Abstract
Objective
To outline a contemporary review of defect classification and reconstructive options.
Design
Review article.
Setting
Tertiary care referral centers.
Results
Although prosthetic rehabilitation remains the standard of care in many institutions, the discomfort of wearing, removing, and cleaning a prosthesis; the inability to retain a prosthesis in large defects; and the frequent need for readjustments often limit the value of this cost-effective and successful method of restoring speech and mastication. However, flap reconstruction offers an option for many, although there is no agreement as to which techniques should be used for optimal reconstruction. Flap reconstruction also involves a longer recovery time with increased risk of surgical complications, has higher costs associated with the procedure, and requires access to a highly experienced surgeon.
Conclusion
The surgeon and reconstructive team must make individualized decisions based on the extent of the maxillectomy defect (eg, the resection of the infraorbital rim, the extent of palate excision, skin compromise) and the need for radiation therapy.
The maxillary defect after ablative tumor surgery typically involves the mucosal lining, the midface osseous framework, and the adjacent soft tissue.1 Reconstruction of this defect remains a considerable challenge because the 3-dimensional architecture of the midface serves both functional and aesthetic roles. Based on these considerations, the final goals for midface reconstruction should ideally be (1) to give support to the orbital content, thus minimizing changes in globe position, orbital volume, and eyelid functions, or treat the exenterated orbit cosmetically; (2) to maintain a patent nasal airway and oronasal separation creating a sufficient platform for mastication, speech quality, and potential dental rehabilitation; and (3) to restore an adequate and symmetric facial contour with the other side of the face.2
Individuals diagnosed as having cancer of the head and neck are overwhelmed by the notion of potential functional sequelae owing to treatment involving surgery, radiation therapy, and chemotherapy. Functional challenges that limit the ability to speak and eat are often apparent and lead to social isolation, loss of employment, and decreased quality of life.3,4 All of this may cumulate to patients’ inability to care for themselves and their families. Loss of employment places a financial burden on society when patients come to rely on social welfare systems. When communicative functions are compromised, the community will often judge social attributes of a speaker very negatively,5 resulting in a distressing impact on quality of life.4,6 With respect to eating, an individual’s intentions to consume food are influenced not only by hunger and physiological mechanisms but also by social and cultural factors.7 When the ability to eat is compromised, as it so often is in head and neck cancer survivors, the impact on socialization can be staggering. Because of the far-reaching social and economic implications of functional deficits after treatment for palatomaxillary cancer, the optimal method for rehabilitating the patient must be carefully considered.
Past techniques have been dominated by prosthetic obturation because this was the only reconstruction option allowing immediate dental restoration without further surgery, but it was limited by problems such as instability, poor retention, and oronasal incompetence. The advent of microsurgery has permitted primary, single-stage reconstruction of these complex facial defects, avoiding the use of various combinations of local and regional flaps that have poorer aesthetic and functional results.1,8 Because maxillectomy is an uncommon operation, single-unit experience is small, and evidence is limited regarding the best reconstructive procedure.9 Although the selection method depends on the extent of the bony and soft-tissue defect, there is no clear or generally accepted recommendation. As a result, controversy remains as to selecting the optimal method of obturation, reconstruction, and rehabilitation.
METHODS
A classification system that concisely groups the wide range of possible tissue losses can potentially serve as a common language, limit reconstructive options, and allow surgeons to compare results.10 The complex 3-dimensional anatomy of this multifunctional region presents not only a technical surgical challenge to restoring the preoperative state but also difficulties with the development of a uniformly accepted classification system to allow the comparison of techniques. As is common with difficult problems, multiple reconstructive algorithms and classification schemes have been applied to maxillary and palatal defects.1–5 Okay et al11 described a maxillectomy defect classification based on biomechanical forces of tissue retentive ability for prosthetic devices, but this scheme is less widely used in the reconstructive literature. Brown et al12 and Cordeiro and Santamaria10 separately developed very similar and accepted classifications (hereinafter Brown class and Cordeiro type, respectively). In the first one, Brown et al12 describe maxillectomy defects by independent vertical and horizontal components (Table). The vertical dimension (classes 1–6) designates the extent of unilateral involvement, with emphasis on the orbit. The horizontal dimension (letters a–d) designates the amount of palate and alveolar ridge sacrificed. Thus, 24 possible designations characterize maxillary defects in this system, in which almost all the possible lesions are incorporated and a systematic reconstructive decision-making algorithm is provided. In the second classification, Cordeiro and Santamaria10 describe a simplified 4-part classification scheme:
| Type 1 | Limited maxillectomy, palate is not involved |
| Type 2 | Subtotal maxillectomy, preservation of orbital floor |
| Type 3 | |
| a | Total maxillectomy with orbital preservation |
| b | Total maxillectomy with orbital exenteration |
| Type 4 | Orbitomaxillectomy, palate is preserved |
Table.
Classification of Maxillary Defects by Brown et ala
| Class or Letter | Defect |
|---|---|
| Vertical Component | |
| Class | |
| 1 | Maxillectomy not causing an oronasal fistula |
| 2 | Not involving the orbit |
| 3 | Involving the orbital adnexae with orbital retention |
| 4 | With orbital enucleation or exenteration |
| 5 | Orbitomaxillary defect |
| 6 | Nasomaxillary defect |
| Horizontal Component | |
| Letter | |
| a | Palatal defect only, not dental alveolus |
| b | Less than or equal to 1/2 of the bilateral or transverse anterior |
| c | Less than or equal to 1/2 of the unilateral |
| d | Greater than 1/2 of the maxillectomy |
Adapted from Brown et al.12
This classification evaluates the surface area to volume requirement, the need for palatal closure, and the need for orbital reconstruction. Although the classification system by Cordeiro and Santamaria10 adequately addresses the 3-dimensional anatomy of the maxilla, it does not clearly select the patients who would be good candidates for dental obturation. The investigators also proposed a reconstructive algorithm, but the main drawback to this scheme is the lack of attention paid to functional dental and nasal reconstruction.
RESULTS
PROSTHETIC REHABILITATION
Prosthetic rehabilitation has long been advocated for the rehabilitation of defects of the soft and hard tissues of the palate. This includes maxillary obturators for defects of the hard palate, pharyngeal obturators for defects of the soft palate, and maxillopharyngeal obturators for defects that include both structures. Several articles describe speech outcomes associated with prosthetic rehabilitation.13–19 In general, prosthetic intervention leads to a restoration of dentition, reduction of hypernasality, 20,21 the most compromised aspect of speech in palatomaxillary resections, and subsequent restoration of speech intelligibility.15–19,21,22 The best results are achieved when the osseous tissues of the palatomaxillary complex are involved.20,21 With respect to eating, one of the most common problems reported by patients is nasal leakage when swallowing liquids or foods, with 25% to 40% of patients reporting moderate to extreme difficulty with this.23–25 However, this perception has not been corroborated by videofluoroscopic study or clinician observation of swallowing behavior.26,27 Other swallowing impairments seem to be rare after rehabilitation with an obturator.27,28
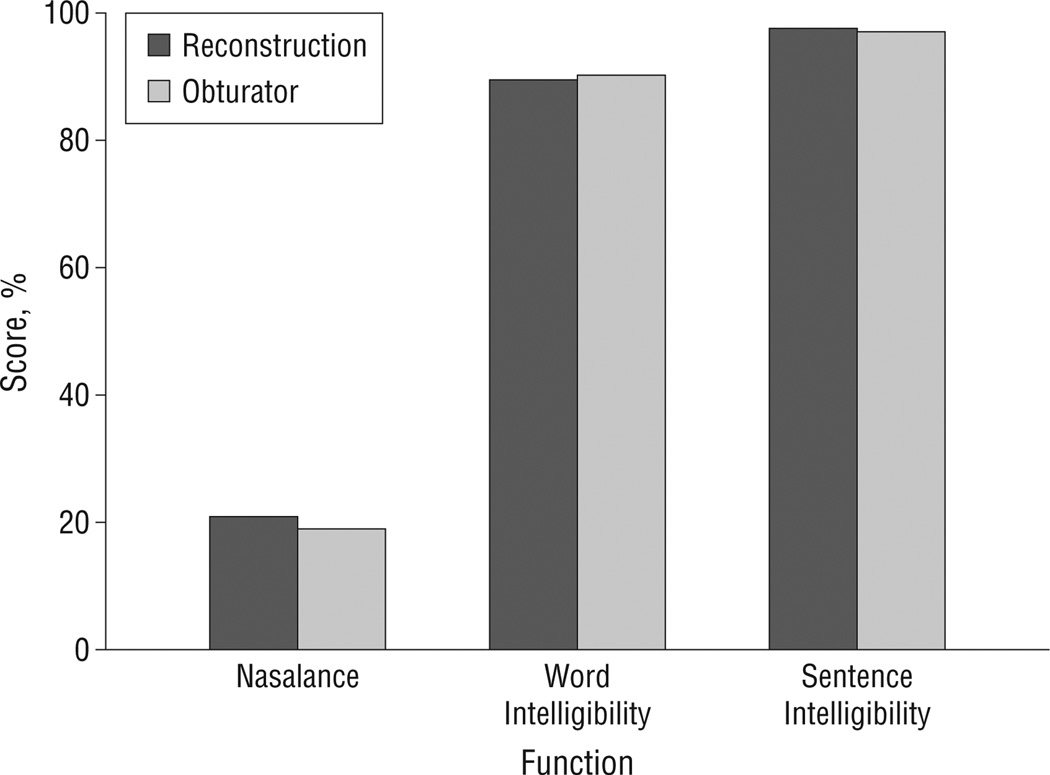
Benefits of rehabilitation with an obturator outside of the functional realm also include the ability to visualize the defect for ongoing cancer surveillance17,29 and restoration of function with minimal surgical intervention in a timely manner.15 However, several disadvantages to a prosthetic approach exist, including (1) the discomfort of wearing a prosthesis, (2) the inconvenience of removing and cleaning the prosthesis, (3) the inability to successfully retain a prosthesis when the defect is large or when dentition is lacking, and (4) the frequent need for readjustments by a prosthodontist.17,30 These drawbacks have led to a search for alternate solutions, the foremost being palatomaxillary reconstruction with microvascular free flaps. Studies that compare prosthetic obturation with reconstruction of a palatomaxillary defect demonstrate that there are some advantages to reconstruction, in particular, quality-of-life issues including comfort, convenience, and decreased feelings of self-consciousness.31 With respect to speech and swallowing function, palatomaxillary rehabilitation outcomes between prosthetic intervention are comparable flap reconstructions32,33 (Figure1). The exception to these findings is when the maxillary defect is extensive or when the anterior palate, including both canines, is resected, in which case patients with free-flap reconstruction have better outcomes than those with a prosthesis.28
Figure 1.
Scores obtained in a study that included 23 patients who underwent obturation and 16 who underwent maxillary reconstruction.34 Normal values for nasalance ranged from 11.9% to 13.7% (average standard deviation, 4.8%); normal values for word and sentence intelligibility were higher than 90%.
While the choice of whether to venture into a prosthetic vs a surgical option for palatomaxillary rehabilitation is based on many considerations, there is one factor that can influence the outcome of either treatment: postradiation xerostomia,35 a condition in which the function of the salivary glands has been altered such that hyposalivation occurs, resulting in subjective complaints of a dry mouth.36 With the use of conventional radiotherapy, salivary dysfunction develops rapidly and permanently. The sequelae of impaired salivary function include mucositis, periodontal disease, discomfort and pain in the oral cavity, dental caries, tongue fissures, loss of teeth, alterations in taste acuity, difficulty wearing dentures or obturator prostheses, and impairments of mastication, deglutition, and speech.34 Thus, the development of salivary dysfunction in patients with palatomaxillary defects is of serious consequence to their nutritional status, their candidacy for advanced dental rehabilitation,37 their capability to speak and interact socially, and their quality of life.38 Salivary function sparing techniques, such as intensity-modulated radiation therapy, should be used whenever feasible to minimize the occurrence of these outcomes and promote successful rehabilitation of the oral cavity after treatment for palatomaxillary tumors.
In summary, the best candidate for prosthetic rehabilitation would be a patient who has not undergone radiation and with a small lateral or posterior palatomaxillary defect and viable dentition to support prosthesis (Brown class 1 or 2a–2b or Cordeiro type 2 defects).
SOFT-TISSUE FREE-FLAP RECONSTRUCTION
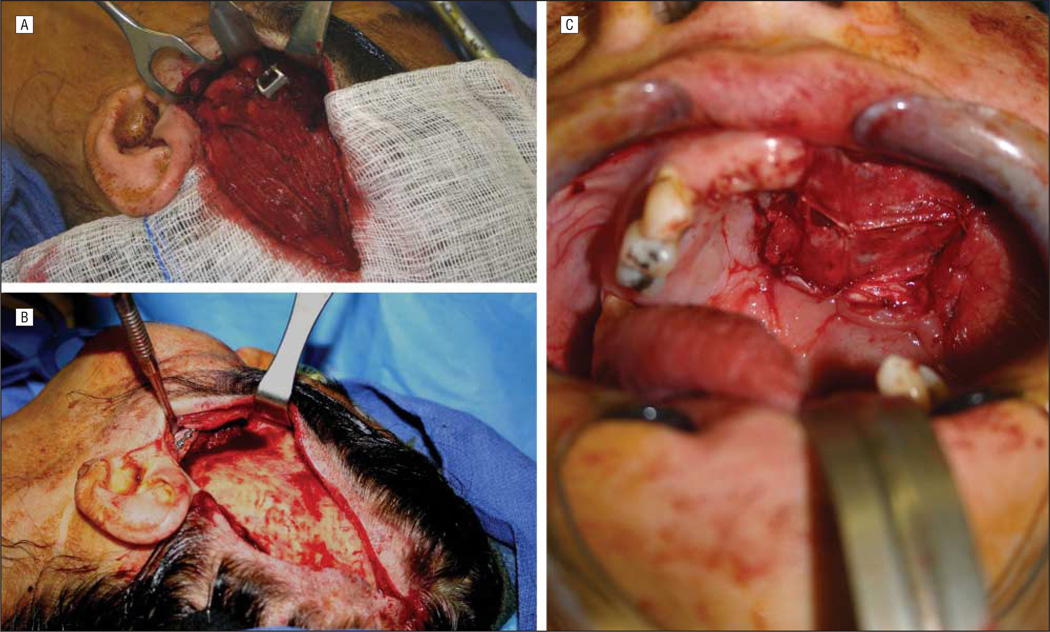
Management of palatal or hemipalatal defects (small to medium sized) with preservation of the orbital floor remains controversial because there are many options that can successfully treat these patients. While dental obturation remains an effective option in patients not expected to receive radiation, the use of local, regional, or distant free flaps all can result in good function and aesthetics, especially in larger defects. Although the obliterated maxillectomy cavity was thought to potentially delay the diagnosis of tumor recurrence, available evidence does not support this hypothesis, which may be related to an increase in the use of anatomic and metabolic imaging strategies in routine follow-up.6 Although free flaps are more commonly used, a temporalis muscle flap may be used for small- and medium-sized palate defects.10,13,14 The use of this flap is more intuitive than other regional options because the harvest can be combined with an approach often needed to expose the infratemporal fossa.14 After harvest through an ipsilateral hemi-coronal incision, the flap can be passed into the maxillectomy/palatectomy cavity by removal (and subsequent replacement) of the zygomatic arcade. The fascial surface is allowed to mucosalize intraorally, often forming more natural intraoral lining than the one provided by skin flaps (Figure 2).
Figure 2.
Temporalis musculofascial rotational flap. The flap is isolated after detachment from the temporal line superiorly (A), passed under the zygomatic arch (B), and into the oral cavity, where it eventually mucosalizes (C).
Patients who underwent reconstruction with radial forearm fasciocutaneous flaps had satisfaction scores for appearance, chewing, and taste that were similar to those of patients who underwent obturation and had higher scores in speech, comfort, convenience, and social interaction compared with the latter group.7 The choice of autologous tissue selection varies on the vascular condition of the donor site, flap surface to volume ratio, pliability of tissues, and pedicle size and length. Because the radial forearm free flap satisfies many of these criteria, it is commonly used for maxillary reconstruction of small volume defects. Other donor sites, including the rectus abdominus (perforator flap), lateral arm, and serratus anterior muscle, have been successfully applied to reconstruct these defects.8,9
Large-volume maxillectomy defects with or without orbital exenteration (Brown class 3 or 4a, 4b, 4c, or 4d or Cordeiro types 3b and 4) typically require microvascular reconstruction. Commonly, multiple skin paddle reconstruction is required for such cases to recreate palatal or external skin defects.15 Use of an additional skin paddle for mucosal lining is controversial and often not necessary. Both vertical rectus myocutaneous and anterolateral thigh free flaps permit development of multiple skin paddles, which is usually accomplished using multiple island perforator flaps or segmental deepithilization.3,16 Analogous to the smaller defects that are reconstructed with temporalis or seratus anterior muscle flaps, the large-volume defects can be reconstructed with muscle-only free flaps, such as rectus abdominus, latissimus dorsi,4,17 or vastus lateralis. In this setting, the vascularized fascia or muscle granulate intraorally or intranasally and eventually mucosalize. In contrast with osteocutaneous flaps, however, the large-volume musculocutaneous flaps suffer from a lack of support that results in soft-tissue flap ptosis into the oral cavity. Several techniques have been described to mitigate this problem, including suture suspension techniques and manipulation of the fascia to prevent intraoral flap prolapse.16,18
In summary, reconstruction with soft-tissue–only flaps may be indicated in 2 settings: small- to medium-sized lower maxillectomy defects with viable dentition to support prosthesis (Brown class 1 or 2a–2b or Cordeiro type 2 defects); or extensive defects in patients with relatively poor oncologic prognosis (Brown class 4 or 5a–5d or Cordeiro type 3b defects).
OSTEOCUTANEOUS FREE-FLAP RECONSTRUCTION
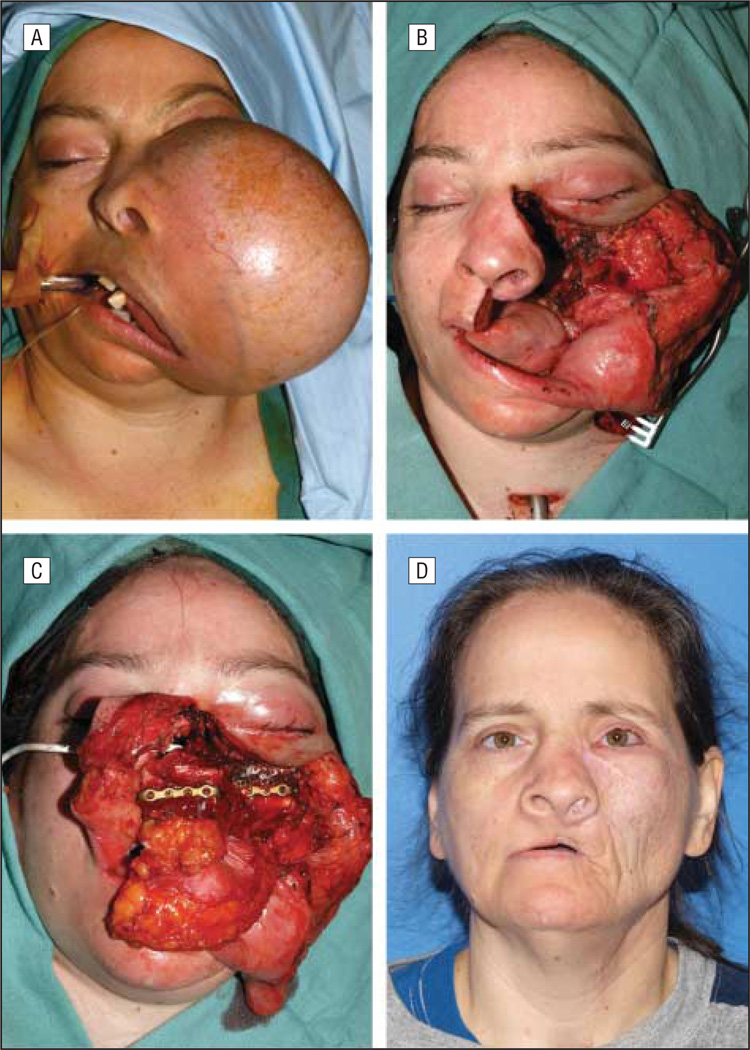
Intermediate-size defects with better survival rates require complete palatal-alveolar-maxillary restoration to maintain the patient’s quality of life, and osteocutaneous free flaps are the best option. Although the selection of the reconstructive method depends on the extent of the bony and soft-tissue defect, there is no clear or generally accepted recommendation. The amount, location, and quality of residual bone of the midface and dentition or denture-bearing alveolar arch largely determine whether a bone-containing flap is necessary. Bone reconstruction should be considered in medium-sized to large maxillectomy defects, with good oncologic prognosis, whenever oral rehabilitation, midface contour, and orbital support are a priority. Although these same defects may also be treated using a prosthetic obturator or a soft-tissue free flap, vascularized bone flaps are often needed to restore midfacial height, width, and projection, as well as to provide adequate bone stock for mastication and osseointegrated implants, which are usually required for the fixation of dental prostheses (Figure 3).28
Figure 3.
Photographs of a patient. A, A woman with a massive maxillary ameloblastoma. B, The resection includes a total left maxillectomy, including the orbital floor, and a partial right maxillectomy, sparing the right orbital floor and region of the malar eminence. C, A fibula osteocutaneous free flap was used to reconstruct this bilateral maxillectomy defect, along with a bone graft secured by titanium mesh, which was used to reconstruct the left orbital floor. D, Postoperative appearance, 1 year later.
The most used composite free tissue flaps for bone and soft-tissue palate and maxillary repair are the osteocutaneous radial forearm (OCRF), fibula, iliac crest, and the subscapular system of flaps. The surgeon needs to establish the extent of missing skin, soft tissue, and bone prior to reconstruction. In the midface, several prerequisites are needed for any type of free-tissue transfer. Ideally, skin, soft tissue, mucosa, and bone would be matched to the characteristics of the appropriate flap before undertaking the reconstructive procedure. In general, it is best to anastomose free flaps to reliable large vessels. These are scarce in the midface and are often resected. Thus, the usual site for donor vessels is the ipsilateral neck. The distance from the ipsilateral neck to the midface is a minimumof 10 to 12 cm. To avoid vein grafting, the flap needs to have a long pedicle with vessels of a good diameter. Other important elements to consider at the time of reconstruction planning include the thickness of the skin, muscle, and subcutaneous fat; the volume of the tissue available; the durability and thickness of the bone; and the morbidity of the donor site.
The OCRF free flap is a reliable flap, easy to harvest in a simultaneous 2-team approach, with a thin bone, a versatile skin paddle, and a long pedicle.30 The bone may be placed horizontally for arch reconstruction,39 obliquely for zygomaticomaxillary restoration, or osteotomized for inferior orbital rim repair.40 The skin paddle can be oriented to repair the mucosal defect and even brought superiorly to pad the midface or fill in a cutaneous defect in the paranasal region. Pathologic donor radial bone fracture has dramatically decreased by prophylactic plating, harvesting less than 40% of radial bone thickness, and use of keel-shaped osteotomies. Patients undergoing inferior maxillectomies with preservation of the infraorbital rim, requiring intraoral lining with small bone defects, are the best candidates for this type of flap (Brown class 2a–2b or Cordeiro type 2). Failure to adequately reconstruct the orbital support can result in considerable postoperative hypophthalmus, which is very difficult to correct secondarily, especially after adjuvant radiotherapy. Use of nonvascularized bone in patients expected to receive radiation is not always successful.
The fibula flap, with its excellent bone stock and soft, pliable skin paddle, can be applied to midface defects, especially those that cross the palatal midline. It has a reliable long pedicle with good vessel diameter. Osteointegrated dental implants may be used immediately or planned after surgery, although this remains a rare event in most head and neck reconstructive practices.41 Multiple osteotomies with severe angles are required to reconstruct the palate, anterior maxillary wall, and infraorbital rim, and the skin island orientation may also be problematic.42 Unfortunately, in large defect reconstruction, a flat cheek is usually obtained, and cosmetic results may be poor.43 For these reason, the best candidates for a fibula flap are those with an inferior maxillectomy that does not compromise the inferior orbital rim, extend across the midline, and necessitates osteointegrated dental implants for oral rehabilitation (Brown class 2a–2d or Cordeiro type 2).
The iliac crest free flap, based on the deep circumflex iliac artery and harvested with internal oblique muscle, provides an complete maxillary reconstruction; one part can restore the alveolus, zygomatic prominence, and infraorbital rim, and the muscle is used for sinus obliteration, oronasal separation, and intranasal lining.44 However, this flap has excessive bulk, restricted soft-tissue mobility in relation to the bone, short pedicle length, and donor-site morbidity.42 Patients undergoing total maxillectomies with or without involvement of the infraorbital rim but with preservation of orbital content are the best candidates for this flap (Brown class 3a–3d, Cordeiro type 3a).
The subscapular system of flaps is another option of composite free tissue transfer for midface reconstruction. The subscapular system of flaps offers perhaps the greatest versatility in flap reconstruction.45 Replacement of the alveolar arch inferiorly with the lateral scapula (supplied by the circumflex scapular artery) and the orbital floor and rim with the scapular tip (supplied by the angular branch of the thoracodorsal artery) suits this reconstruction well. Although all components of this flap can be rotated independently of each other and facilitate insetting, flap elevation cannot occur simultaneously with the extirpative procedure; it requires complex harvesting and has a short pedicle length. As for the iliac crest flap, patients undergoing total maxillectomy with orbital preservation are the best candidates for this flap (Brown class 3a–3d or Cordeiro type 3a).
RECONSTRUCTION OF COMPLEX DEFECTS
Defects that include cheek skin, orbital, and/or the external nose increase the complexity of maxillary reconstruction floor (Brown class 5 or 6c–6b, or Cordeiro type 3b and 4 defects). Augmentation of the reconstructive flap with grafts, implants, or even prostheses may be indicated. In addition, reconstruction might best be delayed or temporized with a prosthesis in select cases, if possible, pending adjuvant treatment or a period of observation to rule out early recurrence, which might undo the work of complex, staged reconstruction.
Patients in whom the cheek skin, in addition to the palate, must be reconstructed are often best served by soft-tissue free flaps that can be designed with more than 1 skin paddle, based on separate cutaneous perforating blood vessels, such as the anterolateral thigh and rectus abdominis myocutaneous free flaps.10 Two or even 3 skin paddles can be designed to resurface the palate, the cheek, and the lateral nasal wall, although this surface does not necessarily need to be lined with skin because it will remucosalize with time. The most complex defects may require multiple free flaps to replace missing bone needed for midfacial projection and mastication as well as line the cheek and palate.
The orbital floor can be reconstructed with vascularized bone flaps, bone grafts, or alloplasts. Vascularized bone flaps have the advantage of being the most resistant to infection and exposure, particularly in patients undergoing radiation. If a vascularized bone flap becomes exposed, it can usually be treated conservatively. However, in most defects, the priority for bony free-flap reconstruction is to restore the midfacial shape and provide a stable surface for mastication. Space in the midface is limited, and it is difficult to configure the free flap to accomplish these goals as well as precisely restore the orbit. In these cases, grafts or alloplasts are commonly used to reconstruct the orbital defect separately.
COMMENT
In the oncologic setting, orbital reconstruction can be more challenging owing to the need for radiation, as mentioned in the previous subsection. Most surgeons agree that bone grafts are resistant to radiation-associated complications, though less so than vascularized bone flaps. If adequate healing has otherwise occurred, infected or exposed bone and hardware can often be partially debrided. Alloplasts, such as titanium mesh or porous polyethylene, have the advantages of having no donor site morbidity and being easier to shape but may need to be completely removed should a complication occur.
Regardless of the method used for orbital reconstruction, accurate replacement of the bony orbit is critical to prevent orbital dystopia, enophthalmos, and exophthalmos. The contralateral eye should be draped into the surgical field to ensure appropriately that the globe and rim are symmetrical. Intraoperatively, they should be palpated to ensure that there is not excessive pressure on the globe, and a forced duction test should be performed to rule out entrapment. Following surgery, the patient should be monitored for changes in vision. In addition, orbital hardware requires coverage by well-vascularized tissues. Replacing or reinforcing scarred or radiated periorbital skin with the reconstructive flap should be considered to minimize the chances of exposure.
Some maxillary resections include the external nose. Aesthetically, the nose dominates the central face and has a profound effect on facial appearance when it is absent or deformed. However, creating a stable, refined, and 3-dimensionally accurate nasal reconstruction remains one of the greatest challenges in reconstructive surgery. Reconstructions need to take into account replacement of the covering skin, bony or cartilaginous skeletal support, and epithelial or mucosal lining.46 As a result, prosthetic rehabilitation is often used to attain excellent aesthetic result for total rhinectomy defects and can eliminate the need for multiple surgical procedures. A prosthesis may also be a good temporary solution while allowing reasonable time to rule out a recurrence or while adjuvant therapy is being given since multistaged reconstruction might delay timely treatment, and radiation-associated tissue changes will compromise the final reconstructive result. Disadvantages associated with prosthetic rehabilitation include the need for periodic replacement, problems with retention, and the sense that the prosthetic nose is not a “natural” part of one’s body.
In conclusion, trends in the provision of prosthetic devices and reconstructive options for maxillary defects will continue to be opinion based rather than evidence based. Multiple reconstructive pathways can be followed in restoring maxillary and midface defects. The surgeon and rest of the reconstructive team must make individualized decisions based on the maxillectomy defect, the resection of the infraorbital rim, extent of palate excision, skin compromise, and radiation therapy as well as the specific needs and concerns of the patient when selecting the optimal reconstructive approach for each individual case.
Footnotes
Author Contributions: Drs Andrades, Hanasono, Rieger, and Rosenthal had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Andrades, Militsakh, Hanasono, Rieger, and Rosenthal. Analysis and interpretation of data: Andrades and Militsakh. Drafting of the manuscript: Andrades, Militsakh, Hanasono, Rieger, and Rosenthal. Critical revision of the manuscript for important intellectual content: Andrades, Militsakh, Hanasono, and Rosenthal. Administrative, technical, and material support: Rosenthal. Study supervision: Andrades, Hanasono, and Rosenthal.
Financial Disclosure: None reported.
Additional Contributions: This work was a product of the American Head and Neck Society Reconstructive Committee.
Contributor Information
Patricio Andrades, Division of Plastic and Maxillofacial Surgery, Department of Surgery, University of Chile Clinical Hospital and Hospital del Trabajador de Santiago, Santiago, Chile.
Oleg Militsakh, Department of Otolaryngology, University of Nebraska Medical Center, Omaha.
Matthew M. Hanasono, Department of Plastic Surgery, The University of Texas M. D. Anderson Cancer Center, Houston.
Jana Rieger, Institute for Reconstructive Sciences in Medicine, Misericordia Hospital, Edmonton, Alberta, Canada.
Eben L. Rosenthal, Department of Surgery, Division of Otolaryngology–Head and Neck Surgery, University of Alabama at Birmingham.
REFERENCES
- 1.Disa JJ, Liew S, Cordeiro PG. Soft-tissue reconstruction of the face using the folded/multiple skin island radial forearm free flap. Ann Plast Surg. 2001;47(6):612–619. doi: 10.1097/00000637-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Santamaria E, Cordeiro PG. Reconstruction of maxillectomy and midfacial defects with free tissue transfer. J Surg Oncol. 2006;94(6):522–531. doi: 10.1002/jso.20490. [DOI] [PubMed] [Google Scholar]
- 3.Ruben RJ. Redefining the survival of the fittest: communication disorders in the 21st century. Laryngoscope. 2000;110(2 pt 1):241–245. doi: 10.1097/00005537-200002010-00010. [DOI] [PubMed] [Google Scholar]
- 4.De Boer MF, McCormick LK, Pruyn JF, Ryckman RM, van den Borne BW. Physical and psychosocial correlates of head and neck cancer: a review of the literature. Otolaryngol Head Neck Surg. 1999;120(3):427–436. doi: 10.1016/S0194-5998(99)70287-1. [DOI] [PubMed] [Google Scholar]
- 5.Rieger J, Dickson N, Lemire R, et al. Social perception of speech in individuals with oropharyngeal reconstruction. J Psychosoc Oncol. 2006;24(4):33–51. doi: 10.1300/J077v24n04_03. [DOI] [PubMed] [Google Scholar]
- 6.Specht L. Oral complications in the head and neck radiation patient: introduction and scope of the problem. Support Care Cancer. 2002;10(1):36–39. doi: 10.1007/s005200100283. [DOI] [PubMed] [Google Scholar]
- 7.de la Rie S, Noordenbos G, Donker M, van Furth E. The patient’s view on quality of life and eating disorders. Int J Eat Disord. 2007;40(1):13–20. doi: 10.1002/eat.20338. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal E, Carroll W, Dobbs M, Scott Magnuson J, Wax M, Peters G. Simplifying head and neck microvascular reconstruction. Head Neck. 2004;26(11):930–936. doi: 10.1002/hed.20076. [DOI] [PubMed] [Google Scholar]
- 9.Brown JS, Jones DC, Summerwill A, et al. Vascularized iliac crest with internal oblique muscle for immediate reconstruction after maxillectomy. Br J Oral Maxillofac Surg. 2002;40(3):183–190. doi: 10.1054/bjom.2001.0774. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro PG, Santamaria E. A classification system and algorithm for reconstruction of maxillectomy and midfacial defects. Plast Reconstr Surg. 2000;105(7):2331–2346. doi: 10.1097/00006534-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Okay D, Genden EM, Buchbinder D, Urken ML. Prosthodontic guidelines for surgical reconstruction of the maxilla: a classification system of defects. J Prosthet Dent. 2001;86(4):352–363. doi: 10.1067/mpr.2001.119524. [DOI] [PubMed] [Google Scholar]
- 12.Brown JS, Rogers SN, McNally DN, Boyle M. A modified classification for the maxillectomy defect. Head Neck. 2000;22(1):17–26. doi: 10.1002/(sici)1097-0347(200001)22:1<17::aid-hed4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Arigbede AO, Dosumu OO, Shaba OP, Esan TA. Evaluation of speech in patients with partial surgically acquired defects: pre and post prosthetic obturation. J Contemp Dent Pract. 2006;7(1):89–96. [PubMed] [Google Scholar]
- 14.Mahanna GK, Beukelman DR, Marshall JA, Gaebler CA, Sullivan M. Obturator prostheses after cancer surgery: an approach to speech outcome assessment. J Prosthet Dent. 1998;79(3):310–316. doi: 10.1016/s0022-3913(98)70243-4. [DOI] [PubMed] [Google Scholar]
- 15.Sakuraba M, Kimata Y, Ota Y, et al. Simple maxillary reconstruction using free tissue transfer and prostheses. Plast Reconstr Surg. 2003;111(2):594–598. doi: 10.1097/01.PRS.0000041941.98504.B6. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan M, Gaebler C, Beukelman D, et al. Impact of palatal prosthodontic intervention on communication performance of patients’ maxillectomy defects: a multilevel outcome study. Head Neck. 2002;24(6):530–538. doi: 10.1002/hed.10095. [DOI] [PubMed] [Google Scholar]
- 17.Triana RJ, Jr, Uglesic V, Virag M, et al. Microvascular free flap reconstructive options in patients with partial and total maxillectomy defects. Arch Facial Plast Surg. 2000;2(2):91–101. doi: 10.1001/archfaci.2.2.91. [DOI] [PubMed] [Google Scholar]
- 18.Umino S, Masuda G, Ono S, Fujita K. Speech intelligibility following maxillectomy with and without a prosthesis: an analysis of 54 cases. J Oral Rehabil. 1998;25(2):153–158. doi: 10.1046/j.1365-2842.1998.00238.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, Furuya Y, Shimodaira K, Kanazawa T, Kataoka R, Takahashi K. Spectral characteristics of hypernasality in maxillectomy patients. J Oral Rehabil. 2000;27(8):723–730. doi: 10.1046/j.1365-2842.2000.00537.x. [DOI] [PubMed] [Google Scholar]
- 20.Bohle G, III, Rieger J, Huryn J, Verbel D, Hwang F, Zlotolow I. Efficacy of speech aid prostheses for acquired defects of the soft palate and velopharyngeal inadequacy: clinical assessments and cephalometric analysis: a Memorial Sloan-Kettering Study. Head Neck. 2005;27(3):195–207. doi: 10.1002/hed.10360. [DOI] [PubMed] [Google Scholar]
- 21.Rieger J, Wolfaardt J, Seikaly H, Jha N. Speech outcomes in patients rehabilitated with maxillary obturator prostheses after maxillectomy: a prospective study. Int J Prosthodont. 2002;15(2):139–144. [PubMed] [Google Scholar]
- 22.Majid AA, Weinberg B, Chalian VA. Speech intelligibility following prosthetic obturation of surgically acquired maxillary defects. J Prosthet Dent. 1974;32(1):87–96. doi: 10.1016/0022-3913(74)90104-8. [DOI] [PubMed] [Google Scholar]
- 23.Irish J, Sandhu N, Simpson C, et al. Quality of life in patients with maxillectomy prostheses. Head Neck. 2009;31(6):813–821. doi: 10.1002/hed.21042. [DOI] [PubMed] [Google Scholar]
- 24.Kornblith AB, Zlotolow IM, Gooen J, et al. Quality of life of maxillectomy patients using an obturator prosthesis. Head Neck. 1996;18(4):323–334. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<323::AID-HED3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Rieger JM, Wolfaardt JF, Jha N, Seikaly H. Maxillary obturators: the relationship between patient satisfaction and speech outcome. Head Neck. 2003;25(11):895–903. doi: 10.1002/hed.10299. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama M, Tsukiyama Y, Koyano K. Objective clinical assessment of change in swallowing ability of maxillectomy patients when wearing obturator prostheses. Int J Prosthodont. 2005;18(6):475–479. [PubMed] [Google Scholar]
- 27.Yontchev E, Karlsson S, Lith A, Almqvist SA, Lindblad P, Engström B. Orofacial functions in patients with congenital and acquired maxillary defects: a fluoroscopic study. J Oral Rehabil. 1991;18(6):483–489. doi: 10.1111/j.1365-2842.1991.tb00069.x. [DOI] [PubMed] [Google Scholar]
- 28.Moreno MA, Sr, Skoracki RJ, Hanna EY, Hanasono MM. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck. 2010;32(7):860–868. doi: 10.1002/hed.21264. [DOI] [PubMed] [Google Scholar]
- 29.Browne JD, Burke AJ. Benefits of routine maxillectomy and orbital reconstruction with the rectus abdominis free flap. Otolaryngol Head Neck Surg. 1999;121(3):203–209. doi: 10.1016/S0194-5998(99)70172-5. [DOI] [PubMed] [Google Scholar]
- 30.Chepeha DB, Moyer JS, Bradford CR, Prince ME, Marentette L, Teknos TN. Osseocutaneous radial forearm free tissue transfer for repair of complex midfacial defects. Arch Otolaryngol Head Neck Surg. 2005;131(6):513–517. doi: 10.1001/archotol.131.6.513. [DOI] [PubMed] [Google Scholar]
- 31.Rogers SN, Lowe D, McNally D, Brown JS, Vaughan ED. Health-related quality of life after maxillectomy: a comparison between prosthetic obturation and free flap. J Oral Maxillofac Surg. 2003;61(2):174–181. doi: 10.1053/joms.2003.50044. [DOI] [PubMed] [Google Scholar]
- 32.Eckardt A, Teltzrow T, Schulze A, Hoppe M, Kuettner C. Nasalance in patients with maxillary defects: reconstruction versus obturation. J Craniomaxillofac Surg. 2007;35(4–5):241–245. doi: 10.1016/j.jcms.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Rieger J, Bohle G, Iii, Huryn J, Tang JL, Harris J, Seikaly H. Surgical reconstruction versus prosthetic obturation of extensive soft palate defects: a comparison of speech outcomes. Int J Prosthodont. 2009;22(6):566–572. [PubMed] [Google Scholar]
- 34.Ohrn KE, Wahlin YB, Sjödén PO. Oral status during radiotherapy and chemotherapy: a descriptive study of patient experiences and the occurrence of oral complications. Support Care Cancer. 2001;9(4):247–257. doi: 10.1007/s005200000214. [DOI] [PubMed] [Google Scholar]
- 35.Guchelaar HJ, Vermes A, Meerwaldt JH. Radiation-induced xerostomia: pathophysiology, clinical course and supportive treatment. Support Care Cancer. 1997;5(4):281–288. doi: 10.1007/s005200050075. [DOI] [PubMed] [Google Scholar]
- 36.Dawes C, Odlum O. Salivary status in patients treated for head and neck cancer. J Can Dent Assoc. 2004;70(6):397–400. [PubMed] [Google Scholar]
- 37.Sugerman PB, Barber MT. Patient selection for endosseous dental implants: oral and systemic considerations. Int J Oral Maxillofac Implants. 2002;17(2):191–201. [PubMed] [Google Scholar]
- 38.List MA, Stracks J. Evaluation of quality of life in patients definitively treated for squamous carcinoma of the head and neck. Curr Opin Oncol. 2000;12(3):215–220. doi: 10.1097/00001622-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Cordeiro PG, Bacilious N, Schantz S, Spiro R. The radial forearm osteocutaneous “sandwich” free flap for reconstruction of the bilateral subtotal maxillectomy defect. Ann Plast Surg. 1998;40(4):397–402. doi: 10.1097/00000637-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Andrades P, Rosenthal EL, Carroll WR, Baranano CF, Peters GE. Zygomatic-maxillary buttress reconstruction of midface defects with the osteocutaneous radial forearm free flap. Head Neck. 2008;30(10):1295–1302. doi: 10.1002/hed.20874. [DOI] [PubMed] [Google Scholar]
- 41.Virgin FW, Iseli TA, Iseli CE, et al. Functional outcomes of fibula and osteocutaneous forearm free flap reconstruction for segmental mandibular defects. Laryngoscope. 2010;120(4):663–667. doi: 10.1002/lary.20791. [DOI] [PubMed] [Google Scholar]
- 42.Futran ND, Mendez E. Developments in reconstruction of midface and maxilla. Lancet Oncol. 2006;7(3):249–258. doi: 10.1016/S1470-2045(06)70616-7. [DOI] [PubMed] [Google Scholar]
- 43.Futran ND, Wadsworth JT, Villaret D, Farwell DG. Midface reconstruction with the fibula free flap. Arch Otolaryngol Head Neck Surg. 2002;128(2):161–166. doi: 10.1001/archotol.128.2.161. [DOI] [PubMed] [Google Scholar]
- 44.Genden EM, Wallace D, Buchbinder D, Okay D, Urken ML. Iliac crest internal oblique osteomusculocutaneous free flap reconstruction of the postablative palatomaxillary defect. Arch Otolaryngol Head Neck Surg. 2001;127(7):854–861. [PubMed] [Google Scholar]
- 45.Bidros RS, Metzinger SE, Guerra AB. The thoracodorsal artery perforator-scapular osteocutaneous (TDAP-SOC) flap for reconstruction of palatal and maxillary defects. Ann Plast Surg. 2005;54(1):59–65. doi: 10.1097/01.sap.0000139561.64564.d7. [DOI] [PubMed] [Google Scholar]
- 46.Winslow CP, Cook TA, Burke A, Wax MK. Total nasal reconstruction: utility of the free radial forearm fascial flap. Arch Facial Plast Surg. 2003;5(2):159–163. doi: 10.1001/archfaci.5.2.159. [DOI] [PubMed] [Google Scholar]