Abstract
Medicinal leeches are aquatic predators that inhabit surface waters during daylight and also leave the water where they might be exposed to less screened light. Whereas the leech visual system has been shown to respond to visible light, leeches in the genus Hirudo do not appear to be as negatively phototactic as one might expect in order to avoid potential ultraviolet radiation (UVR)-induced damage. I used high intensity light emitting diodes to test the hypothesis that leeches could detect and specifically avoid near UVR (395–405 nm). Groups of unfed juvenile leeches exhibited a robust negative phototaxis to UVR, but had no behavioral response to blue or red and only a slight negative phototaxis to green and white light. Individual leeches also exhibited a vigorous negative phototaxis to UVR; responding in 100% of trials compared with modest negative responses to visible light (responding in ~8% of the trials). The responses in fed and unfed leeches were comparable for UVR stimuli. The responses depended upon the stimulus site: leeches shortened away from UV light to the head, and extended away from UV light to the tail. Electrophysiological nerve recordings showed that the cephalic eyes responded vigorously to UVR. Additionally, individual leech photoreceptors also showed strong responses to UVR, and a higher-order neuron associated with shortening and rapid behavioral responses, the S-cell, was activated by UVR, on both the head and tail. These results demonstrate that the leech can detect UVR and is able to discriminate behaviorally between UVR and visible light.
KEY WORDS: Hirudo, Invertebrate, Leech, Light, Escape, Ultraviolet radiation
INTRODUCTION
Virtually all animals have evolved neural circuits to detect light and respond to it in adaptive ways. In addition to visible light detected by the human eye, the solar spectrum contains both longer and shorter wavelengths. The shorter wavelengths [ultraviolet B (UV-B); 280–320 nm] are of particular biological interest because they can cause significant damage to nucleic acids and proteins (Rastogi et al., 2010; Sinha and Häder, 2002), although they are heavily attenuated by atmospheric ozone. Near ultraviolet radiation (UV-A; 320–400 nm; referred to here as UVR) is less attenuated, and is therefore the most available at the surface of the earth, now and over evolutionary periods (Blumthaler and Webb, 2003). Many animals have evolved repair mechanisms to deal with damage caused by UVR (Buma et al., 2003) as well as screening pigments or reflective surfaces that offer protection (Banaszak, 2003). Behaviorally, some animals – especially those that also have effective UVR screening – have also evolved neuronal receptors and circuits that use UVR to gather information that is useful in mate selection (Land et al., 2007), foraging (Kevan et al., 2001) and navigation (Tovée, 1995) behaviors.
Aquatic systems have received less attention regarding UVR, until recently. It has become increasingly clear that significant amounts of UVR can penetrate into the water column (Hargreaves, 2003). Thus, UVR is a potentially significant factor for aquatic vertebrates and invertebrates (Boeing et al., 2004; Leech and Johnson, 2003; Reizopoulou et al., 2000; Tank et al., 2003; Vincent and Roy, 1993). Furthermore, other factors, such as dissolved oxygen levels and turbidity affect aquatic UVR attenuation (Osburn and Morris, 2003) and might therefore be correlated with UVR in the environment.
Aquatic predators such as medicinal leeches can be active in surface waters (Sawyer, 1986) where UVR can be high. Additionally, medicinal leeches of the genus Hirudo are amphibious and leave the water to deposit cocoons on land (Sawyer, 1986). Indeed, never-fed juveniles hatch from cocoons on land and must crawl some distance in order to locate the water. This stage of life (juvenile, hatchling leeches) would seem to be particularly vulnerable to UVR-induced damage. Despite this, leeches in the genus Hirudo are only modestly negatively phototactic when tested with artificial sources of visible light (Mann, 1962). This negative phototaxis to visible light is further reduced by feeding status, such that hungry leeches are somewhat positively phototactic, and have been shown to orient toward moving bars of light associated with water waves that might be produced by potential prey (Carlton and McVean, 1993; Dickinson and Lent, 1984; Harley et al., 2011; Harley et al., 2013). Although there have been a number of studies on the visual system of leeches showing sensitivity to visible wavelengths (Kretz et al., 1976; Laverack, 1969; Walz, 1982), as well as studies characterizing some of the interneuronal targets of photoreceptors within the central nervous system (Laverack, 1969; Peterson, 1984b; Peterson, 1985a; Peterson, 1985b), there has been no report of leeches detecting or responding to UVR.
Medicinal leeches have a specialized visual system with cephalic eyes on the head and multiple simple, ‘sensillar’ eyes along the body surface (Kretz et al., 1976). There are five pairs of pigmented eyecups along the anterior margin of the anterior sucker (Fig. 1). Each of these cephalic eyes contains approximately 50 individual photoreceptors, and the axons of these are projected in bundles that join four cephalic nerves (Kretz et al., 1976). In addition to these cephalic eyes, each midbody segment has seven pairs of small sensilla distributed dorsal to ventral along the central annulus (Kretz et al., 1976). These segmental sensilla contain hair cells used for detection of water wave and other vibrations, as well as photoreceptor cells (Friesen, 1981; Derosa and Friesen, 1981). The sensillar photoreceptors are similar to the cephalic ones in their responses to visible light (Kretz et al., 1976). Leech photoreceptors are phaosomal primary receptor neurons that depolarize in response to light and produce action potentials that are conveyed into the central nervous system (CNS) (Fioravanti and Fuortes, 1972; Lasansky and Fuortes, 1969; Peterson, 1984a). Axons from the sensilla have also been shown to segregate into discrete and highly stereotyped fascicles in the CNS that might correspond to different sensory modalities (Jellies et al., 1994). The cephalic eyes in the head appear to replace several of the segmental sensilla, some of which persist (Mann, 1962; Sawyer, 1986) and may contribute to the activity recorded from nerves. The sensilla in the head region have not yet been characterized, but have been assumed homologous to the segmental sensilla (Sawyer, 1986). Although the opsins in Hirudo have yet to be described, the glossiphoniid leech Helobdella has recently been shown to express multiple opsins consistent with a rhabdomeric origin (Döring et al., 2013). The spectral sensitivities of these opsins have not yet been determined.
Fig. 1.

Medicinal leeches are segmented annelid worms. These worms have suckers at anterior and posterior ends of the body, which they use to attach to their substrate, and five pairs of pigmented cephalic eyes (marked 1–5 on the inset). In addition to the pigmented cephalic eyes there are multiple smaller sensilla in each segment that contain photoreceptor neurons (not shown). The response to visible light for both cephalic eyes and sensilla has been previously described (Kretz et al., 1976).
In this study, I assessed the ability of medicinal leeches to detect and respond to near UVR, and determined whether the responses were directed. I also examined whether known visual pathways and receptor neurons in the leech could be activated by UVR. Additionally, I tested the influence of UVR on a higher-order interneuron known to receive visible light input and to be active during rapid shortening.
List of abbreviations
- APW
artificial pond water
- CNS
central nervous system
- LED
light emitting diode
- LSD
least significant difference
- ND
neutral density
- OD
optical density
- UV
ultraviolet
- UVR
ultraviolet radiation
RESULTS
Group trials can be effective ways of examining avoidance behaviors (Tully and Quinn, 1985). In the present studies, subject groups of small, unfed leeches were exposed to a spot of light from different light emitting diodes (LED) positioned over one quadrant of a transparent dish. Preliminary experiments showed that responses, if they were going to occur at all, would begin within a few seconds, so that a total exposure time of 60 s would easily capture all responses.
Leeches showed a negative phototactic response to near UVR
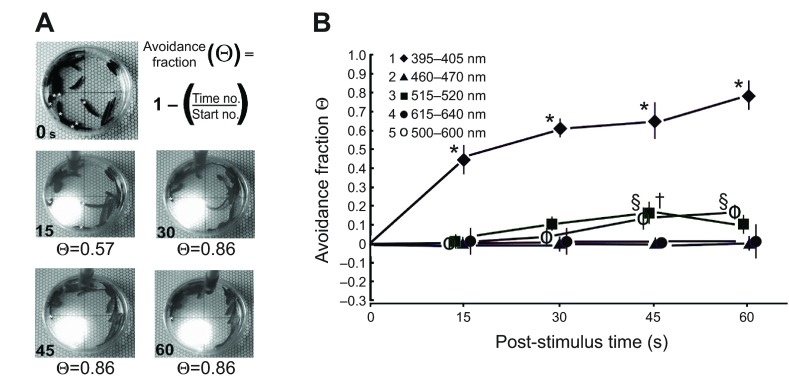
The clearest and most robust response was a negative phototactic reaction to UVR (Fig. 2A). When UVR was shone onto a quadrant, leeches began moving within a few seconds, and by 15 s most of the quadrant was clear of leeches. The avoidance fraction (Θ) quantified the fraction of individuals that left the quadrant. It appeared that – regardless of whether the leech was entering or exiting the quadrant, was stationary or was swimming or crawling – exposure to UVR evoked a change in movement. When the results of all wavelengths were compared across time, it was clear that UVR evoked a robust response (Fig. 2B).
Fig. 2.
Leeches exhibited strong UVR avoidance or escape. (A) For illustration still shots were captured from one trial of UVR exposure. The top left panel shows the distribution of leeches at time 0. Asterisks indicate individuals counted as being in the test quadrant (seven in this example). The avoidance fraction, Θ, allowed for comparisons to be made across time and wavelength. At time 15 s there were three individuals in the quadrant, but only a single individual remained at times 30, 45 and 60 s in this example. (B) The results of subject/group avoidance trials plotted as Θ against time for each of the wavelengths tested (legend shows symbols associated with each wavelength range tested). Sample values of Θ were calculated at 15 s time points. Positive Θ values indicate an avoidance or escape from the test quadrant such that there were fewer individuals at the test time than at time 0. Error bars show ± s.e.m. A two-way repeated measures ANOVA revealed an effect of wavelength (P≤0.001), but not time. Post hoc analysis revealed a significant difference between means, *P≤0.001, §P≤0.005, †P≤0.005.
The two-way repeated measures ANOVA confirmed an effect of wavelength (P≤0.001) but no significant effect was associated with time, or with the interaction of wavelength and time. Post hoc analysis (Fisher's least significant difference; LSD) was conducted to determine which means were likely to be different from each other. Neither red nor blue light evoked a response within the 60 s trial. As expected from inspection, the UVR responses were different from all other wavelengths at all times, except 0 (*P≤0.001) but were not different from each other across time. At 45 s, both green (†) and white (§) light responses were different from red and blue (P≤0.005) and at 60 s, the white light response remained significantly different (P≤0.005). In these group trials it would be possible to miss the behavior of individual leeches, for example if as many individuals entered and remained in the quadrant as left it and stayed out. These experiments assume that such mixing would be random rather than selective. There can be interactions between positive thigmotaxis, negative phototaxis and social relations when groups of leeches are confined (Bisson and Torre, 2011). Although this issue could be investigated further, in the present studies UVR responses appeared to overcome any influences that might keep leeches clumped together over the short time course studied.
Individuals showed a negative phototaxis to different wavelengths of light as predicted by group trials
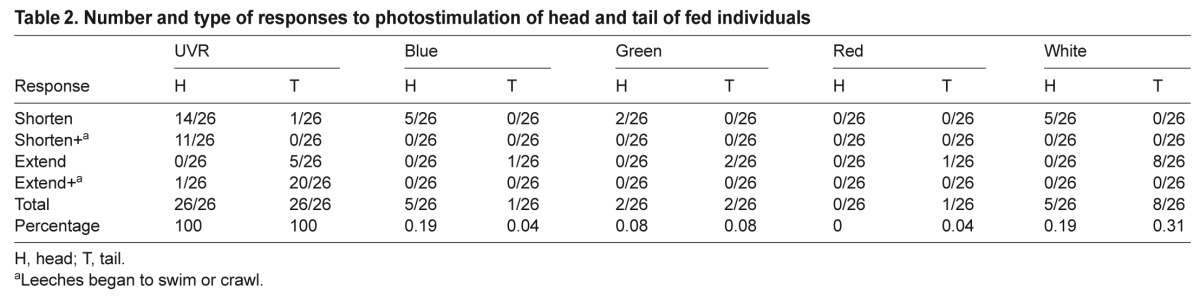
To gain insight into how individual leeches responded to light, and to examine the response as a function of feeding status or age, individual untethered leeches were video-recorded during exposure to different wavelengths. The responses to different wavelengths generally paralleled those of the subject group tests for both younger unfed (Table 1) and older and fed (Table 2) individuals. In both sets of nine individuals across all trials, UVR stimulation resulted in a response in 100% of trials regardless of whether the head or tail was stimulated. In comparison, across all individuals, visible light evoked responses in only approximately 4% of the trials where light was applied to the head, and 11–12% of the trials where light was applied to the tail (~8% overall for head and tail presentations combined). The response frequencies for visible light were slightly larger in the fed group. For the unfed group there were 27 total trials (three each, in each of nine individuals) for each wavelength, making a total of 270 responses (27 trials at the head, 27 at the tail for a total of 54 for each of five wavelengths tested). For the fed group, a single trial was missed for head stimulation in one individual, and one trial was missed during tail stimulation in a second individual, so the total number of trials for that set was 26.
Table 1.
Number and type of responses to photostimulation of head and tail of unfed individuals
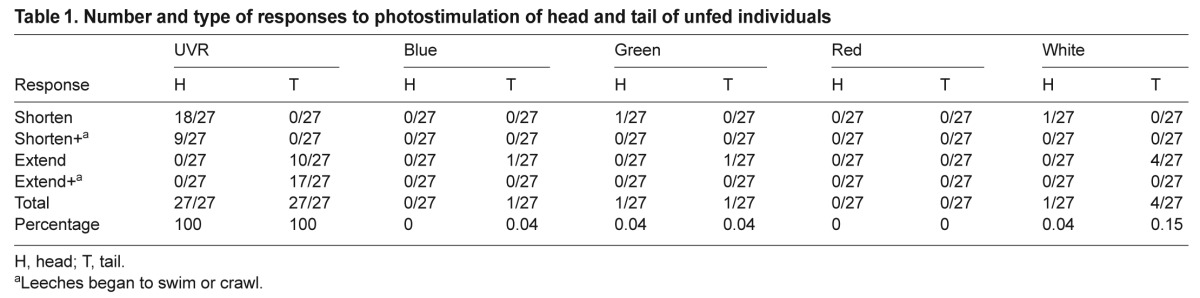
Table 2.
Number and type of responses to photostimulation of head and tail of fed individuals
UVR-evoked avoidance behavior involved different responses depending on whether the head or tail was stimulated
Inspection of the subject group videos revealed that individuals seemed to retract from the quadrant if they entered it with their head, and extend out of it if they were already in it. It was therefore expected that individual leeches would respond to visual stimuli at both head and tail.
The responses to UVR were comparable in both fed and unfed leeches. Individual leeches withdrew the whole body away from the UVR in 100% of the trials in both groups. A set of typical responses is shown in Fig. 3. When the spot of light was directed at the head of a quiescent individual, there was release of the anterior sucker followed by a shortening of the anterior portion of the leech. In this example, the response began within 2 s (Fig. 3E, asterisk). A somewhat different response was seen when stimuli were presented to the tail (Fig. 3, lower panels). The first sign of a response was release of the anterior sucker (Fig. 3P, asterisk; at 2.5 s in this example), but this response was almost always followed by extension of the head away from the stimulus. Then there was reattachment of the anterior sucker followed by release of the posterior sucker and subsequent contraction of the posterior portion of the body. In response to both head and tail presentations, the individual often crawled or swam for many minutes following UVR exposure (shorten+ and extend+ in Tables 1 and 2). In one fed individual that was quiescent but in an already extended state, UVR stimulation of the tail caused it to release the tail sucker and rapidly contract its posterior end; this behavior was scored as a ‘shorten’ response (Table 2). In a different fed individual that was quiescent in a relatively shortened state, head stimulation with UVR caused a rapid extension laterally away from the light; this behavior was scored as an ‘extend’ response (Table 2).
Fig. 3.
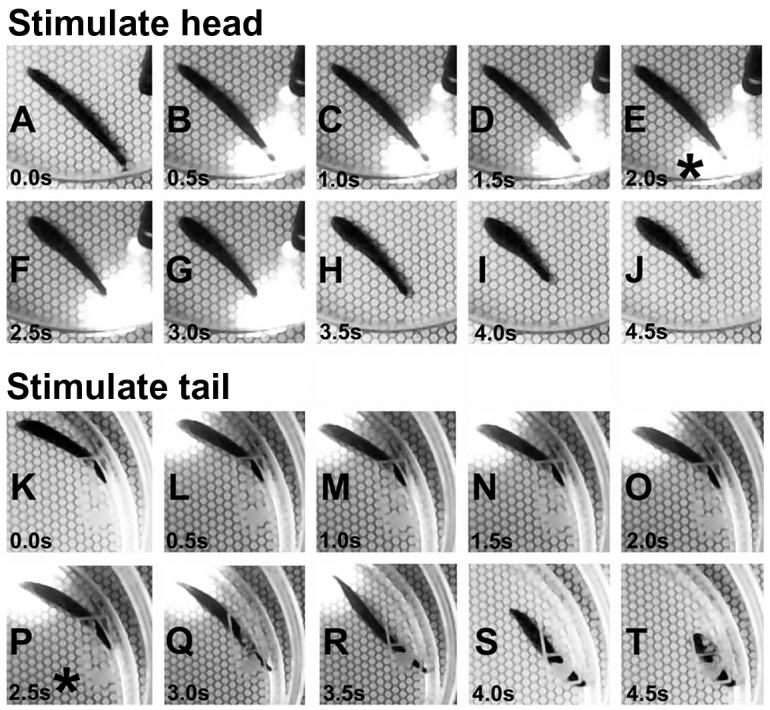
Individuals removed themselves from UVR by shortening, or by extending and then shortening, depending upon stimulus location. Screen shots from the digital videos were grabbed every 0.5 s from a UVR trial involving head stimulation (A–J) and tail stimulation (K–T). Within 2 s of head stimulation with UVR (E,*), the anterior sucker released and shortening was initiated. When the same stimulus was applied to the tail, the anterior sucker again released at approximately 2.5 s (P,*) but this response was immediately followed by extension of the anterior body. Within a few more seconds the tail sucker released and the posterior end shortened away from the stimulus (S,T).
Cephalic nerves responded to near UVR stimulation
Photoreceptor action potentials can be detected by recording from the cephalic nerves. Individual light-evoked spikes are small and overlap with each other to produce complex waveforms that cannot be resolved as individual action potentials (Kretz et al., 1976). The responses may also contain activity from uncharacterized sensilla in the head. Thus, whereas it is possible to demonstrate responses of the eyes to light by nerve recording, it is not possible to determine how many photoreceptors are responding to any given stimulus.
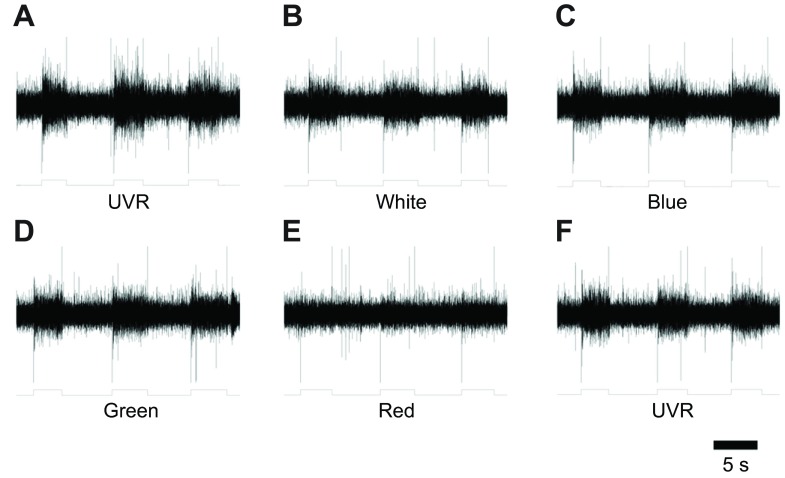
In each of the recordings from the bilaterally paired dorsal-A (DA) and -B (DB) nerves in each of three individual preparations, very similar responses were seen. A representative set of responses is illustrated in Fig. 4. Responses from six DB and five DA nerves were sampled (a single DA nerve was damaged during dissection and not included). Light was presented for 3–5 s with 5–6 s between each of three stimuli at the same wavelength. The different wavelengths were presented in the order: UV, white, blue, green, red and UV, with a period of 10–15 s between each one. No nerve responses to red light were seen (Fig. 4E). The eyes associated with DA and DB exhibited strong responses to UVR (Fig. 4A,F) as well as to white, blue and green light (Fig. 4B–D). In a single case, the smaller DC cephalic nerve was tested and yielded similar responses to light, but no further assessment of different cephalic nerves was made in the present study.
Fig. 4.
Groups of photoreceptors responded to UVR and a range of visible wavelengths. A suction electrode was placed upon the cut end of the DB cephalic nerve that had been severed from the supra-esophageal ganglion. Light from the LED wands was directed at the head and held by hand for a few seconds. Massed photoreceptor responses were complex and slowly adapting as expected (Kretz et al., 1976). The output from a phototransistor (shown below each trace) indicates the timing of the light stimulus and all were delivered at saturating intensities. In each record there was also an instantaneous transient associated with turning the light on (downward) and off (upward). (A) Response to UVR. (B) Response to white light. (C) Response to blue light. (D) Response to green light. (E) Response to red light. (F) Second exposure response to UVR.
Individual photoreceptor neurons responded to near UVR stimulation
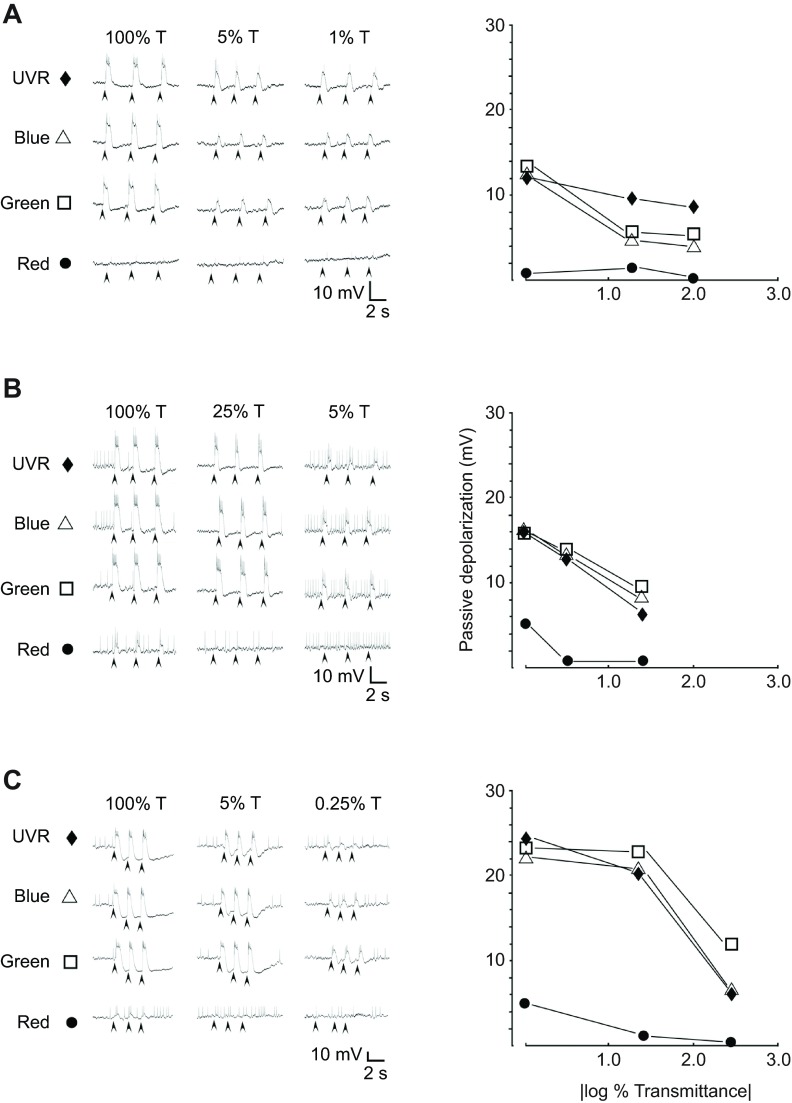
Over 50 individual photoreceptors were sampled from eyes 1–4 and the group exhibited a wide range of response properties, including some that were phasic and others that were tonic. All receptors examined responded to UV, blue and green wavelengths to different extents, and a number also showed weaker responses to the red LED. All but a few cells exhibited action potentials. Qualitatively it appeared that none of the photoreceptors examined so far was narrowly tuned to any of the LED wavelength ranges. Responses from three cells that represent those examined are illustrated in Fig. 5. In all cases, light caused a rapid depolarization. Response parameters such as the time the response lasted beyond the stimulus, the magnitude and duration of the after-hyperpolarization, and the frequency of action potentials evoked, was variable in different cells. By examining the maximal passive depolarization produced, it was possible to compare the responses to different wavelengths across a small range of luminosity produced using neutral density (ND) filters. One neuron (Fig. 5A) appeared slightly more responsive to UVR than blue or green wavelengths, and not at all responsive to red, whereas another (Fig. 5B) was about equally responsive to blue, green and UV wavelengths, and slightly responsive to red. A third (Fig. 5C) was slightly more responsive to green than either blue or UV and slightly responsive to red wavelengths. Thus, although it appears possible that some receptor cells responding to UVR were different from those for visible light, narrowly tuned cells were not found in this brief survey. Rather it was observed that all cephalic photoreceptors examined so far responded to UVR over physiologically relevant ranges of luminosity, similar to the response to visible light.
Fig. 5.
Individual primary photoreceptor neurons responded to ultraviolet and visible light. Activity from each of three different primary photoreceptor neurons from eye 2 (A), eye 3 (B) and eye 2 (C) from three different leeches recorded using a sharp intracellular electrode. The resting potentials in these three examples were −35 to −40 mV. Arrowheads indicate stimulus onset. In all cells (including the three shown here), there were strong responses to UVR. (A) In the neuron shown here there was no response to red light at any luminosity, and apparently equivalent responses to blue, green and UV light at the highest luminosity. As luminosity decreased, the response to UVR remained more robust than the others. (B) In the neuron shown here there was a response to red at 0 OD (|log 100% transmittance|), but the response quickly fell off with decreased luminosity. At 0 OD the cell seemed to have an equal response to blue, green and UV wavelengths, and this response decreased with decreased luminosity. (C) In the neuron shown here there was a response to red light similar to that in the receptor shown in B. Yet, with decreased luminosity the response to green remained more robust than to either blue or UV wavelengths.
A higher-order interneuron responded to UVR
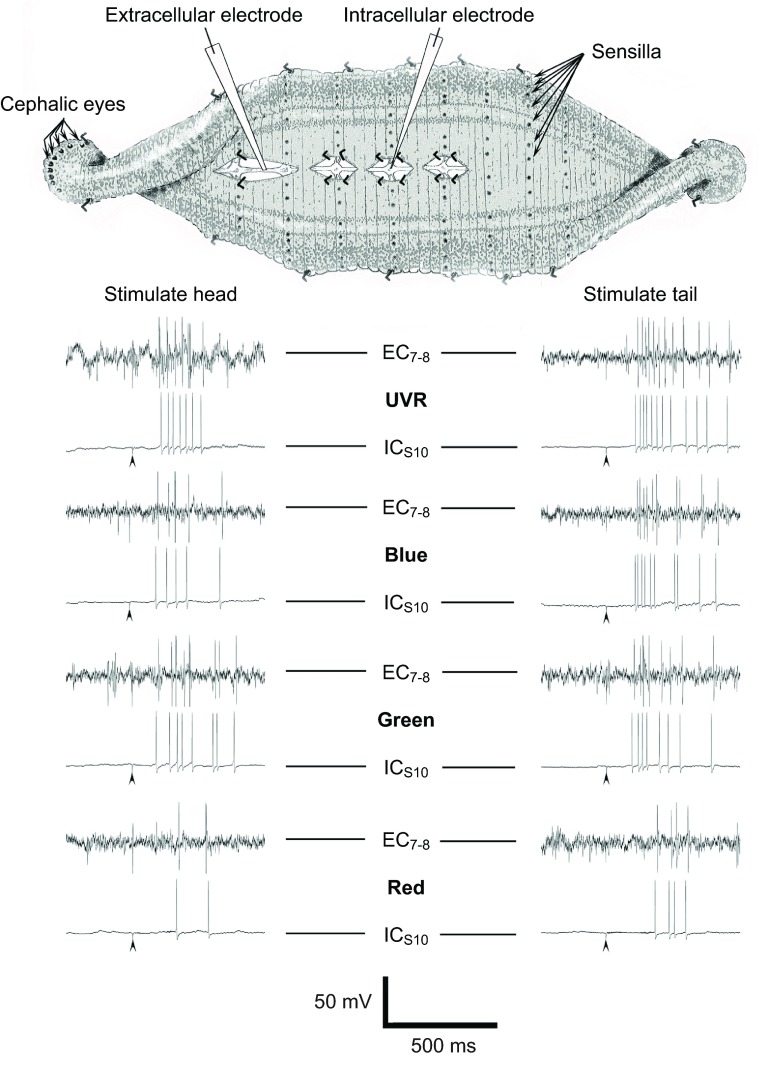
The observation that light stimuli on the tail can evoke an escape response suggested that sensilla also responded to UVR. Kretz et al. demonstrated that the segmental receptors have very similar response properties to the cephalic eyes, but UVR was not tested in those earlier studies (Kretz et al., 1976). A semi-intact preparation with neuronal somata impaled in midbody ganglion 9, 10 or 11 was used to test neuronal responses to light stimuli applied at the head or tail (Fig. 6). In two experimental preparations each of the major mechanosensory neurons (T, P and N) was impaled, as was the heart excitor (HE) motor neuron (Muller et al., 1981). No responses were obtained from these neurons to any wavelength range used here (not shown). However, when the S-cell was examined, it showed strong responses. As expected (Kretz et al., 1976; Sahley et al., 1994) green and blue light evoked bursts of action potentials that adapted rapidly (Fig. 6), and the latency from stimulus onset to the first action potential was short, approximately 100–200 ms. The recording electrode was 2–3 cm from the head and tail. This very short delay was consistent with photo-activation of the S-cell fast-conducting pathway (Kretz et al., 1976). There was more limited and variable S-cell response to red light (Fig. 6). The latency of S-cell spikes in response to red light, when they occurred, was also longer (example shown in Fig. 6).
Fig. 6.
The S-cell, an interneuron of the fast conducting pathway, responded vigorously and with short latency to light stimulation at both the head and the tail. A semi-intact preparation was used to evaluate neuronal responses. It conserved continuity of the CNS and all major nerves from head to tail. See Materials and methods for a detailed description. Representative S-cell intracellular (ICS10) and extracellular (EC7-8) voltage recordings are shown. Arrowheads indicate stimulus onset. The S-cell was impaled in midbody ganglion 10. Ganglia 9 and 11 were routinely exposed for impalement, if necessary. The resting potential was approximately −50 mV and the cell was sporadically active, but generally silent until stimulated with light. The S-cell produced short latency action potentials in response to stimulation using UV, blue and green LEDs. In those instances where the red LED evoked responses, these were longer latency from stimulus onset. These S-cell spikes were always distinguishable in the extracellular (EC) recordings as the largest multiphasic spike that could be related 1:1 with the intracellular (IC) action potential.
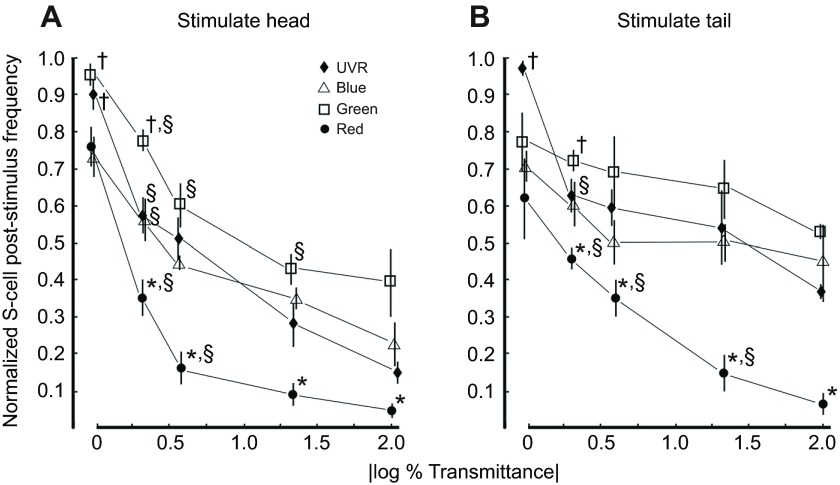
The S-cell responded vigorously to stimulation by UVR (Fig. 6) presented to the head or tail, in all leeches examined. All latencies from UVR stimulus onset to first action potential were in the 100–200 ms range, similar to those observed for responses to blue and green light. To determine whether the S-cell responses to UVR were consistent with expectations for a physiologically relevant stimulus, responses at both head and tail were compared quantitatively across red, green, blue and UV light, also across a range of luminosity (Fig. 7). The repeated measures ANOVA revealed a significant effect for both color of light and optical density (OD; P≤0.001). Post hoc analysis (Fisher's LSD) was conducted to determine which means were different from each other. The most effective stimuli applied to the head (Fig. 7A) were from the green and UV LEDs. They each evoked a comparable S-cell response at full strength (0 OD) and these responses were greater than that evoked by blue or red light (†P≤0.005). At 0.3 OD, the most effective stimulus at the head was the green LED (†P≤0.005). For tail stimuli (Fig. 7B), the most effective stimulus at 0 OD was UVR (†P≤0.005) whereas with the 0.3 OD filter the green was the most effective stimulus (†P≤0.005). Red stimuli were the least effective at both head and tail, being significantly weaker than all other wavelengths, at all ODs other than 0 (*P≤0.001). As might be expected, in many cases for a given color, there was also a reduction in response from one OD filter to the next (§P≤0.005).
Fig. 7.
The S-cell responded strongly, but differentially to visible and UVR stimuli presented to both the head and tail. (A) Light stimuli presented to the head evoked strong responses from the S-cell, with the most robust response evoked equally by green and UV wavelengths. With decreased luminosity, plotted as OD=|log % transmittance|, the response fell off rapidly but differentially. Green light remained an effective stimulus with slightly decreased luminosity (0.3 OD), whereas all others decreased significantly. The response to red light attenuated most readily with decreased luminosity. (B) Light stimuli presented to the tail were also able to evoke strong S-cell responses. At 0 |log % transmittance| the UVR clearly evoked the most robust and consistent response, but as with the head stimuli the UVR response attenuated with the initial decrease in luminosity more so than did the response to the green light. The response to red light attenuated as it did for head stimulation. The responses to UV, blue and green wavelength stimulation on the tail all remained higher across decreased luminosities compared with the same stimuli presented at the head over the same range of |log % transmittance|. A two-way repeated-measures ANOVA revealed an effect of wavelength P≤0.001, and OD P≤0.001. Post hoc analysis revealed a significant difference between means at a given OD, *P≤0.001, †P≤0.005 as well as within wavelength between OD §P≤0.005.
DISCUSSION
These results show that medicinal leeches have a robust and coordinated negative phototactic response to UVR; this behavior removed a leech from UVR regardless of where on the body the stimulus was applied. In addition, I found that cephalic eyes, individual photoreceptor neurons and a higher-order neuron (the S-cell) are all activated by UVR. The responses observed to visible light were similar to those in previous reports (Kretz et al., 1976).
In an extensive study of leech visual cells, Kretz et al. determined that the midrange peak of spectral sensitivity of both cephalic and sensillar photoreceptors is 540 nm (green) with substantial neuronal responses between 500 and 600 nm (Kretz et al., 1976). They also showed that the sensory cells have a lower (but not zero) response to violet and red light. The shortest wavelength tested was 430 nm (Kretz et al., 1976). Leeches appear to be insensitive to near infrared (IR) (Harley et al., 2011) although they can detect and respond to heat (Dickinson and Lent, 1984). I confirmed that IR LEDs with emission peaks at approximately 850 nm evoked no behavioral or neuronal response (J.J., unpublished data). Leeches also use the visible spectrum to orient while swimming, and they respond to shadows, or brief, bright flashes (Kretz et al., 1976). In addition, leeches orient to moving bars of visible light when hungry (Dickinson and Lent, 1984; Carlton and McVean, 1993), and a recent set of elegant experiments have shown that leeches use frequency-modulated (moving bars) visible light in combination with tactile vibration (both of which are related to water disturbances) to orient and locate potential prey (Harley et al., 2011; Harley et al., 2013). Thus, it might be suggested that hirudiniid leeches are not particularly averse to visible light, and under some conditions may use it as an appetitive cue.
Leeches responded to UVR
Leeches were exposed to light both individually and in groups, to examine their untethered responses. As expected, few or muted behavioral responses were found in response to visible light, especially red or blue LEDs. In contrast, robust avoidance or escape responses were evoked when the leeches were exposed to UVR. These UVR responses occurred in 100% of the trials, in both grouped (Fig. 2) and individual animals as well as in unfed and recently fed individuals (Fig. 3, Tables 1, 2).
I included the group of fed individuals expecting that they might show a different response to UVR than the unfed leeches. It has been well established that satiety strongly influences sensory responses (Gaudry and Kristan, 2009) and the probability of evoking certain behaviors, such as swimming or crawling (Gaudry and Kristan, 2010; Misell et al., 1998). Many of the interactions influencing motivation, behavioral choice and responses to stimuli are regulated by amines (Crisp and Mesce, 2006; Esch et al., 2002; Kristan and Nusbaum, 1982-1983; Puhl and Mesce, 2008) and these transmitter systems strongly interact with feeding behavior, swimming and crawling (Brodfuehrer and Friesen, 1984; Lent and Dickinson, 1984; Willard, 1981). Since unfed individuals were expected to be less averse to visible light than fed (consistent with the results for visible light in these studies as well; see Tables 1, 2), I expected that unfed individuals might not exhibit as strong a negative phototaxis to UVR. Instead, the frequency of responses to UVR of unfed leeches was the same (100%) as those of fed leeches.
UVR-evoked behavior involved distinct motor actions integrated to produce avoidance or escape
The responses to UVR were complex and directed. When the head was exposed to UVR, withdrawal of the head by body shortening occurred, whereas when the tail was exposed to UVR, the leeches first extended the head (which involves an opposite set of motor responses) and then released the tail sucker to withdraw from the light. Thus, stimuli detected by the cephalic eyes and possibly the anterior sensilla must activate somewhat different CNS circuits than stimuli transduced by the segmental sensilla of the body wall and tail (Fig. 3). Individual sensilla were not directly examined in this work, but the response of leeches to light shone upon the tail (Fig. 3) as well as the S-cell responses (Figs 6, 7) suggests they are sensitive to UVR. The leech responded in whatever fashion was needed to remove it from the UVR, from whatever starting condition it was in. This result implies a high degree of integration of external and proprioceptive inputs similar to that described for the decision to terminate behaviors, swim or crawl (Mesce and Pierce-Shimomura, 2010).
Rapid withdrawal has been extensively studied in oligochaetes (Drewes and Fourtner, 1989; Zoran and Drewes, 1987; Zoran et al., 1988). A similar behavior has been well documented in leeches when stimulation is applied to the anterior body (Magni and Pelligrino, 1978). When tactile stimuli or electric shocks are applied to the anterior portion of the leech, it shortens rapidly (Kristan et al., 1982; Shaw and Kristan, 1995). Although the S-cell of the fast-conducting pathway is activated during shortening (Shaw and Kristan, 1995), and is required for sensitization of the response (Sahley et al., 1994), it is neither necessary nor sufficient for expression of the behavior (Shaw and Kristan, 1999; Arisi et al., 2001). Rather, there is a parallel network of interneurons that appears to underlie the whole-body shortening (Shaw and Kristan, 1999; Arisi et al., 2001). In the current studies, the S-cell was strongly activated by UVR exposure, confirming that higher-order interneurons involved in rapid movements can be influenced by UVR (Figs 6, 7), but not revealing the details of how UVR evokes escape.
The light-activated circuitry that underlies differential behavioral responses depending upon stimulus location remains to be characterized. The S-cell responses to light at the head (Fig. 7A) and tail (Fig. 7B) are possibly involved because they appear to be asymmetric. For example, when the responses to UV, blue and green wavelengths were examined across luminosity, the overall response to tail stimulation seemed shifted upward (Fig. 7). If tail stimulation evoked a more robust response generally, this would be consistent with the behavioral responses seen in individual leeches (Tables 1, 2) where visible light to the tail evoked a response approximately 12% of the time compared with only 4% when presented at the head. It remains to be determined whether these behavioral and neural responses might be the result of different receptor properties in different locations, or different degrees of convergence of multiple, widely spaced sensillar inputs, or both.
UVR-activated photoreceptors
Cephalic nerve recordings revealed that the photoreceptors of eyes 1 and 2 exhibited a vigorous and sustained response to UVR, one that was comparable to that produced by blue, green and white light LEDs (Fig. 4A–D,F), whereas no response was detected during illumination by the red LED (Fig. 4E). To directly assess how UVR was being detected, I also recorded from a selection of individual primary photoreceptor neurons while stimulating them with light. The responses of individual neurons (Fig. 5) clearly showed that the leech visual system has the ability to effectively transduce UVR and convey that information to the CNS. The S-cell responses to head stimuli were equally sensitive to UVR and green light delivered full strength (Fig. 7A). This would be consistent with responses that have two peaks, the expected one in the green region of the spectrum and an additional peak for UVR. The responses for tail stimulation were consistent with this as well, with maximal sensitivity to UVR at the highest intensity that fell off with decreased luminosity (Fig. 7B) followed by high sensitivity to green wavelengths.
In some way, yet to be determined, the leech is able to distinguish between UV and visible light because it can generate differential behavioral responses to them. Many animals use oil droplets or screening pigments (Honkavaara et al., 2002; Jacobs, 1992) in order to generate UV-tuned neural inputs. UV-selective photopigments and discrete UVR receptor cells are also well known in both vertebrates (Jacobs, 1992) and invertebrates (Salcedo et al., 2003). For example, Daphnia has been shown to have individual ommatidia sensitive to UVR (Smith and Macagno, 1990) and exhibits adaptive responses, including avoidance of ambient UVR (Leech and Williamson, 2001), and Drosophila has a well-described UVR sense mediated by a subset of photoreceptors in the ommatidia (Earl and Britt, 2006; Yamaguchi et al., 2010). It remains to be determined how leeches transduce the UVR and generate discrete inputs for UV and visible light. For example, are there different photopigments that might be expressed in different amounts in different cells or is there a single photopigment with multiple absorption peaks? Recent work with the leech Helobdella has revealed multiple forms of opsin gene expression (Döring et al., 2013). Perhaps there are also multiple opsins in Hirudo. This remains to be determined. Do all receptors exhibit absorption peaks in the UV and green regions? Are there, as yet, undiscovered receptor cells that are tightly tuned to UVR or is the UVR response encoded by populations of receptors with the peak response biased toward UVR? The present results cannot distinguish these possibilities.
These studies show for the first time that medicinal leeches have a discrete, robust and coordinated avoidance and/or escape response to UVR. This response was even more reliably evoked than responses to visible light. Therefore it is probable that the negative phototactic response to UVR would effectively permit leeches to avoid environments with high UVR exposure, or to escape from potentially threatening conditions in the natural environment. Differential UVR penetrance in natural aquatic environments may be a previously under-appreciated factor in the distribution of leeches and may also play an important role in the natural behavior of the animals. Leeches respond vigorously to UVR in the laboratory, but it remains to be determined how UVR is transduced by the photoreceptors and integrated by the nervous system for adaptive behavior.
MATERIALS AND METHODS
Preparations
Medicinal leeches, Hirudo spp. (Siddall et al., 2007), originally obtained from Leeches USA (Westbury, NY, USA) and Niagara Leeches (Cheyenne, WY, USA), were maintained in a breeding colony at room temperature (20°C). Animals were released from their cocoons at ≥30 days and were kept in 60×15 mm Petri dishes with artificial pond water [APW: 0.05% (w/v) Instant Ocean sea salt (Spectrum Brands Inc., Madison, WI, USA) diluted in spring water]. Both unfed animals (109 individuals from 13 sibling cohorts) at 75–90 days of age (juvenile) and twice-fed animals (nine individuals) 6- to 9-months old were used in this study. The unfed juveniles weighed between 30 and 50 mg, fed leeches weighed between 0.45 and 0.8 g.
For group trials, 100 juveniles were divided into five groups of 20 unfed individuals that were selected by combining five individuals from each of four different sibling groups (four different cocoons/group). A total of 10 cocoons were used to establish these five groups, which were then treated as five subjects for testing. Note that individual cocoons have variable numbers of siblings.
The nine juvenile unfed leeches observed individually were selected from a different set of three cocoons that had been kept in individual dishes, and three individuals were selected from each cohort. The individuals selected were kept in separate dishes between trials. The nine fed leeches were older and were selected at random from a group of approximately 200 in the breeding colony. Both subject/groups and individuals were housed in individual 60×15 mm Falcon plastic Petri dishes at room temperature.
Behavioral arena
Untethered leeches were exposed to different conditions in an isolated room with diffuse background lighting maintained by directing a desk lamp with two 15 W fluorescent tubes toward the wall pointing away from the testing area. This resulted in a dim background illumination measured as 5–10 lx at the bench surface (LuxMeterPro iPad 2 application). A test surface was prepared by printing a hexagonal graph paper array on white paper, each unit being 1.5 mm in diameter. A Vivitar digital video camera (508NHD) was used to record all trials using the highest sensitivity and an effective ASA of 400. The video camera was mounted on a small tripod 30 cm above the test surface. Videos were reviewed frame by frame using QuickTime 7 on a MacBook Pro laptop (Apple, Cupertino, CA, USA). To generate the figures, individual screen shots were grabbed at 0.5 s or 5 s intervals and cropped in Photoshop CS3 (Adobe Systems Inc., San Jose, CA, USA). Images were globally adjusted to balance brightness and contrast and converted to gray scale.
LED stimuli
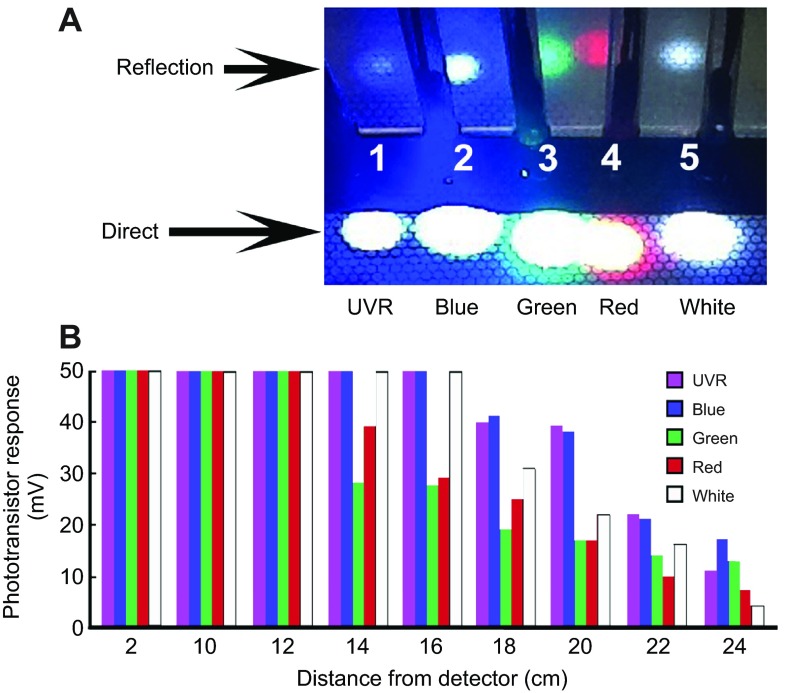
Hand-held wands directed the light from LEDs to the test surface. Each LED was a T-1 3/4 package with a clear, round lens and a viewing angle of 20–25 deg. The five different nominal wavelengths of the LEDs were: UV (395–405 nm), blue (460–470 nm), green (515–520 nm), red (615–640 nm) and white (500–600 nm). The UV (referred to as UVR), red and white LEDs were obtained from Dave's LED Hut (through eBay Store, New Mexico) and the blue and green LEDs were obtained from Longxi Electronics (through eBay store, Shenzen, Chongqing, China). UVR LEDs from three other sources were tested on unfed juveniles and on the semi-intact preparation (not shown) including one with emission at 395–405 nm, one at 380–385 nm and one at 360–365 nm; all of them evoked behavioral and neuronal responses similar to the UVR LED used to acquire the data reported here. Given small variations in forward LED voltage, 9 V through a 300 Ω resistor yielded the following nominal current driving each LED: UVR 19 mA, blue 19 mA, green 19 mA, red 21 mA and white 19 mA. The LEDs all produced spots of light (Fig. 8A) that saturated the camera. In an effort to measure the approximate strengths of stimuli from all five LEDs, their projected spots and reflected images were photographed (Fig. 8A) and each emission was assessed using a phototransistor coupled to a 2 mm diameter fiber optic to gather light. The phototransistor was arranged to gate current through a fixed resistance (Jellies and Kueh, 2012). The relative measures of phototransistor output were a function of several factors including the LED viewing angles and intensity as well as the phototransistor response sensitivity. These measures of response showed that the emissions from all the LEDs were roughly equal (Fig. 8B).
Fig. 8.
LED stimuli were comparably strong over the projected illumination areas. (A) Each wand was held in a plastic bar with a hole drilled through it and pointed downward, 2–3 cm above the test surface to photograph the emission spots. The spots were saturated in the digital image so a perpendicular plastic surface was placed behind the projected spots to simultaneously view the reflected images of each spot, but at a greatly reduced luminance. (B) To examine relative stimulus strength and confirm that no LED was exceptionally strong or weak compared to the others, each was pointed directly at the 2 mm diameter fiber optic face of a transducer coupled to the output of a general IR phototransistor (Jellies and Kueh, 2012). Each spot was then moved away from the transducer by a fixed amount. All wavelengths produced saturating responses out to 12 cm. At 14 cm, the responses to red and green wavelengths began to fall off, then at 18 cm the responses to UV, blue and white light began to fall off.
Group trials
For group trials, a quadrant was drawn on the test surface. Each subject group was placed on top of the quadrant-marked surface. The plastic lids were left on for these studies to keep the animals inside the dishes. The leeches were allowed to settle for approximately 10 min, when most animals were quiescent. An LED was held above a quadrant that was arbitrarily selected with at least four individuals in it (to avoid starting conditions with 0), and then the LED was turned on. The range of starting numbers of leeches in a quadrant was four to 12. The distance was predetermined to be that necessary to center the beam of illumination on the quadrant (3–4 cm) and minimize visible illumination of adjacent quadrants (see Fig. 2 for an example). The light was held in place for at least 60 s, as a video recording was taken. Between trials, the animals were gently stirred with a plastic pipette to detach them from the substrate; they were then allowed to settle, which typically took 2–5 min and never longer than 30 min. The next stimulus was then presented using a different wavelength.
An online random sequence generator (random.org) produced five random sequences that determined the order of presentation of wavelengths. Each group received three repeated trials of all five wavelengths. Time 0 was defined as just before illumination and the number of individuals in the test quadrant was determined at 15, 30, 45 and 60 s. An individual was defined as being in the quadrant if any portion of its body covered the line that defined the quadrant, so that the same animal could be counted in more than one quadrant. The response of each group was then determined as the average of each of the three responses to each of the wavelengths. Results were reported as an avoidance fraction (Θ), which was one minus the number of animals in the quadrant at time x divided by those present at time 0 (Fig. 2A).
Individual animal trials
Each of nine unfed and nine fed individuals was also tested for their responses to illumination on both the head and the tail. For testing, individuals were transferred into a 100 mm plastic Falcon Petri dish that contained a 5 mm depth of APW (approximately 30 ml). Each leech was allowed to become quiescent, which typically took 2–5 min, but rarely was as long as 30 min. To receive a trial the leech had to remain quiescent for at least 30 s. Stimuli to the head (three trials for each wavelength) were delivered first, followed by the same set of stimuli delivered to the tail. The order of presentation of wavelengths for each of the 18 individuals was uniquely determined by an online random sequence generator (random.org).
The plastic lid of the 100 mm Petri dish was held 3.5 cm above the test surface to standardize how close the LED was placed. These were all untethered tests so considerable freedom of movement had to be maintained for stimulus presentation in order to follow subjects at any position. It proved unnecessary to have the lid sitting directly on the dish to keep these individuals inside. Videos were made of head or tail presentation trials for subsequent analysis.
A trial consisted of light being shone upon either the head or tail for 2–5 s. The stimulus was removed before 5 s if a response was initiated. If no response was initiated within 5 s, it was scored as 0 (no response). If a response was initiated within 5 s, movement of the body was classified as either shortening (shorten) or extending (extend). Note was made if any other behavior began within 2–3 s, notably subsequent swimming or crawling (shorten+, extend+).
Electrophysiology
To prepare an animal for dissection and recording, it was first anesthetized with ice-cold Ringer's solution. The leech Ringer's contained (in mmol l−1)
115 NaCl, 4 KCl, 1.8 CaCl2, 1.5 MgCl2, 10 D-glucose, 4.6 Tris maleate and 5.4 Tris base, and had a pH of 7.4 (Nicholls and Baylor, 1968). All electrophysiology experiments were carried out in dim room light (not total darkness) as described above for behavior.
Cephalic nerve recording
To record eye responses from cephalic nerves, the head attached to the first four midbody segments was separated from the rest of the leech and pinned into a 60 mm plastic dish with a 3 mm layer of clear Sylgard resin in it. The main trunks of the two large cephalic nerves (DA and DB) (Kretz et al., 1976) radiating from the underside of the supraesophageal ganglion were surgically exposed. Each DA and DB was cut near where it joined the CNS and a length was cleaned and lifted from the tissue surrounding it. The DA carries axons arising from eye 1 and the DB carries axons from eye 2, along with other axons (Kretz et al., 1976).
A suction electrode was placed on the cut end of a cephalic nerve and signals were amplified using an A-M Systems model 1700 differential amplifier with the gain set at 1000 or 10,000×, low end filter at 10 Hz and high end set at 1000 Hz. The traces were simultaneously viewed on a Tektronix oscilloscope and captured by an ADI PowerLab 26/T (ADInstruments, Colorado Springs, CO, USA) sampling at 10 kHz. Multiple DA and DB recordings were obtained from each of the three preparations, all of which yielded similar responses.
Each LED was presented in arbitrary order to illuminate the anterior sucker for 3–6 s. To monitor presentation of the light, a 2 mm diameter fiber optic-coupled phototransistor that gated current through a 10 kΩ resistor (Jellies and Kueh, 2012) was placed just below where the anterior sucker was placed. A second channel of the PowerLab monitored the voltage across this resistance. For subsequent neuronal recordings comparing the responses to different LED emissions, the white LED was omitted because it had a very broad emission spectrum that overlapped that delivered by the blue and green LEDs.
Intracellular photoreceptor recording
To record from individual cephalic photoreceptors, the eyes were prepared as described elsewhere (Fioravanti and Fuortes, 1972; Kretz et al., 1976; Lasansky and Fuortes, 1969; Peterson, 1984a). Briefly, the dorsal margin of the anterior sucker was dissected away from the leech and pinned dorsal side up in the recording dish. The transparent epithelial covering over each pigmented eyecup was carefully removed using micro-scissors and forceps. When viewed with oblique lighting the individual photoreceptor somata were clearly visible as spherical cells.
Intracellular recordings from individual primary receptor neurons in eyes 1–4 were obtained using glass microelectrodes (resistances of 25–45 MΩ, filled with 1 mol l−1 potassium acetate) using a standard recording amplifier (World Precision Instruments, Sarasota, FL, USA; 773 preamplifier). Signals were simultaneously viewed on a Tektronix oscilloscope and digitized by a PowerLab 26/T or a PowerLab 4/35 (ADInstruments, Colorado Springs, CO, USA) at 10 or 20 kHz. All cells had resting membrane potentials in the range of −35 to −48 mV and were held in stable penetrations for variable times. To examine responses across luminosity levels a set of four neutral density (ND) filters was used. The filters were 25.4 mm in diameter; nickel-chromium-coated fused silica (7980) suitable for both UV and visible wavelengths. They were obtained from an online eBay vendor (caprice47, Loveland, CO, USA). A simple holder was fashioned from stiff foam pipe insulation that could hold an LED wand, and which had a slit cut into it allowing the ND filters to be interposed between the light and the preparation. In most cases, single filters were interposed, but in a few cases two filters were stacked to yield intermediate or higher OD. For these studies, over 50 cells were successfully impaled and stimulated with light. However, there was considerable variability in the responses of different cells to different LEDs [consistent with previous studies on visible light and leech receptors (see Peterson, 1984a)] and a more complete quantitative characterization will be the topic of future studies. For these studies I was able to obtain stable recordings in eight cells for the extended time required to present red, green, blue and UVR LEDs, across at least two interposed ND filters in addition to using no filter. As a result, recordings from three representative cells (from eye 2 from two different leeches and eye 3) were selected for presentation.
Simultaneous intracellular and extracellular recording
To record from individual midbody ganglionic neurons during presentation of stimuli, the leech was dissected using cold Ringer as described above. All leeches used were 5–6 cm long when dissected. The head and tail suckers were twisted and pinned with the dorsal side oriented upward and the midbody segments were pinned ventral upward (Fig. 6). Small incisions were made to expose ganglia in segments 7–11 in much the same way as described by Kretz et al. (Kretz et al., 1976). A suction electrode was placed on the intersegmental connective between segments 7 and 8 such that the central portion containing Faivre's nerve (Fernandez, 1978) was beneath the electrode opening. Recordings were obtained en passant but otherwise were similar to the methods described above. Once the largest extracellular spike was identified by 1:1 correlation with the intracellular record, it could then be used to quantify S-cell responses even if the intracellular impalement was lost.
Intracellular recordings from individual neurons in ganglion 9, 10 or 11 were obtained as described above. Light beams from LEDs covered the anterior or posterior sucker and the adjacent three to four segments with diminishing intensity. Multiple recordings from various identified neurons were obtained from each of two preparations during presentation of light stimuli, including the mechanoreceptors and HE neurons (Muller et al., 1981) as well as unidentified neurons. The S-cell was readily identifiable as a small (<20 μm) soma in the central packet, with fast, overshooting action potentials that adapted rapidly (Gardner-Medwin et al., 1973; Sahley et al., 1994). Although several as yet unidentified cells showed responses to light, a survey of UVR effects on neurons generally was beyond the scope of the present study. Instead, the S-cell was used to examine the effects of UVR in more detail because it is a known (visible) light-responsive, higher order interneuron. An additional five preparations were used to examine the response of the S-cell to UVR and visible light across levels of luminosity. Light pulses of 0.5–1.0 s were delivered in groups of three with a 2–3 s interpulse interval and 10–15 s between presentations of different wavelengths. A 15–30 s interval was allowed to elapse between stimulation sequences at different luminosities (0, 0.3, 0.6, 1.3 and 2.0 OD). Stimuli were presented in the order red, green, blue and UV, with and without ND filters interposed. This order was selected because preliminary studies revealed that the UVR stimulus sometimes evoked long-lasting tonic responses in cephalic photoreceptors, from the S-cells and in sensillar nerve recordings (J.J., unpublished). It seemed prudent to place the UVR stimulus before the longer interval in stimulus presentations to allow longer recovery. Responses were computed as the spiking frequency in the first 500 ms after the stimulus onset, normalized to the peak frequency within that individual for that wavelength range and OD.
Data analysis
All statistical analyses on the avoidance fraction (Θ) in the group response experiments were based on the average number of values from three trials at each wavelength across four time points in each of the five subjects (n=5 groups of animals). All data were analyzed using a two-way repeated-measures ANOVA, with the two factors being wavelength and time. A post hoc test (Fisher's LSD) was conducted to examine possible differences between means when significant results were found. Statistical significance was defined as P<0.05 for all tests. The data on S-cell responses to light presented to the head and tail across wavelength range (color of LED emission) and luminosity (interposed ND filters) were analyzed in a similar fashion. All individual responses were the average values from three pulses delivered at each wavelength at each condition of luminosity. Data from five individuals was then averaged to yield the summary graphs presented here (n=5). All figures were generated with Photoshop CS3 (Adobe Systems) and all statistical analyses were performed with SPSS (IBM, Armonk, NY, USA).
ACKNOWLEDGEMENTS
I thank Rachel King, Christopher Rousch and Gerardo Zayas for their assistance in gathering initial electrophysiology data and their leech husbandry assistance. I also thank Daniel Kueh for his advice on statistics and comments on a draft of the manuscript, and William B. Kristan, Jr for reading the manuscript and offering many helpful comments and editorial suggestions.
FOOTNOTES
Competing interests
The author declares no competing financial interests.
Funding
This work was supported by a Faculty Research and Creative Activities Award from the Office of the Vice President for Research, Western Michigan University [grant number W2013-007]; a grant to Western Michigan University from the Howard Hughes Medical Institute through the Undergraduate Science Education Program [grant number 52006962]; and a Research Experiences for Undergraduates Grant, no. DBI-1062883, National Science Foundation. Deposited in PMC for release after 6 months.
References
- Arisi I., Zoccolan D., Torre V. (2001). Distributed motor pattern underlying whole-body shortening in the medicinal leech. J. Neurophysiol. 86, 2475-2488 [DOI] [PubMed] [Google Scholar]
- Banaszak A. T. (2003). Photoprotective physiological and biochemical responses of aquatic organisms. In UV Effects in Aquatic Organisms and Ecosystems (ed. Helbling E. W., Zagarese H.), pp. 331-358 Cambridge: The Royal Society of Chemistry; [Google Scholar]
- Bisson G., Torre V. (2011). Statistical characterization of social interactions and collective behavior in medicinal leeches. J. Neurophysiol. 106, 78-90 [DOI] [PubMed] [Google Scholar]
- Blumthaler M., Webb A. R. (2003). UVR climatology. In UV Effects in Aquatic Organisms and Ecosystems (ed. Helbling E. W., Zagarese H.), pp. 23-58 Cambridge: The Royal Society of Chemistry; [Google Scholar]
- Boeing W. J., Leech D. M., Williamson C. E., Cooke S., Torres L. (2004). Damaging UV radiation and invertebrate predation: conflicting selective pressures for zooplankton vertical distribution in the water column of low DOC lakes. Oecologia 138, 603-612 [DOI] [PubMed] [Google Scholar]
- Brodfuehrer P. D., Friesen W. O. (1984). A sensory system initiating swimming activity in the medicinal leech. J. Exp. Biol. 108, 341-355 [DOI] [PubMed] [Google Scholar]
- Buma A. G. J., Boelen P., Jeffrey W. H. (2003). UVR-induced damage in aquatic organisms. In UV Effects in Aquatic Organisms and Ecosystems (ed. Helbling E. W., Zagarese H.), pp. 293-327 Cambridge: The Royal Society of Chemistry; [Google Scholar]
- Carlton T., McVean A. (1993). A comparison of the performance of two sensory systems in host detection and location in the medicinal leech Hirudo medicinalis. Comp. Biochem. Physiol. 104, 273-277 [DOI] [PubMed] [Google Scholar]
- Crisp K. M., Mesce K. A. (2006). Beyond the central pattern generator: amine modulation of decision-making neural pathways descending from the brain of the medicinal leech. J. Exp. Biol. 209, 1746-1756 [DOI] [PubMed] [Google Scholar]
- Derosa Y. S., Friesen W. O. (1981). Morphology of leech sensilla: Observations with the scanning electron microscope. Biol. Bull. 160, 383-393 [Google Scholar]
- Dickinson M. H., Lent C. M. (1984). Feeding behavior of the medicinal leech, Hirudo medicinalis L. J. Comp. Physiol. A 154, 449-455 [Google Scholar]
- Döring C., Gosda J., Tessmar-Raible K., Hausen H., Arendt D., Purschke G. (2013). Evolution of clitellate phaosomes from rhabdomeric photoreceptor cells of polychaetes – a study in the leech Helobdella robusta (Annelida, Sedentaria, Clitellata). Front. Zool. 10, 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes C. D., Fourtner C. R. (1989). Hindsight and rapid escape in a freshwater Oligochaete. Biol. Bull. 177, 363-371 [DOI] [PubMed] [Google Scholar]
- Earl J. B., Britt S. G. (2006). Expression of Drosophila rhodopsins during photoreceptor cell differentiation: insights into R7 and R8 cell subtype commitment. Gene Expr. Patterns 6, 687-694 [DOI] [PubMed] [Google Scholar]
- Esch T., Mesce K. A., Kristan W. B. (2002). Evidence for sequential decision making in the medicinal leech. J. Neurosci. 22, 11045-11054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J. (1978). Structure of the leech nerve cord: distribution of neurons and organization of fiber pathways. J. Comp. Neurol. 180, 165-191 [DOI] [PubMed] [Google Scholar]
- Fioravanti R., Fuortes M. G. F. (1972). Analysis of responses in visual cells of the leech. J. Physiol. 227, 173-194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen W. O. (1981). Physiology of water motion detection in the medicinal leech. J. Exp. Biol. 92, 255-275 [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin A. R., Jansen J. K. S., Taxt T. (1973). The “giant” axon of the leech. Acta Physiol. Scand. 87, 30A-31A [Google Scholar]
- Gaudry Q., Kristan W. B., Jr (2009). Behavioral choice by presynaptic inhibition of tactile sensory terminals. Nat. Neurosci. 12, 1450-1457 [DOI] [PubMed] [Google Scholar]
- Gaudry Q., Kristan W. B., Jr (2010). Feeding-mediated distention inhibits swimming in the medicinal leech. J. Neurosci. 30, 9753-9761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves B. R. (2003). Water column optics and penetration of UVR. In UV Effects in Aquatic Organisms and Ecosystems (ed. Helbling E. W., Zagarese H.), pp. 61-105 Cambridge: The Royal Society of Chemistry; [Google Scholar]
- Harley C. M., Cienfuegos J., Wagenaar D. A. (2011). Developmentally regulated multisensory integration for prey localization in the medicinal leech. J. Exp. Biol. 214, 3801-3807 [DOI] [PubMed] [Google Scholar]
- Harley C. M., Rossi M., Cienfuegos J., Wagenaar D. (2013). Discontinuous locomotion and prey sensing in the leech. J. Exp. Biol. 216, 1890-1897 [DOI] [PubMed] [Google Scholar]
- Honkavaara J., Koivula M., Korpimäki E., Siitari H., Viitala J. (2002). Ultraviolet vision and foraging in terrestrial vertebrates. Oikos 98, 505-511 [Google Scholar]
- Jacobs G. H. (1992). Ultraviolet vision in vertebrates. Am. Zool. 32, 544-554 [Google Scholar]
- Jellies J., Kueh D. (2012). Centrally patterned rhythmic activity integrated by a peripheral circuit linking multiple oscillators. J. Comp. Physiol. A 198, 567-582 [DOI] [PubMed] [Google Scholar]
- Jellies J., Johansen K., Johansen J. (1994). Specific pathway selection by the early projections of individual peripheral sensory neurons in the embryonic medicinal leech. J. Neurobiol. 25, 1187-1199 [DOI] [PubMed] [Google Scholar]
- Kevan P. G., Chittka L., Dyer A. G. (2001). Limits to the salience of ultraviolet: lessons from colour vision in bees and birds. J. Exp. Biol. 204, 2571-2580 [DOI] [PubMed] [Google Scholar]
- Kretz J. R., Stent G. S., Kristan W. B., Jr (1976). Photosensory input pathways in the medicinal leech. J. Comp. Physiol. A 106, 1-37 [Google Scholar]
- Kristan W. B., Jr, Nusbaum M. P. (1982-1983). The dual role of serotonin in leech swimming. J. Physiol. (Paris) 78, 743-747 [PubMed] [Google Scholar]
- Kristan W. B., Jr, McGirr S. J., Simpson G. V. (1982). Behavioural and mechanosensory neurone responses to skin stimulation in leeches. J. Exp. Biol. 96, 143-160 [Google Scholar]
- Land M. F., Horwood J., Lim M. L. M., Li D. (2007). Optics of the ultraviolet reflecting scales of a jumping spider. Proc. Biol. Sci. 274, 1583-1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Fuortes M. G. F. (1969). The site of origin of electrical responses in visual cells of the leech, Hirudo medicinalis. J. Cell Biol. 42, 241-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverack M. S. (1969). Mechanoreceptors, photoreceptors and rapid conduction pathways in the leech, Hirudo medicinalis. J. Exp. Biol. 50, 129-140 [DOI] [PubMed] [Google Scholar]
- Leech D. M., Johnsen S. (2003) Behavioral responses – UVR avoidance and vision. In UV Effects in Aquatic Organisms and Ecosystems (ed. Helbling W., Zagarese H.), pp. 457-481 Cambridge: Royal Chemistry Society; [Google Scholar]
- Leech D. M., Williamson C. E. (2001). In situ exposure to solar UV radiation alters the depth distribution of Daphnia. Limnol. Oceanogr. 46, 416-420 [Google Scholar]
- Lent C. M., Dickinson M. H. (1984). Serotonin integrates the feeding behavior of the medicinal leech. J. Comp. Physiol. A 154, 457-471 [Google Scholar]
- Magni F., Pellegrino M. (1978). Neural mechanisms underlying the segmental and generalized chord shortening reflexes in the leech. J. Comp. Physiol. 124, 339-351 [Google Scholar]
- Mann K. H. (1962). Leeches (Hirudinea) Their Structure, Physiology, Ecology and Embryology. New York, NY: Pergamon Press; [Google Scholar]
- Mesce K. A., Pierce-Shimomura J. T. (2010). Shared strategies for behavioral switching: understanding how locomotor patterns are turned on and off. Front. Behav. Neurosci. 4, 1-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misell L. M., Shaw B. K., Kristan W. B., Jr. (1998). Behavioral hierarchy in the medicinal leech, Hirudo medicinalis: feeding as a dominant behavior. Behav. Brain Res. 90, 13-21 [DOI] [PubMed] [Google Scholar]
- Muller K. J., Nicholls J. G., Stent G. S. (1981). Neurobiology of the Leech. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; [Google Scholar]
- Nicholls J. G., Baylor D. A. (1968). Specific modalities and receptive fields of sensory neurons in CNS of the leech. J. Neurophysiol. 31, 740-756 [DOI] [PubMed] [Google Scholar]
- Osburn C. L., Morris D. P. (2003). Photochemistry of chromophoric dissolved organic matter in natural waters. In UV Effects in Aquatic Organisms and Ecosystems (ed. Helbling E. W., Zagarese H.), pp. 187-217 Cambridge, The Royal Society of Chemistry; [Google Scholar]
- Peterson E. L. (1984a). Photoreceptors and visual interneurons in the medicinal leech. J. Neurobiol. 15, 413-428 [DOI] [PubMed] [Google Scholar]
- Peterson E. L. (1984b). Two stages of integration in a leech visual interneuron. J. Comp. Physiol. A 155, 543-557 [Google Scholar]
- Peterson E. L. (1985a). Visual interneurons in the leech brain II. The anterior visual cells of the supraesophageal ganglion. J. Comp. Physiol. A 156, 707-717 [Google Scholar]
- Peterson E. L. (1985b). Visual interneurons in the leech brain III. The unpaired H cell. J. Comp. Physiol. 156, 719-727 [Google Scholar]
- Puhl J. G., Mesce K. A. (2008). Dopamine activates the motor pattern for crawling in the medicinal leech. J. Neurosci. 28, 4192-4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi R. P., Richa H., Kumar A., Tyagi M. B., Sinha R. P. (2010). Molecular mechanisms of ultraviolet radiation-induced damage and repair. J. Nucleic Acids. 10.4061/2010/592980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizopoulou S., Santas P., Danielidis D., Häder D.-P., Santas R. (2000). UV effects on invertebrate and diatom assemblages of Greece. J. Photochem. Photobiol. 56, 172-180 [DOI] [PubMed] [Google Scholar]
- Sahley C. L., Modney B. K., Boulis N. M., Muller K. J. (1994). The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening. J. Neurosci. 14, 6715-6721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo E., Zheng L., Phistry M., Bagg E. E., Britt S. G. (2003). Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 23, 10873-10878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. T. (1986). Leech Biology and Behaviour: Feeding Biology, Ecology and Systematics. Oxford: Clarendon Press; [Google Scholar]
- Shaw B. K., Kristan W. B., Jr (1995). The whole-body shortening reflex of the medicinal leech: motor pattern, sensory basis, and interneuronal pathways. J. Comp. Physiol. A 177, 667-681 [DOI] [PubMed] [Google Scholar]
- Shaw B. K., Kristan W. B., Jr (1999). Relative roles of the S cell network and parallel interneuronal pathways in the whole-body shortening reflex of the medicinal leech. J. Neurophysiol. 82, 1114-1123 [DOI] [PubMed] [Google Scholar]
- Siddall M. E., Trontelj P., Utevsky S. Y., Nkamany M., Macdonald K. S., III (2007). Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc. Biol. Sci. 274, 1481-1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. P., Häder D.-P. (2002). UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1, 225-236 [DOI] [PubMed] [Google Scholar]
- Smith K. C., Macagno E. R. (1990). UV photoreceptors in the compound eye of Daphnia magna (Crustacea, Branchiopoda). A fourth spectral class in single ommatidia. J. Comp. Physiol. A 166, 597-606 [DOI] [PubMed] [Google Scholar]
- Tank S. E., Schindler D. W., Arts M. T. (2003). Direct and indirect effects of UV radiation on benthic communities: epilithic food quality and invertebrate growth in four montane lakes. Oikos 103, 651-667 [Google Scholar]
- Tovée M. J. (1995). Ultra-violet photoreceptors in the animal kingdom: their distribution and function. Trends Ecol. Evol. 10, 455-460 [DOI] [PubMed] [Google Scholar]
- Tully T., Quinn W. G. (1985). Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. A 157, 263-277 [DOI] [PubMed] [Google Scholar]
- Vincent W. F., Roy S. (1993). Solar ultraviolet-B radiation and aquatic primary production: damage, protection, and recovery. Environ. Rev. 1, 1-12 [Google Scholar]
- Walz B. (1982). Ca2+-sequestering smooth endoplasmic reticulum in an invertebrate photoreceptor. I. Intracellular topography as revealed by OsFeCN staining and in situ Ca accumulation. J. Cell Biol. 93, 839-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard A. L. (1981). Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J. Neurosci. 1, 936-944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Desplan C., Heisenberg M. (2010). Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc. Natl. Acad. Sci. USA 107, 5634-5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoran M. J., Drewes C. D. (1987). Rapid escape reflexes in aquatic oligochaetes: Variations in design and function of evolutionarily conserved giant fiber systems. J. Comp. Physiol. A 161, 729-738 [Google Scholar]
- Zoran M. J., Drewes C. D., Fourtner C. R. (1988). The lateral giant interneurons of the tubificid worm, Branchiura sowerbyi: Structural and functional asymmetry in a paired giant fiber system. J. Comp. Neurol. 275, 76-86 [DOI] [PubMed] [Google Scholar]