Abstract
Ventilator-associated pneumonia (VAP) represents one of the most common infections in patients requiring endotracheal tubes with mechanical ventilation. It is a major healthcare burden measured by increased hospital costs, greater number of ICU days, longer duration of mechanical ventilation, and higher mortality. However, despite widely accepted recommendations for interventions designed to reduce rates of VAP, there are surprisingly few studies that validate the ability of these interventions to improve patient outcomes, namely fewer intensive care unit (ICU) or hospital days, and mortality. Possible reasons for this absence of convincing data include the inability to correctly diagnose VAP and/or an overly expansive interpretation of what the evidence in the literature supports. As advances in our understanding of VAP improve, and new technologies to reduce VAP become available, studies should directly assess patient outcomes before the health care community broadly requires specific prevention approaches in clinical practice.
Case Presentation
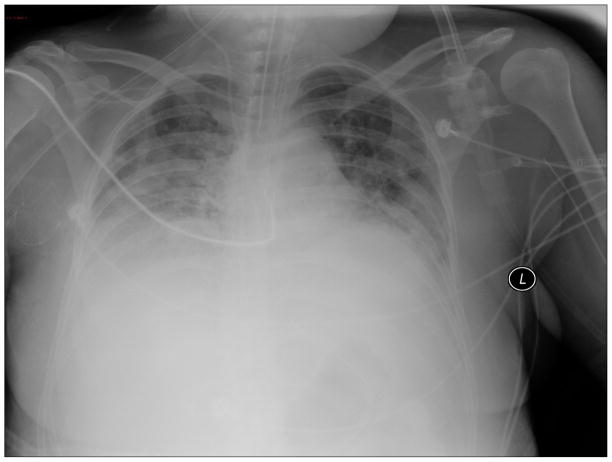
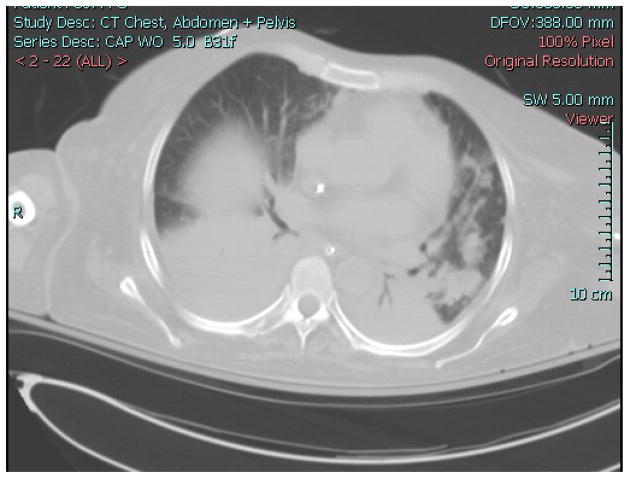
A woman was admitted to the National Institutes of Health Clinical Center because of worsening diarrhea and abdominal pain approximately one year after a matched-related stem cell transplant (SCT) for a hematological malignancy. Her prior clinical course had been complicated by graft-versus host disease, Clostridium difficile infection, and re-activation of cytomegalovirus infection. She developed abdominal pain and distention, tachypnea and tachycardia over the next 24 hours. A CT scan of the chest and abdomen revealed bilateral pulmonary infiltrates and dilatation of the transverse colon. She was admitted to the intensive care unit (ICU), intubated, and started empirically on vancomycin, meropenem, levofloxacin and anidulafungin. A bronchoalveolar lavage (BAL) recovered no respiratory pathogens. She remained intubated for eight days, improved, and was then extubated. However, she failed to ventilate adequately and was re-intubated on ICU day 10. A chest x-ray and CT scan showed worseninginfiltrates (Figure 1). Her antibiotics were changed to linezolid, meropenem, levofloxacin, colistin, tigecycline, anidulafungin and foscarnet. She underwent BAL that again revealed no pathogens. The following day a tracheal aspirate grew multidrug resistant (MDR) Acinetobacter baumannii. She developed septic shock, worsening hypoxia, and died several days later. On autopsy, the lung pathology showed bacterial pneumonia (Figure 2) and lung culture grew MDR Acinetobacter baumannii. The antibiotic susceptibility pattern matched the tracheal aspirate.
Figure 1.
Figure 2.
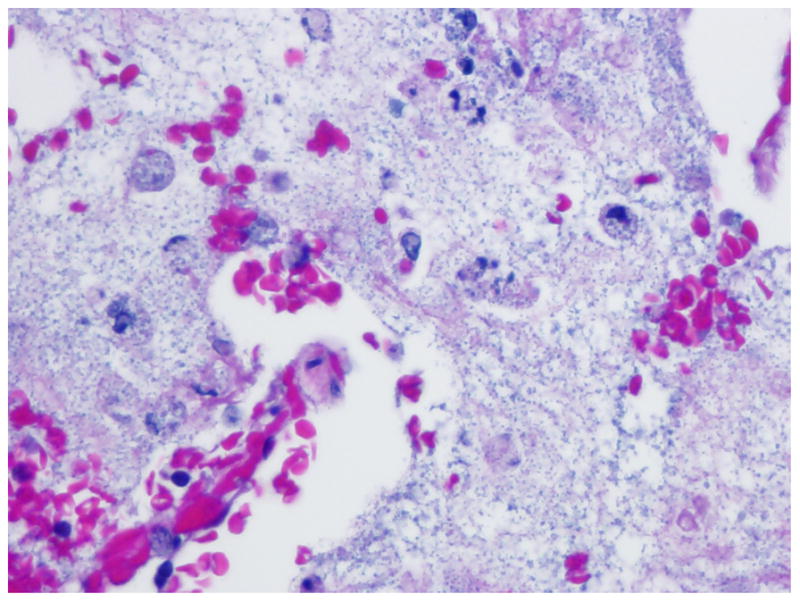
Lung Pathology.
Lung parenchyma showing abundant bacterial elements (hematoxylineosin stain; original magnification _40×).
This case illustrates the difficulty in diagnosing VAP. While this was a complicated patient, making the diagnosis of VAP is difficult even in less complex patients. Negative pre-mortem BAL cultures in patients with VAP are well documented and attributed to concurrent antimicrobial therapy, collection of specimens from noninvolved areas of the lung, and dilution of the specimen resulting in an insufficient quantity of bacteria.1 It is no surprise that given the difficulty in making a clinical diagnosis of VAP, definitive studies on management and prevention of VAP have yielded inconclusive or inconsistent results.
This Grand Rounds will review the clinical impact and pathogenesis of VAP, the role of the oral microbiome in the development of VAP, and the strategies recommended in the guidelines published by the American Thoracic Society and the Infectious Disease Society 2 that provide the basis for the Institute of Healthcare Improvement’s (IHI) bundle to prevent ventilator associated adverse events.
Clinical Impact of Ventilator Associated Pneumonia
Defining the incidence of VAP is complicated because there is often overlap with other lower respiratory infections such as ventilator-associated tracheobronchitis.2 The incidence varies and depends on the surveillance strategy employed, case mix, case definition, diagnostic procedures, and the way in which rates are expressed.3
VAP is one of the major causes of nosocomial infection in intubated patients.4 In studies examining the association between VAP and patient outcomes, VAP has been associated with increased lengths of ICU stay and higher mortality, although the attributable mortality due to VAP is controversial.5 Hospital costs are higher in patients with VAP.6 Lastly, the isolation of MDR organisms from intubated patients with pulmonary dysfunction suggests that management of VAP is becoming more difficult.7 Despite the general agreement about the morbidity, excess cost, and the need to prevent VAP, controversy exists about definitions for VAP and strategies for its prevention.
Two organizations have issued separate definitions of VAP, which use different timelines to meet criteria (Table 1). It is important to note that these are surveillance definitions, which are less rigorous than clinical definitions. Most institutions use the less rigorous surveillance definitions to monitor their rates, which have been devised to simplify and standardize the diagnosis of VAP. However, even with careful adherence to the definition, there is high inter-observer variability in the interpretation of evidence for VAP.1 The difference in the timelines is also problematic when comparing institutional rates against benchmarks. For example, the rate of VAP will be higher if the NHSN definition is used because it will include cases that occur within the first 48 hours of intubation, which the ATS/IDSA definition excludes. Therefore, inter-institutional comparisons and benchmarks may not be valid.
Table 1.
Definitions of VAP
| CDC National Healthcare Safety Network 54 | American Thoracic Society and Infectious Disease Society of America2 | |
|---|---|---|
| Timeline | Pneumonia in persons who had a device to assist or control respiration continuously through a tracheostomy or by endotracheal intubation within the 48-hour period before the onset of infection, inclusive of the weaning period | Pneumonia that occurs more than 48- 72 hours after intubation |
| Clinical signs | Change in pulmonary secretions or impaired gas exchange and systemic signs of infection | Change in pulmonary secretions or impaired gas exchange and systemic signs of infection |
| Radiographic evidence | New or progressive opacities | New or progressive opacities |
| Microbiologic evidence | None required | None required |
Despite the uncertainties of the diagnosis, hospitals and clinicians are under pressure from legislators and regulatory agencies to implement VAP prevention strategies. This comes as legislators consider requiring hospitals to publicly report VAP rates and the Centers for Medicare and Medicaid consider adding VAP to the list of hospital-acquired conditions that are non-reimbursable. Yet reporting rates of VAP will not be meaningful until measured rates are reliable and prevention strategies produce concomitant reductions in meaningful clinical outcomes.
Risk Factors for VAP
Although any patient with an endotracheal tube (ETT) in place is at risk for VAP, certain patients are at higher risk. Across numerous studies that use distinct definitions for VAP, consistent risk factors include underlying chronic lung disease, age >70, depressed levels of consciousness, aspiration, elevating gastric pH, and previous antibiotic exposure.8–10 Poor prognostic indictors include inappropriate antibiotic therapy, high severity of underlying disease, the presence of bacteremia, and time to onset of VAP.11, 12 Patients with late onset VAP (>7days) have higher mortality rates than patients with early onset VAP. The distribution of MDR organisms are significantly more frequent in patients with late-onset VAP.11, 12 This difference in distribution pattern is linked to the frequency in which patients have had prior antimicrobial therapy and to the local epidemiology.
Microbiology
Accurate data on the microbiology of VAP are limited by the absence of standardized criteria for diagnosis. The prevalence of pathogens vary considerably, depending on the patient population, the duration of hospitalization and mechanical ventilation (MV), prior exposure to antibiotics, and criteria used for diagnosis.13 The etiology will depend on the patient’s risk for MDR pathogens and the hospital’s local microbiology, and may be suggested by knowledge of microorganisms with which the patient is colonized.
Pathogenesis
VAP is directly linked to colonization of the oral cavity with potential respiratory pathogens that are aspirated. Leaking of the oral flora around the ETT occurs in virtually all patients (Figure 3).14, 15 Biofilms on the ETT provide a direct route for bacteria into the lower airways.16 Other pathways such as sinus or gastric colonization, inhalation of aerosolized bacteria from contaminated healthcare devices, and bacteremia with hematogenous spread play much smaller roles.2
Recent investigations have closely examined the oral microbiome as a means to understand how changes in the microbial community can contribute to the development of VAP. Knowledge of the oral microbiome had been limited in the past by lack of sensitive and specific microbiologic methods to identify bacteria that cannot be cultured by routine methods. This obstacle has become less limiting recently, with the use of next-generation sequencing to characterize microbial communities.17
VAP and Oral Microbiome
The human oral microbiome is composed of all microbial species in the oral cavity. The teeth, gingival, tongue and oral mucosa all support unique and distinctive bacteria in healthy individuals.18 Individuals with illness, taking antibiotics or immunosuppressants may have very different flora. This results from a reduction in microbial diversity from antibiotic selection that contributes directly to pathogen selection through loss of microbial competition.19
In order to characterize the bacteria in the oral pharynx and in the concominant secretions from the lungs of intubated patients, investigators have studied oral swabs from the tongues and BAL from intubated patients.20 Clone libraries, molecular sequencing, and standard cultures were performed and demonstrated that the majority of VAP patients had the same bacteria in their oral cavaties as in their lungs, suggesting that the mouth flora was the source of bacteria in the lungs. Importantly, they also found that more than half of the pathogens found in the lung were not identified by routine culture.
In a similar study another group of investigators sampled supragingival plaque from 100 intubated critically ill trauma patients.21 Tracheal aspirates were collected, and in 30 patients, BAL fluid was obtained. In 60% of the patients, respiratory pathogens were identified from oral specimens. Eighteen of the 30 who had BALs had further analysis using pulse-field gel electropheresis (PFGE) to identify potential respiratory pathogens. These patients had more than one respiratory pathogen identified in all three sampled sites. The majority of isolates that were recovered from the plaque genetically matched the organisms from the BAL by PFGE pattern.
Taken in total, these studies suggest that when a patient develops VAP, the bacteria in their lung secretions are the similar to the bacteria that are present in their oral flora, and are more numerous than previously demonstrated. Further, pneumonia may be caused by either previously recognized organisms or by pathogens not previously identified by standard culture methods. If bacterial community changes in the mouth could be monitored using more comprehensive culture-independent methods in a timely and cost-effective manner, surveillance of the mouth for reduction in microbial diversity and pathogen selection may help predict and guide therapy for patients who develop VAP.
PREVENTION STRATEGIES FOR VAP
Bundles
Bundles are a set of processes of care that, when instituted as a group, provide more robust results than when each process is instituted individually. This is particularly true when components interact with each other synergistically or when partial execution fails to achieve the desired result.22 Credit for delivering each component can only be obtained if the entire bundle is executed correctly. In other words, credit for delivery is all or none.
In order for a bundle to be effective, each component must have an explicit rationale.22 There should be a logical relationship between the elements (additive and not antagonistic), and there should be strong evidence that each component improves the targeted outcome.22 The Institute for Healthcare Improvement (IHI) has developed a ventilator-bundle that incorporates several strategies to prevent morbidity associated with the ventilator. Three elements of this bundle target VAP, while two other elements target stress ulcer prevention and prevention of thromboembolic disease (Table 2).23 The IHI ventilator bundle has been broadly adopted by many hospitals as part of an effort to reduce VAP, and is often mistakenly called a VAP bundle. It is instead a “ventilator associated adverse-event” bundle, with the element requiring histamine-2-antagonsists for stress ulcer prophylaxis at odds with preventing VAP,24 and no relationship between the element requiring deep venous thrombosis prophylaxis and preventing VAP.
Table 2.
Components of the IHI Ventilator Bundle
| Intervention | Target for Prevention |
|---|---|
| Elevation of the head of the bed to 45 degrees | VAP |
| Daily sedation vacations and assessment of readiness to extubate | VAP |
| Daily oral care with chlorhexidine | VAP |
| Proton pump inhibitors or H2 receptor antagonists | Peptic Ulcer Disease |
| Anticoagulants or compression devices | Deep Venous Thrombosis |
Elevation of the Head of Bed
Elevating the head of the bed to 45° at all times is one of the recommendations to prevent VAP. The strategy is based on the observation that gastric reflux and aspiration of gastric contents into the lung may be prevented by placing the patient in a semi-recumbent position with the head of the bed elevated to 30–45°.25 In two small studies, aspiration and VAP were reduced by almost three-fold for patients with the head of the bed at 45°, compared to patients who were supine.10, 25 Elevating the head of the bed is better than having the patient supine, but a 45° elevation is difficult to achieve,26 because patients who are sedated are unable to maintain their head in an upright position. Further, patients who are hypotensive cannot have the head of the bed elevated to 45°. Lastly, semi-recumbency fails to take into account the gravitational forces that facilitate leakage of pooled secretions around the ETT,27 and may increase the risk of thromboembolism and hemodynamic instability.28
The benefit for the semi-recumbent position was shown in studies in which the controls were supine. A study comparing 45° to standard-of care (10°) showed no difference in rates of VAP, although 45° was rarely achieved (28.1° was achieved).26 It is not clear that 45° are necessary. It is possible that 10° are sufficient compared to being supine, and that additional elevation makes no difference.
Daily Interruption of Sedation
Using daily interruptions of sedation to assess the patient’s readiness to extubate has been incorporated into the IHI ventilator bundle because it reduces the duration of MV.29 Spontaneous awakening trials added to spontaneous breathing trials improve ventilator weaning, and shorten the number of ICU and hospital days.30 Since duration of MV is a risk for the development of VAP, reducing the number of days of MV should correlate to a reduced rate of VAP.23 However, there are no published studies that demonstrate a reduction in VAP rates utilizing this strategy. Further, this strategy is not without risks. Reducing a patient’s sedation increases the risk for pain, anxiety and self-extubation. Neither study enrolled surgical or trauma patients whose needs for analgesia may be different than medical patients. For this reason, daily interruption of sedation may be best applied to selected patients in whom the benefit outweighs these risks.
Chlorhexidine Oral Care
Because the pathophysiology of VAP involves aspiration of contaminated secretions into the respiratory tract, efforts have been made to decontaminate the mouth with chlorhexidine in order to prevent VAP. Although chlorhexidine has been shown to be useful in preventing VAP in the trauma and cardiac surgery patient population, it has not shown benefit for any other patient group.31–33 Given that the trauma and cardiac surgery patient populations studied had short periods of intubation, it is thought that chlorhexidine may simply delay the onset of VAP. Chlorhexidine mouth care had no mortality benefit, and had no impact on the number of ventilator days or days in the ICU, but was added to the IHI bundle for all ventilated patients, despite the paucity of evidence showing benefit for most patients.
Another decontamination strategy studied to reduce VAP is selective decontamination of the digestive tract (SDD). This strategy uses a combination of non-absorbable antibiotics in the oropharynx and the gastrointestinal tract combined with an intravenous cephalosporin.
Several meta-analyses have demonstrated that the use of SDD is associated with significant reductions in the incidence of VAP and lower rates of hospital mortality, particularly among surgical patients.34–36 However, in the largest multicenter study published to date, there was no difference in crude mortality in patients receiving SDD compared to controls.37 Only after adjusting for covariates was there a reduction in mortality by 3.5% for patients receiving SDD. In this study, SDD had marked effects on the bacterial ecology in the ICUs, with rising rates of ceftazadime resistance in the respiratory tract during the intervention and continued resistance in the gastrointestinal tract after discontinuation of SDD.38 Whether SDD is effective and safe remains controversial.
Novel Strategies Not Included in the Current Ventilator Bundle
Positioning
A new approach to positioning has been evaluated in an animal model of VAP, and is now being studied in humans. In the first published animal study, sheep were randomized to either a head-up position to mimic a semi-recumbent position, or head-down position with the ETT/trachea below the horizontal plane 39. Half of the sheep in the head-down position received enteral feedings and half did not. Sheep in the head-down position were placed in a rotational system that allowed turning the animal from side to side at a timed interval. Animals were ventilated for 72 hours and then sacrificed. Histologic and microbiologic evaluation demonstrated that sheep in the head-up position had heavy bacterial colonization of the lungs and a significant decrease in PaO2/Fio2. The sheep in the head down position had no evidence of bacterial colonization, VAP or impaired PaO2/FiO2.39
The first clinical trial in humans to study the relationship of positioning and bacterial colonization randomized 60 intubated infants to either supine or lateral position (keeping the orientation of the ETT/trachea at or below the horizontal plane).40 After 5 days of MV, tracheal cultures were positive in 26/30 infants in the supine group and 9/30 in the lateral group (p<0.05). Maintaining a ventilated adult patient in a lateral position has been tested successfully in a pilot study 41 for feasibility, and a larger randomized multi-center study has been designed to assess whether the lateral head-down position may prevent VAP in adults.42
These studies reinforce the importance of patient positioning but suggest that the current practice of elevating the head of the bed may not be the best strategy as aspiration and gravitational forces create a continuous bacterial challenge to the ETT. Using gravity to help move secretions away from the ETT cuff may make sense if this positioning can be maintained safely in critically ill patients.
Subglottic Suctioning
Secretions in the upper airways of intubated patients pool above the ETT cuff, allowing for leakage of contaminated secretions into the lower airway. In several studies, the effect of using an ETT that has a separate dorsal lumen, which allows continuous aspiration of the subglottic secretions, was compared with that of a conventional ETT.43–45 Although studies showed a beneficial effect of continuous suctioning of subglottic secretions on the incidence of VAP, none showed a corresponding effect on mortality rate, length of stay in the ICU, or duration of MV. Additionally, the aspiration port clogs easily and the continuous suction has the potential to injure the oropharynx and proximal airway.
Preventing Biofilm Formation
Biofilms form on surfaces of ETTs when bacteria encounter surfaces and “settle” on that surface, up-regulating genes involved in matrix production.46 The recognition of these biofilms has resulted in the development of several approaches aimed at limiting their formation. One approach is to incorporate an antiseptic such as silver onto the intra-luminal surface of the ETT. Some studies have shown a reduction in the incidence of VAP and delayed time to VAP, however, reductions in days of ventilation, hospital stays, or mortality have not been demonstrated.47
Mucus Shaver
A novel approach to keeping the ETT free from secretions is the mucus shaver, a concentric inflatable catheter for removal of mucus from the interior lumen of the ETT.42 The normal method for cleaning and preventing occlusion of the ETT is to insert a small flexible suction catheter to the distal portion of the ETT and withdraw it slowly while maintaining suction. This method leaves residual secretions, which may organize into biofilms. Residual bacteria may also spread to the lower airways via micro-aspiration causing pneumonia.
To improve the process, a group of investigators designed a molded silicon rubber tube with 2 shaving rings that allow for a complete cleaning of the ETT with one pass. The device is inserted to the distal portion of the ETT, inflated such that the shaver’s edge is in contact with the interior lumen of the ETT, and withdrawn over a period of 3–6 seconds, removing all accumulated mucus. This device was first tested in mechanically ventilated sheep.48 The ETTs that were cleaned with the mucus shaver showed no colonization or residual secretions. The device has subsequently been tested in humans in a small, randomized controlled trial.49 At the time of extubation, 1/12 tubes in the mucus shaver group was colonized, while 10/12 in the control group was colonized (p<0.001).
This approach would represent an intriguing advance in our ability to clean and maintain an ETT free from mucus and biofilms if larger trials show efficacy.
Summary
Despite broad implementation of a bundled strategy aimed at preventing ventilator-associated adverse events in many hospitals, there are no convincing published reports of reductions in VAP using this bundle. Two studies that have reported on the effectiveness of implementing the ventilator bundle did not report adherence rates to the bundle.50, 51 Another study evaluating this bundle reported a 95% compliance with the bundle and an associated reduction in VAP, but investigators acknowledged that the reduction may have resulted from a confounding concurrent improvement program that focused on care of the ventilated patient with multidisciplinary teams and daily goal-setting rather than adherence to the bundle.52
No large randomized study has demonstrated that reducing VAP using any VAP prevention strategy, including those in the IHI bundle, translates into any meaningful clinical outcome including reduced mortality (Supplemental Table 3). This likely stems from the non-specificity of VAP definitions, resulting in mislabeling benign events as VAP and misdiagnosing non-infectious pulmonary dysfunction as VAP.53
Before clinicians broadly adopt a bundled strategy in all patients, the evidence should first demonstrate that implementing the bundle would improve patient outcomes. While bundles are an important advance in healthcare delivery and have the potential to improve clinical outcomes, healthcare providers should rely on rigorous studies demonstrating that a bundle is effective and not harmful, so that resources can be directed to the most productive strategies for preventing complications and limiting morbidity.
Supplementary Material
References
- 1.Rea-Neto A, Youssef NC, Tuche F, et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care. 2008;12(2):R56. doi: 10.1186/cc6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ATS. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb 15;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Eggimann P, Hugonnet S, Sax H, Touveneau S, Chevrolet JC, Pittet D. Ventilator-associated pneumonia: caveats for benchmarking. Intensive Care Med. 2003 Nov;29(11):2086–2089. doi: 10.1007/s00134-003-1991-9. [DOI] [PubMed] [Google Scholar]
- 4.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009 Dec;37(10):783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bekaert M, Timsit JF, Vansteelandt S, et al. Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med. 2011 Nov 15;184(10):1133–1139. doi: 10.1164/rccm.201105-0867OC. [DOI] [PubMed] [Google Scholar]
- 6.Warren DK, Shukla SJ, Olsen MA, et al. Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med. 2003 May;31(5):1312–1317. doi: 10.1097/01.CCM.0000063087.93157.06. [DOI] [PubMed] [Google Scholar]
- 7.Park DR. The microbiology of ventilator-associated pneumonia. Respir Care. 2005 Jun;50(6):742–763. discussion 763–745. [PubMed] [Google Scholar]
- 8.Chastre J, Trouillet JL, Vuagnat A, et al. Nosocomial pneumonia in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1165–1172. doi: 10.1164/ajrccm.157.4.9708057. [DOI] [PubMed] [Google Scholar]
- 9.Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008 Oct;29(Suppl 1):S31–40. doi: 10.1086/591062. [DOI] [PubMed] [Google Scholar]
- 10.Kollef MH. Ventilator-associated pneumonia. A multivariate analysis. JAMA. 1993 Oct 27;270(16):1965–1970. [PubMed] [Google Scholar]
- 11.Agbaht K, Diaz E, Munoz E, et al. Bacteremia in patients with ventilator-associated pneumonia is associated with increased mortality: A study comparing bacteremic vs. nonbacteremic ventilator-associated pneumonia. Crit Care Med. 2007 Sep;35(9):2064–2070. doi: 10.1097/01.CCM.0000277042.31524.66. [DOI] [PubMed] [Google Scholar]
- 12.Luna CM, Aruj P, Niederman MS, et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J. 2006 Jan;27(1):158–164. doi: 10.1183/09031936.06.00049105. [DOI] [PubMed] [Google Scholar]
- 13.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002 Apr 1;165(7):867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 14.Cook D, De Jonghe B, Brochard L, Brun-Buisson C. Influence of airway management on ventilator-associated pneumonia: evidence from randomized trials. Jama. 1998 Mar 11;279(10):781–787. doi: 10.1001/jama.279.10.781. [DOI] [PubMed] [Google Scholar]
- 15.du Moulin GC, Paterson DG, Hedley-Whyte J, Lisbon A. Aspiration of gastric bacteria in antacid-treated patients: a frequent cause of postoperative colonisation of the airway. Lancet. 1982 Jan 30;1(8266):242–245. doi: 10.1016/s0140-6736(82)90974-6. [DOI] [PubMed] [Google Scholar]
- 16.Inglis TJ, Millar MR, Jones JG, Robinson DA. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989 Sep;27(9):2014–2018. doi: 10.1128/jcm.27.9.2014-2018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuster SC. Next-generation sequencing transforms today’s biology. Nat Methods. 2008 Jan;5(1):16–18. doi: 10.1038/nmeth1156. [DOI] [PubMed] [Google Scholar]
- 18.Mager DL, Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Distribution of selected bacterial species on intraoral surfaces. J Clin Periodontol. 2003 Jul;30(7):644–654. doi: 10.1034/j.1600-051x.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 19.Flanagan JL, Brodie EL, Weng L, et al. Loss of bacterial diversity during antibiotic treatment of intubated patients colonized with Pseudomonas aeruginosa. J Clin Microbiol. 2007 Jun;45(6):1954–1962. doi: 10.1128/JCM.02187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahrani-Mougeot FK, Paster BJ, Coleman S, et al. Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 2007 May;45(5):1588–1593. doi: 10.1128/JCM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo SM, Haase EM, Lesse AJ, Gill SR, Scannapieco FA. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis. 2008 Dec 15;47(12):1562–1570. doi: 10.1086/593193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolan T, Berwick DM. All-or-none measurement raises the bar on performance. JAMA. 2006 Mar 8;295(10):1168–1170. doi: 10.1001/jama.295.10.1168. [DOI] [PubMed] [Google Scholar]
- 23.IHI. [accessed Jan 24, 2012];Implement the IHI Ventilator Bundle. http://www.ihi.org/knowledge/Pages/Changes/ImplementtheVentilatorBundle.aspx.
- 24.Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004 Aug 17;141(4):305–313. doi: 10.7326/0003-4819-141-4-200408170-00011. [DOI] [PubMed] [Google Scholar]
- 25.Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999 Nov 27;354(9193):1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 26.van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit Care Med. 2006 Feb;34(2):396–402. doi: 10.1097/01.ccm.0000198529.76602.5e. [DOI] [PubMed] [Google Scholar]
- 27.Berra L, Panigada M, De Marchi L, et al. New approaches for the prevention of airway infection in ventilated patients. Lessons learned from laboratory animal studies at the National Institutes of Health. Minerva Anestesiol. 2003 May;69(5):342–347. [PubMed] [Google Scholar]
- 28.Niel-Weise BS, Gastmeier P, Kola A, Vonberg RP, Wille JC, van den Broek PJ. An evidence-based recommendation on bed head elevation for mechanically ventilated patients. Crit Care. 2011;15(2):R111. doi: 10.1186/cc10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000 May 18;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 30.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008 Jan 12;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 31.Labeau SO, Van de Vyver K, Brusselaers N, Vogelaers D, Blot SI. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis. 2009 Nov;11(11):845–854. doi: 10.1016/S1473-3099(11)70127-X. [DOI] [PubMed] [Google Scholar]
- 32.Chan EY. Oral decontamination with chlorhexidine reduced ventilator associated pneumonia in patients needing mechanical ventilation for >/=48 hours. Evid Based Nurs. 2007 Jan;10(1):19. doi: 10.1136/ebn.10.1.19. [DOI] [PubMed] [Google Scholar]
- 33.Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2007 Feb;35(2):595–602. doi: 10.1097/01.CCM.0000253395.70708.AC. [DOI] [PubMed] [Google Scholar]
- 34.D’Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A. Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. Bmj. 1998 Apr 25;316(7140):1275–1285. doi: 10.1136/bmj.316.7140.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev. 2009;(4):CD000022. doi: 10.1002/14651858.CD000022.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathens AB, Marshall JC. Selective decontamination of the digestive tract in surgical patients: a systematic review of the evidence. Arch Surg. 1999 Feb;134(2):170–176. doi: 10.1001/archsurg.134.2.170. [DOI] [PubMed] [Google Scholar]
- 37.de Smet AM, Kluytmans JA, Cooper BS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med. 2009 Jan 1;360(1):20–31. doi: 10.1056/NEJMoa0800394. [DOI] [PubMed] [Google Scholar]
- 38.Oostdijk EA, de Smet AM, Blok HE, et al. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med. 2010 Mar 1;181(5):452–457. doi: 10.1164/rccm.200908-1210OC. [DOI] [PubMed] [Google Scholar]
- 39.Panigada M, Berra L, Greco G, Stylianou M, Kolobow T. Bacterial colonization of the respiratory tract following tracheal intubation-effect of gravity: an experimental study. Crit Care Med. 2003 Mar;31(3):729–737. doi: 10.1097/01.CCM.0000049943.01252.E5. [DOI] [PubMed] [Google Scholar]
- 40.Aly H, Badawy M, El-Kholy A, Nabil R, Mohamed A. Randomized, controlled trial on tracheal colonization of ventilated infants: can gravity prevent ventilator-associated pneumonia? Pediatrics. 2008 Oct;122(4):770–774. doi: 10.1542/peds.2007-1826. [DOI] [PubMed] [Google Scholar]
- 41.Mauri T, Berra L, Kumwilaisak K, et al. Lateral-horizontal patient position and horizontal orientation of the endotracheal tube to prevent aspiration in adult surgical intensive care unit patients: a feasibility study. Respir Care. 2010 Mar;55(3):294–302. [PubMed] [Google Scholar]
- 42.Berra L, Sampson J, Fumagalli J, Panigada M, Kolobow T. Alternative approaches to ventilator-associated pneumonia prevention. Minerva Anestesiol. 2011 Mar;77(3):323–333. [PubMed] [Google Scholar]
- 43.Bouza E, Perez MJ, Munoz P, Rincon C, Barrio JM, Hortal J. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest. 2008 Nov;134(5):938–946. doi: 10.1378/chest.08-0103. [DOI] [PubMed] [Google Scholar]
- 44.Kollef MH, Skubas NJ, Sundt TM. A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients. Chest. 1999 Nov;116(5):1339–1346. doi: 10.1378/chest.116.5.1339. [DOI] [PubMed] [Google Scholar]
- 45.Valles J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995 Feb 1;122(3):179–186. doi: 10.7326/0003-4819-122-3-199502010-00004. [DOI] [PubMed] [Google Scholar]
- 46.Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest. 2003 Nov;112(10):1466–1477. doi: 10.1172/JCI20365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. Jama. 2008 Aug 20;300(7):805–813. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 48.Kolobow T, Berra L, Li Bassi G, Curto F. Novel system for complete removal of secretions within the endotracheal tube: the Mucus Shaver. Anesthesiology. 2005 May;102(5):1063–1065. doi: 10.1097/00000542-200505000-00028. [DOI] [PubMed] [Google Scholar]
- 49.Berra L, Coppadoro A, Bittner EA, et al. A clinical assessment of the Mucus Shaver: A device to keep the endotracheal tube free from secretions*. Crit Care Med. 2012 Jan;40(1):119–124. doi: 10.1097/CCM.0b013e31822e9fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berriel-Cass D, Adkins FW, Jones P, Fakih MG. Eliminating nosocomial infections at Ascension Health. Jt Comm J Qual Patient Saf. 2006 Nov;32(11):612–620. doi: 10.1016/s1553-7250(06)32079-x. [DOI] [PubMed] [Google Scholar]
- 51.Unahalekhaka A, Jamulitrat S, Chongsuvivatwong V, Ovretveit J. Using a collaborative to reduce ventilator-associated pneumonia in Thailand. Jt Comm J Qual Patient Saf. 2007 Jul;33(7):387–394. doi: 10.1016/s1553-7250(07)33044-4. [DOI] [PubMed] [Google Scholar]
- 52.Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf. 2005 May;31(5):243–248. doi: 10.1016/s1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 53.Klompas M. The paradox of ventilator-associated pneumonia prevention measures. Crit Care. 2009;13(5):315. doi: 10.1186/cc8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008 Jun;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.