Abstract
Objective
Postlaryngectomy stricture formation and dysphagia negatively affect quality of life and result in nutritional compromise. Understanding risk factors and successful treatment strategies may improve treatment outcomes.
Study Design
Historical cohort study.
Setting
Tertiary care medical center.
Subjects and Methods
Patients at a tertiary care center who underwent a total laryngectomy between 2003 and 2009 (N = 263) were evaluated in a retrospective manner. Patient demographics, comorbidities, tobacco and alcohol usage, dietary outcomes, feeding tube dependence, and treatment modalities were assessed. Management strategies and outcomes were evaluated.
Results
Strictures developed in 19% (n = 49) of patients, and the majority (82%) occurred in the first year. Incidences of stricture formation were similar for primary (19%) and salvage laryngectomy (19%) patients. Patients undergoing salvage laryngectomy were 2 times more likely to be reconstructed with a free flap, whereas those undergoing a primary laryngectomy were 3 times more likely to be closed primarily. Tubed flap reconstruction significantly increased the incidence of stricture formation compared to primary closure (P = .02) in salvage laryngectomy cases. In primary laryngectomy patients, stricture formation did not correlate with flap reconstruction (P = .34) or adjuvant radiation therapy (P = .79). Patients who required a single dilation had better dietary outcomes compared to patients who required serial dilations (P = .14). There was no difference in overall disease-free survival in primary vs salvage laryngectomy patients (P = .95).
Conclusion
Rates of stricture formation were the same in patients undergoing salvage compared to primary total laryngectomy.
Keywords: stricture, squamous cell carcinoma, head and neck, survival
Advanced laryngeal cancer requires aggressive treatment with either chemotherapy and radiation therapy or surgical ablation. In addition, it is common for patients initially treated with chemotherapy and radiation therapy to require salvage surgical ablation for disease persistence or recurrence. When determining the best course of treatment, patient quality of life is important to consider. Pharyngeal, hypopharyngeal, and esophageal stricture formation are potential sequelae of both organ preservation modalities and a total laryngectomy. Stricture formation can negatively affect patient quality of life and compromise nutritional status.
Factors previously implicated in stricture formation include type of reconstruction and history of neoadjuvant or adjuvant radiation.1,2 When resecting a malignancy of the oropharynx and larynx, there is always the potential for swallowing dysfunction, which can limit oral intake and increase the risk for aspiration. Even in the realm of organ preservation modalities, such as radiation therapy and combination chemotherapy and radiation therapy, there are known functional sequelae.1,2 These include pharyngeal, hypopharyngeal, and esophageal stenosis and swallowing dysfunction. It is recommended that free flap closure be used for radiation therapy failures, but there is minimal outcome data on this practice in the literature. Furthermore, a comparison of free flap reconstruction for salvage laryngectomy patients vs primary laryngectomy patients has been unfavorable.3,4 In addition, there are no recent publications from institutions where free flap closures are routinely performed in this patient population. We hypothesize that outcomes are now equivalent between these 2 groups. This study was designed to evaluate the risk factors of stricture formation following total laryngectomy and to survey the differences between patients who received neoadjuvant or concurrent radiation vs surgery as initial treatment.
Materials and Methods
Patient Selection
Following approval by the University of Alabama at Birmingham Institutional Review Board for Human Use, a retrospective cohort review was performed. Patients who underwent a total laryngectomy at the University of Alabama at Birmingham between January 2003 and December 2009 were included (N = 263). Tumors were staged according to the American Joint Committee on Cancer (AJCC) guidelines,5 and histology was confirmed by pathology. Each patient underwent a total laryngectomy, and the majority had either neoadjuvant or adjuvant radiation therapy (Table 1). Barium swallows were performed postoperatively to confirm patency of the pharynx and that no leaks were present.
Table 1.
Baseline Characteristics of Stricture and Nonstricture Groups
| Characteristic | Stricture Group (n = 49) | Nonstricture Group (n = 214) | P Value |
|---|---|---|---|
| Age, mean, y | 60 | 59 | |
| Gender | |||
| Male | 78 (38) | 82 (176) | .42 |
| Female | 22 (11) | 18 (38) | |
| Initial treatment | |||
| Radiation | 31 (15) | 29 (61) | .80 |
| Chemo/radiation | 22 (11) | 27 (58) | |
| Surgery | 47 (23) | 44 (95) | |
| Adjuvant therapy | |||
| Radiation | 18 (9) | 22 (47) | .39 |
| Chemo/radiation | 14 (7) | 10 (22) | |
| Initial tumor site | |||
| Oropharynx | 0 | 4 (8) | .40 |
| Hypopharynx | 27 (13) | 16 (34) | |
| Larynx | |||
| Supraglottic | 27 (13) | 29 (61) | |
| Glottic | 43 (21) | 49 (105) | |
| Subglottic | 2 (1) | 1 (2) | |
| Other | 2 (1) | 2 (4) | |
| T classification | |||
| 1 | 2 (1) | 2 (5) | .59 |
| 2 | 4 (2) | 11 (23) | |
| 3 | 37 (18) | 34 (73) | |
| 4 | 51 (25) | 49 (105) | |
| TMN stage | |||
| I | 0 | 2 (4) | .24 |
| II | 2 (1) | 7 (15) | |
| III | 22 (11) | 19 (41) | |
| IV | 71 (35) | 68 (145) | |
| Regional metastasis | |||
| No positive nodes | 61 (30) | 61 (131) | 1.00 |
| Positive nodes | 39 (19) | 39 (83) | |
| Distant metastasis | |||
| No metastasis | 84 (41) | 85 (182) | .82 |
| Positive metastasis | 16 (8) | 15 (32) | |
| Follow-up, mo, median (mean) | 18 (26) | 9 (17) | |
| Tobacco history | |||
| No | 6 (3) | 5 (11) | .73 |
| Yes | 86 (42) | 86 (183) | |
| Unknown | 8 (4) | 10 (21) | |
| Ethanol history | |||
| None | 29 (14) | 32 (69) | .04 |
| Light | 10 (5) | 11 (24) | |
| Moderate | 20 (10) | 11 (24) | |
| Heavy | 27 (13) | 19 (41) | |
| Unknown | 14 (7) | 27 (57) | |
Values presented as No. (%) unless otherwise specified. P <.05 was considered statistically significant (bold) by Fisher exact test or χ2 test.
The mean age of the patients was 59 years, with the majority of the patients being male (81%; n = 214). The most common tumor site was the glottic larynx (48%; n = 126), followed by supraglottic larynx (28%; n = 74) and hypopharynx (18%; n = 47). Forty percent had evidence of nodal metastasis (n = 102), and 15% had distant metastasis (n = 40). The majority were stage III (20%; n = 52) or IV (68%; n = 180). The median follow-up time was 10 months (mean of 18 months). Salvage laryngectomy was performed on the majority of patients (55%; n = 145). One-third had either adjuvant radiation or chemoradiation (n = 85). Alcohol and tobacco history was also examined (Table 1). A history of alcohol use was defined as light (average of 3 or less drinks per week), moderate (average of 4–14 drinks per week), and heavy (average of more than 14 drinks per week). There was no difference in tobacco history between the 2 groups. However, there was a higher incidence of moderate and heavy alcohol consumption in the stricture group (Table 1).
Patients were divided into 2 cohorts: stricture vs nonstricture. Strictures were identified by either barium swallow or the surgeon via an exam under anesthesia. Patients with pre-existing strictures prior to total laryngectomy or those strictures that were the result of recurrent cancer were excluded from the stricture group. Information concerning the patients’ swallowing ability and diet was obtained from reviewing their clinical charts. Various comorbidities (diabetes mellitus, history of non–head and neck malignancies, congestive heart failure, hypothyroidism, chronic obstructive pulmonary disease/emphysema, gastroesophageal reflux disease, abnormal thyroid-stimulating hormone [TSH] value) were included for analysis in this study. In addition, primary vs salvage laryngectomy and the surgical closure of the defect (primary closure vs flap reconstruction) were also analyzed (Table 2).
Table 2.
Comparison of Surgical Treatments in the Stricture and Nonstricture Groups
| Type of Surgery | Stricture Group (n = 49), No. (%) | Nonstricture Group (n = 214), No. (%) | P Value | Odds Ratio (95% CI) |
|---|---|---|---|---|
| Primary TL | 45 (22) | 45 (96) | 1.00 | 1.00 (0.54–1.87) |
| Salvage TL | 55 (27) | 55 (118) | ||
| Flap | ||||
| None | 20 (10) | 37 (79) | <.0001 | 0.13 (0.06–0.28) |
| Jejunal | 4 (2) | 3 (7) | .94 | 1.07 (0.21–5.35) |
| RFFF | 67 (33) | 51 (108) | .42 | 1.57 (0.67–3.67) |
| Tubed | 29 (14) | 11 (24) | .03a | 2.58 (1.13–5.89) |
| Patch | 39 (19) | 39 (84) | ||
| Pectoralis | 4 (2) | 5 (10) | 1.00 | 0.71 (0.15–3.39) |
| Rectus | 2 (1) | 8 (16) | .13 | 0.21 (0.03–1.62) |
| ALT | 6 (3) | 3 (6) | .45 | 1.61 (0.40–6.54) |
| Fibula | 0 | 1 (2) | 1.00 | 0.71 (0.03–15.1) |
| Multiple flaps | 6 (3) | 7 (14) | 1.00b | 1.00 (0.27–3.72) |
Abbreviations: ALT, anterior lateral thigh; CI, confidence interval; RFFF, radial forearm free flap; TL, total laryngectomy. P <.05 was considered statistically significant (bold) by Fisher exact test.
Tubed RFFF compared to patch RFFF.
Multiple flaps compared to single flaps.
Statistical Analysis
Descriptive variables were summarized by mean for continuous variables and n (%) for categorical variables. The relationships between the stricture group and nonstricture group and the various outcomes were calculated using a 2-tailed Fisher exact test. In addition, the odds ratio (OR) with 95% confidence interval (CI) was also calculated. A multivariate analysis of the relationship between stricture formation and various demographics, comorbidities, and operative interventions was analyzed as well. The relationship between patient- and cancer-specific survival was calculated by using the Kaplan-Meier method. Survival time was calculated as the interval from date of surgery to date of death or date of last follow-up. Deaths due to other causes were censored for these analyses. Disease-free survival was calculated from date of surgery to time of recurrence or the last follow-up indicating the patient had no evidence of disease. A P value of <.05 was considered statistically significant. Statistical analysis was performed using GraphPad software (GraphPad, San Diego, California) and JMP 9 software (SAS, Cary, North Carolina).
Results
Cohort Comparison
Baseline characteristics were found to be the same for the 2 cohorts (Table 1). The cohorts had similar T classifications (P = .59), TMN staging (P = .24), incidence of regional disease (P = 1.00), and incidence of distant metastasis (P = .82). In addition, a significant smoking history was present in both groups. The stricture group did have a slightly higher known history of alcohol consumption (P = .04). The median follow-up was greater in duration in the stricture (18 months) compared to the nonstricture group (9 months).
Stricture Formation Rates
Pharyngeal, hypopharyngeal, or esophageal strictures were observed in 19% of the patients (n = 49). Most strictures occurred in the first year following total laryngectomy (82%; n = 40) (Figure 1). There was not a statistically significant difference in dietary outcome or management requirements for strictures that occurred earlier compared to those that occurred later. Overall, 35% of patients (n = 91) experienced dysphagia, and in 48% of those patients, stricture formation (n = 44) was the source.
Figure 1.
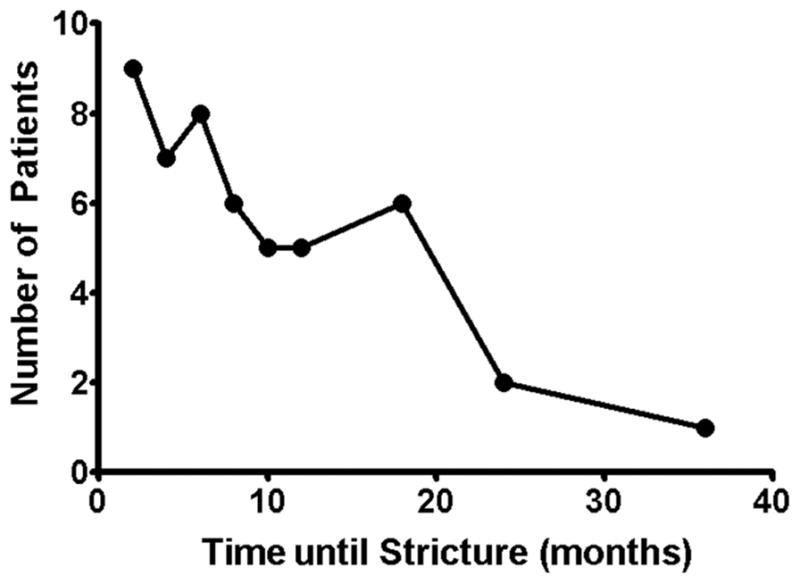
The rate of stricture formation following a total laryngectomy in patients with head and neck cancer.
Risk Factors
On univariate and multivariate analysis, there was no statistically significant relationship between gender, race, medical comorbidities (diabetes mellitus, congestive heart failure, hypothyroidism, chronic obstructive pulmonary disease/emphysema, gastroesophageal reflux disease), fistula formation, prior/continued tobacco or continued alcohol usage, and stricture formation. However, on univariate analysis, there was a correlation with previous alcohol usage and stricture formation (P = .04; Table 1).
Outcomes
On multivariate analysis (R2 = 0.03, P = .03), there was a statistically significant relationship between stricture formation and myocutaneous free flap reconstruction (P = .02); however, there was no relationship between primary and salvage total laryngectomies (P = .50) or neck dissections (P = .17) and stricture formation. Additional multivariate analysis (R2 = 0.06, P = .03) found no relationship between neoadjuvant or adjuvant radiation therapy, chemotherapy or combination therapy, and stricture formation. Furthermore, multivariate analysis (R2 = 0.5, P = .04) found no relationship between margin status, perineural invasion, lymphovascular invasion, and stricture formation; however, those patients with evidence of cartilage invasion were less likely to develop a stricture (P = .01).
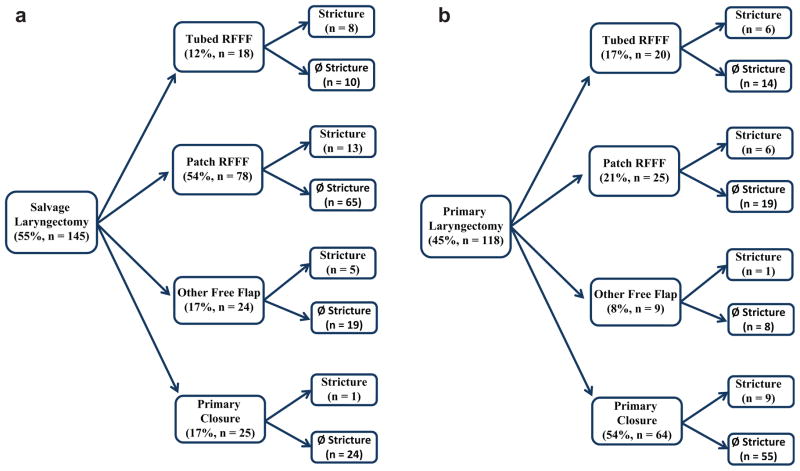
In patients undergoing primary total laryngectomy, there was no correlation with flap reconstruction (P = .34), adjuvant radiation therapy (P = .79), or chemoradiation therapy (P = 1.00) and stricture formation (Table 3). Primary closure was associated with decreased rates of stricture formation compared to flap reconstruction (P <.001; OR = 0.13; 95% CI, 0.06–0.28; Table 2). There was not a statistically significant difference in the incidence of stricture formation in those patients who received primary total laryngectomy compared to those who received a salvage total laryngectomy (P = 1.00; OR = 1.00; 95% CI, 0.54–1.87; Table 2). Of the patients who underwent salvage laryngectomy (n = 145), 19% developed strictures (n = 27), which was equivalent to stricture rates (19%; n = 22) in those patients who underwent primary laryngectomy (n = 118). Patients undergoing salvage laryngectomy were 2 times more likely to be reconstructed with a free flap, whereas those patients undergoing a primary laryngectomy were 3 times more likely to be closed primarily.
Table 3.
Comparison of Surgical Treatments in Patients Undergoing Primary Total Laryngectomy and ± Adjuvant Radiation Therapy or Chemoradiation Therapy
| Type of Closure | Stricture Group (n = 22), No. (%) | Nonstricture Group (n = 96), No. (%) | P Value |
|---|---|---|---|
| Primary | 41 (9) | 57 (55) | |
| None | 56 (5) | 47 (26) | |
| XRT | 22 (2) | 38 (21) | .69 |
| Chemo/XRT | 22 (2) | 15 (8) | 1.00 |
| RFFF-tubed | 27 (6) | 15 (14) | |
| None | 17 (1) | 0 | |
| XRT | 67 (4) | 64 (9) | .36 |
| Chemo/XRT | 17 (1) | 36 (5) | .29 |
| RFFF-patch | 27 (6) | 20 (19) | |
| None | 17 (1) | 5 (1) | |
| XRT | 33 (2) | 53 (10) | .40 |
| Chemo/XRT | 50 (3) | 42 (8) | 1.00 |
| Other | 5 (1) | 8 (8) | |
| None | 0 | 25 (2) | |
| XRT | 20 (1) | 38 (3) | 1.00 |
| Chemo/XRT | 0 | 38 (3) | 1.00 |
Abbreviations: RFFF = radial forearm free flap; XRT, radiation therapy. P value was calculated using Fisher exact test and comparing either XRT or combination therapy to neither.
In patients undergoing salvage laryngectomy, primary closure was less likely to result in a stricture formation compared to tubed radial forearm free flap (RFFF) reconstruction (P = .002; OR = 0.05; 95% CI, 0.01–0.47; Figure 2a). Furthermore, reconstruction with a tubed RFFF significantly increased the incidence of stricture formation compared to reconstruction with patch RFFF (P = .03; OR = 2.58; 95% CI, 1.13–5.89). In patients undergoing primary laryngectomy, there was no correlation with surgical repair of the defect and stricture formation (P = .34; Figure 2b).
Figure 2.
A comparison of the surgical repair for a laryngectomy defect in patients undergoing a salvage (a) vs primary (b) total laryngectomy. RFFF, radial forearm free flap.
Most patients who underwent tubed RFFF reconstruction had poor swallowing, with 50% (n = 7) on a limited diet and 7% (n = 1) percutaneous endoscopic gastrostomy (PEG) tube dependent. Whereas 42% of patients with strictures who underwent patch RFFF reconstruction had normal swallowing (n = 8), 21% had a limited diet (n = 4) and 21% were PEG tube dependent (n = 4). Strictures were managed with both single dilation (47%; n = 23) and serial dilation (45%; n = 22). Patients who required a single dilation had better dietary outcomes (semisolid) compared to patients who were not treated with dilation (P = .10) or who had serial dilations (P = .14). Patients who had undergone primary closure were more likely to be successfully managed with a single dilation alone (70%; n = 7), compared to those who had patched RFFF (56%; n = 9; P = .68) or tubed RFFF (43%; n = 6; P = .24).
Survival Characteristics
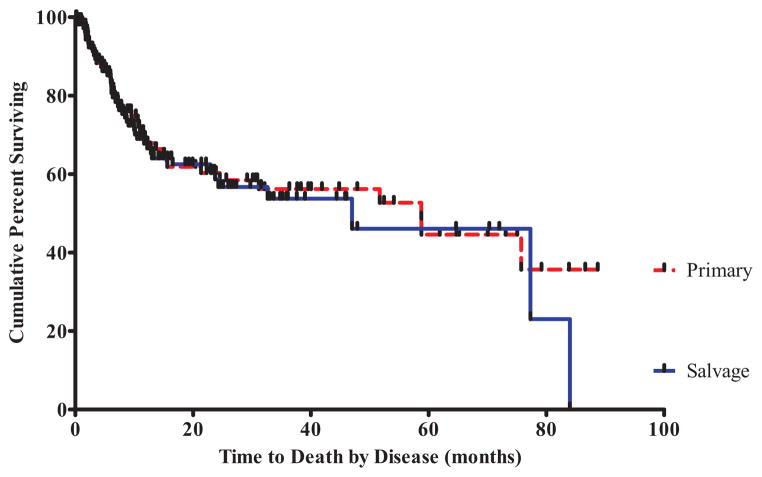
There was no difference in overall disease-free survival for those patients who underwent a salvage vs primary total laryngectomy (P = .95; Figure 3). The 2-year and 5-year survival rates were similar for salvage laryngectomy patients (57% and 46%, respectively) compared to primary laryngectomy patients (59% and 45%, respectively).
Figure 3.
Kaplan-Meier survival analysis found no difference in overall disease-free survival for patients undergoing a salvage vs primary total laryngectomy (P = .95).
Discussion
In patients with advanced laryngeal cancer, a total laryngectomy is often required to improve prognosis and survival. It is common for patients to experience dysphagia following a total laryngectomy. Dysphagia negatively affects the patient’s quality of life and is a known cause of decreased nutritional intake, particularly when it is the result of stricture formation.6,7 With a trend toward more aggressive therapies, the incidence of dysphagia is on the rise.6
In this study, 35% of patients presented with dysphagia postoperatively, which is lower than previous reports of rates as high as 50% to 72%.7,8 Interestingly, only half of these patients had radiographic or physical evidence of a stricture. This is likely the result of reconstructing the pharyngeal defect with a free flap because it lacks the muscular architecture of the normal pharynx and can subsequently result in pharyngeal dysmotility. In a previous study, it was found that even primary closure resulted in lower pharyngeal pressures, especially in patients who underwent mucosa-alone closures compared to mucosa and muscle closures.7
In this study, the majority of strictures occurred within the first year of surgery. Following pharyngeal reconstruction, stricture formation rates have been cited to range from 13% to 50%.3,4,9–12 Our stricture rates fell on the low end of this range, with 19% of total laryngectomy patients possessing evidence of stricture formation. This is likely due to a multitude of factors, including the high frequency of free flap reconstruction performed at our institution in this patient population (77%), the type of free flap used, how the free flap is inset, and the criteria for free flap reconstruction. In a study citing a similar rate of free flap reconstruction (91%), stricture rates were similar (15%).10 However, another study reported free flap reconstruction rates of only 35% and still had a low rate of stricture formation rate (16%). When analyzed closer, they found 21% of primary pharyngeal closures compared to only 5% of free flap reconstructions resulted in stricture formation. In addition, the study found stricture formation occurred in 33% of salvage laryngectomies vs only 8% in primary laryngectomies.4 Another study investigating outcomes of RFFF reconstruction only found that 78% of the strictures occurred in salvage patients.3 In contrast to these studies, our study found no difference between rates of stricture formation in patients undergoing a salvage vs a primary total laryngectomy. This is potentially due to the majority of salvage laryngectomy patients being reconstructed with a patch RFFF (54%) vs primary closure (17%) or a tubed RFFF (12%). In contrast, the pharynx was closed primarily (54%) in the majority of patients undergoing a primary total laryngectomy (Figure 2). In the studies investigating outcomes of RFFF reconstruction only, stricture rates are cited between 13% and 36%.3,12 Our stricture rates in patients undergoing RFFF reconstruction (23%) were similar.
Our institution has found that myocutaneous free flap reconstruction is associated with reduced donor site morbidity and allows for more substantial protection from leaks and vessel exposure, particularly in salvage laryngectomy patients, where radiation therapy has altered the elasticity of the tissue. Furthermore, the marginal microvasculature in salvage patients may necessitate free flap reconstruction over primary closure. In deciding on the type of reconstruction to perform, several factors are taken into consideration. If a posterior, oncologically sound mucosal strip at least 2 cm wide is available, a patch radial forearm free flap will be used for the reconstruction. If this mucosal strip is absent or cannot be closed circumferentially around the surgeon’s finger without tension, a tubed RFFF is used for the reconstruction. In addition, the quality of the mucosal strip needs to be taken into consideration; often in salvage cases, the mucosa may appear normal but fail to handle the stretch required for a patch reconstruction or primary closure, leading to increased stricture rates.
In this study, it was found tubed RFFF reconstruction led to higher rates of stricture formation; therefore, whenever a free flap is required, we advocate the use of a patch free flap if feasible. The higher rate of stricture formation in tubed reconstructions is likely the result of having to circumferentially inset the flap. The lack of mucosa strip creates a closed ring, which can contract over time. In addition, the mucosa strip has the ability to stretch, unlike the skin and musculature of the free flap. A previous publication has advocated for interdigitation of the flap inset to prevent the circumferential closure and subsequent contracture.13
Compared to other publications, we did not see a correlation between radiation therapy or chemoradiation therapy and stricture formation.11,12,14,15 Treatment with chemoradiation, radiation alone, or surgery did not increase the odds of stricture formation. The majority of patients received their radiation therapy at outside establishments not associated with our tertiary care center; therefore, dosages of radiation and fraction frequency were unknown for the majority of this patient population. This made drawing any conclusions or relationships between radiation dose and stricture formation unfeasible. Although previous studies found a correlation with comorbidities, nodal disease, and gender in development of strictures, we did not.12,15
Interestingly, patients with a known history of alcohol consumption had higher rates of stricture formation. Although the explanation for this pathophysiology is not fully understood, it may be the result of epithelial changes in the mucosa, decreasing its elasticity and ability to heal normally. This may result in increased contraction of the scar and decreased flexibility of the neopharynx. In addition, it was found that cartilage invasion was associated with decreased rates of stricture formation. We hypothesize this is associated with the biology of the disease. Those tumors with cartilage invasion tend to have a deep, exophytic growth pattern, and therefore the posterior mucosa can be saved, allowing them to be closed primarily or reconstructed with a patch free flap. In contrast, those tumors without cartilage invasion tend to grow along the mucosa, requiring excision of the posterior mucosa. This results in the absence of a posterior mucosal strip and reconstruction with a tubed RFFF.
Stricture formation is a difficult complication to manage, often requiring multiple dilations to allow for adequate nutritional intake.3,14 In this study, strictures were managed with both single dilation (45%) and serial dilation (47%). Patients with a stricture who had undergone primary closure were more likely to be successfully managed with a single dilation alone (70%), compared to those who had tubed RFFF (56%) or patch RFFF (43%). Similarly, a previous study found 56% of patients who had undergone RFFF reconstruction and required dilation for stenosis were able to be managed with single dilation alone.3 The stricture patients who required only a single dilation had better dietary outcomes compared to patients who were not treated with dilation or who had serial dilations. Among stricture patients, only 27% of patients in this study achieved normal swallowing following dilation.
Conclusion
Dysphagia is a known complication following treatment for laryngeal malignancies. It negatively affects quality of life and limits nutritional intake. It is particularly devastating if the dysphagia is the result of stricture formation. In our study examining retrospectively the incidence and outcomes associated with stricture formation following total laryngectomy, we found about one-third of the patients experienced some form of dysphagia, whereas 19% developed strictures. Strictures typically formed within the first year following surgery, and rates were similar between the salvage and primary total laryngectomy patients. Among patients undergoing a salvage laryngectomy, higher rates of stricture formation were see for primary closure vs flap reconstruction and for tubed RFFF compared to patch RFFF. There was no correlation with comorbidities, gender, continued tobacco or alcohol usage, or radiation treatments (neoadjuvant or adjuvant) and stricture formation. About half of the strictures could be managed with a single dilation, whereas the majority of those remaining required serial dilations to maintain nutritional intake. In addition, 2% of patients were PEG tube dependent at the conclusion of the study.
Acknowledgments
Sponsorships: None.
Funding source: The National Institutes of Health (2T32 CA091078-09) provided funding for NIH research fellows working on the project.
Footnotes
Reprints and permission: sagepub.com/journalsPermissions.nav
Author Contributions
Larissa Sweeny, conception and design, analysis and interpretation of data, drafting the article, final approval of the version to be published; J. Blake Golden, conception and design, analysis and interpretation of data, editing of the article, final approval of the version to be published; Hilliary N. White, conception and design, analysis and interpretation of data, editing of the article, final approval of the version to be published; J. Scott Magnuson, conception and design, analysis and interpretation of data, editing of the article, final approval of the version to be published; William R. Carroll, conception and design, analysis and interpretation of data, editing of the article, final approval of the version to be published; Eben L. Rosenthal, conception and design, analysis and interpretation of data, drafting the article, final approval of the version to be published.
Competing interests: None.
This article was presented at the 2011 AAO-HNSF Annual Meeting & OTO EXPO; September 11–14, 2011; San Francisco, California.
References
- 1.Smith RV, Kotz T, Beitler JJ, Wadler S. Long-term swallowing problems after organ preservation therapy with concomitant radiation therapy and intravenous hydroxyurea: initial results. Arch Otolaryngol Head Neck Surg. 2000;126:384–389. doi: 10.1001/archotol.126.3.384. [DOI] [PubMed] [Google Scholar]
- 2.Mittal BB, Pauloski BR, Haraf DJ, et al. Swallowing dysfunction—preventative and rehabilitation strategies in patients with head-and-neck cancers treated with surgery, radiotherapy, and chemotherapy: a critical review. Int J Radiat Oncol Biol Phys. 2003;57:1219–1230. doi: 10.1016/s0360-3016(03)01454-8. [DOI] [PubMed] [Google Scholar]
- 3.Scharpf J, Esclamado RM. Reconstruction with radial forearm flaps after ablative surgery for hypopharyngeal cancer. Head Neck. 2003;25:261–266. doi: 10.1002/hed.10197. [DOI] [PubMed] [Google Scholar]
- 4.Tsou YA, Lin MH, Hua CH, et al. Comparison of pharyngeal stenosis between hypopharyngeal patients undergoing primary versus salvage laryngopharyngectomy. Otolaryngol Head Neck Surg. 2010;143:538–543. doi: 10.1016/j.otohns.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York: Springer; 2010. [Google Scholar]
- 6.Francis DO, Weymuller EA, Jr, Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119:391–397. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]
- 7.Maclean J, Szczesniak M, Cotton S, Cook I, Perry A. Impact of a laryngectomy and surgical closure technique on swallow biomechanics and dysphagia severity. Otolaryngol Head Neck Surg. 2011;144:21–28. doi: 10.1177/0194599810390906. [DOI] [PubMed] [Google Scholar]
- 8.Maclean J, Cotton S, Perry A. Post-laryngectomy: it’s hard to swallow: an Australian study of prevalence and self-reports of swallowing function after a total laryngectomy. Dysphagia. 2009;24:172–179. doi: 10.1007/s00455-008-9189-5. [DOI] [PubMed] [Google Scholar]
- 9.Robertson SM, Yeo JC, Dunnet C, Young D, Mackenzie K. Voice, swallowing, and quality of life after total laryngectomy: results of the west of Scotland laryngectomy audit. Head Neck. 2011 Mar 17; doi: 10.1002/hed.21692. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Clark JR, Gilbert R, Irish J, et al. Morbidity after flap reconstruction of hypopharyngeal defects. Laryngoscope. 2006;116:173–181. doi: 10.1097/01.mlg.0000191459.40059.fd. [DOI] [PubMed] [Google Scholar]
- 11.Murray DJ, Novak CB, Neligan PC. Fasciocutaneous free flaps in pharyngolaryngo-oesophageal reconstruction: a critical review of the literature. J Plast Reconstr Aesthetic Surg. 2008;61:1148–1156. doi: 10.1016/j.bjps.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Cho BC, Kim M, Lee JH, et al. Pharyngoesophageal reconstruction with a tubed free radial forearm flap. J Reconstr Microsurg. 1998;14:535–540. doi: 10.1055/s-2008-1040771. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara T, Shih HS, Chen CC, et al. Interdigitation of the distal anastomosis between tubed fasciocutaneous flap and cervical esophagus for stricture prevention. Laryngoscope. 2011;121:289–293. doi: 10.1002/lary.21289. [DOI] [PubMed] [Google Scholar]
- 14.Scharpf J, Esclamado RM. Analysis of recurrence and survival after hypopharyngeal ablative surgery with radial forearm free flap reconstruction. Laryngoscope. 2005;115:429–432. doi: 10.1097/01.mlg.0000157856.54767.bf. [DOI] [PubMed] [Google Scholar]
- 15.Francis DO, Weymuller EA, Jr, Parvathaneni U, Merati AL, Yueh B. Dysphagia, stricture, and pneumonia in head and neck cancer patients: does treatment modality matter? Ann Otol Rhinol Laryngol. 2010;119:391–397. doi: 10.1177/000348941011900605. [DOI] [PubMed] [Google Scholar]