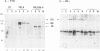Abstract
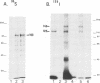
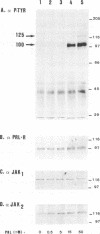
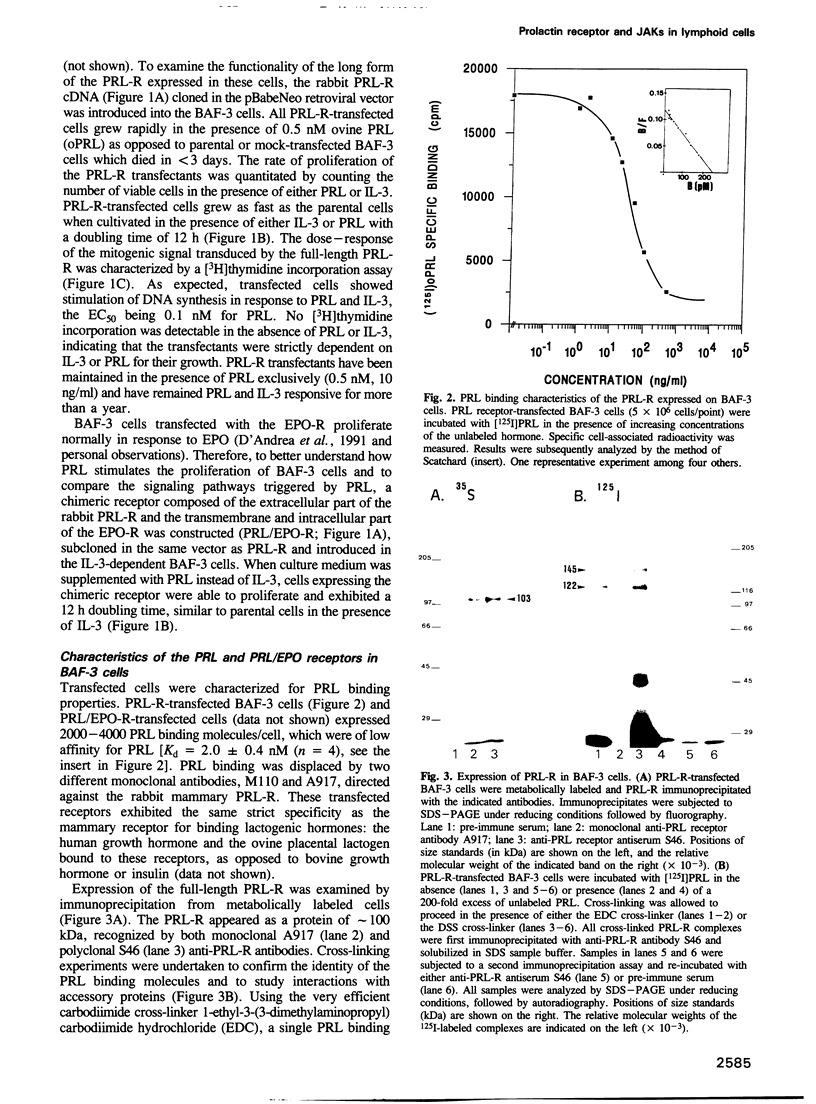
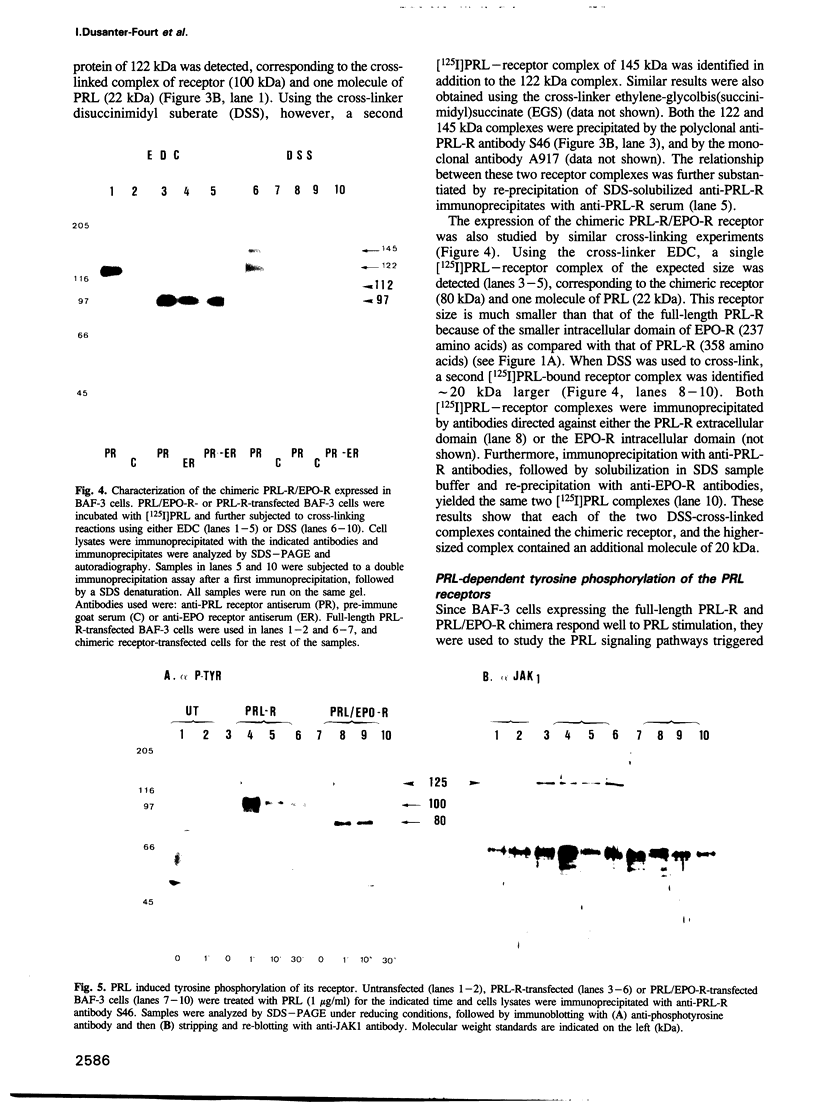
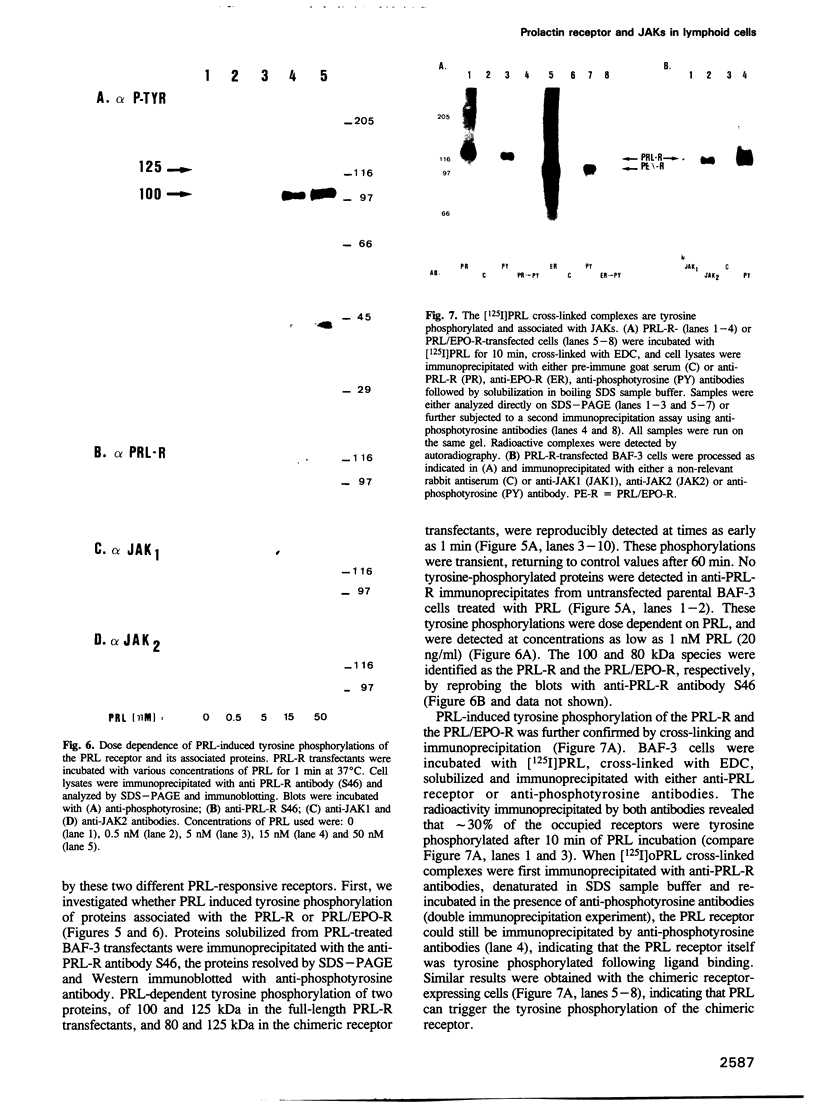
The mechanism of action of prolactin (PRL) was studied in murine lymphoid BAF-3 cells transfected with either the long form of the PRL receptor (PRL-R), or a chimeric receptor consisting of the extracellular domain of the PRL-R and the transmembrane and intracellular domain of the erythropoietin receptor (PRL/EPO-R). PRL sustained normal and long-term proliferation of BAF-3 cells expressing either the PRL-R or the hybrid PRL/EPO-R. Upon [125I]PRL cross-linking, both types of BAF-3 transfectants were shown to express two [125I]PRL cross-linked species differing in size by 20 kDa. These cross-linked complexes, after denaturation, were recognized by antibody against the PRL-R, indicating that they contain the transfected receptor. PRL induced rapid and transient tyrosine phosphorylation of both the PRL-R and the PRL/EPO-R in BAF-3 transfectants. Furthermore, PRL induced rapid tyrosine phosphorylation of the Janus kinase 2 (JAK2) which was already physically associated with the PRL-R or the PRL/EPO-R in the absence of ligand. JAK1 was also associated with PRL-R and PRL/EPO-R in the absence of ligand. However, only in BAF-3 cells expressing the PRL-R does PRL induce rapid and transient tyrosine phosphorylation of JAK1. These results demonstrate that JAK protein tyrosine kinases couple PRL binding to tyrosine phosphorylation and proliferation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S., Pellegrini I., Kelly P. A. A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem. 1991 Oct 25;266(30):20110–20117. [PubMed] [Google Scholar]
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin J. M., Jolicoeur C., Okamura H., Gagnon J., Edery M., Shirota M., Banville D., Dusanter-Fourt I., Djiane J., Kelly P. A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988 Apr 8;53(1):69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- Cahoreau C., Petridou B., Cerutti M., Djiane J., Devauchelle G. Expression of the full-length rabbit prolactin receptor and its specific domains in baculovirus infected insect cells. Biochimie. 1992 Dec;74(12):1053–1065. doi: 10.1016/0300-9084(92)90003-w. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carter-Su C., Stubbart J. R., Wang X. Y., Stred S. E., Argetsinger L. S., Shafer J. A. Phosphorylation of highly purified growth hormone receptors by a growth hormone receptor-associated tyrosine kinase. J Biol Chem. 1989 Nov 5;264(31):18654–18661. [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Casadevall N., Lacombe C., Muller O., Billat C., Fischer S., Mayeux P. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992 May 25;267(15):10670–10675. [PubMed] [Google Scholar]
- Dusanter-Fourt I., Kelly P. A., Djiane J. Immunological recognition of the prolactin receptor: identification of a single binding unit of molecular weight approximately 42,000. Biochimie. 1987 Jun-Jul;69(6-7):639–646. doi: 10.1016/0300-9084(87)90183-0. [DOI] [PubMed] [Google Scholar]
- Edery M., Jolicoeur C., Levi-Meyrueis C., Dusanter-Fourt I., Pétridou B., Boutin J. M., Lesueur L., Kelly P. A., Djiane J. Identification and sequence analysis of a second form of prolactin receptor by molecular cloning of complementary DNA from rabbit mammary gland. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2112–2116. doi: 10.1073/pnas.86.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuh G., Colosi P., Wood W. I., Wells J. A. Mechanism-based design of prolactin receptor antagonists. J Biol Chem. 1993 Mar 15;268(8):5376–5381. [PubMed] [Google Scholar]
- Gala R. R. Prolactin and growth hormone in the regulation of the immune system. Proc Soc Exp Biol Med. 1991 Oct;198(1):513–527. doi: 10.3181/00379727-198-43286b. [DOI] [PubMed] [Google Scholar]
- Hooghe R., Delhase M., Vergani P., Malur A., Hooghe-Peters E. L. Growth hormone and prolactin are paracrine growth and differentiation factors in the haemopoietic system. Immunol Today. 1993 May;14(5):212–214. doi: 10.1016/0167-5699(93)90165-h. [DOI] [PubMed] [Google Scholar]
- Ishizaka-Ikeda E., Fukunaga R., Wood W. I., Goeddel D. V., Nagata S. Signal transduction mediated by growth hormone receptor and its chimeric molecules with the granulocyte colony-stimulating factor receptor. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):123–127. doi: 10.1073/pnas.90.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M., Djiane J., Kelly P. A. Monoclonal antibodies against rabbit mammary prolactin receptors. Specific antibodies to the hormone binding domain. J Biol Chem. 1985 Sep 25;260(21):11422–11429. [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Kono T., Hatakeyama M., Minami Y., Miyazaki T., Perlmutter R. M., Taniguchi T. Functional coupling of the src-family protein tyrosine kinases p59fyn and p53/56lyn with the interleukin 2 receptor: implications for redundancy and pleiotropism in cytokine signal transduction. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4201–4205. doi: 10.1073/pnas.90.9.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesueur L., Edery M., Ali S., Paly J., Kelly P. A., Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnekin D., Evans G., Michiel D., Farrar W. L. Characterization of a 97-kDa phosphotyrosylprotein regulated by multiple cytokines. J Biol Chem. 1992 Nov 25;267(33):23993–23998. [PubMed] [Google Scholar]
- Mayeux P., Lacombe C., Casadevall N., Chretien S., Dusanter I., Gisselbrecht S. Structure of the murine erythropoietin receptor complex. Characterization of the erythropoietin cross-linked proteins. J Biol Chem. 1991 Dec 5;266(34):23380–23385. [PubMed] [Google Scholar]
- Miyajima A., Kitamura T., Harada N., Yokota T., Arai K. Cytokine receptors and signal transduction. Annu Rev Immunol. 1992;10:295–331. doi: 10.1146/annurev.iy.10.040192.001455. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993 Oct 1;82(7):1960–1974. [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Nicoll C. S. Ontogeny and evolution of prolactin's functions. Fed Proc. 1980 Jun;39(8):2563–2566. [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Rillema J. A., Campbell G. S., Lawson D. M., Carter-Su C. Evidence for a rapid stimulation of tyrosine kinase activity by prolactin in Nb2 rat lymphoma cells. Endocrinology. 1992 Aug;131(2):973–975. doi: 10.1210/endo.131.2.1639035. [DOI] [PubMed] [Google Scholar]
- Rui H., Djeu J. Y., Evans G. A., Kelly P. A., Farrar W. L. Prolactin receptor triggering. Evidence for rapid tyrosine kinase activation. J Biol Chem. 1992 Nov 25;267(33):24076–24081. [PubMed] [Google Scholar]
- Sakamaki K., Wang H. M., Miyajima I., Kitamura T., Todokoro K., Harada N., Miyajima A. Ligand-dependent activation of chimeric receptors with the cytoplasmic domain of the interleukin-3 receptor beta subunit (beta IL3). J Biol Chem. 1993 Jul 25;268(21):15833–15839. [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silva C. M., Weber M. J., Thorner M. O. Stimulation of tyrosine phosphorylation in human cells by activation of the growth hormone receptor. Endocrinology. 1993 Jan;132(1):101–108. doi: 10.1210/endo.132.1.7678212. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Witthuhn B. A., Quelle F. W., Cleveland J. L., Yi T., Ihle J. N. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992 Dec;6(15):3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- Veals S. A., Schindler C., Leonard D., Fu X. Y., Aebersold R., Darnell J. E., Jr, Levy D. E. Subunit of an alpha-interferon-responsive transcription factor is related to interferon regulatory factor and Myb families of DNA-binding proteins. Mol Cell Biol. 1992 Aug;12(8):3315–3324. doi: 10.1128/mcb.12.8.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Venkitaraman A. R., Cowling R. J. Interleukin 7 receptor functions by recruiting the tyrosine kinase p59fyn through a segment of its cytoplasmic tail. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12083–12087. doi: 10.1073/pnas.89.24.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling D., Guschin D., Müller M., Silvennoinen O., Witthuhn B. A., Quelle F. W., Rogers N. C., Schindler C., Stark G. R., Ihle J. N. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993 Nov 11;366(6451):166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G., Kurban R. R., Ralph S. J., Zürcher G., Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991 Apr;11(4):2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Yu-Lee L. Y., Hrachovy J. A., Stevens A. M., Schwarz L. A. Interferon-regulatory factor 1 is an immediate-early gene under transcriptional regulation by prolactin in Nb2 T cells. Mol Cell Biol. 1990 Jun;10(6):3087–3094. doi: 10.1128/mcb.10.6.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]