Abstract
S-(3,4-dichlorobenzyl)isothiourea (A22) disrupts the actin cytoskeleton of bacteria, causing defects of morphology and chromosome segregation. Previous studies have suggested that the actin homolog MreB itself is the target of A22, but there has been no direct observation of A22 binding to MreB and no mechanistic explanation of its mode of action. We presently show that A22 binds MreB with at least micromolar affinity in its nucleotide binding pocket in a manner that is sterically incompatible with simultaneous ATP binding. A22 negatively affects both the timecourse and extent of MreB polymerization in vitro in the presence of ATP. A22 prevents MreB assembly into long, rigid polymers, as determined by both fluorescence microscopy and sedimentation assays. A22 increases the critical concentration of ATP-bound MreB assembly from 500 nM to approximately 2000 nM. We therefore conclude that A22 is a competitive inhibitor of ATP binding to MreB. A22-bound MreB is capable of polymerization, but with assembly properties that more closely resemble those of the ADP-bound state. Because the cellular concentration of MreB is in the low micromolar range, this mechanism explains the ability of A22 to largely disassemble the actin cytoskeleton in bacterial cells. It also represents a novel mode of action for a cytoskeletal drug and the first biochemical characterization of the interaction between a small molecule inhibitor of the bacterial cytoskeleton and its target.
Despite prior assumptions to the contrary, bacteria have cytoskeletons comprised of tubulin (1, 2), actin (3, 4), and intermediate filament (5) homologs. While the cellular functions of these molecules differ significantly from their canonical behaviors in eurkaryotic cells, they are increasingly understood to control the morphology and division of a wide variety of bacteria (6). The bacterial actin, MreB, is required for establishment and maintenance of the characteristic rod shape (7), cell division (8) (3), chromosome segregation (9–11), cell wall morphogenesis (12), cell polarity (13), and even plays a role in the localization of the chromosome replication machinery (14) in species ranging from Gram-negative (E. coli) to Gram-positive (B. subtilis and C. crescentus) bacteria.
S-(3,4-dichlorobenzyl)isothiourea (A22) was identified by virtue of its ability to induce spherical anucleate cells in the rod-shaped bacterium, E. coli (15). The treatment of cells with A22 disrupts the helical localization of MreB in vivo and appears to cause its disassembly, which leads to the diffuse distribution of MreB in the cytoplasm and loss of rod-shaped cell morphology and viability (16, 17). Despite the widespread use of A22 to disrupt MreB and bacterial morphology (16–28), to date there has been no experimental evidence for a direct interaction between A22 and MreB and no mechanistic studies of the mode of action of A22.
We sought to test and characterize the interaction between A22 and MreB and to discern its mechanism of action. We found that A22 is a competitive inhibitor of ATP binding by MreB and that its binding induces a state in MreB capable of polymerizing, but at a greatly reduced affinity for polymerization. These results explain the disassembly of MreB polymers by A22 in cells. They also represent a novel mode of action for any cytoskeletal drug and the first biochemical characterization of the interaction of any drug with the bacterial cytoskeleton.
Experimental Procedures
Proteins
Untagged Thermotoga maritima MreB1 was overexpressed and purified from E. coli as previously described (29) and stored in CaG8 buffer without DTT (2 mM TrisHCl, pH 8.0, 0.1 mM CaCl2, 200 µM ATP, 0.02% NaN3). ADP-MreB was prepared by Dowex treatment as previously described (29). Cys332 substituted and Alexa488-labeled MreB were produced as previously described (29). T. maritima MreB1-his was constructed as follows: mreB1 was amplified from genomic DNA using forward and reverse primers 5’ GGGAATTCCATATGTTGAGAAAAGACATAGGAATAGAT C 3’ and 5’ ATAAGAATGTCGACCCCGGCACCCTGAAGCTTCTTC 3’, digested with NdeI/SalI, and ligated into the NdeI/XhoI site of pET23a. MreB1-his was expressed in BL21(DE3) cells, extracted in NiG8 buffer, purified by Ni-agarose chromatography, eluted with imidazole in CaG8 buffer, dialyzed against 10 mM TrisHCl, pH 8.0 without nucleotide, and stored at −80 °C until use. All proteins were quantified by SDS-PAGE/Coomassie Blue densitometry in ImageJ using rabbit actin as a standard. With the exception of NMR, all experiments herein were performed using untagged MreB1.
MreB polymerization
Unless stated otherwise, non-protein reaction components were mixed on ice in proportions such that the final reactions contained 200uM ATP, 10mM imidazole pH 7.0, 20 mM KCl, 1mM MgCl2 and 1mM EGTA (KMEI). Separately on ice, MreB in CaG8 storage buffer was mixed with 1/9th volume of 10× cation exchange buffer (1 mM MgCl2, 10 mM EGTA) and incubated on ice for one minute. Polymerization was initiated by combining the two samples with gentle mixing.
Light Scattering
90° perpendicular light scattering experiments were carried out as previously described (29) using a PC1 spectrofluorometer (ISS) equipped with a temperature control jacket and under control of Vinci software (ISS) v1.4.9.5. Excitation and emission monochrometers were set at 400nm and slit pairs (typically 1 mm) were employed in both excitation and emission paths. Data was collected at 1 s intervals, analyzed in Excel and plotted using Kaleidagraph.
Critical concentration
MreB was polymerized as described above at varying protein concentrations. Reactions were equilibrated for one hour at the experimental temperature prior to measuring light scattering intensity as described above. Light scattering values were the average of one minute of acquired signal. Critical concentrations were determined by the intersection of two linear fits to the data points above and below the apparent inflection point in the graph as determined by visual inspection.
Sedimentation
MreB was polymerized as described and centrifuged either at 4 °C for 30 minutes at 100k × g in a Beckman tabletop ultracentrifuge or at room temperature for 10 minutes at 100k × g in a Beckman airfuge. Supernatant and pellet fractions were separated and analyzed by SDS-PAGE, stained with Coomassie blue and quantitated by densitometry.
Fluorescence microscopy
Alexa488-labeled L332C MreB and unlabeled native MreB were mixed and polymerized at room temperature for 1h in standard buffer conditions at 1 µM total concentration. Samples were applied directly to coverslips and viewed on an Olympus IX-71 epifluorescence microscope using a 60× 1.45 n.a. objective lens and fluorescein filter set (Chroma). Images were acquired using a Hamamatsu Orca 285 camera under control of Wasabi software. Image manipulation, limited to contrast and brightness enhancement, was carried out in Adobe Photoshop CS2. Polymer lengths were measured manually and analyzed using Excel and Kaleidagraph.
A22
A22 was synthesized from 3,4-dichlorobenzyl chloride and thiourea and purified by recrystallization from diethyl ether three times. Spectral data for the compound matched that reported previously (15).
STD NMR
NMR experiments were performed at 10 °C on a 750 MHz Bruker DMX spectrometer equipped with a 5-mm triple-resonance cryogenic probe. A saturation transfer difference pulse sequence with a saturation frequency at −1 ppm and an off-resonance saturation frequency at −40 ppm was used with a standard 1-D proton spectrum as described in the literature (30). NMR samples consisted of: 600 µL of a solution of His-tagged MreB in Tris buffer (10 mM, pH 8.0), 70 µL of D2O, and 30 µL of a solution of A22 in DMSO-d6; the total sample volume was 700 µL. The final concentration of A22 in each NMR sample was 5 mM; the concentration of MreB in the samples varied from 0–12.8 µM. Deuterium oxide (D2O) was purchased from Cambridge Isotope Laboratories, Inc (Andover, MA) and (methyl sulfoxide)-d6 (DMSO-d6) from Sigma-Aldrich (St. Louis, MO).
We processed NMR data using the NutsPro software (Acorn NMR). The free induction decay was filtered with a 90° phase-shifted, sine-squared function, and the resulting data was zero-filled and transformed using a Fourier function. A baseline correction was performed on the transformed spectra. We used line-fitting scripts to determine the area of the peaks that corresponded to the three aromatic protons of A22. We fit data using Prism 4.0 (GraphPad Software) to a one-site binding model of the form:
where Y is the integrated area under the NMR peak, X is the concentration of MreB (µM), Kd is the dissociation constant, and Bmax is the theoretical maximum intensity at saturation binding. The individual data sets were normalized between their integrated peak areas at zero protein concentration and their fitted Bmax values, resulting in a response range from 0 to 1.0. To determine the lower-limit of the Kd of A22 bound to MreB the data sets were combined and fit to the one-site binding model described above.
Docking
The starting point for the docking studies was the crystal structure of MreB bound with AMPPNP (pdb 1JCG) (4) where we modified the AMPPNP to make ATP. For A22, we constructed the compound in Sybyl 6.8 (Tripos Inc., St. Louis, MO) and assigned Gasteiger-Marsili charges to MreB, ATP and A22. We performed two sets of docking studies – one to place A22 on the MreB-ATP complex and one to dock A22 to the nucleotide free form of MreB. Both docking runs were carried out using AutoDock 3.0 (31) with 0.375 Å spacing and 50 independent runs using the Lamarckian genetic algorithm with 10 million energy evaluations. The docking results were clustered using a 1.0 Å RMSD and analyzed both for their predicted docking energy and cluster size. Additional docking studies were carried out using MreB mutants T158A and V315A as well as (2,3-dichlorobenzyl) and (2,4,6-trichlorobenzyl) derivatives of A22.
Results
A22 binds MreB with micromolar affinity
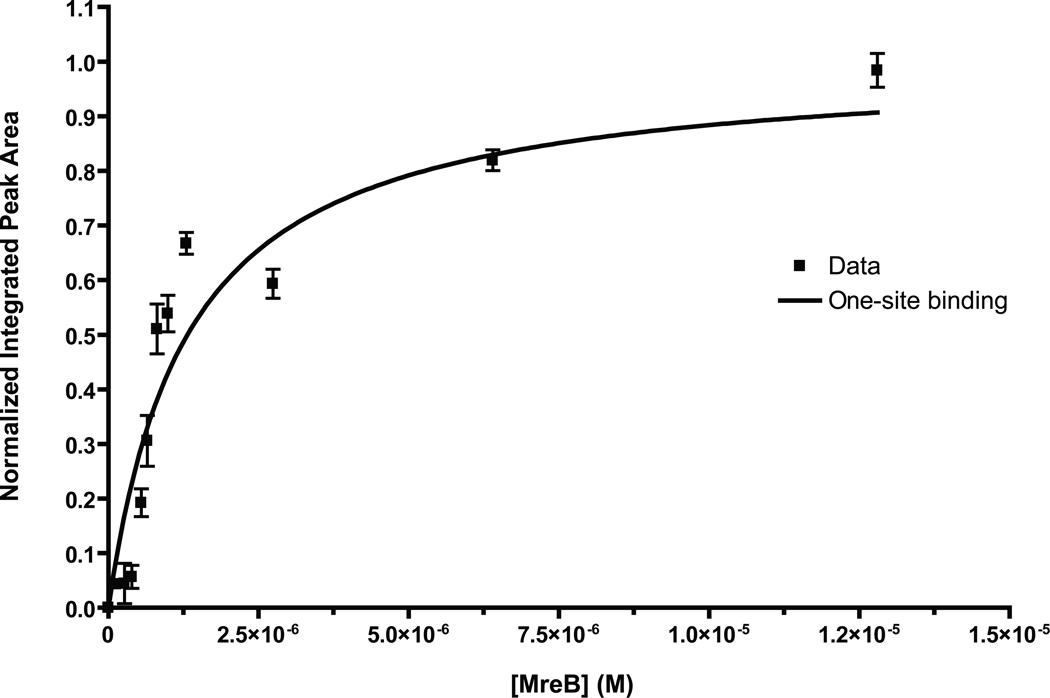
We used saturation transfer difference (STD) NMR to test for a direct interaction between A22 and MreB-his in the absence of free ATP. Because MreB is not monomeric at very high concentrations, even in low-salt storage buffer, we used a high concentration of A22 (5 mM) and varied the concentration of MreB over a range from 0–12.8 µM. We chose the three aromatic protons of A22 for our STD analysis because they were well resolved from the other peaks in the NMR spectra (e.g. buffer, solvents). We collected and normalized NMR data sets, plotted the peak areas of the three aromatic protons versus MreB concentration, and fit it to a one-site binding model to estimate the Kd (Figure 1). The curve-fit yielded a Kd of 1.32 ± 0.14 µM (R2 = 0.89) for A22 binding to MreB. Because this experiment measures a saturation transfer difference, the observed peak areas are amplified by a factor equal to koff (i.e. observed peak area = koff × [PL]). An apparent larger value of protein-ligand complex would result in an apparent smaller Kd (since Kd = [P][L]/[PL]). Therefore, the estimated Kd that we report represents a lower limit for the affinity.
Figure 1. A22-MreB interaction observed by NMR.
5 mM A22 was incubated with varying concentrations of MreB in 10 mM Tris-HCl, pH 8.0 at 10°C. Normalized integrated peak areas collected for the three aromatic protons in A22 were plotted versus protein concentration and fitted to a one-site binding model. The Kd was found to be 1.32 ± 0.14 µM (R2 = 0.89).
A22 binding site overlaps with nucleotide
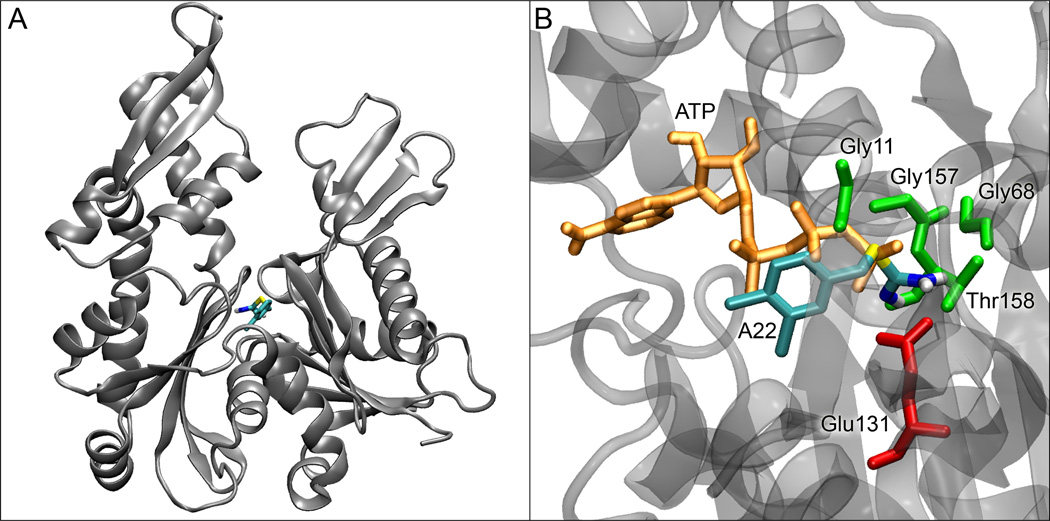
In order to determine where A22 binds on the MreB structure, we performed a series of small molecule docking studies. When we used the ATP-bound form of MreB, A22 docked in a non-specific, non-reproducible manner over the surface of MreB with indeterminate binding affinity. When we removed the nucleotide and repeated the docking studies, A22 bound with high affinity (~0.19 µM) and specificity in the nucleotide binding site (see Figure 2). A22 interacts primarily with E131 and T158 of MreB, both highly conserved. The aromatic ring and thiourea group of A22 overlap with the positions that would be occupied by the nucleotide's beta and gamma phosphates, respectively. Together, these results suggest that A22 functions as competitive inhibitor of ATP. Docking studies using T158A or V315A MreB mutants binding to A22 or MreB binding to (2-3-dichlorobenzyl) or (2,4,6-trichlorobenzyl) derivatives of A22 yielded uniformly weaker binding (not shown).
Figure 2. A22 docking in the MreB nucleotide cleft.
(A) Docking site of A22 in the nucleotide-free form of MreB. (B) Details of the binding site of A22 in MreB with residues within 2.5 Å of A22 shown with their sidechains. ATP is shown in its crystal position, illustrating the clashes that would occur between its beta and gamma phosphates and A22.
Fluorescence microscopy: A22 prevents MreB polymer assembly
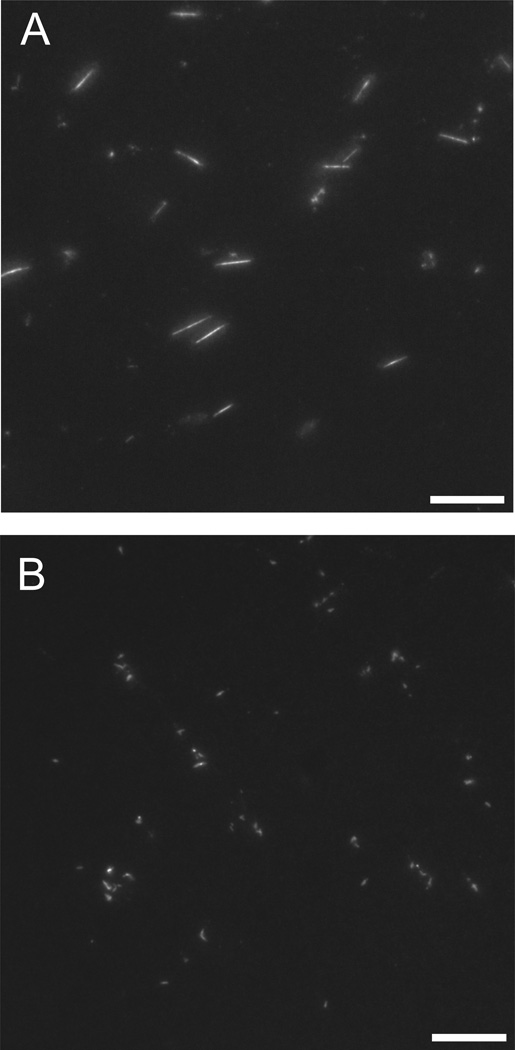
We investigated the effects of A22 on the assembly and morphology of MreB polymers by fluorescence microscopy. We made use of C332-substituted MreB covalently labeled with Alexa-488 to visualize polymers by epifluorescence microscopy. When 1 µM MreB was incubated under standard polymerizing conditions for one hour and applied directly to glass sides, the product of the reaction was long, rigid polymers (Figure 3A) as previously observed (29). When identical reactions were carried out in the presence of 300 µM A22, however, no linear structures were observed but instead, only small (< 1 µm), amorphous fluorescent structures.
Figure 3. A22-mediated inhibition of MreB assembly visualized by fluorescence microscopy.
1 µM MreB (20% Alexa-488 labeled) was polymerized in 10 mM imidazole, pH 7.0, 1 mM MgCl2, 1 mM EGTA, 20 mM KCl and 200 µM ATP at 20° C for 1 hr in the absence (A) or presence (B) of 300 µM A22 and imaged directly by epifluorescence microscopy. Scale bar = 10 µm.
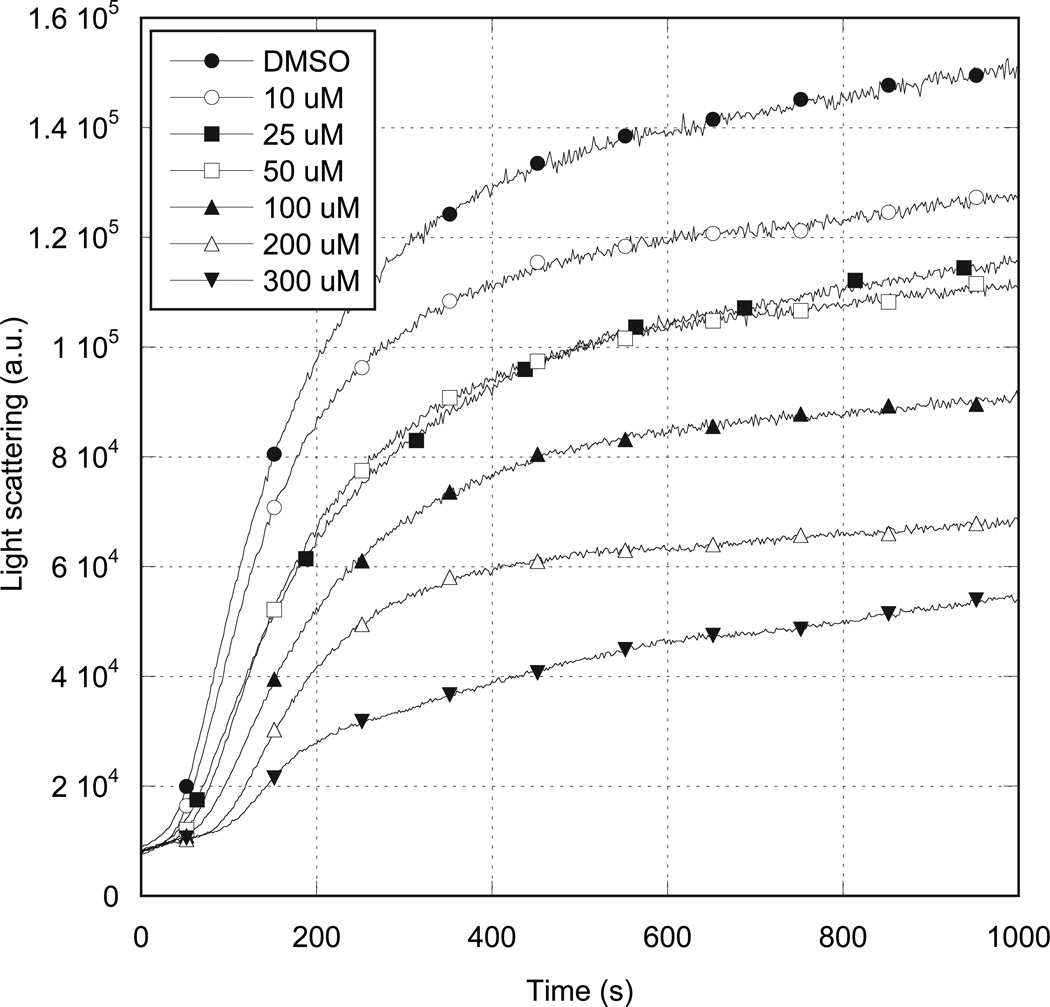
Light scattering timecourse: A22 affects MreB assembly timecourse
We further studied the effects of A22 on MreB by following the timecourse of polymerization by light scattering (Figure 4). In the absence of A22, and under standard polymerization conditions, 5 µM ATP-bound MreB assembly followed a timecourse that clearly exhibited the lag (nucleation), linear (polymerization) and plateau (steady-state) phases of actin assembly. When A22 was included at increasing concentrations, the rate and extent of MreB assembly were reduced. Maximal inhibition was observed at an A22 concentration of 300 µM. At higher A22 concentrations, nonspecific, secondary binding of A22 to MreB caused protein aggregation and massive, rapid increases in light scattering intensity (not shown). Similar experiments using ADP-bound MreB produced no observable effect over the same concentration range (Supplementary Figure 1).
Figure 4. Effects of A22 on MreB polymerization timecourse.
5 µM MreB was polymerized in 10 mM imidazole, pH 7.0, 20 mM KCl, 1 mM MgCl2 and 200 µM ATP at 20° C in the presence of varying concentrations of A22. MreB polymerization timecourse was followed by 400 nm right angle light scattering. A22 concentrations are as specified in the inset.
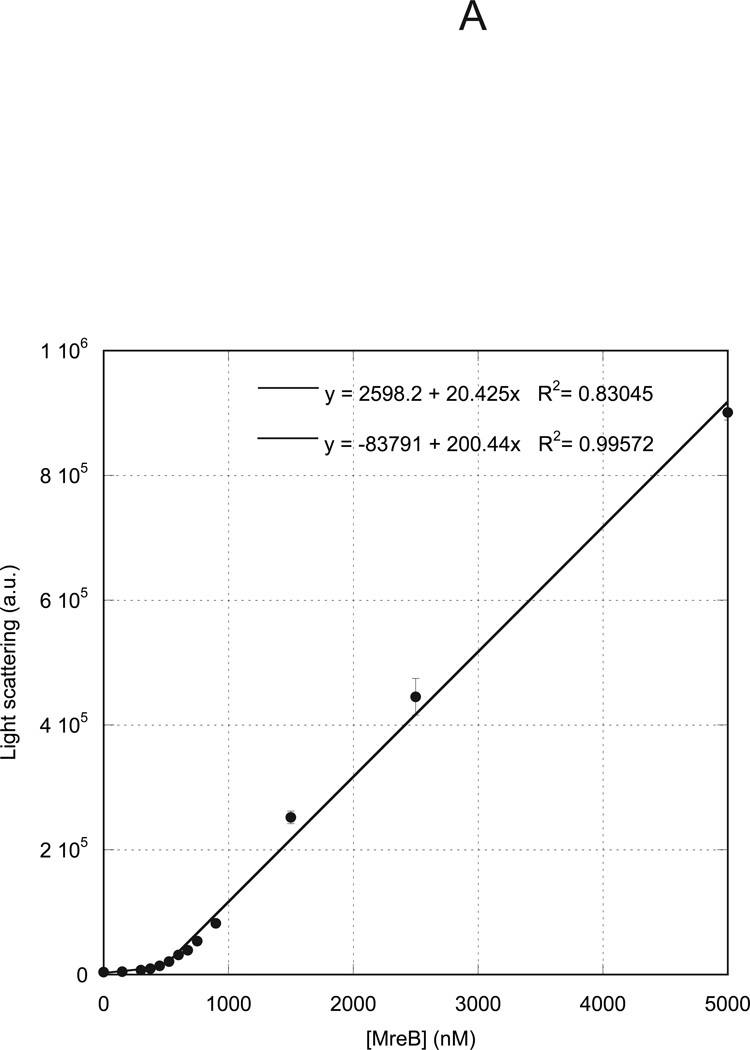
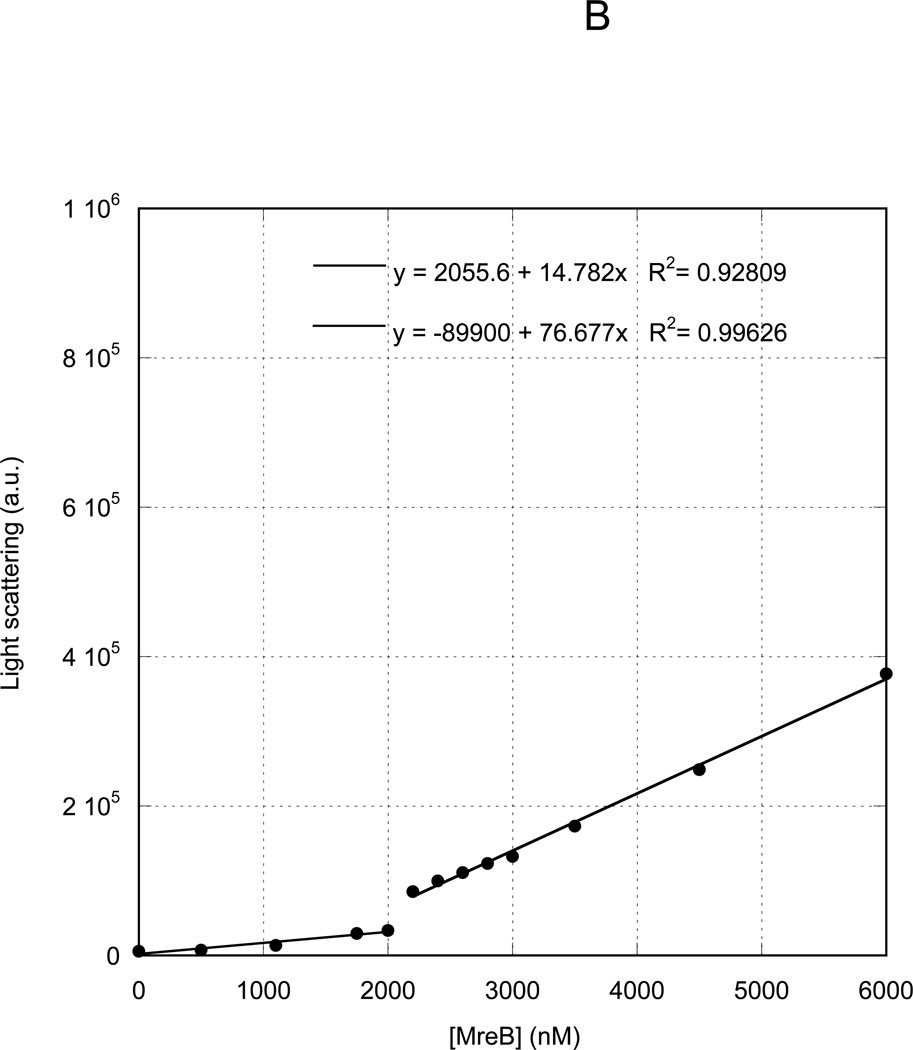
A22 increases critical concentration for MreB assembly
We previously measured the critical concentration for assembly of MreB under varying nucleotide, temperature and buffer conditions (29). We presently investigated the effects of A22 on the critical concentration of MreB. We allowed MreB to assemble to steady state at varying concentrations under standard reaction conditions in the presence of ATP and measured the light scattering intensity. A plot of light scattering intensity versus protein concentration (Figure 5A) demonstrated a characteristic biphasic behavior, with two independent linear distributions of data points. The concentration at which the data inflect upward represents the critical concentration, equivalent to the affinity of monomers for polymers, or the concentration above which polymerization occurs. In the absence of A22, in ATP at 20 °C, MreB exhibited a critical concentration of 500 nM. When A22 was included at its maximal inhibitory concentration of 300 µM, the critical concentration was shifted to approximately 2000 nM (Figure 5B).
Figure 5. Effect of A22 on MreB critical concentration.
Varying concentrations of MreB were polymerized at 4° C overnight in 10 mM imidazole, pH 7.0, 1 mM MgCl2, 1 mM EGTA, 20 mM KCl and 200 µM ATP in the absence (A) or presence (B) of 300 µM A22. Samples were equilibrated to 20° C for 1 h and the 400 nm light scattering intensity was measured. Linear fits to the data yielded critical concentrations of 500 nM (A) and 2000 nM (B), respectively.
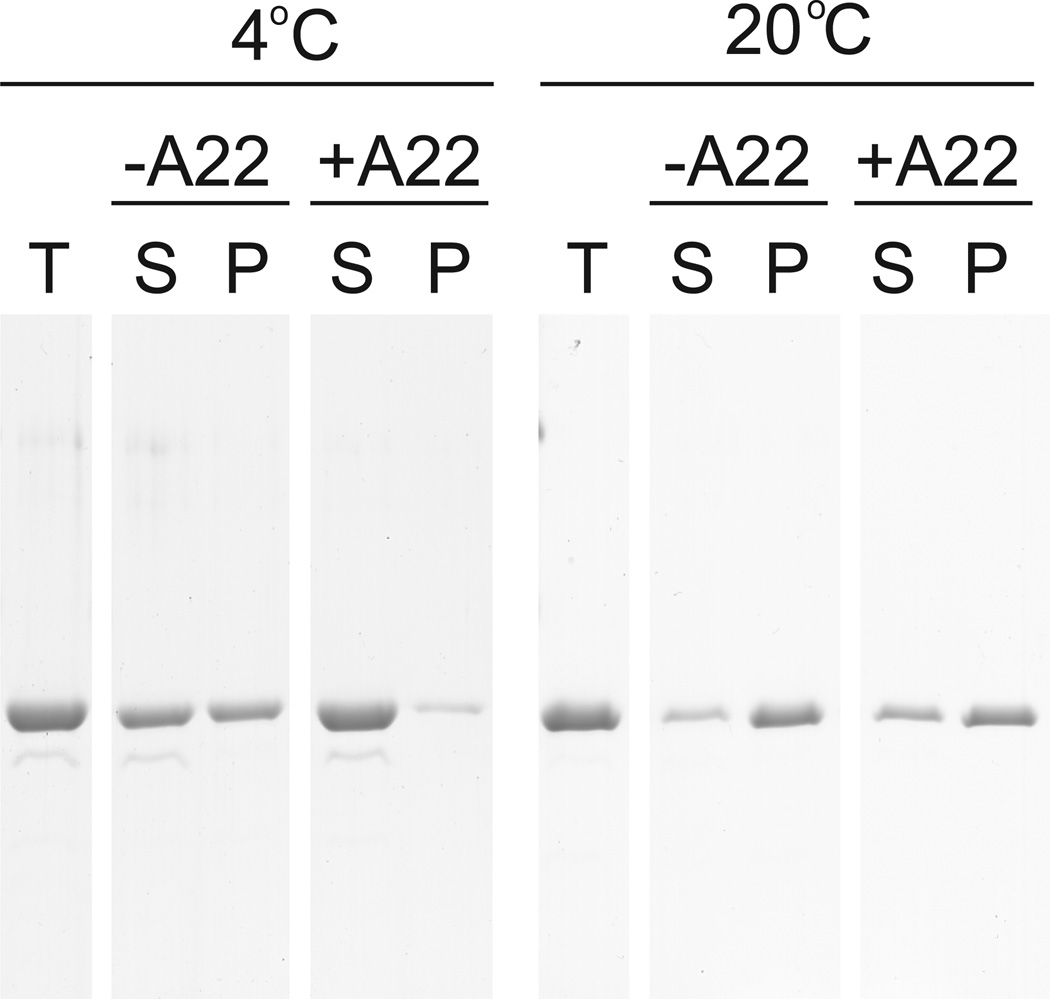
A22 shifts monomer-polymer equilibrium in sedimentation assay
MreB polymers pellet when subjected to ultracentrifugation, but monomers remain in solution. We polymerized 5 µM MreB in standard buffer conditions in the presence of ATP at 4 °C or 20 °C in the presence or absence of 300 µM A22. At 4 °C, approximately 40% of MreB pelleted in the absence of A22, consistent with the previously determined critical concentration of 2100 nM under identical conditions (Figure 6). In the presence of A22, however, virtually all MreB remained in the supernatant at 4 °C, consistent with a temperature-dependent increase in critical concentration. At 20 °C, approximately 90% of MreB pelleted in the absence of A22, consistent with the previously measured critical concentration of 500 nM. Addition of A22 decreased the fraction of MreB recovered in the pellet to approximately 60%, which is consistent with the presently determined critical concentration of approximately 2000 nM. In parallel experiments in the presence of ADP, A22 produced no observable change in the fraction of MreB pelleted (Supplemental Figure 2).
Figure 6. Effects of A22 on sedimentation of MreB.
5 µM MreB was polymerized for 1 h at 4° C or 20° C in 10 mM imidazole, pH 7.0, 1 mM MgCl2, 1 mM EGTA, 20 mM KCl and 200 µM ATP in the presence or absence of 100 µM A22. Samples were centrifuged at 4° C or 20° C for 30 min at 100000g. Equivalent volumes of total (T), supernatant (S) and pellet (P) samples are shown by SDS-PAGE and Coomassie blue.
Discussion
Gitai et al., isolated seven A22-resistant missense mutants in C. crescentus (16) and found that each mapped to the nucleotide-binding cleft of MreB. This observation made a strong implicit case for MreB as the target of A22, but did not rule out alternative explanations and provided no explanation for the mechanism of A22’s possible effect on MreB.
The NMR experiments described in this paper represent the first evidence for a direct interaction between A22 and MreB. We found that A22 binds nucleotide-free MreB with a Kd of not greater than 1.3 µM. This value is much lower than the concentrations of A22 typically used in cellular assays and the measured minimum inhibitory concentration for A22 and its derivatives (22). This observation is likely due to the competitive binding of A22 and ATP and the high cellular ATP concentration. MreB binds ATP with sub-micromolar affinity (unpublished observations). Therefore in cytoplasm or experiments containing millimolar ATP, A22 must be used at concentrations much greater than its Kd to saturate binding.
Our docking studies suggest that A22 binds with high affinity and specificity in the nucleotide binding pocket of MreB, overlapping with the position that would otherwise be occupied by the beta and gamma phosphates of ATP. The two MreB residues that interact most directly with A22 are E131 and T158. Both residues are conserved among all MreBs, but not in eukaryotic actins or the plasmid-encoded actin ParM. Significantly, the A22-resistance mutation most frequently found in C. crescentus by Gitai, et al. was T176A in C. crescentus, which corresponds to T158 in T. maritima. Thus, the mechanism of resistance in that mutation is likely to be a simple failure of the mutant MreB to bind A22. Viewed in concert, these results explain the failure of A22 to affect eukaryotic actins and ParM.
We observed the direct disruption of MreB assembly by A22 in a variety of biochemical and microscopic assays. By epifluorescence microscopy, we found that A22 inhibits the normal assembly of MreB into large, rigid polymers, producing instead only small, poorly defined clumps of fluorescence. We also observed A22 to inhibit the timecourse of MreB assembly in a bulk light scattering assay, with maximal inhibition at 300 µM A22. Competition with tightly bound ATP accounts for the high maximal inhibition concentration, similar to that observed in cells. At higher concentrations, A22 caused precipitation of MreB. The low-affinity, nonspecific association of A22 to the MreB surface, identified in the docking studies may explain both this precipitation and the amorphous clumps observed by fluorescence microscopy.
Our measurement of the critical concentration for MreB polymerization in the absence and presence of A22 revealed that A22-binding causes a reduced affinity between MreB monomers and polymers. The critical concentration under polymerizing conditions at 20 °C shifted from 500 nM when bound to ATP to approximately 2000 nM when bound to A22. This value is similar to the value of 1700 nM measured for ADP-bound MreB (29). In all assays tested, A22 had no significant effects on MreB in the presence of ADP. We therefore hypothesize that A22-bound MreB is functionally, if not structurally equivalent to the ADP-bound form. Experimental validation of this hypothesis will require atomic structures of both A22- and ADP-bound MreB.
The significance of the critical concentration increase is illustrated by our polymerization timecourse, sedimentation and microscopy results. The timecourse of polymerization of 5 µM MreB was significantly reduced by A22, but not completely inhibited because the effective, polymerizable monomer concentration was effectively reduced from 4.5 µM to ~3 µM. Similarly, in the sedimentation assay, the fraction of MreB polymerized was reduced by A22 by approximately 1.5 uM, the difference between the critical concentrations with and without the drug. These experiments are directly relevant to the physiological effects of A22. Unlike eukaryotic actins, which are present in cytoplasm at concentrations of several hundred micromolar, MreB is present only in the low micromolar range (3). Thus, subtle shifts in the critical concentration can cause complete disassembly of the bacterial actin cytoskeleton. This predicts that the effects of A22 could be overcome by significant increases in the cellular MreB concentration. This is in contrast to the effects of known actin toxins, which either stabilize filaments by binding multiple protomers or by capping filaments or sequestering monomers from the polymerizable pool (32). Because the effects of A22 are physiologically and biochemically subtle, it is possible that derivatives of the molecule may be identified whose structural effects, not only affinities, make them more attractive candidates for development as clinical antibiotics.
Supplementary Material
Acknowledgments
We wish to thank Dr. Milo Westler (NMRFAM) and Ryan Marscheschi for expert technical assistance with saturation transfer NMR experiments and data processing and Joshua Mayer for many helpful discussions.
This work was supported by an American Heart Association Scientist Development Award (#0430162N) and a National Science Foundation Award (#0818946) to K.J.A.; awards by the Searle Scholar Program and 3M Corporation and a Human Frontiers Science Program grant to D.B.W.; and a National Institutes of Health grant (GM067246) to D.S.. This study made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH grants P41RR02301 (BRTP/ NCRR) and P41GM66326 (NIGMS). Additional equipment was purchased with funds from the University of Wisconsin, the NIH (RR02781, RR08438), the NSF (DMB-8415048, OIA-9977486, BIR-9214394), and the USDA.
Abbreviations
- ATP
Adenosine triphosphate
- ADP
Adenosine diphosphate
- GTP
Guanosine triphosphate
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- EGTA
ethylene glycol tetraacetic acid
- A22
S-(3,4-dichlorobenzyl)isothiourea
Footnotes
Supporting Information Available
A22 had no observable effect on the timecourse or sedimentation behavior of MreB in the presence of ADP. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Bramhill D, Thompson CM. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J. Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones LJF, Carballido-Lopez R, Errington J. Control of Cell Shape in Bacteria: Helical, Actin-like Filaments in Bacillus subtilis. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 4.van den Ent F, Amos LA, Lowe J. Prokaryotic origin of the actin cytoskeleton. Nature. 2001;413:39–44. doi: 10.1038/35092500. [DOI] [PubMed] [Google Scholar]
- 5.Ausmees N, Kuhn JR, Jacobs-Wagner C. The Bacterial Cytoskeleton: An Intermediate Filament-Like Function in Cell Shape. Cell. 2003;115:705–713. doi: 10.1016/s0092-8674(03)00935-8. [DOI] [PubMed] [Google Scholar]
- 6.Pogliano J. The bacterial cytoskeleton. Current Opinion in Cell Biology. 2008;20:19–27. doi: 10.1016/j.ceb.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Doi M, Wachi M, Ishino F, Tomioka S, Ito M, Sakagami Y, Suzuki A, Matsuhashi M. Determinations of the DNA sequence of the mreB gene and of the gene products of the mre region that function in formation of the rod shape of Escherichia coli cells. J. Bacteriol. 1988;170:4619–4624. doi: 10.1128/jb.170.10.4619-4624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wachi M, Matsuhashi M. Negative control of cell division by mreB, a gene that functions in determining the rod shape of escherichia coli cells. J. Bacteriol. 1989;171:3123–3127. doi: 10.1128/jb.171.6.3123-3127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kruse T, Moller-Jensen J, Lobner-Olesen A, Gerdes K. Dysfunctional MreB inhibits chromosome segregation in Escherichia coli. EMBO J. 2003;22:5283–5292. doi: 10.1093/emboj/cdg504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soufo HJD, Graumann PL. Actin-like Proteins MreB and Mbl from Bacillus subtilis Are Required for Bipolar Positioning of Replication Origins. Current Biology. 2003;13:1916–1920. doi: 10.1016/j.cub.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Kruse T, Gerdes K. Bacterial DNA segregation by the actin-like MreB protein. Trends in Cell Biology. 2005;15:343–345. doi: 10.1016/j.tcb.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol. 2004;51:1321–1332. doi: 10.1111/j.1365-2958.2003.03936.x. [DOI] [PubMed] [Google Scholar]
- 13.Gitai Z, Dye N, Shapiro L. An actin-like gene can determine cell polarity in bacteria. PNAS. 2004;101:8643–8648. doi: 10.1073/pnas.0402638101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defeu Soufo HJ, Graumann P. Bacillus subtilis actin-like protein MreB influences the positioning of the replication machinery and requires membrane proteins MreC/D and other actin-like proteins for proper localization. BMC Cell Biology. 2005;6:10. doi: 10.1186/1471-2121-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwai N, Nagai K, Wachi M. Novel S-benzylisothiourea compound that induces sperical cells in Escherichia coli probably by acting on a rod-shape-determining protein(s) other than penicilin-binding protein 2. Biosci Biotechnol Biochem. 2002;66:2658–2662. doi: 10.1271/bbb.66.2658. [DOI] [PubMed] [Google Scholar]
- 16.Gitai Z, Dye NA, Reisenauer A, Wachi M, Shapiro L. MreB Actin-Mediated Segregation of a Specific Region of a Bacterial Chromosome. Cell. 2005;120:329–341. doi: 10.1016/j.cell.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Divakaruni AV, Ogorzalek Loo RR, Xie Y, Loo JA, Gober JW. The cell-shape protein MreC interacts with extracytoplasmic proteins including cell wall assembly complexes in Caulobacter crescentus. PNAS. 2005;102:18602–18607. doi: 10.1073/pnas.0507937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dye NA, Pincus Z, Theriot JA, Shapiro L, Gitai Z. Two independent spiral structures control cell shape in Caulobacter. PNAS. 2005;102:18608–18613. doi: 10.1073/pnas.0507708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilsen T, Yan AW, Gale G, Goldberg MB. Presence of multiple sites containing polar material in spherical Escherichia coli cells that lack MreB. J. Bacteriol. 2005;187:6187–6196. doi: 10.1128/JB.187.17.6187-6196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse T, Blagoev B, Lobner-Olesen A, Wachi M, Sasaki K, Iwai N, Mann M, Gerdes K. Actin homolog MreB and RNA polymerase interact and are both required for chromosome segregation in Escherichia coli. Genes Dev. 2006;20:113–124. doi: 10.1101/gad.366606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Divakaruni A, Baida C, White C, Gober JW. The cell shape proteins MreB and MreC control cell morphogenesis by positioning cell wall synthetic complexes. Molecular Microbiology. 2007;66:174–188. doi: 10.1111/j.1365-2958.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 22.Iwai N, Fujii T, Nagura H, Wachi M, Kitazume T. Structure-activity relationship study of the bacterial actin-like protein MreB inhibitors: effects of stubstitution of benzyl group in S-benzylisothiourea. Biosci Biotechnol Biochem. 2007;71:246–248. doi: 10.1271/bbb.60443. [DOI] [PubMed] [Google Scholar]
- 23.Karczmarek A, Martínez-Arteaga R, Alexeeva S, Hansen FG, Vicente M, Nanninga N, den Blaauwen T. DNA and origin region segregation are not affected by the transition from rod to sphere after inhibition of Escherichia coli MreB by A22. Molecular Microbiology. 2007;65:51–63. doi: 10.1111/j.1365-2958.2007.05777.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Trimble MT, Brun YV, Jensen GJ. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 2007;26:4694–4708. doi: 10.1038/sj.emboj.7601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson GT, Doyle TB, Du Q, Duncan L, Mdluli KE, Lynch AS. A novel indole compound that inhibits Pseudomonas aeruginosa growth by targeting MreB is a substrate for MexAB-OprM. J. Bacteriol. 2007;189:6870–6881. doi: 10.1128/JB.00805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava P, Demarre G, Karpova TS, McNally J, Chattoraj DK. Changes in Nucleoid Morphology and Origin Localization upon Inhibition or Alteration of the Actin Homolog, MreB, of Vibrio cholerae. J. Bacteriol. 2007;189:7450–7463. doi: 10.1128/JB.00362-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noguchi N, Yanagimoto K, Nakaminami H, Wakabayashi M, Iwai N, Wachi M, Sasatsu M. Anti-infectious effects of S-benzylisothiourea compound A22, which inhibits the actin-like protein, MreB, in Shigella flexneri. Biological and Pharmaceutical Bulletin. 2008;31:1327. doi: 10.1248/bpb.31.1327. [DOI] [PubMed] [Google Scholar]
- 28.Uehara T, Park JT. Growth of Escherichia coli: Significance of Peptidoglycan Degradation during Elongation and Septation. J. Bacteriol. 2008;190:3914–3922. doi: 10.1128/JB.00207-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bean GJ, Amann KJ. Polymerization Properties of the Thermotoga maritima Actin MreB: Roles of Temperature, Nucleotides, and Ions. Biochemistry. 2008;47:826–835. doi: 10.1021/bi701538e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer M, Meyer B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew. Chem. Int. Ed. 1999;38:1784–1788. doi: 10.1002/(SICI)1521-3773(19990614)38:12<1784::AID-ANIE1784>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart W, Belew RK, Olson AJ. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of Computational Chemistry. 1999;19:1939–1662. [Google Scholar]
- 32.Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nature Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.