Abstract
Objectives/Hypothesis
To evaluate survival outcomes in patients undergoing temporal bone resection.
Study Design
Retrospective review.
Methods
From 2002 to 2009 a total of 65 patients underwent temporal bone resection for epithelial (n = 47) and salivary (n = 18) skull base malignancies. Tumor characteristics, defect reconstruction, and postoperative course were assessed. Outcomes measured included disease-free survival and cancer recurrence.
Results
The majority of patients presented with recurrent (65%), advanced stage (94%), cutaneous (72%), and squamous cell carcinoma (57%). Thirty-nine patients had perineural invasion (60%) and required facial nerve resection; 16 (25%) had intracranial extension. Local (n = 6), regional (n = 2), or free flap (n = 46) reconstruction was required in 80% of patients. Free flap donor sites included the anterolateral thigh (31%), radial forearm free flap (19%), rectus (35%), and latissimus (4%). The average hospital stay was 4.9 days (range, 1–28 days). The overall complication rate was 15% and included stroke (n = 4), cerebrospinal fluid leak (n = 2), hematoma formation (n = 1), infection (n = 1), flap loss (n = 1), and postoperative myocardial infarction (n = 1). A total of 22 patients (34%) developed cancer recurrence during the follow-up period (median, 10 months), 17 (77%) of whom presented with recurrent disease at the time of temporal bone resection. Two-year disease-free survival was 68%, and 5-year disease-free survival was 50%.
Conclusions
Aggressive surgical resection and reconstruction is recommended for primary and recurrent skull base malignancies with acceptable morbidity and improved disease-free survival.
Keywords: Temporal bone resection, free flap reconstruction, squamous cell carcinoma, external auditory canal, middle ear obliteration
INTRODUCTION
Lateral skull base malignancy, although rare, has traditionally been associated with a poor prognosis and high rate of cancer recurrence. Prior to the 1950s, cancer of the skull base was primarily treated with combined radical mastoidectomy and radiotherapy.1 It was not until 1954 that en bloc total temporal bone resection was first described by Parsons and Lewis2 and later popularized as a single-stage approach.3 Since that time, advancements in skull base surgery have led to more aggressive tumor excision and improved local control.4 However, intracranial extension with involvement of the dura has traditionally been considered a relative contra-indication to surgical excision. Outcomes in patients undergoing aggressive surgical resection remains undefined. In the present study we review outcomes for patients with skull base malignancies following lateral or subtotal temporal bone resection and discuss reconstructive options. To our knowledge this is the first study to address survival rates in a large population of patients presenting with recurrent, advanced-stage disease.
MATERIALS AND METHODS
A retrospective review of all patients who presented for lateral temporal bone resection between October 2002 and November 2009 was performed at the University of Alabama at Birmingham following institutional review board approval. A total of 65 patients were identified who underwent temporal bone resection for primary or recurrent epithelial and salivary skull base malignancies. Patients with mesenchymal skull base tumors (chondrosarcomas, chordomas, hemangiopericytomas) were excluded from this study in order to allow for a more accurate assessment of survival outcomes.
Tumors were staged by cancer type according to the American Joint Committee on Cancer guidelines, and histology was confirmed by pathology. All patients underwent lateral or subtotal temporal bone resection as determined by tumor involvement of surrounding structures and proximity to the tympanic membrane. No classic total en block temporal bone resections were performed.3 In cases in which the lesion was limited to the ear canal, a lateral temporal bone resection with removal of the entire ear canal, tympanic membrane, and immediate ossicular chain was performed en bloc, following mastoidectomy.5 In select cases, the external auditory canal (EAC) or a portion of the canal was preserved and contents of the middle ear were maintained. When lesions extended medial to the tympanic membrane, a subtotal temporal bone resection was performed, consisting of lateral temporal bone resection and local excision of the involved portion of the temporal bone.5 In cases in which en bloc resection could not be cleanly performed, piecemeal skull base resection was performed in an aggressive and sequential manner. With this method the vast majority of the tumor was removed en bloc followed by aggressive skull base dissection consisting of drilling through tumor involved skull base with methodical re-excision until negative margins were achieved. When possible, additional removal of uninvolved skull base with preservation of all neurovascular structures was performed to insure successful complete tumor removal.
Involvement of cranial nerves or dura with or without intracranial extension was managed by complete resection followed by rehabilitative procedures. It is our institution’s practice to perform immediate cable nerve grafting when proximal and distal facial nerve is available to graft.6 Static procedures including gold weight and facial sling were performed in all patients requiring facial nerve reconstruction, and additional procedures ranging from canthopexy and brow lift to repeat facial sling were performed during subsequent operations. Following reconstruction, facial nerve function was scored according to the House-Brackmann (H-B) scale7 where 1 is normal and 6 is complete facial nerve paralysis.
The majority of patients required neck dissection and postoperative radiation. Selective or modified neck dissection was performed at the time of lateral temporal bone resection or in a staged procedure. Neck dissection was indicated when nodal metastasis was suspected on preoperative imaging or when the patient presented with an advanced T stage lesion of either squamous cell epithelial or salivary histology. Postoperative radiation was recommended for patients with 1) large skull base malignancies, 2) when more than one positive node was identified on neck dissection, or 3) in the presence of perineural, lymphovascular, or intracranial invasion.
Free flap reconstruction was required for large skull base or cutaneous defects following tumor ablation. Defects were classified based on the lateral temporal bone reconstructive algorithm proposed by Rosenthal et al.8 Class I defects required minimal auditory canal reconstruction with preservation of the EAC, and class II defects involved partial auricular reconstruction. Class III defects were the result of complete auriculectomy following lateral temporal bone resection and often required a larger cutaneous based flap for reconstruction.
Demographic characteristics, including patient age, gender, race, and previous head and neck cancer treatment were recorded. Postoperative complications, length of hospital stay, and the need for adjuvant radiation were evaluated. Outcomes measured consisted of disease-specific survival and cancer recurrence.
Descriptive variables are reported as means (± standard deviation) and categorical variables as percentages. Descriptive statistics were compared by general linear models for normally distributed variables or the Kruskal-Wallis test for otherwise. The χ2 test with exact option was used to compare categorical variables by group. The relationship between patient clinical and treatment factors and disease-specific survival was calculated using the Kaplan-Meier method. Survival time was calculated as the interval from date of surgery to date of death or date of last follow-up. Deaths due to other causes were censored for these analyses. The Cox regression model was used to calculate risk of recurrence. For all analyses, a P value of <.05 was considered statistically significant. Statistical analysis was performed using SAS verion 9.2 software (SAS Institute, Inc., Cary, NC).
RESULTS
From 2002 to 2009, a total of 65 patients underwent temporal bone resection for primary (n = 23) or recurrent (n = 42) skull base malignancies. Patient demographics and tumor characteristics are presented in Table I. The majority of patients were male, between the ages of 50 and 75 years, and presented with advanced-stage (stage III or IV) (93.8%), cutaneous (72.3%) squamous cell carcinoma (56.9%). Median overall tumor size was 3.5 cm (range, 0.5–11 cm). Previous treatment consisted of surgery (n = 22), surgery and radiation (n = 15), radiation and chemotherapy (n = 2), or a combination of surgery and chemoradiation (n = 3).
TABLE I.
Patient Characteristics.
| Characteristic | No. (%) |
|---|---|
| Age, yr | |
| Mean (range) | 65.2 (34–88) |
| Gender | |
| Male | 50 (77) |
| Female | 15 (23) |
| Race | |
| Caucasian | 59 (91) |
| African-American | 6 (9) |
| T classification | |
| T1 | 2 (3) |
| T2 | 2 (3) |
| T3 | 3 (5) |
| T4 | 58 (89) |
| Stage | |
| I | 2 (3) |
| II | 2 (3) |
| III | 38 (59) |
| IV | 23 (35) |
| Histopathology | |
| Epithelial | 47 (72) |
| SCC | 37 |
| BCC | 11 |
| Salivary | 18 (28) |
| Adenocarcinoma | 2 |
| Adenoid cystic | 5 |
| Acinic cell | 2 |
| Mucoepidermoid | 2 |
| Carcinoma ex pleomorphic adenoma | 6 |
SCC = squamous cell carcinoma; BCC = basal cell carcinoma.
Extent of temporal bone resection ranged from subtotal or lateral resection to limited resection with preservation of the EAC (n = 20) or without middle ear obliteration (n = 16). A total of 39 patients (60%) demonstrated perineural invasion. All (100%) required facial nerve resection followed by immediate reanimation procedures including gold weight and facial sling. Cable nerve grafting was performed in 30% of patients, whereas 70% underwent static procedures alone. Further reanimation procedures including canthopexy, brow lift, or repeat facial sling were performed during subsequent operations as needed. One patient required resection of cranial nerves X and XII due to tumor invasion without reconstruction. Intracranial extension occurred in 16 patients (24.6%). Three had persistent local disease despite aggressive surgical resection where resection extended into the temporal or occipital lobe in some cases.
A total of 47 patients (72.3%) underwent selective or modified radical neck dissection. Overall rate of nodal metastasis was 36.1% (n = 17). Of those patients, 40% demonstrated no evidence of neck disease on preoperative clinical exam (N0). Patients with salivary skull base malignancies were more likely to demonstrate positive nodal metastasis on surgical histology (71.4%, n = 7/27) when compared to patients with epithelial squamous cell carcinoma (26.0%, n = 10/14) (P = .01). Five patients did not undergo neck dissection despite advanced T stage squamous cell or salivary lesions early in this series, largely due to surgeon preference.
Reconstruction
Lateral or subtotal temporal bone resection with partial or total auriculectomy (n = 31) results in considerable skull base and cutaneous defects. A total of 54 local (n = 6), regional (n = 2), or free tissue (n = 46) flaps were required for 52 patients following tumor ablation. Defects were classified according to the degree of periauricular and temporal bone resection (Table II) (Fig. 1).8 There were 18 patients with class I defects, 11 of whom underwent 13 flap reconstructive procedures. The majority of patients required local flap coverage (30.7%), with one patient undergoing combined sternocleidomastoid, temporalis, and cervicofacial flap reconstruction. Of 21 patients with class II defects, 16 required free flap reconstruction. The anterolateral thigh (ALT) (31.2%) and radial forearm free flap (37.5%) were most commonly utilized in class II defect reconstruction, whereas patients with class III defects (n = 26) typically required reconstruction with ALT (32%) or rectus free flaps (48%) (Table III). In all cases in which flap reconstruction was not employed, defects were closed either by primary repair or split thickness skin graft coverage.
TABLE II.
Defect Classification.
| Class I | EAC and auricle preservation, anterior skin loss, temporal bone resection |
| Class II | Middle ear obliterated, partial auriculectomy, lateral or subtotal temporal bone resection |
| Class III | Middle ear obliterated, total auriculectomy, lateral or subtotal temporal bone resection |
EAC = external auditory canal.
Fig. 1.
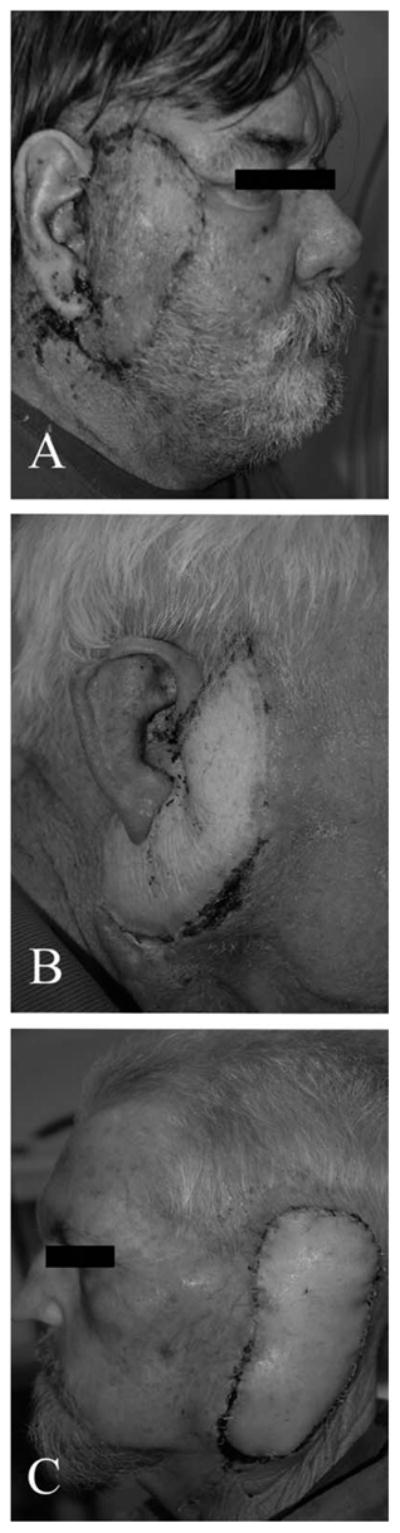
(A) Class I defect following reconstruction with an anterolateral thigh (ALT) flap and preservation of the auricle. (B) Class II defect with partial auriculectomy and free flap reconstruction. (C) Class III defect in which the patient has had a complete auriculectomy and middle ear obliteration following lateral temporal bone resection.
TABLE III.
Reconstruction of Cutaneous and Lateral Temporal Bone Defects.
| Defect Class | No. Patients | Reconstruction Method
|
Total | |||||
|---|---|---|---|---|---|---|---|---|
| ALT | Rectus | RFFF | Latissimus | Regional | Local | |||
| Class I | 18 | 3 | 3 | 2 | 0 | 1 | 4 | 13 |
| Class II | 21 | 5 | 3 | 6 | 0 | 0 | 2 | 16 |
| Class III | 26 | 8 | 12 | 2 | 2 | 1 | 0 | 25 |
| Total | 52 | 16 | 18 | 10 | 2 | 2 | 6 | 54 |
Class I: external auditory canal preserved, skin loss; Class II: middle ear obliteration, partial auriculectomy; Class III: total auriculectomy. Rotational flaps include the pectoralis flap and local flaps include cervicofacial, temporalis, and sternocleidomastoid flaps.
ALT = anterolateral thigh; RFFF = radial forearm free flap.
Average hospital stay was 4.9 days (range, 1–28 days). The overall complication rate was 15.3% (n = 10), including stroke (n = 4), cerebrospinal fluid (CSF) leak (n = 2), hematoma formation (n = 1), infection (n = 1), and postoperative myocardial infarction (n = 1). Both CSF leaks were noted intraoperatively. In one case an ALT flap was placed without evidence of leak postoperatively. In the other case, a temporalis flap was secured over a small leak at the point of the mastoid tegmen. Postoperatively, the patient required lumbar drain placement. No CSF leak was observed after 5 days, and the drain was clamped and removed. In addition, one patient developed flap failure with central necrosis nearly a year after ALT reconstruction followed by postoperative radiation. Despite serial debridements followed by wet to dry dressings, the patient eventually required repeat free flap reconstruction with a radial forearm free flap.
Outcomes
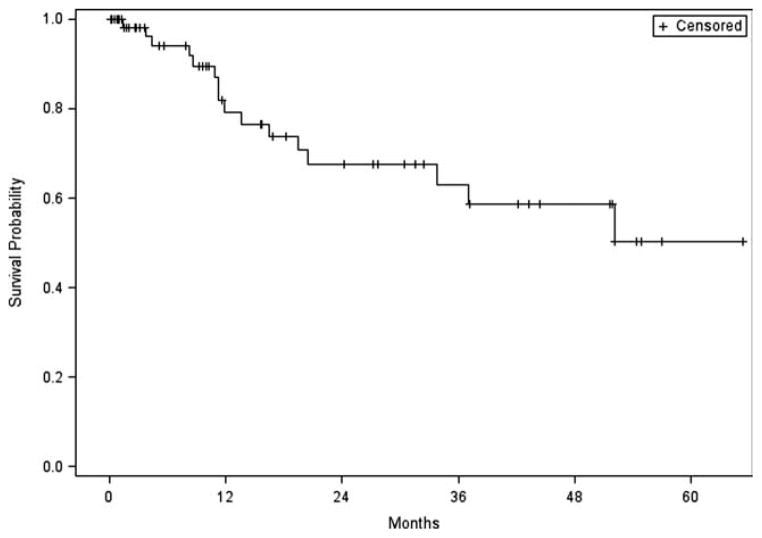
Follow-up ranged from 1 month to over 5 years with a median of 10 months. At the time of last follow-up, 32 patients were disease free, 8 were alive with disease, 9 had died of other causes, and 16 had died of disease. Two-year disease-free survival was 67.6%, and 5-year disease-free survival was 50.2% (Fig. 2).
Fig. 2.
Five-year disease-free survival for patients with lateral temporal bone malignancies.
Patients were classified into groups based on the treatment each received: surgery alone (n = 11), surgery and preoperative radiation (n = 8), surgery and postoperative radiation (n = 34), or surgery and both pre- and postoperative radiotherapy (n = 12). Local disease control was maintained in all patients (100%) who underwent surgery as their primary treatment and 62.5% of patients who underwent radiotherapy prior to resection. Most patients required postoperative radiation (72.3%) as a result of advanced-stage disease. Out of 12 patients treated with both pre- and postoperative radiotherapy, local disease control was maintained in 40%.
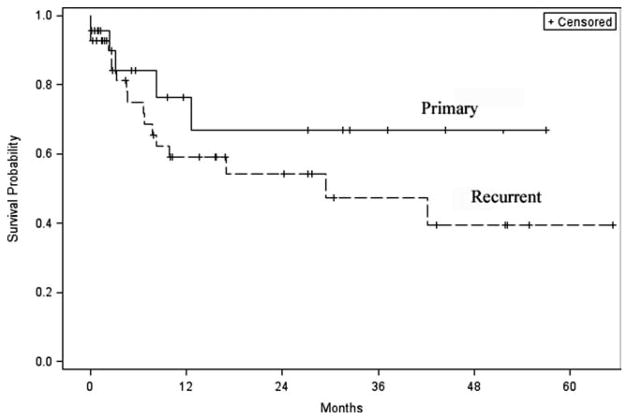
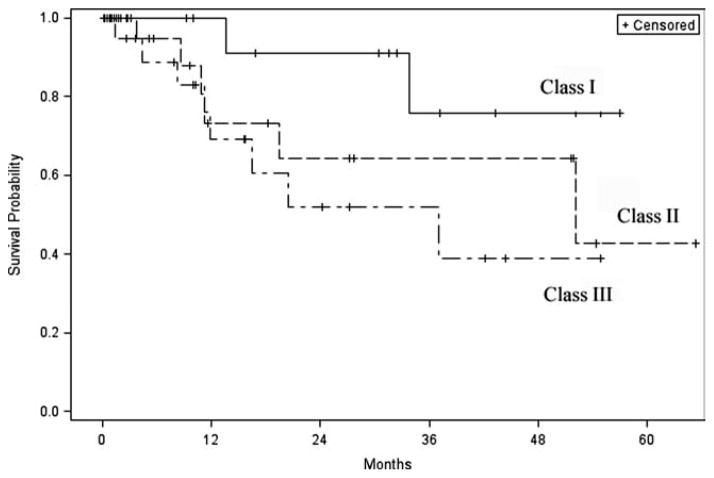
Patients with recurrent disease were nearly twice as likely to recur as those with primary disease (hazard ratio [HR] 1.8; 95% confidence interval [CI], 0.66–4.87]. Out of the 22 patients (33.8%) who recurred during the follow-up period, the majority (77.2%) had presented with recurrent disease. One-year disease-free survival for patients presenting with a primary skull base malignancy was 76.4% compared to 59% for those who presented with recurrent disease, and at 2 years 66.8% versus 54.1% (P = .24) (Fig. 3). Patient characteristics including age, gender, race, and previous treatment had no significant impact on survival (P > .05). Disease-free survival was 100% for patients (n = 4) treated for early stage (stage I or II) disease. Median tumor size (3.5 cm) was used as a cut point for statistical comparison. Patients with a tumor measuring ≥3.5 cm were twice as likely to die from disease (HR 1.93; 95% CI, 0.58–6.46) when compared to those with tumors of a smaller size. Not surprisingly, patients with class I defects tended to have better survival rates compared to class II defects, whereas patients with class III defects faired the worst (P = .14) (Fig. 4).
Fig. 3.
Disease-free survival for recurrent versus primary disease.
Fig. 4.
Disease-free survival by class defect.
Intracranial tumoral extension in 16 patients did not have an effect on disease-free survival (P = .86). This includes three patients who had incomplete resections; in the remaining 13, local control was maintained in 10 (76.9%). Local control rates were similar between patients with (76.9%) and without intracranial extension (71.7%) following resection and postoperative radiation (P = .72). A total of 39 patients had perineural involvement and required facial nerve resection. Three patients had persistent disease and two had distant metastasis. Out of the remaining 34, local control was maintained in 23 (67.6%). There was no difference between local control rates for patients who required facial nerve resection due to perineural invasion (67.6%) and those who did not (76.9%) (P = .13). Cable nerve grafting or static facial reanimation procedures were performed on all patients requiring facial nerve reconstruction. Most (97%) demonstrated some return of facial nerve function during the postoperative period with a median H-B score of 3 (range, 2–6). Patients who required facial nerve resection trended toward lower disease-free survival rates, although no significant difference was observed (P = .11).
In contrast, positive nodal metastasis was associated with poor prognosis. Two-year disease-free survival for patients with nodal disease was 34.1% compared to 81.1% for those without disease, and at 5 years 17.1% versus 73% (P = .005). Nearly 55% of patients who presented with nodal metastasis developed local (n = 4) or regional (n = 2) recurrence. Two patients had persistent disease and one developed lung metastasis. Mean time to recurrence was 4 months (± 2.31 months). Despite a diagnosis of advanced T stage squamous cell or salivary carcinoma, five patients did not undergo neck dissection. Of those patients, 40% (2/5) developed regional metastasis during the follow-up period.
DISCUSSION
Lateral skull base malignancies, although rare, remain a challenge for the head and neck surgeon. Significant advancements in skull base surgery have led to more aggressive excision of temporal bone lesions resulting in complete tumor excision4,9 and improved local control.10,11 Most studies to date have assessed outcomes for patients with primary disease. In the present study we review survival rates for patients with both primary and recurrent advanced-stage skull base malignancies.
Survival rates for patients with temporal bone carcinoma range from 46% to 85% in reported cases.12–16 By in large these studies are limited to patients treated for primary squamous cell carcinoma either by lateral temporal bone resection alone or in combination with postoperative radiotherapy. The majority of patients seen at our institution were treated for recurrent carcinoma (65%). Overall 2-year disease-free survival was 68%, and 5-year disease-free survival was 50%. Local control was maintained in 63% of patients. These results are similar to those previously published by McGrew et al.,5 where local control was maintained in 74% of patients treated for primary epithelial, salivary, and mesenchymal skull base malignancies. Tumors of mesenchymal origin are generally less aggressive and have been associated with improved local control rates when compared to other malignancies17; therefore, this tumor type was excluded from the present study to allow for a more accurate assessment of survival outcomes.
Patients who presented with recurrent disease at the time of surgical resection were twice as likely to recur as those who presented with primary disease. Two-year disease-free survival for patients with a primary skull base malignancy was 83% compared to 61% for those who presented with cancer recurrence. Previous studies have demonstrated recurrence rates as high as 50% for some patients with primary advanced-stage disease.18 Here we demonstrate an overall recurrence rate of 34% with the majority of cases occurring in patients who presented with recurrent disease (77%). No other study to date has reported survival rates for such a large population of patients with recurrent skull base malignancies. Traditional en bloc resections of the skull base have not achieved this level of tumor control or survival even when combined with radiation therapy. Piecemeal resection when aggressive and done in a sequential manner appears to be more effective than traditional en bloc skull base resection.
The incidence of neck metastasis in patients with temporal bone squamous cell carcinoma is relatively rare and has been estimated to be 10% to 23%.19 Here we demonstrate a 36% overall rate of nodal metastasis for patients with both epithelial and salivary skull base malignancies and a risk of occult metastasis approaching 40%. Although previous studies have demonstrated that nearly all cases of nodal metastasis can be detected with preoperative imaging,20 it is this institution’s practice to now perform a neck dissection on all patients with advanced-stage skull base malignancies. This allows for staging of the disease for postoperative radiation, and exposed vessels can then be preserved for anastomosis during free tissue transfer. However, even with neck dissection the presence of nodal metastasis carries with it a poor prognosis. In a study by Moncrieff et al.,21 no patient with nodal metastasis survived beyond 30 months. The rate of recurrence for patients with nodal metastasis in this study was 55%, and 2- and 5-year disease-free survival 34% and 17%, respectively. Just as aggressive surgical resection is warranted to improve local control for advanced-stage disease, so is neck dissection to reduce the risk of recurrence.
Postoperative radiation has been shown to increase survival rates for patients with temporal bone squamous cell carcinoma and is advocated in most if not all cases.10,11 The majority of patients in this study underwent postoperative radiation therapy as a result of advanced disease. Local control was maintained in 73% of patients who underwent resection followed by radiation. Patients treated with preoperative radiation tended to have lower local control rates (63%) as did patients treated with both preoperative and postoperative radiation (40%). In previous studies in which skull base malignancies were treated with primary radiotherapy,17,22 survival rates were poor and ranged from 0% to 22%.4,17,22 Whether patients who underwent both pre- and postoperative radiation had more aggressive tumor types and therefore lower control rates or worse outcomes as a result of initial radiation therapy remains unknown. However, these findings further support surgical resection as initial treatment for both early and advanced-stage disease with postoperative radiotherapy reserved for patients with more extensive skull base malignancies.
Overall recurrence rates and disease-free survival demonstrated for this patient population reflect the aggressive approach to surgical resection our institution holds for patients with advanced skull base disease. Previous studies have cited intracranial invasion as a contraindication to surgery.12,23 In the present study, 16 patients with intracranial extension underwent aggressive surgical resection with dura or in some cases occipital or temporal lobe resection. The 2-year disease-free survival rate was 65%; this compared to a survival rate of 0% in a study by Moody et al.23 No perioperative deaths occurred, two patients had postoperative cerebrovascular accidents, and one developed a CSF leak, which resolved following lumbar drain placement. Intracranial tumoral extension did not have an effect on disease-free survival (P = .86). This finding was unexpected given that intracranial extension has historically been associated with poor prognosis. In all cases, intracranial extension was managed by aggressive resection of tumor involving the dura with a diamond burr, followed by flap reconstruction and postoperative radiation when feasible. Failure to show that intracranial extension has an effect on disease-free survival might be related to improved oncologic and reconstructive strategies as compared to historical controls.
Similarly, local control rates were not different between patients requiring facial nerve resection and those without facial nerve involvement (P = .13). Previous studies have shown that preoperative facial nerve dysfunction is a poor indicator of overall survival.24 Perineural invasion did not significantly influence disease-free survival following facial nerve resection in this patient population (P = .11), and acceptable postoperative facial nerve function was achieved either by cable nerve grafting or static reanimation procedures. Although no significant difference in survival was observed, patients with perineural invasion trended toward worse outcomes. These results are consistent with previous studies, which have demonstrated that local control rates are equivalent when cases of perineural involvement are treated with aggressive facial nerve resection and postoperative radiation,5 and suggest resection is warranted in some cases.
Advances in skull base surgery have allowed for en bloc resection of both primary and recurrent advanced-stage disease. As more aggressive surgical resection is warranted so is the need for defect reconstruction. Nearly 80% of patients required free flap reconstruction for lateral skull base and cutaneous defects. The majority of patients with class I defects required local flap coverage (31%), whereas patients with class II and III defects underwent rectus (37%), ALT (31%), and radial forearm free flap reconstruction (20%). Not surprisingly, patients with class I defects tended to have better survival rates compared to class II defects, whereas patients with class III defects faired the worst (P = .14). Cervicofacial, temporalis, and other local flaps have been utilized for small superficial periauricular cutaneous defects for decades; however, their use is limited by the small tissue volume they provide. Regional flaps provide considerable volume but are limited by length and the amount of tension that can be placed on the vascular pedicle. Free flap reconstruction has become the standard of care for definitive management of large cutaneous and skull base defects. The radial forearm free flap is reliable and well suited for class I defects, though more tissue volume is required for class II and III defects, especially for patients who will undergo postoperative radiation. In larger defects the rectus or ALT flap are ideal for bony and soft tissue coverage.
CONCLUSION
Lateral temporal bone malignancy, although rare, remains a challenging disease. Initial therapy includes surgery followed by postoperative radiation for treatment of advanced-stage disease. Aggressive surgical resection, including neck dissection is warranted even in the presence of intracranial extension or cranial nerve involvement to allow for complete tumor resection, decreased recurrence, and improved survival.
Acknowledgments
This work was supported by grants from the National Cancer Institute (NCI K08CA102154) and the National Institutes of Health (2T32 CA091078-06).
Footnotes
Given as an oral presentation at the 2010 Triological Society Combined Sections Meeting, Orlando, Florida, U.S.A., February 6, 2010.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Level of Evidence: 2b
BIBLIOGRAPHY
- 1.Ward GE, Loch WE, Lawrence W., Jr Radical operation for carcinoma of the external auditory canal and middle ear. Am J Surg. 1951;82:169–178. doi: 10.1016/0002-9610(51)90316-9. [DOI] [PubMed] [Google Scholar]
- 2.Parsons H, Lewis JS. Subtotal resection of the temporal bone for cancer of the ear. Cancer. 1954;7:995–1001. doi: 10.1002/1097-0142(195409)7:5<995::aid-cncr2820070524>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JS. Temporal bone resection. Review of 100 cases. Arch Otolaryngol. 1975;101:23–25. doi: 10.1001/archotol.1975.00780300027006. [DOI] [PubMed] [Google Scholar]
- 4.Goodwin WJ, Jesse RH. Malignant neoplasms of the external auditory canal and temporal bone. Arch Otolaryngol. 1980;106:675–679. doi: 10.1001/archotol.1980.00790350017006. [DOI] [PubMed] [Google Scholar]
- 5.McGrew BM, Jackson CG, Redtfeldt RA. Lateral skull base malignancies. Neurosurg Focus. 2002;12:e8. doi: 10.3171/foc.2002.12.5.9. [DOI] [PubMed] [Google Scholar]
- 6.Iseli TA, Harris G, Dean NR, Iseli CE, Rosenthal EL. Outcomes of static and dynamic facial nerve repair in head and neck cancer. Laryngoscope. 2010;120:478–483. doi: 10.1002/lary.20789. [DOI] [PubMed] [Google Scholar]
- 7.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal EL, King T, McGrew BM, et al. Evolution of a paradigm for free tissue transfer reconstruction of lateral temporal bone defects. Head Neck. 2008;30:589–594. doi: 10.1002/hed.20744. [DOI] [PubMed] [Google Scholar]
- 9.Moore MG, Deschler DG, McKenna MJ, et al. Management outcomes following lateral temporal bone resection for ear and temporal bone malignancies. Otolaryngol Head Neck Surg. 2007;137:893–898. doi: 10.1016/j.otohns.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Hahn SS, Kim JA, Goodchild N, et al. Carcinoma of the middle ear and external auditory canal. Int J Radiat Oncol Biol Phys. 1983;9:1003–1007. doi: 10.1016/0360-3016(83)90388-7. [DOI] [PubMed] [Google Scholar]
- 11.Spector JG. Management of temporal bone carcinomas: a therapeutic analysis of two groups of patients and long-term followup. Otolaryngol Head Neck Surg. 1991;104:58–66. doi: 10.1177/019459989110400112. [DOI] [PubMed] [Google Scholar]
- 12.Kunst H, Lavieille JP, Marres H. Squamous cell carcinoma of the temporal bone: results and management. Otol Neurotol. 2008;29:549–552. doi: 10.1097/MAO.0b013e31816c7c71. [DOI] [PubMed] [Google Scholar]
- 13.Cristalli G, Manciocco V, Pichi B, et al. Treatment and outcome of advanced external auditory canal and middle ear squamous cell carcinoma. J Craniofac Surg. 2009;20:816–821. doi: 10.1097/SCS.0b013e3181a14b99. [DOI] [PubMed] [Google Scholar]
- 14.Kinney SE, Wood BG. Malignancies of the external ear canal and temporal bone: surgical techniques and results. Laryngoscope. 1987;97:158–164. doi: 10.1288/00005537-198702000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Pensak ML, Gleich LL, Gluckman JL, et al. Temporal bone carcinoma: contemporary perspectives in the skull base surgical era. Laryngoscope. 1996;106:1234–1237. doi: 10.1097/00005537-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Prasad S, Janecka IP. Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone: a literature review. Otolaryngol Head Neck Surg. 1994;110:270–280. doi: 10.1177/019459989411000303. [DOI] [PubMed] [Google Scholar]
- 17.Manolidis S, Pappas D, Jr, Von Doersten P, et al. Temporal bone and lateral skull base malignancy: experience and results with 81 patients. Am J Otol. 1998;19:S1–S15. [PubMed] [Google Scholar]
- 18.Chang CH, Shu MT, Lee JC, et al. Treatments and outcomes of malignant tumors of external auditory canal. Am J Otolaryngol. 2009;30:44–48. doi: 10.1016/j.amjoto.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Moffat DA, Wagstaff SA. Squamous cell carcinoma of the temporal bone. Curr Opin Otolaryngol Head Neck Surg. 2003;11:107–111. doi: 10.1097/00020840-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Gillespie MB, Francis HW, Chee N, et al. Squamous cell carcinoma of the temporal bone: a radiographic-pathologic correlation. Arch Otolaryngol Head Neck Surg. 2001;127:803–807. [PubMed] [Google Scholar]
- 21.Moncrieff MD, Hamilton SA, Lamberty GH, et al. Reconstructive options after temporal bone resection for squamous cell carcinoma. J Plast Reconstr Aesthet Surg. 2007;60:607–614. doi: 10.1016/j.bjps.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Barrs DM. Temporal bone carcinoma. Otolaryngol Clin North Am. 2001;34:1197–1218. x. doi: 10.1016/s0030-6665(05)70374-1. [DOI] [PubMed] [Google Scholar]
- 23.Moody SA, Hirsch BE, Myers EN. Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Am J Otol. 2000;21:582–588. [PubMed] [Google Scholar]
- 24.Lobo D, Llorente JL, Suarez C. Squamous cell carcinoma of the external auditory canal. Skull Base. 2008;18:167–172. doi: 10.1055/s-2007-994290. [DOI] [PMC free article] [PubMed] [Google Scholar]