Abstract
The present study used a cohort-sequential design to examine developmental changes in children's ability to bind items in memory during early and middle childhood. Three cohorts of children (aged 4, 6, or 8 years) were followed longitudinally for three years. Each year, children completed a source memory paradigm assessing memory for items and binding. Results suggest linear increases in memory for individual items (facts or sources) between 4 and 10 years of age, but that memory for correct fact/source combinations (indicative of binding) showed accelerated rates of change between 5 and 7 years. Taken together, these results suggest differences exist in developmental trajectories between the development of memory processes underlying successful item memory and processes underlying binding. Implications of these findings are discussed in relation to longitudinal research examining autobiographical memory.
Keywords: binding, source memory, memory development, longitudinal investigation
Memories for personally-experienced events, or autobiographical memories, are important for defining who we are as individuals. Although adults can recall numerous memories from late childhood and adulthood, they tend to have few, if any, memories from early in life. This decrease in memory for life events cannot be accounted for simply by the passage of time (Wetzler and Sweeney, 1986), and thus is referred to as a unique phenomenon: infantile/childhood amnesia. Although multiple conceptual changes have been proposed to contribute to the offset of infantile amnesia and the development of autobiographical memory (e.g., self-concept, autonoetic awareness, language, narrative ability, see Bauer 2007 for review), developmental changes in basic mnemonic processes likely contribute as well. Specifically, recall of autobiographical memories depends on binding together a rich array of various kinds of information about an event. Not only is memory for individual aspects of the event important (e.g., who, what), but also the spatio-temporal context surrounding the event (e.g., when, where). The ability to remember such contextual details has been suggested to undergo significant developmental change during childhood (see Newcombe, Lloyd, & Balcomb, 2012 for review).
A number of different experimental paradigms have been used to examine developmental changes in children's ability correctly recall contextual details associated with an item or event, including relational memory paradigms, binding paradigms, and/or source memory paradigms. In each, the critical element is children's ability to recall the individual item in its original context (i.e., item in context). This can only be achieved if the item and context are “bound” together. For example, Sluzenski and colleagues (2006) investigated children and adults' ability to bind items and locations in memory. In their study, 4- to 6-year-old children and adults viewed pictures of 1) animals, 2) backgrounds, and 3) animals on backgrounds and, after a brief delay, were asked to recall either the individual aspects of pictures (e.g., animals or background) or combinations (animals on specific backgrounds). Results suggested an improvement in memory for the combinations (animals on specific backgrounds) between ages of 4 and 6 years, but no improvement for memory of the isolated parts (animals or backgrounds individually). Importantly, performance on this memory task predicted free recall of a naturalistic event, suggesting binding processes (as measured in the laboratory) are related to memory for real life events.
Drummey and Newcombe (2002) also investigated children's ability to bind aspects in memory using a source memory task modeled after a novel fact paradigm in the adult literature (Schacter, Harbluk, & McLachlan, 1984). Specifically, 4-, 6-, and 8-year-old children were taught novel facts in the laboratory by a puppet or experimenter. After a one-week delay, children were asked to recall the facts and source from whom the facts were learned. These served as measures of item and source memory, respectively. In this study accurate source memory reflects binding as accurate source memory was conditionalized on accurate item memory. Results suggested that memory for facts increased from 4 to 8 years (with particular gains in recall between 6 and 8 years) and that source memory increased between 4 and 6 years. This latter finding directly overlaps with the findings of Sluzenski and colleagues (2006) described above.
This developmental “shift” in binding abilities appears to be a general phenomenon, as it has also been shown in reality monitoring paradigms (Lindsay, Johnson, & Kwon, 1991; Sluzenski, Newcombe, & Ottinger 2004; Welch-Ross, 1995) and paradigms requiring children to remember the location of previous real-life experiences (Bauer, Doydum, Pathman, Larkina, Guler, & Burch, 2012). However, one distinct advantage of the source task used by Drummey & Newcombe (2002) over other paradigms is that it involved a free recall procedure. Thus the types of errors children made could be examined in order to shed more insight into the age-related differences. Towards this end, the authors examined how often children displayed source amnesia (indicting the source was outside the experimental setting, i.e., an extra-experimental error) versus source forgetting (indicating an incorrect source within the experimental setting, i.e., intra-experimental error). Their results showed that 4 year olds' were more likely than 6 or 8 year olds to nominate an extra-experimental source, whereas 6 and 8 year olds showed few such errors, suggesting a unique, qualitative change in memory that was not simply a consequence of degraded or fragmented memory (Schacter et al., 1984).
Taken together, laboratory-based studies suggest that the binding of items and contexts shows significant developmental change during childhood. However, to date, these studies have been cross-sectional in nature. Although time consuming, longitudinal designs are vital because they allow for the detection of different rates of change both as a function of age and task. In addition, longitudinal data are needed to address questions regarding the nature of this shift (e.g., is the development in binding sudden or gradual). To our knowledge, the only existing longitudinal experimental studies of memory to date have focused on development of memory for individual items (or related abilities such as strategy use) as opposed to binding specifically (e.g., Schneider, in press; Schneider & Bjorklund, 1998; Weinert & Schneider, 1999).
Thus, the goal of the present study was to longitudinally assess developmental changes in binding processes using a source memory task in early and middle childhood. To achieve this we used a cohort sequential design in which 3 groups of children (aged 4, 6, or 8 years) were followed longitudinally for 3 years. Each year, participants completed a source memory task. This task was chosen because it is especially “diagnostic” of binding processes. The combination of this task with the cross sequential design with 4, 6, and 8 year olds, allowed for the examination of developmental changes in: 1) memory for items (facts or sources not conditionalized on fact recall) and 2) binding or memory for items in context (operationalized as: source memory conditionalized on fact recall) between 4 – 10 years of age. The source memory task was modeled after the ones used by Schacter and colleagues (1984) and Drummey and Newcombe (2002). However, modifications were made to accommodate the longitudinal nature of the design and extend the conclusions that could be drawn based on the types of errors children made. First, we expanded the number of to-be-remembered novel facts so that each year children learned different facts and utilized a video presentation (versus live) in order to maintain consistency across 3 years of data collection. Second, we expanded the acceptable responses to the source memory question to include “guessing” or simply “knowing”. This response option was included in the original paper by Schacter and colleagues (1984) as adults commonly claimed to be “guessing” or said they had “deduced” or “figured out” the answer based on their previous knowledge. We thought it was important to include this as an option as this type of error may differ from extra-experimental errors, which are distinctively associated with patients with frontal lobe dysfunction and other disorders associated with confabulation (Moscovitch, 1989; Schacter et al 1984; see Burgess & Shallice, 1996 for discussion). Finally, we analyzed source memory that was not conditionalized on fact recall (i.e., correctly stating the source of the fact in absence of recalling the correct answer to the fact). Examination of this variable was important as it allowed for the investigation of how memorable an individual source may have been on a given trial, without regard to memory for the fact.
Method
Participants
Participants included 135 children (73 female, 62 male) enrolled in a longitudinal investigation of memory development. At the first visit to the lab (wave 1, visit 1) 48 participants (21 female, 27 male) were 4 years of age (M = 4.18 years, SD = 21 days), 44 participants (25 female, 19 male) were 6 years of age (M = 6.19 years, SD = 19 days), and 43 participants (27 female, 16 male) were 8 years of age (M = 8.20 years, SD = 16 days). All participants were recruited from a participant pool maintained by faculty at a large University in the Midwestern United States. The participant pool consists of names of children whose parents were contacted by mail shortly after their children's births who subsequently returned postcards stating their desired involvement in research. This sample was representative of the community from which it was drawn; 93% of participants reported being of Caucasian, non-Hispanic descent. Although 135 children were enrolled in the longitudinal study, sample sizes for participants who completed the source memory task varied at each Wave due to factors such as attrition (n=26), insufficient time to complete the protocol (n=18), video equipment failure (n=1), experimenter error (n=3), and refusal to complete the task (n=3). Actual sample sizes are reported in Table 1. A University institutional review board approved the protocol prior to the star to the study and written parental consent was obtained for each child. At the end of the second visit at each wave, children received a small toy and parents were given a gift certificate to a local merchant for participating.
Table 1.
Sample sizes by Cohort and Wave.
| Wave 1 | Wave 2 | Wave 3 | |
|---|---|---|---|
| 4-year | 35 | 36 | 36 |
| 6-year | 43 | 40 | 34 |
| 8-year | 42 | 32 | 30 |
Materials and Procedure
Participants visited the laboratory annually; these are referred to as “waves” of data collection. The average delay between Wave 1 and Wave 2 was 364 days (SD = 35 days, range 270–442 days) and the average delay between Wave 2 and Wave 3 was 329 days (SD = 26 days, range 264–403 days). At each wave, participants visited the lab on 2 different occasions approximately one week apart (Wave 1: M = 7 days, SD = 1 day, range 5–14 days; Wave 2: M = 7 days, SD = 2 days, range 5–21 days; Wave 3: M = 8 days, SD = 3 days, range = 4–28 days); these are referred to as visits. At each wave, participants were shown a source memory video (details below) at the end of the first visit and were asked to recall material from this source memory video during the second visit. In addition, measures of general cognitive abilities were obtained from standardized assessments. Specifically, non-verbal IQ was measured by the Test of Nonverbal Intelligence (TONI-3, Brown, Sherbenou, & Johnsen, 1992) at Wave 2 for the 6-and 8-year-old Cohorts and at Wave 3 for the 4-year-old Cohort. Verbal comprehension (Wave 1 only), processing speed (Visual Matching, Pair Cancellation, all Waves), and working memory (Numbers Reversed, all Waves) were measured by the Woodcock-Johnson tests of Cognitive Abilities (Woodcock, McGrew, & Mather, 2001). Although children also participated in other memory and cognitive paradigms (e.g., autobiographical memory interviews), how performance on these tasks varied as a function of age is not part of the present report. Children also visited the laboratory for a fourth wave of data collection, however the source memory task was not administered at that visit due to 1) time constraints of the session and 2) sufficient overlap between cohorts was achieved at wave 3 to address issues such as practice effects. Each visit lasted approximately 1–1.5 hours. The author and 8 additional female adults were trained to adhere to the procedure, which was outlined in a written protocol. The researchers reviewed and discussed videotaped sessions on a regular basis throughout the entire study in order to ensure the procedures were carried out in an identical manner.
At visit 1, children were taught 12 new facts that we anticipated children would not routinely learn in school (e.g., “Cheetahs are the only big cats that can't roar,” or “A group of rhinos is called a crash”) by way of videotape from one of two different sources (i.e, a female adult or a clownfish puppet; see Drummey & Newcombe, 2002 for a similar paradigm). Participants were assigned one of three different fact lists, each of which had two different random presentation orders. All of the lists contained similar types of questions (cf. “A group of kangaroos is called a mob”, “A group of goats is called a tribe,” see Appendix). To bolster performance, presentation from each source was blocked such that children learned all 6 facts from the first source and then all 6 facts from the second source. The source that the children saw first (person or puppet) was randomized across participants. The experimenter instructed the children to watch the videos and learn the new facts because they would be asked about them later. However, memory for the source of the information was incidental (i.e., no instructions were given to the children regarding remembering the source of the facts). To ensure the children understood the statements and maintained interest in the video, the experimenter commented on each fact, regardless of whether it was presented by the person or puppet, by repeating a portion (but not all) of the fact (e.g., “Oh, a tribe”).
Following presentation of the facts, each child was asked whether they knew any of the facts prior to watching the video. If the children responded yes, these items were excluded from the analyses. At each phase, the majority of children knew only one or two facts, and thus the average number of valid facts was 10 (SD = 3) for wave 1, 10 (SD = 2) for wave 2, and 10 (SD = 2) for wave 3.
During the second visit at each wave, children were asked to answer 24 `trivia' questions and state from whom they learned the information. Facts queried were equally distributed between four conditions: 1) facts that had been presented by the person on the videotape, 2) facts that had been presented by the puppet on the videotape, 3) facts commonly known by children (e.g, “What color is grass”), 4) facts children typically do not know and which had not been taught on the videotape (e.g., “What is the colored part of your eye called?”). For each of the three trivia lists there were two different random presentation orders of the questions that were counterbalanced across participants. Children were instructed to ask for “hints” (i.e., four multiple choice options, see example below) if they did not know an answer to a question. Because the multiple choice options for the source task were always the same, at the beginning of the task, children were made aware of the fact that some of the items they learned from the videotape, some of the items they learned from outside the laboratory (e.g., from a teacher or parent), and some items they might not know.
Each question was presented and the child was given the opportunity for free recall (e.g., “What are the only big cats that can't roar?”). If the child indicated s/he did not know the answer, four plausible multiple choice options were given (e.g., “Cheetahs, Panthers, Tigers, Leopards”). After the child responded, regardless of whether the answer was correct or incorrect, the experimenter asked from whom the child learned the information (e.g., From who did you learn that?). Acceptable responses from the children also included that they “just knew” the answer to the question or that they “guessed” at the answer (as this may have been the case with the questions regarding commonly known facts and questions regarding facts that children did not know). If the child did not respond to the source question during this free recall period or indicated that they did not know where they learned the information, five multiple choice options were given: parent, teacher, person on the videotape, puppet on the videotape, or “just knew”/guessed (these 5 options were included in the instructions as examples prior to any questions being asked). Correct responses that were given immediately after the trivia question were considered free recall. Correct responses that were given after the multiple choice options were considered recognition; however, given that some children did not have recognition responses, recall and recognition were collapsed to form an index of total fact memory (see Drummey & Newcombe, 2002 for similar approach). Separate tallies were made based on recall only (i.e., fact recall) and on recall-plus-recognition (i.e., fact total). Reponses to the source memory questions from the video were grouped into 1 of 5 categories: correct responses, extra-experimental errors (i.e., responses indicating parents, teachers, or friends), intra-experimental errors (i.e., responses indicating the incorrect source from the videotape), “guessed/always knew” responses, and “I don't know” responses. Since the multiple choice options for the source memory question were given at the beginning of the task and did not change between questions, source recall and source recognition were collapsed to form an index of total source memory (i.e., source total, see Drummey & Newcombe, 2002 for similar approach).
Given that data regarding facts each child knew prior to watching the videotape were excluded, each child may have had a different number possible on the fact recall. On average each child knew 1 of the facts prior to the session, SD = 1.45 (although the number of facts previously known did increase slightly as a function of age, with 4 year olds knowing less than 1 fact and 10 year olds knowing 3.75 facts). Therefore, proportions were used in analyses for both fact and source memory. A minimum of 7 valid responses to the source memory question were required for inclusion in the dataset.1
Analytic approach
A total of 303 data points were available for analysis. Missing data points were determined to be “missing at random” (Little's MCAR test, χ2 (2) = 4.60, p = .10); therefore, maximum likelihood estimation (a technique recommended for handling missing data in longitudinal studies, see Jelicic, Phelps, & Lerner, 2009) was used to impute missing outcome values based on wave, cohort, and gender. This approach allowed us to test our hypotheses with improved power over listwise deletion and less biased parameter estimates than other techniques including listwise deletion, mean substitution, and multiple regression estimation (Graham, 2009).
The resulting dataset was analyzed using 3 (cohort) × 3 (wave/time of measurement) Generalized Estimating Equations (GEEs), an extension of generalized linear models. GEEs account for both potential correlations among repeated observations as well as missing data, and are not restricted to normally distributed data sets (Ballinger, 2004; Hardin & Hilbe, 2003; Zeger, Liang, & Albert, 1988). Moreover, GEEs represent one approach to examining effects from three conceptually distinct sources of developmental influence: Age, Cohort, and Time of Measurement, which are unavoidably intertwined in developmental research. For example, in cross-sectional research although time of measurement is held constant, age and cohort effects are confounded. Conversely, in longitudinal designs because there is only one cohort, time of measurement and age are confounded. In the current analyses, ANOVAs are applied to the cohort sequential design with Cohort as a between subjects factor and Time of Measurement as a within-subjects factor. In this analysis age is not directly tested (because it is confounded with Cohort and Time of Measurement), but contributes to each of the main effects (as the Cohorts differ in chronological age at each Time of Measurement). Thus, age effects are reflected in main effects for both Cohort and Time of Measurement. However, if one main effect is observed, then that effect is not likely to be due to age-related changes, but rather likely to cohort effects or historical change. A significant interaction between Cohort and Time of Measurement indicates a difference in the rate of change between the cohorts.
Practice effects are important to consider in the current study because all Cohorts had repeated experience with this task. As a result, although the source memory task was incidental at Wave 1, it was not incidental at the subsequent waves. Thus, practice effects may have modified performance in subsequent years either as a result of repeated experience (i.e., `practice') with the task or increased knowledge of the task as a result of the previous experience with it or both. In the present study, practice effects would be revealed when 1) terminal performance of the 4-year-olds is higher than enrollment performance of the 6-year-olds and 2) terminal performance of the 6-year-olds is higher than enrollment performance of the 8-year-olds. Although this pattern could also indicate Cohort effects, these were less of a concern because of the cohort-sequential design and analytic approach utilized. In the context of GEE analysis, practice effects would revealed by significant differences in the pairwise comparisons of these groups that overlap in age. However, we assume that practice effects, when present, will influence groups in the same manner since previous experience with the task was identical across groups. Therefore, practice effects would not result in differential rates of learning within Cohorts and would not account for interactions between Cohort and Time of Measurement (which suggest differences in the rate of change, or slope, within groups). In addition, pairwise comparisons could also be used to address whether knowledge of the nature of the task (i.e., incidental at Wave 1 but not Waves 2 and 3) influenced performance. Specifically, comparing change from Wave 1 to Wave 2 (i.e., going from incidental to potentially intentional) to change from Wave 2 to wave 3 (i.e., in both cases the task is intentional) would address effects of knowledge of task on performance.
We specified an unstructured correlation matrix and conducted our significance tests using the Type III sum of squares approach and one-tailed, directional tests because we hypothesized that item and source memory would improve with age. The output of the GEE analysis consists of Wald Chi Square values for main e ects and interactions within a given model and estimated marginal means that can then be examined with pairwise comparisons. We probed significant main effects and interactions using the least significant difference method for pairwise comparisons of estimated marginal means.
Results
Results of the GEE analyses for fact recall, fact total, source total, extra-experimental errors, intra-experimental errors, and guessed/knew responses are summarized in Table 2. Differences within Cohorts and between Cohorts tested at the same chronological age, as indicated by pairwise comparisons, are presented in Table 3.
Table 2.
Summary of GEE results. Bold denotes significant effects.
| Time of Measurement | Cohort | Time of Measurement × Cohort | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Dependent measure | Wald Chi Square | p-value | Wald Chi Square | p-value | Wald Chi Square | p-value |
| Fact recall | 94.13 | <.001 | 199.35 | <.001 | 1.09 | 0.45 |
| Fact total | 99.38 | <.001 | 229.77 | <.001 | 4.4 | 0.18 |
| Source total | 19.91 | <.001 | 16.6 | <.001 | 7.82 | <.05 |
| Extra-experiment errors | 1.19 | 0.2765 | 1.26 | 0.266 | 5.53 | 0.12 |
| Intra-experiment errors | 35.43 | <.001 | 67.33 | <.001 | 2.85 | 0.29 |
| Guessed/Knew responses | 40.63 | <.001 | 49.19 | <.001 | 8.56 | <.05 |
| Unconditionalized source total | 42.6 | <.001 | 34.23 | <.001 | 2.66 | 0.31 |
Table 3.
| A) Summary of differences within each Cohort for all dependent measures. Bold denotes significant effects. | ||||||||||||
| Wave | Cohort | Actual Age (years) | Wave | Cohort | Actual Age (years) | Fact Recall | Fact Total | Source Total | Extra-experimental Errors | Intra-experimental Errors | Guessed/Knew Responses | Unconditionalized Source Knowledge |
| 1 | 4-year | 4 | 2 | 4-year | 5 | <.001 | <.001 | .39 | .11 | .02 | .07 | .03 |
| 2 | 4-year | 5 | 3 | 4-year | 6 | <.001 | .07 | .03 | .27 | .24 | .15 | .02 |
| 1 | 6-year | 6 | 2 | 6-year | 7 | <.001 | <.001 | .01 | .30 | <.001 | <.001 | <.001 |
| 2 | 6-year | 7 | 3 | 6-year | 8 | .04 | .02 | .11 | .30 | .15 | .03 | .01 |
| 1 | 8-year | 8 | 2 | 8-year | 9 | <.001 | <.001 | .07 | .03 | .12 | .26 | .16 |
| 2 | 8-year | 9 | 3 | 8-year | 10 | .02 | .01 | .31 | .34 | .06 | .01 | .02 |
| B) Summary of differences between Cohorts tested at the same chronological age (i.e., 6 and 8 years). Bold denotes significant effects. | ||||||||||||
| Wave | Cohort | Actual Age (years) | Wave | Cohort | Actual Age (years) | Fact Recall | Fact Total | Source Total | Extra-experimental exp Errors | Intra-experimental Errors | Guessed/Knew Responses | Unconditionalized Source Knowledge |
| 3 | 4-year | 6 | 1 | 6-year | 6 | .15 | .12 | .02 | .23 | .19 | .12 | .01 |
| 3 | 6-year | 8 | 1 | 8-year | 8 | .46 | .48 | .03 | .20 | .14 | .01 | .04 |
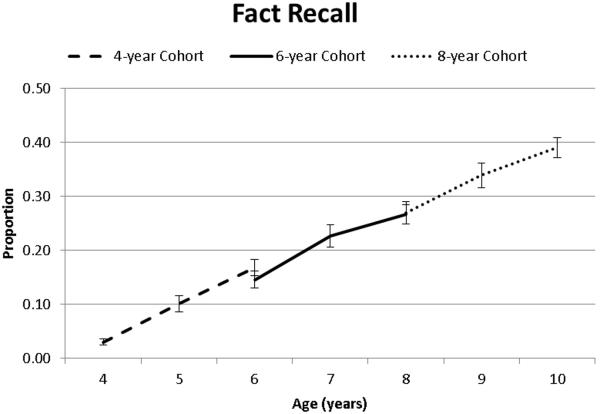
Fact recall increased as a function of age as indicated by main effects of both Time of Measurement and Cohort (see Table 2, Figure 1). Pairwise comparisons (Table 3) and inspection of the means (Figure 1) suggested a linear increase in fact recall abilities between 4 to 10 years of age and that there were no practice effects within Cohorts.
Figure 1.
Fact recall as a function of age and cohort.
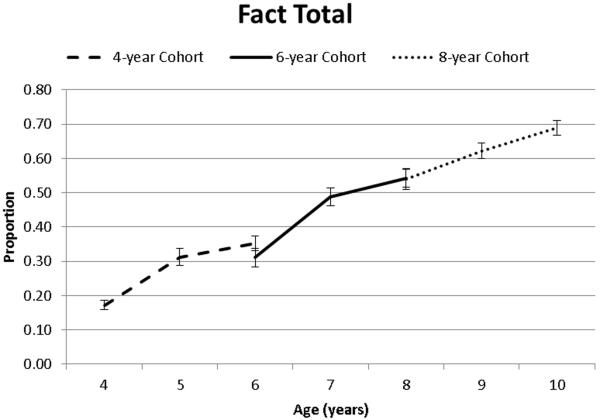
Fact total (Fact recall plus Fact recognition) also differed as a function of age, as indicated by main effects of both Time of Measurement and Cohort (see Table 2, Figure 2). No practice effects were observed within Cohorts and there was a relatively linear increase in performance (although increases between 5 and 6 years were marginal, see Figure 2 and Table 3).
Figure 2.
Fact total as a function of age and cohort.
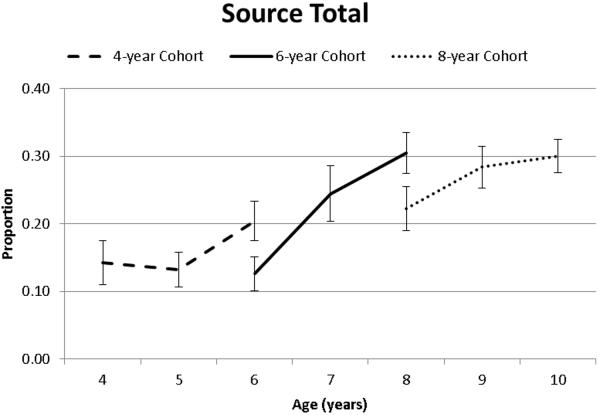
Source memory increased as a function of age as indicated by main effects of both Time of Measurement and Cohort. However, these main effects were qualified by a significant interaction between Time of Measurement and Cohort (Table 2, Figure 3). As illustrated in Figure 3, the rate of change differed between the cohorts. Overall, the 4- and 8-year cohorts are changed more slowly (i.e., the slopes were flatter). This is especially apparent between waves 1 and 2 for the 4 year olds and between waves 2 and 3 for the 8 year olds. In contrast, the rate of change in the 6-year group was high between all waves (i.e., they changed more quickly across the 3 waves).
Figure 3.
Source total as a function of age and cohort.
This interpretation is consistent with pairwise comparisons (Table 3). Focusing exclusively on within Cohort change, the following differences are observed: for the 4-year Cohort, there was no change between Wave 1 and 2 (4 to 5 years) but a significant increase in source total between Wave 2 and 3 (5 to 6 years). For the 6-year Cohort, there was a significant increase in source total between Wave 1 and 2 (6 and 7 years) but no change between Wave 2 and 3 (7 to 8 years). Finally, for the 8-year Cohort there was a marginal increase from Wave 1 to Wave 2 (8 to 9 years) but no increase from Wave 2 to 3 (9 to 10 years). Taken together with the significant interaction between Cohort and Time of Measurement, these findings suggest different rates of age-related change in source memory between the 3 Cohorts and that the period between 5 to 7 years is an important time for developmental improvements in source memory.
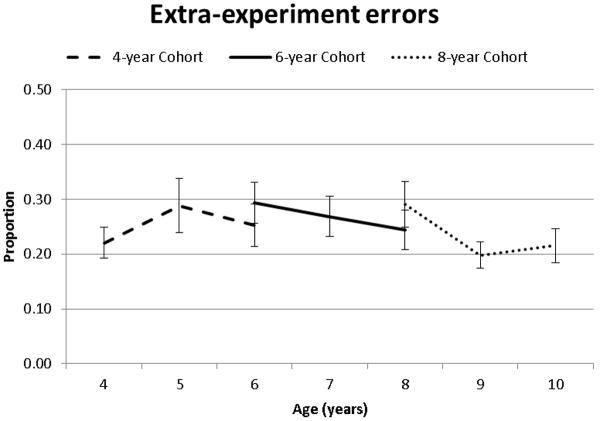
Extraexperiment errors (i.e., indicating an external experimental source such as a parent, teacher, book, etc when the fact was learned in the experiment) did not differ as a function of age as indicated by the lack of a main effect of Time of Measurement and Cohort (Tables 2 and 3, Figure 4).
Figure 4.
Extra-experimental errors as a function of age and cohort.
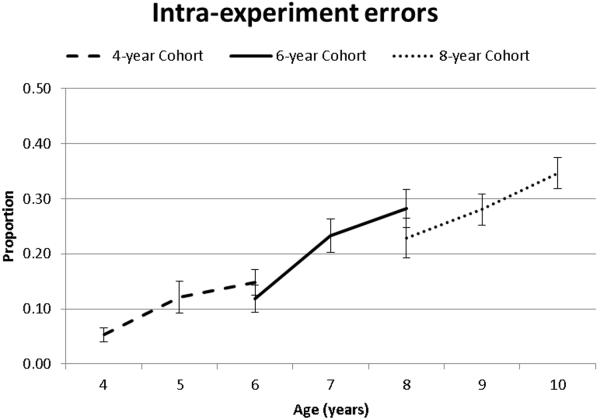
Intraexperiment errors (i.e., indicating the wrong experimental source) differed as a function of age as indicated significant main effects of both Time of Measurement and Cohort (Table 2, Figure 5). Pairwise comparisons suggested there were no practice effects for this variable and that Intraexperiment errors increased between 4 to 5 years and 6 to 7 years (Table 3)..
Figure 5.
Intra-experimental errors as a function of age and cohort.
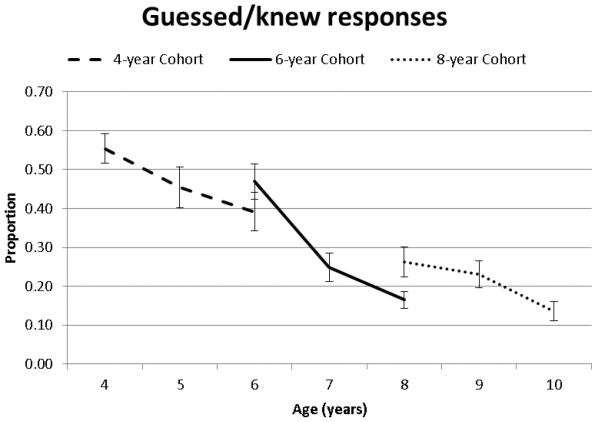
Guessed/Knew responses differed as a function of age as indicated by main effects of both Time of Measurement and Cohort, which were qualified by a significant interaction between Time of Measurement and Cohort (Table 2, Figure 6). Pairwise comparisons indicated no practice effects for the 4-year Cohort and that guessed/knew responses decreased between 6 to 7, 7 to 8, and 9 to 10 years of age.
Figure 6.
Guessed/knew responses as a function of age and cohort.
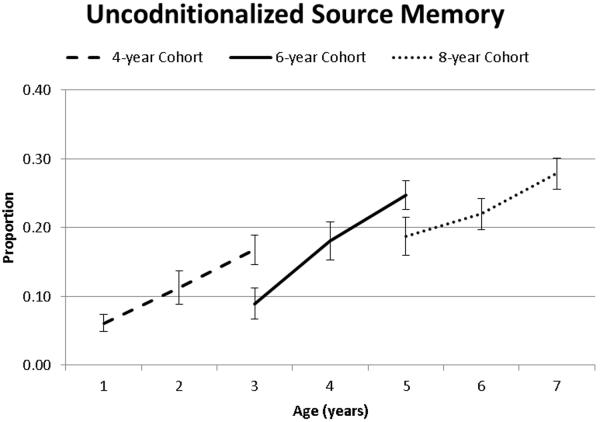
Finally, we also analyzed source memory responses that were not conditionalized on fact memory. This analysis is critical for determining if general memory for “individuals” (i.e., the puppet or person) was improving versus memory for the particular source of a particular piece of information (i.e., binding of person/puppet to the item). These analyses revealed that unconditionalized source memory responses increased as a function of age, as there was a main effect of Time of Measurement and a main effect of Cohort (Table 2, Figure 7). However unlike measures of source memory conditionalized on item memory, the interaction between Time of Measurement and Cohort was not significant (p=.31, see Table 2). Pairwise comparisons (Table 3) indicated practice effects were present and that unconditionalized source memory performance increased as a function of age (with the exception of 8 to 9 years).
Figure 7.
Source memory responses that were not conditionalized on item memory as a function of age and cohort.
Finally, because the period between 5 to 7 years was identified as a time of accelerated change in source memory ability, we sought to explore the possible mechanisms driving this change. We examined whether measures of non-verbal IQ, verbal comprehension, processing speed, or working memory were significant predictors of change during this period. Specifically, non-parametric correlations (Kendall's Tau-b) were computed between non-verbal IQ, verbal comprehension, processing speed, and working memory and changes in fact and source memory. When possible, predictors were obtained from the Wave 2 dataset for the 4-year Cohort and from the Wave 1 dataset for the 6-year Cohort as these were the time points that preceded the accelerated change. For measures that were only obtained once during the study (i.e., non-verbal IQ and verbal comprehension) these measures were obtained from the only dataset available and used for both Cohorts. Results revealed that both verbal comprehension and processing speed (as measured by Visual Matching) were related to improvements in fact memory, r(70) = .19, p<.05 and r(83) = .16, p<.05 respectively. However, none of the variables examined were related to improvements in source memory (all ps >.45).
Discussion
To our knowledge, this study represents the first longitudinal investigation of binding processes during early and middle childhood. Using a source memory paradigm within a cohort-sequential design, we examined developmental changes in memory for individual items (facts or sources) and memory for correct fact/source combinations (indicative of binding) between 4 and 10 years of age. Findings suggested steady increases in memory for individual items but rapid improvements in memory for correct fact/source combinations between 5 to 7 years. Specifically, only source responses contingent on successful item recall (i.e., conditionalized source responses) showed differences in rate of change between the groups. Thus, 5 to 7 may be particularly important period for the development of binding processes in memory.
These findings illustrate, within a longitudinal sample, different developmental trajectories may exist for binding and item memory. This is consistent with the growing body of literature using cross-sectional designs to examine the development of binding processes in laboratory-based settings (e.g., Drummey & Newcombe, 2002; Lindsay et al., 1991; Sluzenski et al., 2006). However, due to the longitudinal nature of our design, we were able to distinguish with increased specificity the period over which binding shows the most rapid development (5 to 7 years) and contrast that to development in item memory (which showed steady improvements during this same period).
Our results also suggest that different mechanisms are driving age-related changes in binding and item memory during this period. Specifically, verbal comprehension and processing speed were related to changes in item memory, but were not related to changes in binding. In fact, none of the general cognitive abilities assessed in this study (IQ, verbal ability, processing speed, or working memory) predicted changes in binding between 5 to 7 years. There are several likely candidates that should be investigated in future studies, including: development of brain regions involved in memory and children's transition into the classroom. In terms of brain development, regions in the both medial temporal lobe and prefrontal cortex are known to contribute to memory performance in older children and adults. Both these regions undergo significant change during childhood (e.g., Gogtay et al., 1999; Giedd et al., 1999; see Ghetti & Bunge 2012, for review in school-age children). An important question for future research is if behavioral changes are due to changes in medial temporal lobe structures known to be important for memory (e.g., Ghetti, DeMaster, Yonelinas, & Bunge, 2010; see Riggins, 2012 for a conceptual argument) or prefrontal regions implicated in cognitive control processes associated with targeting remembering (e.g., Sprondel, Kipp, & Mecklinger, , 2012; see also Ghetti, Lyons, & DeMaster, 2012). Interestingly, within the medial temporal lobe, synaptic connectivity within the hippocampus (a structure that is critical for binding) reaches mature levels around 5 years of age postnatally (Serres, 2001). Although this likely has strong implications for functional development and, ultimately, behavior, the significance of this developmental change has not yet been empirically established (see Bachevalier & Vargha-Khadem, 2005 for discussion).
In terms of formal schooling, research has shown that experience in the classroom alters children's memory abilities (e.g., Ornstein, Grammer, Coffman, 2010). For example, first and second graders exposed to memory-rich teaching exhibit greater levels of strategic knowledge and engage in more sophisticated strategy use in a memory task involving instructional content than do students exposed to low memory instruction (Grammer, Coffman, and Ornstein, 2013). Identification of the mechanisms underlying changes in binding abilities between 5 to 7 years is an important topic for future research.
The reported findings of accelerated change in binding between 5–7 years, is particularly exciting as this period overlaps in ontogenetic time with important developmental changes identified in research on the development of autobiographical memory. Autobiographical memory requires binding in order to encode and subsequently retrieve spatio-temporal context associated with experience (e.g., where and when the event occurred). Data from cross-sectional studies suggest rapid changes in autobiographical memory between 3 and 6–7 years of age as the number of events that children recall increases linearly and the amount of information that is recalled doubles (Bauer, Burch, Scholin, & Guler, 2007; Bauer, 2007; Howe & Courage, 1993, 1997). A recent longitudinal study clarified these effects by asking children to nominate their 3 earliest memories and tracking them over a 2-year period (Peterson et al., 2011). Although younger children had access to earlier first memories than older children, over time, the age of children's earliest memories shifted to a later period of their lives. In terms of content, there was almost no consistency in the memories reported for 4–6 year olds (either the specific events recalled or the details of the events); only after 7 years of age did children identify the same memories consistently. In fact, authors reported that many of the memories previously provided by children younger than 7 years of age were subsequently forgotten. Thus, in younger children memories are fragile and vulnerable to forgetting, whereas memories in older children are more consolidated and robust (Peterson et al., 2011). Based on these findings the authors argued that it is not until age 7 that the distribution of autobiographical memories appears to be adult-like and the memories that remain show some stability. Developmental changes in binding and the ability to recall contextual details between 5 to 7 years may be the mechanism underlying these effects (see Sluzenski et al., 2006 for similar argument).
Findings from the present study extend previous research on binding in several ways. First, we specifically examined how source memory irrespective of fact recall (i.e., unconditionalized source memory) improved as a function of age. Previous research has suggested relatively stable increases in fact recall between 4 and 8 years of age (cf. Drummey & Newcombe, 2002). However, how memory for individual sources changed over time is not commonly explored. This distinction is important as it can distinguish what is improving in memory development: memory for individual units or binding two (or more) individual units together. For example, one strength of the study by Sluzenski and colleagues was that memory for individual animals or backgrounds did not change between 4 and 6 years, only memory for animal-background combinations did (Slulzenski et al., 2006). A real-life example of this may be the following: a professor walks into their office to retrieve a pen, but upon arrival, does not remember what they walked in their office to get. In this scenario, a spatio-temporal detail (i.e., location = office) is recalled correctly. However, because it was not successfully bound to the item (i.e., pen), it is simply a memory for an individual spatial location as opposed to a bound memory representing the union of a location and a specific item (see Ranganath, 2010 for elaboration). Our results show relatively steady increases in unconditionalized source memory (memory for individual sources) between 4 and 10 years of age, a pattern that was similar to that for memory for individual facts.
Second, results of the present study help clarify the types of errors made by children since it allowed them to state that they “guessed” or “just knew” the facts. The previous cross-sectional source memory study by Drummey and Newcombe (2002) reported that extra-experimental errors decreased dramatically with age and suggested that this response pattern resembled confabulations by patients with frontal lobe dysfunction. In contrast, findings in the present study suggest that extra-experimental errors remain fairly consistent between 4 and 10 years of age, when participants are given the opportunity to indicate they “guessed” or simply “knew” the fact. The finding that children appropriately used the “guess”/“know” option is particularly surprising given the known improvements in metacognition during early and middle childhood (e.g., Flavell, 1999). However, results of the current study suggest that all children in the study were aware when they could not localize a specific source and responded accordingly (i.e., they accurately stated they guessed the response as opposed to nominating an extra-experimental source). They did not inaccurately recall or imagine an erroneous extra-experimental source. An interesting avenue for future research would be to examine the connection between metacognition and different categories of responses on source memory tasks (i.e., to assess understanding of “guessing” and “knowing” and its relation to memory).
Results from the present study revealed practice effects were not present for measures of Fact memory (i.e., Fact Recall or Fact Total, see Table 3B). This suggests that previous experience with the task did not significantly alter children's ability to learn and remember new facts. However, there were practice effects for some measures of Source memory (i.e., Source Total, Unconditionalized Source Total, and, for 8 year olds, Guessed/Knew responses; see Table 3B), suggesting that previous experience with the task increased participants' likelihood of generating a correct source response. This is reflected by significant differences in the pairwise comparisons in Table 2 and Figures 3, 6, and 7, as the starting points for each cohort are shifted slightly downward compared to the same aged children from the younger cohorts. However, practice effects were not observed for Extra-or Intra-experiment errors (see Figures 4 and 5). Although practice effects suggest some effect of completing the task multiple times, they cannot account for the Cohort × Time of Measurement interaction as practice effects would not result in differential rates of learning within Cohorts. Thus, the conclusion that Source Memory shows accelerated change between 5 – 7 years is valid even in the context of practice effects. If the pattern of change in Source Memory was solely attributable to practice effects, the slopes would remain the same. (i.e., there would be no Cohort × Time interaction), which is the pattern observed for Unconditionalized Source Memory. In short, previous experiences with this task did lead to participants being more likely to nominate a correct source (both when it was recalled with or without the correct Fact), however this does not account for differences in rates of change between Cohorts. Related, the pairwise comparisons between Waves 1 and 2 can also be contrasted with those between Waves 2 and 3 to determine whether knowledge of the nature of the task (incidental versus intentional) mattered (Table 3A). Given that no consistent pattern emerged in terms of effects that were present between Waves 1 and 2 but not 2 and 3 (or vise versa), we conclude that the impact of knowledge regarding the nature of the task was minimal in this dataset.
Given the increased specificity regarding ages when source memory shows rapid development, there are multiple avenues for future research. In particular, future investigations should begin to address what changes to account for the improvement in binding, such as changes in neural mechanisms or formal schooling.. In addition, closer inspection of changes in memory processes is warranted as well. For instance, a question of importance is whether binding of items and contexts did not occur because they were not encoded initially, because they were not bound together with the fact information, or that the two kinds of information were not retrieved together (i.e., whether developmental improvements in binding can be attributed to changes at encoding, consolidation/storage, or retrieval, see Bauer, Larking, & Doydum, in press; Bauer, 2006, for a conceptual argument). Previous cross-sectional studies (Howe, 1995; Howe & O'Sullivan 1997; Lloyd, Doydum, & Newcombe, 2009) suggest the largest portion of age-related variance in children's recall is accounted for by failure at the level of consolidation and storage, as opposed to encoding or retrieval; however, additional work examining this question is needed.
In conclusion, findings in the present report suggest 5 to 7 years of age is a time of important change in memory binding, which stands in contrast to relatively steady changes observed for memory for items. These results are exciting as they coincide with longitudinal research examining autobiographical memory that suggests this same period marks the transition from fragile to robust memories for personal life events (Peterson et al., 2011) and may account for part of this transition.
Acknowledgments
This research was supported by in part by a Doctoral Dissertation fellowship from the University of Minnesota, grants from the National Institutes of Health (HD-R01-28425, PI: Patricia J. Bauer, and HD-R03-067425, PI: Tracy Riggins), and the Department of Psychology at the University of Maryland, College Park. The author would like to thank Patricia J. Bauer for her generous support of this work, the members of the Cognition in Transition Laboratory at University of Minnesota, in particular Marina Larkina, the members of the Neurocognitive Development Lab at the University of Maryland, especially Victoria Smith and Jennifer Sloane, the Design and Statistical Analysis Lab (DASL) at the University of Maryland, particularly Laura Sherman, as well as the families who participated in this study. Portions of these data were presented at the meeting of the Society for Research Child Development in Atlanta, GA, April 2005 and in DeBoer, T. (2005). A neurobehavioral investigation of autobiographical memory development: Contributions of source memory and memory for temporal order. (Doctoral dissertation, University of Minnesota).
Appendix
Source Memory Task Stimuli
List 1
-
1
Cheetahs are the only big cats that can't roar.
-
2
Honey bees communicate with each other by dancing.
-
3
A group of goats is called a tribe.
-
4
A baby kangaroo is called a Joey.
-
5
Alaska is the largest state in America.
-
6
Venus is the brightest planet in the sky.
-
7
The California state flower is called the Golden Poppy.
-
8
Glass is made from sand.
-
9
The flute is the oldest musical instrument in the world.
-
10
A hummingbird is the only bird that can fly backwards.
-
11
Hair is the fastest growing part of the human body.
-
12
An airplane mechanic invented the Slinky.
List 2
-
1
Dolphins talk to each other by squeaking and clicking.
-
2
A honey bee fly can fly up to 15 miles an hour.
-
3
A group of kangaroos is called a mob.
-
4
A baby turtle is called a hatchling.
-
5
The Nile is the longest river in the world.
-
6
Jupiter is the largest planet in the solar system.
-
7
The Idaho state tree is the white pine.
-
8
The most popular name for a pet in America is Max.
-
9
The leader of Canada is called the Prime Minister.
-
10
Butterflies taste things with their feet.
-
11
The Common Flicker is a bird.
-
12
Tokyo, Japan has more people than any other city in the world.
List 3
-
1
A giraffe cannot make any sounds.
-
2
A honey bee has 4 wings.
-
3
A group of rhinos is called a crash.
-
4
A baby frog is called a tadpole.
-
5
The largest ocean in the world is the Pacific Ocean.
-
6
Mercury is the closest planet to the sun.
-
7
The Wisconsin State flower is the wood violet.
-
8
A two-person bicycle is called a tandem bike.
-
9
Bananas grow in bunched called hands.
-
10
A crocodile cannot stick its tongue out.
-
11
China has more people than any other country in the world.
-
12
Paper money is made from cotton.
Footnotes
1Data from 24 assessments were excluded because children answered fewer than 7 source questions (most of these participants nominated the video as the source of the information but refused to state whether it was from the person on the video or puppet on the video, thus rendering the response invalid).
References
- Ballinger GA. Using Generalized Estimating Equations for Longitudinal Data Analysis. Organizational Research Methods. 2004;7(2):127–150. doi:10.1177/1094428104263672. [Google Scholar]
- Bachevalier J, Vargha-Khadem F. The primate hippocampus: ontogeny, early insult and memory. Current Opinion in Neurobiology. 2005;15(2):168–174. doi: 10.1016/j.conb.2005.03.015. doi:10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Constructing a past in infancy: A neuro-developmental account. Trends in Cognitive Sciences. 2006;10:175–181. doi: 10.1016/j.tics.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Remembering the times of our lives: Memory in infancy and beyond. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. [Google Scholar]
- Bauer PJ, Burch MM, Scholin SE, Güler OE. Using cue words to investigate the distribution of autobiographical memories in childhood. Psychological Science. 2007;18:910–916. doi: 10.1111/j.1467-9280.2007.01999.x. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Doydum AO, Pathman T, Larkina M, Güler OE, Burch M. It's all about location, location, location: Children's memory for the “where” of personally experienced events. Journal of experimental child psychology. 2012;113(4):510–522. doi: 10.1016/j.jecp.2012.06.007. doi:10.1016/j.jecp.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Larkina M, Doydum AO. Explaining variance in long-term recall in 3-and 4-year-old children: The importance of post-encoding processes. Journal of Experimental Child Psychology. 2012;113:195–210. doi: 10.1016/j.jecp.2012.05.006. DOI: 10.1016/j.jecp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4:359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of Nonverbal Intelligence: A language-free measure of cognitive ability. 3rd PRO-ED; Austin, TX: 1992. [Google Scholar]
- Drummey AB, Newcombe NS. Developmental changes in source memory. Developmental Science. 2002;5:502–513. [Google Scholar]
- Flavell JH. Cognitive Development: Children's Knowledge About the Mind. Annual Review of Psychology. 1999;50(1):21–45. doi: 10.1146/annurev.psych.50.1.21. doi:10.1146/annurev.psych.50.1.21. [DOI] [PubMed] [Google Scholar]
- Graham JW. Missing Data Analysis: Making It Work in the Real World. Annual Review of Psychology. 2009;60(1):549–576. doi: 10.1146/annurev.psych.58.110405.085530. doi:10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Developmental cognitive neuroscience. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. doi:10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge SA. Developmental Differences in Medial Temporal Lobe Function during Memory Encoding. The Journal of Neuroscience. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. doi:10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Lyons KE, DeMaster DM. The development of subjective remembering. In: Ghetti S, Bauer PJ, editors. Origins and development of recollection: Perspectives from psychology and neuroscience. Oxford University Press; New York, NY: 2012. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. doi:10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. doi:10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Grammer J, Coffman JL, Ornstein P. The Effect of Teachers' Memory-Relevant Language on Children's Strategy Use and Knowledge. Child Development. 2013 doi: 10.1111/cdev.12100. doi:10.1111/cdev.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin JW, Hilbe JM. Generalized Estimating Equations. CRC Press; 2003. [Google Scholar]
- Howe ML. Interference effects in young children's long-term retention. Developmental Psychology. 1995;31(4):579–596. doi:10.1037/0012-1649.31.4.579. [Google Scholar]
- Howe ML, Courage ML. On resolving the enigma of infantile amnesia. Psychological Bulletin. 1993;113:305–326. doi: 10.1037/0033-2909.113.2.305. [DOI] [PubMed] [Google Scholar]
- Howe ML, Courage ML. The emergence and early development of autobiographical memory. Psychological Review. 1997;104:499–523. doi: 10.1037/0033-295x.104.3.499. [DOI] [PubMed] [Google Scholar]
- Howe ML, O'Sullivan JT. What Children's Memories Tell Us about Recalling Our Childhoods: A Review of Storage and Retrieval Processes in the Development of Long-Term Retention. Developmental Review. 1997;17(2):148–204. doi:10.1006/drev.1996.0428. [Google Scholar]
- Jelicic H, Phelps E, Lerner RM. Use of missing data methods in longitudinal studies: The persistence of bad practices in developmental psychology. Developmental Psychology. 2009;45(4):1195–1199. doi: 10.1037/a0015665. [DOI] [PubMed] [Google Scholar]
- Lindsay DS, Johnson MK, Kwon P. Developmental changes in source monitoring. Journal of Experimental Child Psychology. 1991;53(3):297–318. doi: 10.1016/0022-0965(91)90065-z. doi:10.1016/0022-0965(91)90065-Z. [DOI] [PubMed] [Google Scholar]
- Lloyd ME, Doydum AO, Newcombe NS. Memory binding in early childhood: evidence for a retrieval deficit. Child Development. 2009;80(5):1321–1328. doi: 10.1111/j.1467-8624.2009.01353.x. doi:10.1111/j.1467-8624.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Multiple dissociations of function in amnesia. In: Cermak LS, editor. Human Memory and Amnesia. Erlbaum Associates; Hillsdale, NJ: 1982. pp. 337–370. [Google Scholar]
- Newcombe NS, Lloyd ME, Balcomb F. Contextualizing the development of recollection: Episodic memory and binding in young children. In: Ghetti S, Bauer PJ, editors. Origins and development of recollection: Perspectives from psychology and neuroscience. Oxford University Press; 2012. pp. 73–100. [Google Scholar]
- Ornstein PA, Grammer JK, Coffman JL. Teachers' “mnemonic style” and the development of skilled memory. In: Waters HS, Schneider W, editors. Metacognition, strategy use, and instruction. Guilford Publications; New York: 2010. pp. 23–53. [Google Scholar]
- Peterson C, Warren KL, Short MM. Infantile amnesia across the years: a 2-year follow-up of children's earliest memories. Child Development. 2011;82(4):1092–1105. doi: 10.1111/j.1467-8624.2011.01597.x. doi:10.1111/j.1467-8624.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. doi:10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Riggins T. Building blocks of recollection. In: Ghetti S, Bauer PJ, editors. Origins and Development of Recollection: Perspectives from Psychology and Neuroscience. Oxford University Press; New York, NY: 2012. [Google Scholar]
- Serres L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. MIT Press; Cambridge, MA: 2001. pp. 45–58. [Google Scholar]
- Schacter DL, Harbluk JL, McLachlan DR. Retrieval without recollection: An experimental analysis of source amnesia. Journal of Verbal Learning and Verbal Behavior. 1984;23(5):593–611. doi:10.1016/S0022-5371(84)90373-6. [Google Scholar]
- Schneider W, Bjorklund DF. Cognitive, language, and perceptual development. In: Kuhn D, Siegler RS, Damon W, editors. Handbook of child psychology. 5th Ed. Vol. 2. Wiley; New York: 1998. pp. 467–521. Memory. [Google Scholar]
- Schneider W. Individual differences in memory development and educational implications: Cross-sectional and longitudinal evidence. In: Bauer PJ, Fivush R, editors. Handbook on the Development of Children's Memory. Wiley-Blackwell; in press. [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs S. Binding, relational memory and recall of naturalistic events: A developmental perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, Ottinger W. Changes in reality monitoring and episodic memory in early childhood. Developmental Science. 2004;7:225–245. doi: 10.1111/j.1467-7687.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- Sprondel V, Kipp KH, Mecklinger A. Electrophysiological evidence for late maturation of strategic episodic retrieval processes. Developmental Science. 2012;15(3):330–344. doi: 10.1111/j.1467-7687.2011.01130.x. doi:10.1111/j.1467-7687.2011.01130.x. [DOI] [PubMed] [Google Scholar]
- Wetzler SE, Sweeney JA. Childhood amnesia: An empirical demonstration. In: Rubin DC, editor. Autobiographical memory. Cambridge University Press; New York: 1986. pp. 191–201. [Google Scholar]
- Weinert FE, Schneider W. Individual Development from 3 to 12: Findings From the Munich Longitudinal Study. Cambridge University Press; 1999. [Google Scholar]
- Welch-Ross MK. Developmental changes in preschoolers' ability to distinguish memories of performed, pretended, and imagined actions. Cognitive Development. 1995:421–441. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson Tests of Cognitive Abilities. Riverside; Itasca, IL: 2001. [Google Scholar]
- Zeger SL, Liang K-Y, Albert PS. Models for Longitudinal Data: A Generalized Estimating Equation Approach. Biometrics. 1988;44(4):1049–1060. doi:10.2307/2531734. [PubMed] [Google Scholar]