulted in inadequate power to detect statistically significant findings. Poor item-item correlations for the constipation and laryngeal items may have been influenced by data collection methods. Parents and/or children were asked to report constipation or hoarseness severity experienced a week or more in the past. Retrospective data of this nature is less valid. In addition, other factors, such as opioid use, could have influenced constipation incidence.
Another possible limitation has to do with the feasibility of translating these findings to clinical practice. Rigorous methods were used to train all neuropathy assessors located at several academic centers throughout the mid-and eastern United States. A train-the-trainer approach helped nurses, physician assistants, and hematologists to learn new VIPN assessment skills, and the scores obtained were similar to those obtained from pediatric neurologists. This suggests that broader implementation of the V-Rex assessment approach to community and academic settings is feasible, but may require some type of widespread educational effort, possibly offered at professional meetings.
In conclusion, the TNS-PV is a reliable and valid instrument for measuring vincristine-related neuropathy in children with leukemia. It is sensitive to change over time (15 weeks) and feasible for use in children ≥ 6 years of age. The main drawback of the 5- and 7-item TNS-PV is that scoring of most of its items is not possible when used in children less than five years, and some of the individual items are not responsive to change over time. As an alternative, the simple 2-item V-Rex measure, combined with the FACES pain scale, could be used to assess VIPN in all children ≥ 6 years of age. Future consideration should be given to revising the TNS-PV scoring criteria to be more consistent with distal to proximal VIPN extension as neuropathy worsens. The National Cancer Institute Common Terminology Criteria and Balis scales have significant floor effects and therefore may underestimate VIPN severity. In addition, grading scale construct validity is inferior when compared to the TNS-PV.
Implications for Practice
As a means for optimizing chemotherapy efficacy while minimizing toxicity, an important goal will be to implement into clinical practice the use of sensitive, responsive, reliable, and valid tools to assess toxicities. The TNS-PV may be a useful tool for assessing vincristine toxicity in children with acute lymphoblastic leukemia.
Acknowledgments
Special thanks are extended to the following contributors: Patricia Robertson, MD; Alexandra Scott, MS, PA; Mary Fedewa, MSN, FNP; Angela Stovall, BS; Bidisha Ghosh, MS; Joshua Waymire, MBA; Brianna Freedman, BSN, RN; James Kelly; Karin Thomas, Julie Albert, PNP; Jessica Nance, MD; and Christopher Watson.
Funding for This Work:
Smith, Lang, Hutchinson, Robertson, Ho, Burnette, Wells, Bridges, Renbarger (NCI RO1 PAR-08-248-0132428); Smith, Li, Renbarger (3UL 1RR025761-02S5, CA146882-01)
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Vainionpaa L, Kovala T, Tolonen U, Lanning M. Vincristine therapy for children with acute lymphoblastic leukemia impairs conduction in the entire peripheral nerve. Pediatr Neurol. 1995;13(4):314–8. doi: 10.1016/0887-8994(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 2.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. Journal of the Peripheral Nervous System. 2009;14(3):184–189. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty P, Cata J, Burton A, Vu K, Weng H. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage. 2007;33(2):166–79. doi: 10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 5.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa T, Mimura Y, Kato H, Ootsubo S, Murakoshi M. The usefulness of rabbits as an animal model for the neuropathological assessment of neurotoxicity following the administration of vincristine. Neurotoxicology. 2000;21(4):501–11. [PubMed] [Google Scholar]
- 7.Visovsky C, Daly BJ. Clinical evaluation and patterns of chemotherapy-induced peripheral neuropathy. J Am Acad Nurse Pract. 2004;16(8):353–359. doi: 10.1111/j.1745-7599.2004.tb00458.x. [DOI] [PubMed] [Google Scholar]
- 8.Reinders Messelink HA, Schoemaker MM, Hofte M, et al. Fine motor and handwriting problems after treatment for childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;27(6):551–5. doi: 10.1002/(SICI)1096-911X(199612)27:6<551::AID-MPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Bakitas MA. Background noise: The experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323–331. doi: 10.1097/01.NNR.0000289503.22414.79. [DOI] [PubMed] [Google Scholar]
- 10.Meyer-Rosberg K, Kvarnstrom A, Kinnman E, Gordh T, Nordfors LO, Kristofferson A. Peripheral neuropathic pain--a multidimensional burden for patients. Eur J Pain. 2001;5(4):379–389. doi: 10.1053/eujp.2001.0259. [DOI] [PubMed] [Google Scholar]
- 11.Nail L. Symptoms and physical function following treatment among people with chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2011;38(1):A48–A49. [Google Scholar]
- 12.Berger A, Dukes E, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. The Journal of Pain. 2004;5(3):143–9. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Cavaletti G, Jann S, Pace A, et al. Multi-center assessment of the total neuropathy score for chemotherapy-induced peripheral neurotoxicity. Journal of the Peripheral Nervous System. 2006;11(2):135–141. doi: 10.1111/j.1085-9489.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 14.Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9(7):739–744. doi: 10.1023/a:1008344507482. [DOI] [PubMed] [Google Scholar]
- 15.Kuroi K, Shimozuma K, Ohashi Y, et al. Prospective assessment of chemotherapy-induced peripheral neuropathy due to weekly paclitaxel in patients with advanced or metastatic breast cancer (CSP-HOR 02 study) Support Care Cancer. 2009;17:1071–1080. doi: 10.1007/s00520-008-0550-x. [DOI] [PubMed] [Google Scholar]
- 16.Lavoie Smith EM, Cohen JA, Pett MA, Beck SL. The validity of neuropathy and neuropathic pain measures in patients with cancer receiving taxanes and platinums. Oncol Nurs Forum. 2011;38(2):133–142. doi: 10.1188/11.ONF.133-142. [DOI] [PubMed] [Google Scholar]
- 17.Cavaletti G, Cornblath DR, Merkies IS, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann Oncol. 2013;24(2):454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith K, Merkies ISJ, Hill E, Cornblath D. Measures of chemotherapy-induced peripheral neuropathy: A systematic review of psychometric properties. Journal of the Peripheral Nervous System. 2010;15(4):314–25. doi: 10.1111/j.1529-8027.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 19.Gilchrist LS. Measuring chemotherapy-induced peripheral neuropathy in children: Development of the ped-mTNS and pilot study results. Rehabilitation Oncology. 2009;27(3):7–15. [Google Scholar]
- 20.Gilchrist LS, Tanner L. The pediatric-modified total neuropathy score: A reliable and valid measure of chemotherapy-induced peripheral neuropathy in children with non-CNS cancers. Support Care Cancer. 2013;21(3):847–856. doi: 10.1007/s00520-012-1591-8. [DOI] [PubMed] [Google Scholar]
- 21.Stinson J, Kavanagh T, Yamada J, Gill N, Stevens B. Systematic review of the psychometric properties, interpretability and feasibility of self-report pain intensity measures for use in clinical trials in children and adolescents. Pain. 2006;125(1–2):143–57. doi: 10.1016/j.pain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 22.von Baeyer CL, Spagrud LJ. Systematic review of observational (behavioral) measures of pain for children and adolescents aged 3 to 18 years. Pain. 2007;127(1–2):140–150. doi: 10.1016/j.pain.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 23.McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. Journal of Pain. 2008;9(9):771–783. doi: 10.1016/j.jpain.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Smith EL, Beck SL, Cohen J. The total neuropathy score (TNS): A tool for measuring chemotherapy-induced peripheral neuropathy. Oncol Nurs Forum. 2008;35(102):96–102. doi: 10.1188/08.ONF.96-102. [DOI] [PubMed] [Google Scholar]
- 25.Smith EM, Cohen JA, Pett MA, Beck SL. The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–183. doi: 10.1097/NCC.0b013e3181c989a3. [DOI] [PubMed] [Google Scholar]
- 26.Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: Validation and reliability study. Neurology. 1999;53(8):1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhry V, Eisenberger MA, Sinibaldi VJ, Sheikh K, Griffin JW, Cornblath DR. A prospective study of suramin-induced peripheral neuropathy. Brain. 1996;119(Pt 6):2039–2052. doi: 10.1093/brain/119.6.2039. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: Clinical and electrophysiological studies. Ann Neurol. 1994;35(3):304. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhry V, Cornblath D, Corse A, Freimer M, Simmons-O’Brien E, Vogelsang G. Thalidomide-induced neuropathy. Neurology. 2002;59:1872–1875. doi: 10.1212/01.wnl.0000037480.59194.85. [DOI] [PubMed] [Google Scholar]
- 30.Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp LS. The modified total neuropathy score: A clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. Supportive Oncology. 2006;4(8):W9–W16. [Google Scholar]
- 31.Postma TJ, Heimans JJ. Grading of chemotherapy-induced peripheral neuropathy. Ann Oncol. 2000;11(5):509–513. doi: 10.1023/a:1008345613594. [DOI] [PubMed] [Google Scholar]
- 32.Hockenberry MJ, Wilson D, Winkelstein ML, editors. Wong’s essentials of pediatric nursing. 7. St. Louis, MO: Elsevier Mosby; 2005. [Google Scholar]
- 33.Ferketich S. Focus on psychometrics: Internal consistency estimates of reliability. Res Nurs Health. 1990;13(6):437–440. doi: 10.1002/nur.4770130612. [DOI] [PubMed] [Google Scholar]
- 34.Devillis RF. Scale development: Theory and applications. 2. Thousand Oaks: Sage Publications; 2003. [Google Scholar]
- 35.Strickland OL. Internal consistency analysis: Making the most of what you’ve got. J Nurs Meas. 1996;4(1):3–4. [PubMed] [Google Scholar]
- 36.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 37.Williams PD, Williams AR, Kelly KP, et al. A symptom checklist for children with cancer: The therapy-related symptom checklist-children. Cancer Nurs. 2012;35(2):89–98. doi: 10.1097/NCC.0b013e31821a51f6. [DOI] [PubMed] [Google Scholar]