Abstract
Objective
To assess the feasibility of panitumumab in real-time fluorescent imaging and histologic processing of cutaneous squamous cell carcinoma (cSCC) in mice.
Design
A near-infrared (NIR) fluorescent probe (IRDye800-CW) was covalently linked to a monoclonal antibody–targeting epidermal growth factor receptor (panitumumab) or nonspecific IgG and injected into mice bearing flank xenografts from a cSCC cell line (SCC-13 or SRB-12; n = 7), human split-thickness skin grafts (STSGs; n = 3), or a human tumor explant (n = 1). The tumor and lymph nodes were imaged and dissected using fluorescence guidance with the SPY imaging system and verified with a charge-coupled NIR system. An NIR scanning device (Odyssey) was used to measure fluorescence intensity in histological sections.
Subjects
Immunodeficient mice.
Setting
In vivo and in vitro imaging lab.
Results
Tumor tissue could be delineated from the human STSG with tumor-to-background ratios of 4.5 (Pearl) and 3.4 (SPY). Tumor detection was substantially improved with panitumumab-IRDye800 compared with IgG-IRDye800. Biopsies positive for fluorescence were assessed by histology and immunohistochemistry (n = 18/18) to confirm the presence of tumor, yielding a 100% sensitivity. Biopsies of non-fluorescent tissue negative for malignancy (n = 18/18) yielded a specificity of 100%. Furthermore, the SPY system was able to detect residual disease as small as 200 μm in diameter. In addition, the Odyssey confirmed fluorescence of microscopic disease (in tumor samples of frozen and paraffin-embedded histologic specimens) but not in adjacent noncancerous tissue.
Conclusions
These data suggest panitumumab-IRDye800 may have clinical utility in detection and removal of subclinical cSCC using Food and Drug Administration–approved imaging hardware.
Keywords: optical imaging, cutaneous cancer, head and neck carcinoma, panitumumab
Cutaneous squamous cell carcinoma (cSCC) is one of the most commonly diagnosed malignancies in the United States. There has been an alarming increase in the incidence of cSCC over the past 20 years, and now there are more than 1 million cases reported each year. Most of these cancerous lesions can be identified and treated successfully by Mohs, cryosurgery, curettage, or topical therapy. However, the literature reports that 6% to 16% of cSCC are incompletely excised after primary excision. Furthermore, for reexcision of those lesions that were previously incompletely excised, there is a 60% risk of leaving residual tumor behind.1 Most of those incompletely excised lesions had involvement of the deep margins. If excision is incomplete, there is an increased risk for recurrence and metastasis. Incompletely excised cSCCs have the potential to metastasize to regional lymph nodes as well as distant organs. The chance for recurrence in 5 years after primary excision is between 6% and 8%.2 Currently, the recommended excision margins vary between 2 and 15 mm.1,3 Recurrent disease presents a challenge since the desire to limit resection of normal tissues, especially around the head and neck, conflicts with the need to obtain negative margins.
While Mohs micrographic surgery minimizes the amount of uninvolved tissue taken and offers high cure rates, histological sectioning of the entire margin is costly and time-consuming. A real-time imaging modality has the potential to decrease the rate of positive margins and also spare uninvolved tissue by guiding surgical resection while being more time efficient and possibly less costly. Although there are several methods to image large cancers preoperatively, including computed tomography (CT) and positron emission tomography–CT, these have limited application in smaller cancers and do not represent a real-time method to image tumors intraoperatively.
The purpose of this study was to demonstrate the feasibility of disease detection with the fluorescently-labeled monoclonal antibody panitumumab. Overexpression of epidermal growth factor receptor (EGFR) is present in up to 79% of cSCC of the head and neck.4 In addition, we investigated the use of fluorescence imaging (Odyssey scanner; LI-COR Biosciences, Lincoln, NE) for detecting disease in frozen histological sections. This technology would provide a more efficient and accurate modality for intraoperative and histological detection of cancer.
Materials and Methods
Cell Lines and Tissue Culture
Two cutaneous head and neck SCC cell lines were used, SCC-13 and SRB-12. SRB-12 was derived from biopsies of primary SCC from patients at the University of Texas M.D. Anderson Cancer Center. The SRB-12 cell line was a kind gift from Dr Janet Price (Department of Cell Biology, M.D. Anderson Cancer Center). The SCC-13 cell line was kindly received from the laboratory of Santosh Katiyar, PhD (University of Alabama at Birmingham, Birmingham, AL). These cell lines were grown and maintained as previously published.5
Reagents
Panitumumab (Vectibix; Amgen, Thousand Oaks, CA; 147 kDa), a recombinant and fully humanized monoclonal antibody that binds to the extracellular domain of human EGFR, was the antibody used. Protein A–purified IgG antibody (Ir-Hu-Gf, No. 30010BM; Innovative Research, Novi, MI; 146 kDa) was used as a control antibody.
Near-Infrared Fluorescence Agents
IRDye800CW (IRDye800CW-N-hydroxysuccinimide ester; LI-COR Biosciences) was the fluorescent probe. It has an near-infrared (NIR) absorption and emission peak of 778 nm/794 nm. When conjugated to either panitumumab or IgG, the absorbance and emission maximums decrease slightly (774 nm/789 nm).6 Panitumumab and IgG were labeled as previously published.5
Antigen Detection Assay
To determine binding efficacy of IRDye800CW-labeled panitumumab, an antigen detection assay was performed as previously published.5
Cell-Binding Assay
Cutaneous SCC cell lines, SCC-13 and SRB-12, were plated at 2 × 105 per well in 96-well clear-bottom black culture plates. Cells were then allowed to reach confluence and then incubated for 2 hours with 2 μg of labeled panitumumab and imaged with the Pearl Imager.
Animal Models
Nude (nu/nu) and SCID female mice (Charles River Laboratories, Hartford, CT), aged 4 to 6 weeks, were obtained and housed in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines. This study was reviewed and approved by the animal care and use committee, and all experiments were conducted and the mice euthanized according to IACUC guidelines.
For the flank model, nude mice (n = 7) received subcutaneous injections of SCC-13 or SRB-12 cells (2 × 106) suspended in media (200 μL). Tumor size was monitored weekly until they reached 25 mm2. The mice were then systemically injected with fluorescently labeled panitumumab or IgG (50–100 μg). For the human split-thickness skin graft (STSG) model (n = 3), the skin from the back of SCID mice was excised and the STSG sutured in place. The human tumor explant model (n = 1) was performed by transplanting a human tumor graft from a patient with cSCC into the flank of a nude mouse.
Fluorescence Imaging and Measurement
The SPY imaging system captures fluorescent light using a charge-couple device video camera at 30 frames/second and displays it on a computer monitor.7 The SPY system was used to guide tumor and lymph node resections by real-time fluorescence guidance 48 to 96 hours following injection of the panitumumab-IRDye800. Following resections, the excised tissues were placed in tissue cassettes and imaged with both the Pearl and SPY.
In vivo fluorescence detected with the SPY machine was quantified using ImageJ (http://rsb.info.nih.gov/ij/). Fluorescence intensity was measured by selecting approximately 5 regions of interest within the tumor. The mean fluorescence was calculated, which represented the “tumor” fluorescence. Nonfluorescent tissue within 1 cm of the tumor was similarly measured, and the mean value was used to represent background fluorescence. Subsequently, a tumor-to-background ratio (TBR) was calculated based on the fluorescence intensity from a sample of normal tissue adjacent to the tumor border.
The Pearl Impulse small animal imager was used to verify the TBR measured by the SPY because the Pearl is designed to image IRDye800, whereas the SPY was designed to image indocyanine green. The overlapping emission and absorption spectra of these fluorescent probes allows both of them to be detected by either imaging modality.
Fluorescent microscopy of histologic sections was performed using the Odyssey scanner (LI-COR Biosciences). Co-localization of the fluorescent signal with tumor was performed by overlaying the Odyssey-acquired fluorescent image with the microscopic hematoxylin and eosin (H&E) image.
Immunohistochemistry
Immunohistochemical analysis with CD147 (Millipore, clone 1S9-2A, mouse monoclonal IgG2ak) and cytokeratin (clone: AE1+AE3; Ventana, Tuscan, AZ) was performed to confirm the presence of tumor cells as according to our previously published methods.5
Analysis
Because of the descriptive and exploratory nature of this study and small sample size, no formal statistical comparisons were performed. TBRs are reported as means with standard deviations.
Results
Detection of Panitumumab-IRDye800
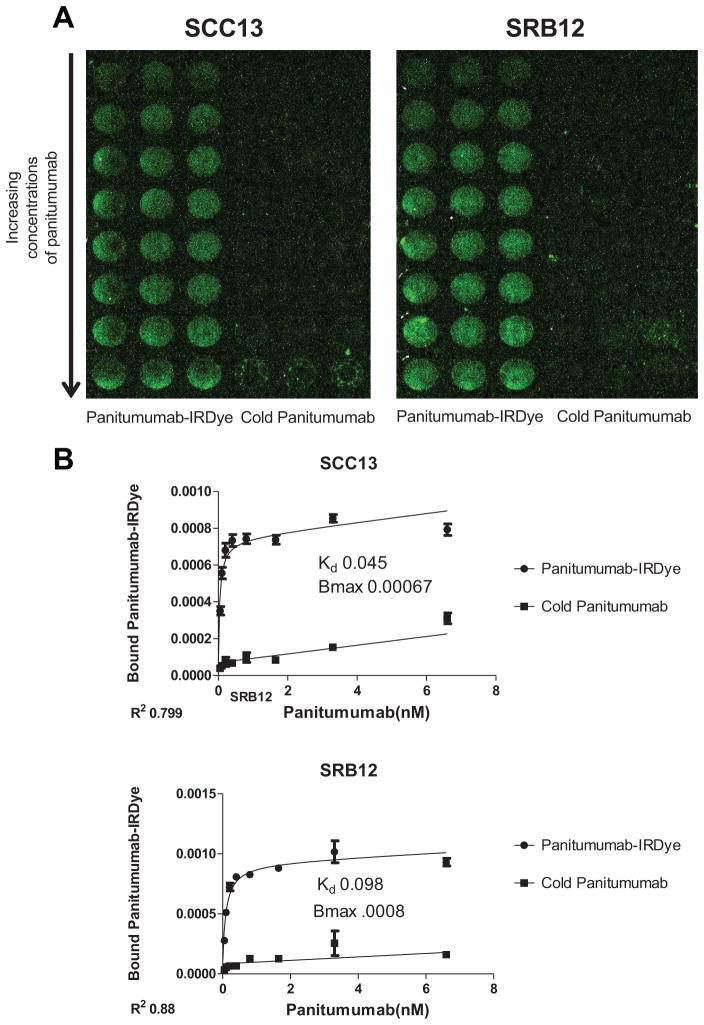
We first performed in vitro experiments that demonstrated that the antibody-fluorophore bioconjugate maintained its specificity to EGFR as described previously.5,8 Western analysis of both the SCC-13 and the SRB-12 cell lines showed expression of EGFR (Figure 1). Next, an in vitro cell-binding assay showed binding of the pantimumumab-IRDdye800 in both cell lines (Figure 2A and B).
Figure 1.
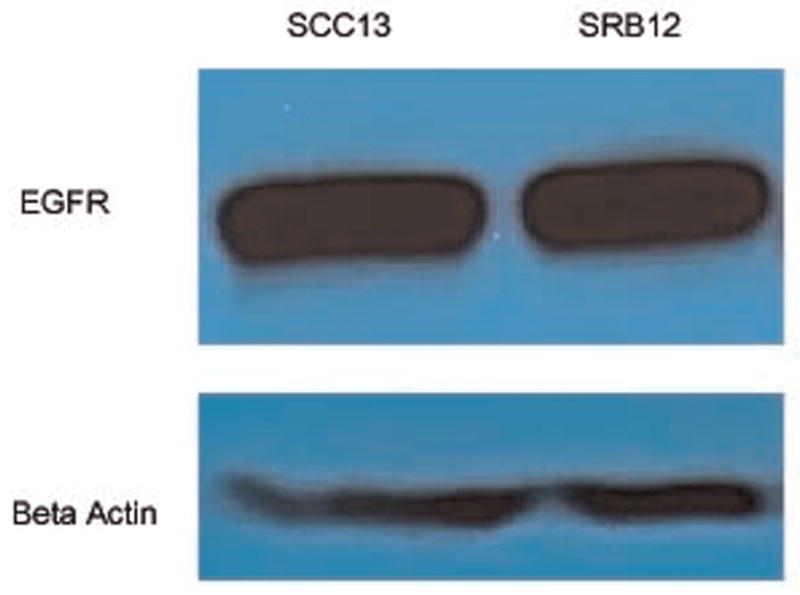
Western analysis demonstrating epidermal growth factor receptor protein expression in SCC13 and SRB12 cutaneous cell lines.
Figure 2.
Quantitative (A) and qualitative (B) analysis of in vitro cell-binding assay with panitumumab-IRDye and unlabeled (cold) panitumumab.
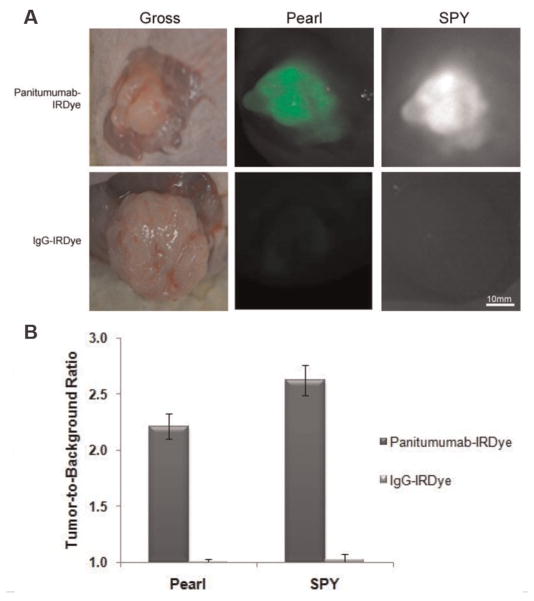
Next, we evaluated in vivo specificity of panitumumab-IRDye800 for the SCC-13 and SRB-12 cell lines by comparing panitumumab-IRDye800 uptake to nonspecific antibody IgG-IRDye800 uptake in mice with SCC-13 or SRB-12 flank tumors (Figure 3A). Tumor fluorescence was evaluated using the SPY intraoperative imaging system designed for indocyanine green imaging and compared with the gold standard for IRDye800 imaging, a small-animal optical imaging system. A significantly greater degree of fluorescence was detected in the tumors of mice injected with panitumumab-IRDye800 versus IgG-IRDye800. The uptake of the nonspecific, control dye resulted in a TBR of 1.0 for both imaging modalities, indicating equivalent fluorescent intensity between the tumor tissue and healthy background tissue. The uptake of panitumumab-IRDye800 resulted in a TBR of 2.2 (Pearl) and 2.6 (SPY). Therefore, the panitumumab-IRDye800 demonstrated an appreciably higher TBR versus control (Figure 3B) and provides for sufficient contrast between tumor and background necessary to guide surgical resection.
Figure 3.
Panitumumab-IRDye800 versus aspecific control (IgG-IRDye800). Uptake of near-infrared fluorescence agent in an immunodeficient mouse with cutaneous flank SCC-13 tumor. Representative intraoperative image using SPY is shown.
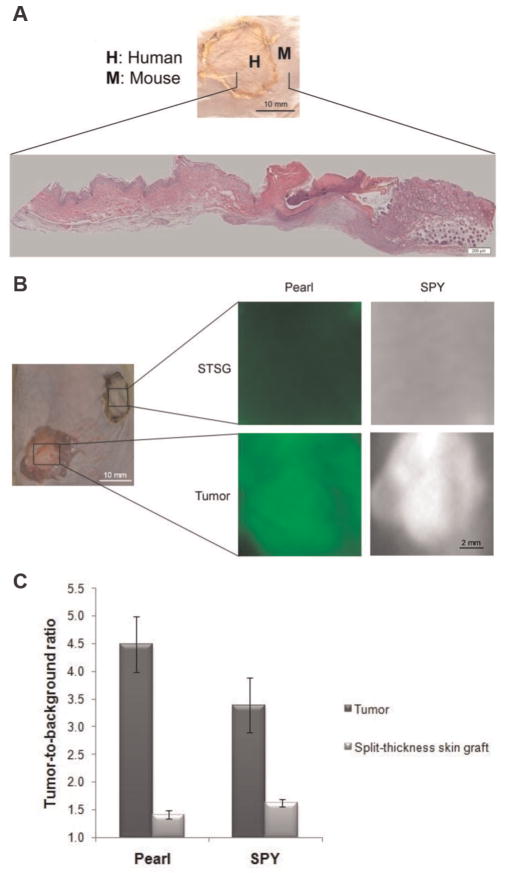
Since the antigenic properties of rodent EGFR are slightly different than human EGFR, we analyzed the uptake of panitumumab-IRDye800 in human split-thickness skin grafts (STSGs; n = 3) to determine the expected background fluorescence of this bioconjugate if it were to be used in humans. Histological sections of the native rodent skin juxtaposed to the human STSG demonstrated successful grafting and viability (Figure 4A). Relative fluorescence between tumors was assessed by comparing the fluorescence intensity of the SCC-13 and SRB-12 tumor to the STSG (Figure 4B). The tumor had a TBR of 4.5 ± 1.8 (Pearl) and 3.4 ± 0.2 (SPY). The STSG had a TBR of 1.4 ± 0.03 (Pearl) and 1.6 ± 0.2 (SPY; Figure 4C). With such an appreciable difference, this suggests that if panitumumab-IRDye800 was used in humans, it would achieve a sufficient TBR to be differentiated from normal surrounding epithelium.
Figure 4.
Analysis of the human skin graft. (A) Gross representation of the split-thickness skin graft (STSG) and conventional hematoxylin and eosin histology of the mouse and human skin. (B) Comparison of panitumumab-IRDye800 uptake in tumor versus STSG. (C) Tumor-to-background ratio of tumor and STSG are shown for each imaging modality.
Resection under NIR Fluorescence-Guided Imaging
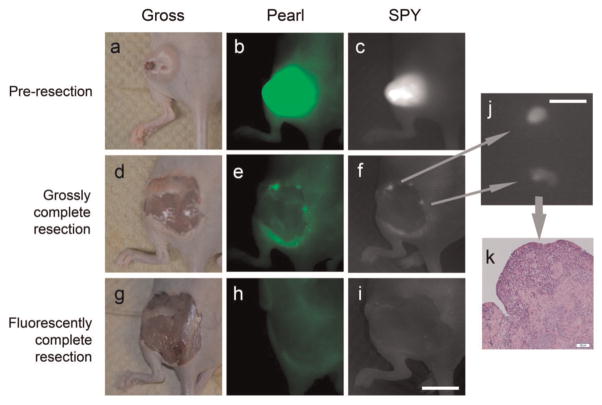
The aim of the next experiment was to use panitumumab-IRDye800 with cSCC to enhance the visualization of margin resection to ensure negative margins by resecting tumor boundaries that would otherwise appear grossly normal. Initially, the primary tumor was resected by conventional methods; grossly palpable tumor was resected with a 1- to 2-mm margin of healthy tissue. Next, we identified areas of residual disease that could not be clearly identified by palpation or visual inspection but could be detected by fluorescence on the SPY. The primary tumor as well as the margins and residual areas of positive fluorescence were resected and then imaged and sent for histological analysis (Figure 5a–i). In the mice receiving panitumumab-IRDye800, histological analysis was done on primary tumor resections (n = 4, average diameter: 8 mm, range: 5–11 mm), and residual pieces were taken from the margin (n = 18, average diameter: 4.5 mm, range: 2–9 mm). All primary tumor resections demonstrated gross tumor infiltration by conventional H&E. In the residual pieces that were fluorescently positive but grossly ambiguous for cancer, 18 of 18 samples had confirmed tumor by H&E, yielding a sensitivity of 100% (Figure 5j–k). Samples of negatively fluorescent tissue (n = 18) located within 1 cm of the margin were also taken for histological analysis for comparison. Of these, 18 of 18 were negative for the presence of tumor, yielding a specificity of 100%. All 36 positive and negative samples confirmed the presence or absence of tumor using histological and immunohistochemical analysis.
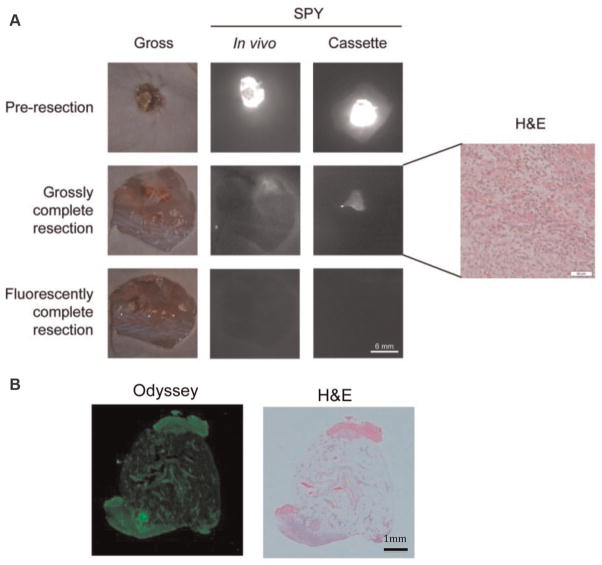
Figure 5.
Detection of microscopic residual disease. Uptake of near-infrared panitumumab-IRDye800 before resection (a–c), after grossly complete resection (d–f), and after fluorescently complete resection (g–i). Fluorescence imaging of microscopic residual disease with the SPY (j) is confirmed by histology with hematoxylin and eosin staining (k). Scale in (i) is 10 mm. Scale in (j) is 3 mm.
Optical Imaging of Microscopic Disease to Confirm Antibody Specificity for Tumor on Histological Assessment
Using an optical imaging scanning instrumentation (Odyssey), we imaged the histological sections from the cutaneous flank tumors after embedding them in optimal cutting temperature medium or paraffin. The fluorescence detected correlated with the presence of tumor by routine histology. Small islands of tumor could be identified with fluorescence and correlated with areas of tumor as confirmed by conventional H&E. The Odyssey confirmed tumor-specific localization of fluorescence detected by the SPY and Pearl imaging systems, as the Odyssey was capable of detecting microscopic islands of tumor cells as small as 200 μm in diameter within a region of normal tissue (Figure 6).
Figure 6.
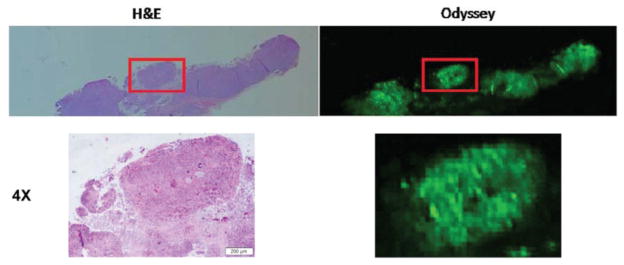
Fluorescent microscopy using the Odyssey scanner to detect malignancy. Areas of fluorescence detected with the Odyssey scanner correlate to tumor presence on hematoxylin and eosin histology.
Furthermore, we compared a known positive from the SCC-13 tumor edge and a known negative from the adjacent muscle from the adjacent muscle, and each questionable sample was determined to be negative or positive for tumor based on fluorescence. The samples were again correlated with H&E (Figure 7).
Figure 7.
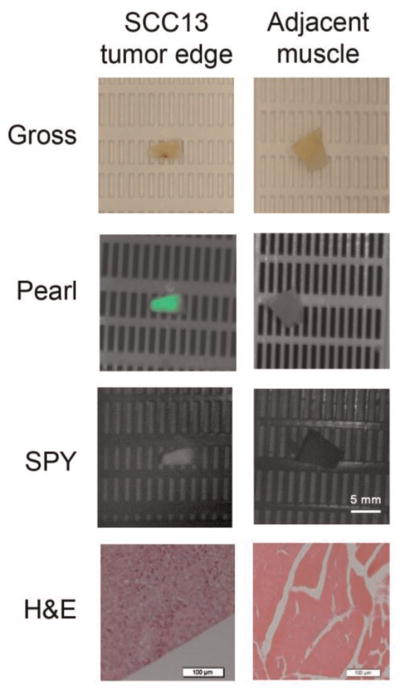
Analysis of positive and negative samples. Samples of paraffin-embedded tumor and negative margin were imaged with the Pearl and SPY along with a known positive and known negative. The samples were correlated with hemotoxylin and eosin.
Optical Imaging of a Human Tumor Explant Model
To demonstrate the application of optical imaging to human tumors, we transplanted a human tumor graft from a patient with cSCC into the flank of a nude mouse and systemically administered panitumumab-IRDye800. The human tumor explant had a mean TBR of 3.9 (Pearl) and 2.3 (SPY). As described above, the SPY system was used to guide resection of both the primary tumor and residual tumor that was not grossly apparent or palpable (Figure 8A). Tumor pathology was confirmed by histology and correlated to fluorescence detected by fluorescence imaging of the histological sections. Areas of fluorescence correlated with tumor (Figure 8B).
Figure 8.
Human explant tumor model. (A) Gross and SPY images following injection of panitumumab-IRDye800 before and after grossly complete and fluorescently complete resection. (B) Histological section of residual tumor with Odyssey and hematoxylin and eosin, demonstrating co-localization of the probe signal with tumor cells.
Discussion
The current method for intraoperative assessment of high-risk cSCC is Mohs surgery, which relies on the dermatologist to resect a thin margin of tissue around the cancerous lesion, evaluate by frozen section H&E analysis, and, if positive, harvest additional margins. Although thorough, this mapping technique for clearing margins can be very time-consuming as well as costly and still results in positive margins in approximately 6% of cases. Systemic administration of fluorescently labeled antibodies targeting cSCC may permit real-time cSCC-specific imaging within the clinic and operating room, and the use of optical imaging may improve sampling error. We propose that systemic administration of EGFR targeting monoclononal antibody may improve in vivo and histological assessment of margins. Successful imaging and tumor identification were demonstrated in both xenografted cell lines with uniform EGFR expression and in human tumors with a mosaic pattern of EGFR protein expression and composed of stromal elements. Disease that was subclinical in nature or not grossly palpable could be imaged in real time and subsequently confirmed as tumor by histology.
The value of this technique will be only as accurate as the intraoperative imaging hardware employed for imaging, yet there is currently no consensus on which hardware is best suited for this purpose. Developing novel camera systems to detect IRDye800 fluorescence in real time has been done by a few groups,9–11 but their application to humans is complicated by development costs, overlapping intellectual property rights, large-scale manufacturing, and Food and Drug Administration (FDA) approval.12,13 Intraoperative fluorescence imaging systems have been developed for detecting blood flow with indocyanine green and are readily available for intraoperative use; these include the SPY system developed by Novadaq for plastic surgery procedures and next-generation robotic consoles (Firefly, Intuitive Surgical, DaVinci) developed for guiding surgical resections. Because of the overlapping emission and absorption spectra of indocyanine green and IRDye800, we undertook the current study to determine if operative imaging systems designed for indocyanine green can be used for IRDye800 imaging. Using IRDye800, we assessed the potential of the SPY system for detecting primary and distant tumors in an in vivo mouse model. The SPY system is currently a clinical system that is designed to visualize indocyanine green for perfusion studies in the operating room. Since IRDye800 has similar fluorescent properties as indocyanine green, we sought to determine if this hardware, which is already in place in the operating room, can be used to detect cSCC using a labeled antibody specific for EGFR.
We found that the SPY system could detect both macro and microscopic disease. We were able to use this imaging modality to resect cutaneous tumors in mice and obtain negative margins and then detect residual disease and metastatic lymph nodes as small as 1 mm in diameter. Following resection of the primary tumor, we performed histologic analysis on all positive and negative slides that were prepared as frozen specimens and as paraffin-embedded specimens. The histological analysis with the Odyssey system was able to detect islands of residual tumor as small as 200 μm in diameter and confirm localization of tumor. Although histological analysis can be performed by a trained pathologist, use of this technology may improve the threshold of detection, improve the speed of detection, or limit the level of experience needed for analyzing frozen sections, which are time-consuming and can be reversed on final pathology in as many as 2% to 10% of cases.14,15
Selective delivery of pantitumumab-IRDye800 to the tumor has the additional benefit of improving postresection assessment. Once removed, the tumor can be assessed on the “back table” with the SPY system for confirmation of complete resection. In addition, fluorescence imaging with the Odyssey scanner of frozen histological sections can aid in identification of small islands of disease, as shown in our study and other preclinical models.7 Such imaging could be used by pathologists to augment intraoperative margin evaluation and to improve sensitivity and specificity.
Panitumumab is a therapeutic antibody that blocks the EGFR and inhibits tumor growth.16,17 We chose to use this antibody in our studies because it is fully humanized and FDA approved for use in humans, and it can potentially be easily translated to the clinic. In the current study, we used subtherapeutic doses of panitumumab to visualize the tumors, and we believe dosing will minimize any of the rare side effects that are seen with EGFR inhibitors, including skin rash and anaphylaxis. There are currently clinical trials in Europe evaluating the potential of targeting monoclonal antibodies labeled with fluorescent probes in oncological surgery.13,18,19 One trial is testing the safety of therapeutic, monoclonal labeled antibody to perform the studies we demonstrated in a preclinical model here: to delineate tumor margins in the operating room, to detect residual microscopic disease during resection, and to identify lymph node metastases.19 In our previously published studies, we used cetuximab (EGFR antibody) conjugated to the fluorophore Cy5.5 (excitation, 678 nm; emission, 703 nm) to detect primary tumors and distant metastases.8 We chose to use IRDye800 in the present study because it has better fluorescent properties and consistent protein labeling when compared with cy5.5.20
Although histology remains the gold standard for identification of tumor in clinical settings, improving detection of subclinical or nonpalpable disease may limit sampling error by the surgeon or pathologist and thereby substantially improve the ability to obtain negative margins. In conclusion, we were able to use the SPY imager, a modality currently clinically available, in conjunction with panitumumab-IRDye800 to fluorescently visualize tumors and guide resection to ensure negative margins.
Acknowledgments
Sponsorships: The SPY equipment was donated by Novadaq.
Funding source: This work was supported by a grant from the NIH (T32CA091078).
Footnotes
Author Contributions
C. Hope Heath, design, experiments, write up, editing, and review of the study; Nicholas L. Deep, experiments and editing; Lauren N. Beck, experiments and editing; Kristine E. Day, write up and editing; Larissa Sweeny, editing; Kurt R. Zinn, design and experiments; Conway C. Huang, design; Eben L. Rosenthal, design, experiments, write up, editing, and review of the study.
Disclosures
Competing interests: None.
This article was presented at the 2012 AAO-HNSF Annual Meeting & OTO EXPO; September 9-12, 2012; Washington, DC.
References
- 1.Tan PY, Ek E, Su S, Giorlando F, Dieu T. Incomplete excision of squamous cell carcinoma of the skin: a prospective observational study. Plast Reconstr Surg. 2007;120:910–916. doi: 10.1097/01.prs.0000277655.89728.9f. [DOI] [PubMed] [Google Scholar]
- 2.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–983. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg. 2003;112:57–63. doi: 10.1097/01.PRS.0000067479.77859.31. [DOI] [PubMed] [Google Scholar]
- 4.Ch’ng S, Low I, Ng D, et al. Epidermal growth factor receptor: a novel biomarker for aggressive head and neck cutaneous squamous cell carcinoma. Hum Pathol. 2008;39:344–349. doi: 10.1016/j.humpath.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Heath CH, Deep NL, Sweeny L, Zinn KR, Rosenthal EL. Use of panitumumab-IRDye800 to image microscopic head and neck cancer in an orthotopic surgical model. Ann Surg Oncol. 2012;19:3879–3887. doi: 10.1245/s10434-012-2435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LI-COR Biosciences. IRDye(R) 800CW Protein Labeling Kit-High MW. Lincoln, NE: LI-COR Biosciences; 2007. pp. 1–9. [Google Scholar]
- 7.Reuthebuch O, Haussler A, Genoni M, et al. Novadaq SPY: intraoperative quality assessment in off-pump coronary artery bypass grafting. Chest. 2004;125:418–424. doi: 10.1378/chest.125.2.418. [DOI] [PubMed] [Google Scholar]
- 8.Gleysteen JP, Newman JR, Chhieng D, Frost A, Zinn KR, Rosenthal EL. Fluorescent labeled anti-EGFR antibody for identification of regional and distant metastasis in a preclinical xenograft model. Head Neck. 2008;30:782–789. doi: 10.1002/hed.20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sevick-Muraca EM. Translation of near-infrared fluorescence imaging technologies: emerging clinical applications. Annu Rev Med. 2012;63:217–231. doi: 10.1146/annurev-med-070910-083323. [DOI] [PubMed] [Google Scholar]
- 10.van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 11.Swijnenburg RJ, Crane LM, Buddingh KT, van de Velde CJ, Vahrmeijer AJ, van Dam GM. Intraoperative imaging using fluorescence [in Dutch] Ned Tijdschr Geneeskd. 2012;156:A4316. [PubMed] [Google Scholar]
- 12.Themelis G, Yoo JS, Soh KS, Schulz R, Ntziachristos V. Real-time intraoperative fluorescence imaging system using light-absorption correction. J Biomed Opt. 2009;14:064012. doi: 10.1117/1.3259362. [DOI] [PubMed] [Google Scholar]
- 13.Keereweer S, Kerrebijn JD, van Driel PB, et al. Optical image-guided surgery—where do we stand? Mol Imaging Biol. 2011;13:199–207. doi: 10.1007/s11307-010-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandour-Edwards RF, Donald PJ, Lie JT. Clinical utility of intraoperative frozen section diagnosis in head and neck surgery: a quality assurance perspective. Head Neck. 1993;15:373–376. doi: 10.1002/hed.2880150502. [DOI] [PubMed] [Google Scholar]
- 15.Remsen KA, Lucente FE, Biller HF. Reliability of frozen section diagnosis in head and neck neoplasms. Laryngoscope. 1984;94:519–524. doi: 10.1288/00005537-198404000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu T, Izumi H, Oga A, et al. Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous-cell carcinoma of the skin. Dermatology. 2001;202:203–206. doi: 10.1159/000051637. [DOI] [PubMed] [Google Scholar]
- 17.Kundu SK, Nestor M. Targeted therapy in head and neck cancer. Tumour Biol. 2012;33:707–721. doi: 10.1007/s13277-012-0350-2. [DOI] [PubMed] [Google Scholar]
- 18.Keereweer S, Sterenborg HJ, Kerrebijn JD, Van Driel PB, de Jong RJ, Lowik CW. Image-guided surgery in head and neck cancer: current practice and future directions of optical imaging. Head Neck. 2012;34:120–126. doi: 10.1002/hed.21625. [DOI] [PubMed] [Google Scholar]
- 19.Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med. 2011;52:1778–1785. doi: 10.2967/jnumed.111.092833. [DOI] [PubMed] [Google Scholar]
- 20.Adams KE, Ke S, Kwon S, Liang F, Fan Z, Lu Y, et al. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007;12:024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]