Abstract
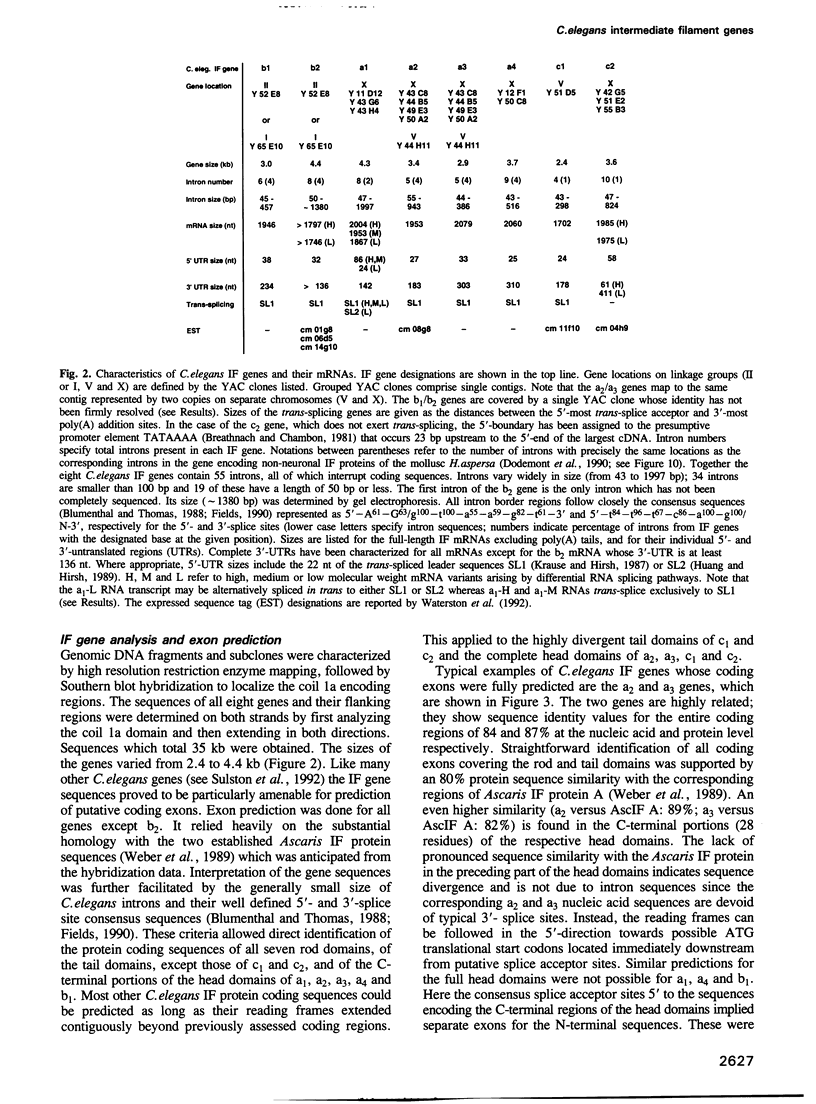
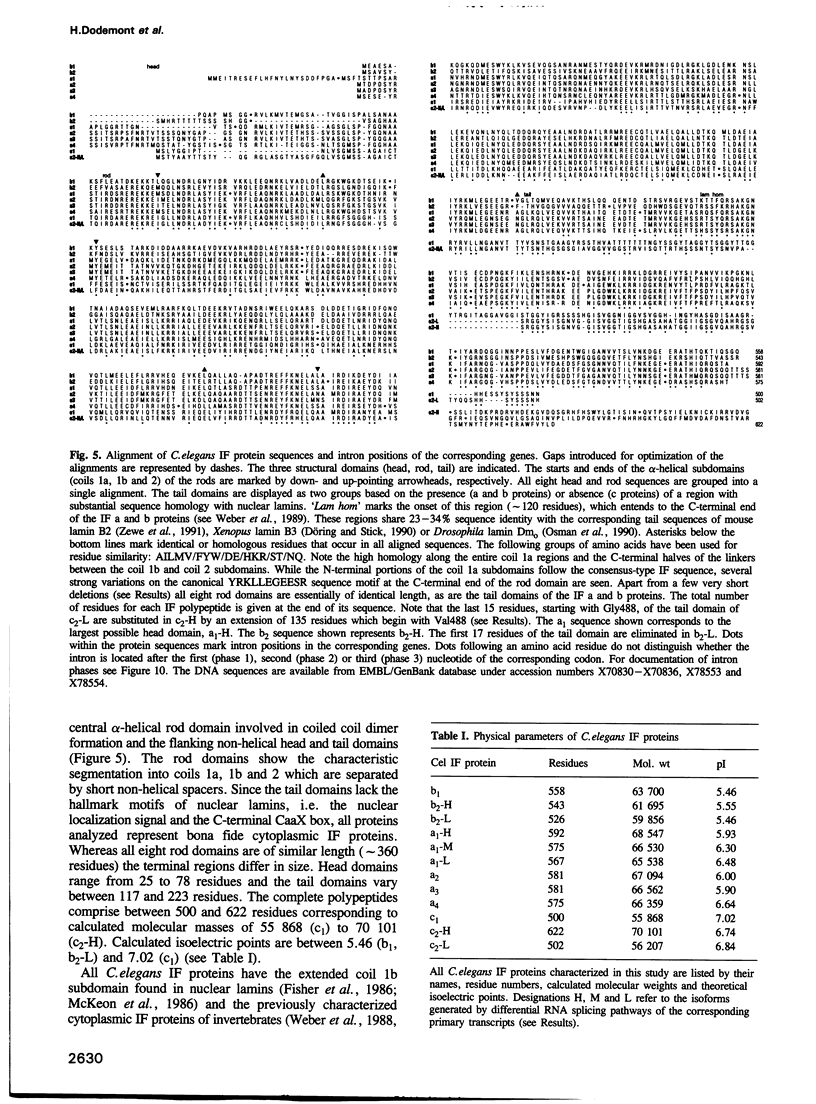
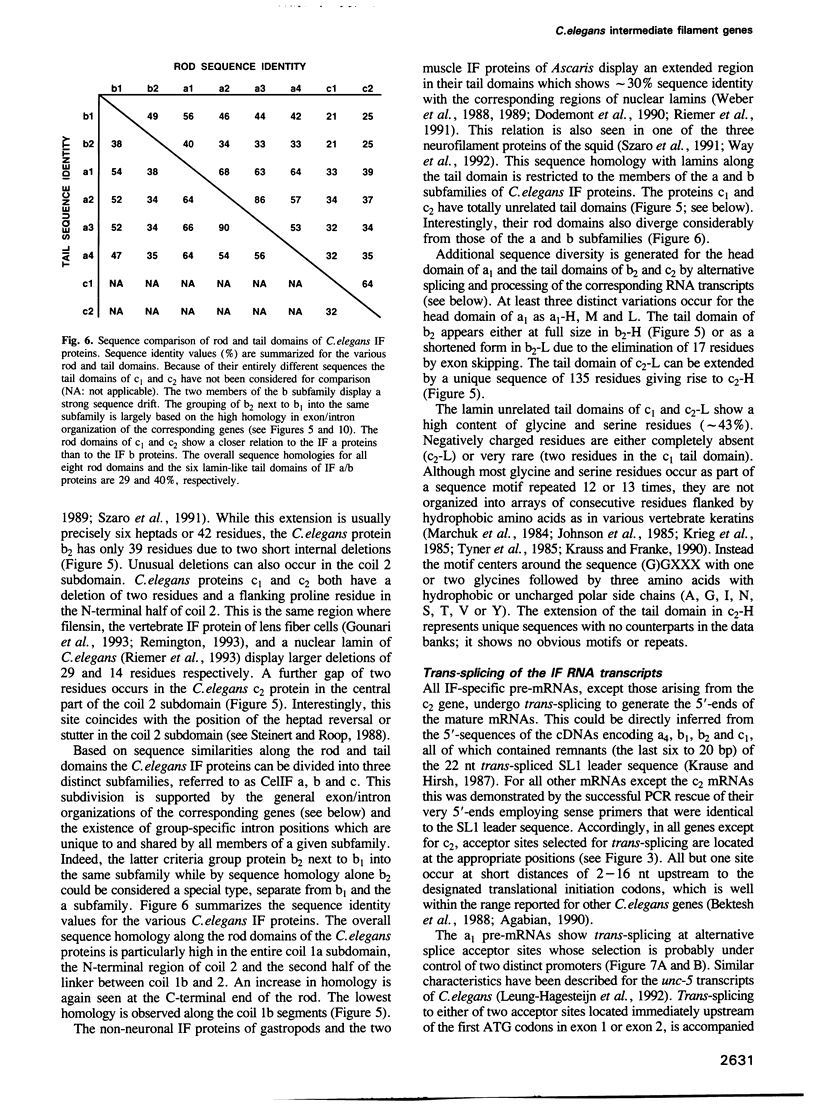
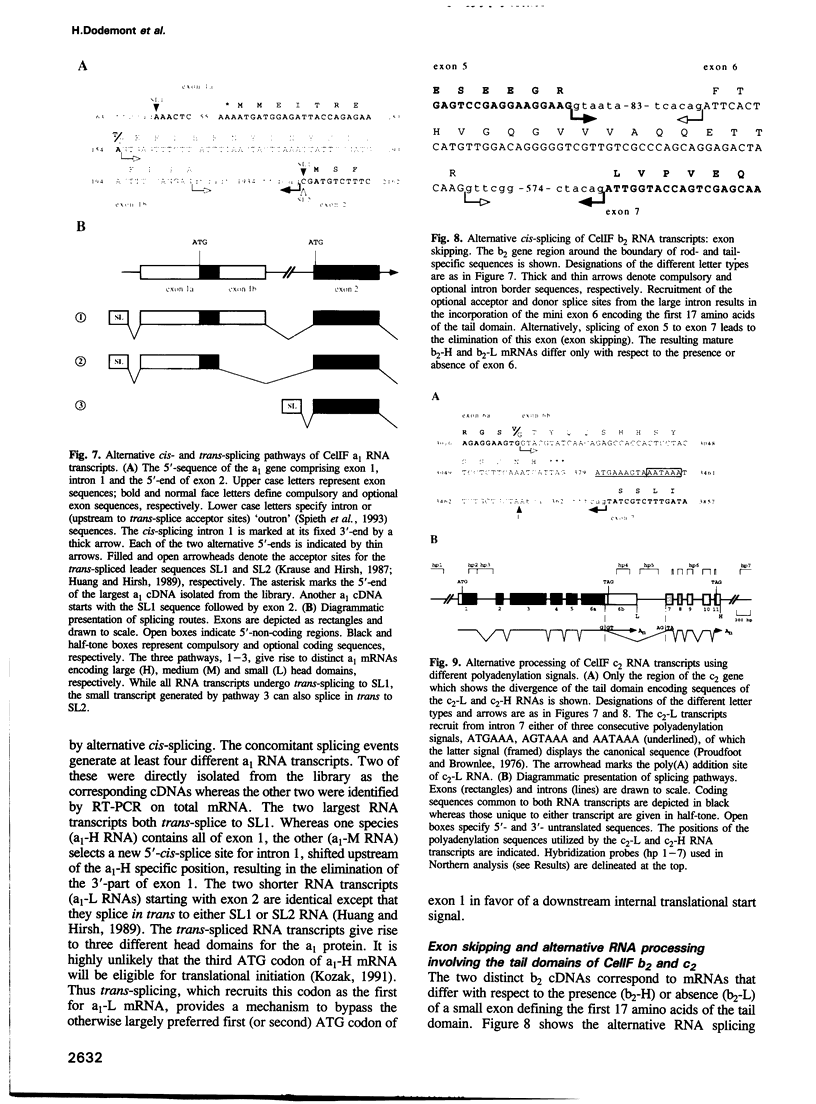
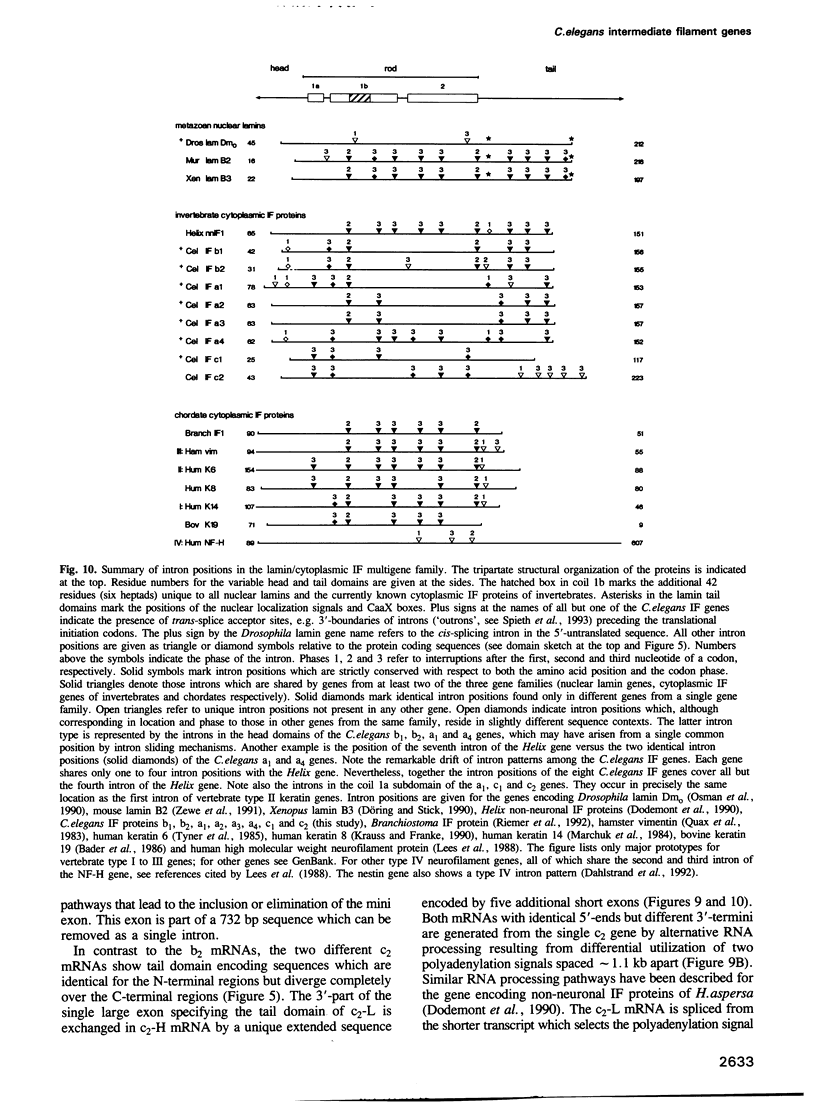
Cytoplasmic intermediate filament (IF) proteins of Caenorhabditis elegans are encoded by a dispersed multigene family comprising at least eight genes which map to three linkage groups. Exon sequences and intron patterns define three distinct subfamilies. While all eight IF genes display the long coil 1b subdomain of nuclear lamins, only six genes (a1-a4, b1 and b2) retain a lamin-like tail domain. Two genes (c1 and c2) have acquired entirely novel tail domains. The overall sequence identity of the rod domains is only 29%. The gene structures show a strong drift in number and positions of introns, none of which are common to all genes. Individual genes share only one to four intron locations with the Helix aspersa IF gene, but all eight nematode genes together account for nine of the 10 introns of the gastropod gene. All C.elegans IF genes are transcribed and all except gene c2 produce trans-spliced mRNAs. Alternatively spliced mRNAs arise from genes a1, b2 and c2 through several mechanisms acting at the transcriptional and posttranscriptional levels. These involve the alternative use of distinct promoters, polyadenylation sequences and both cis and trans RNA splice sites. The resulting sequence variations are restricted to the non-helical end domains. Minimally 12 distinct IF proteins are encoded by the various mRNAs. Different abundances in mixed-stage nematode populations suggest cell type- and/or stage-specific expression of individual mRNAs.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N. Trans splicing of nuclear pre-mRNAs. Cell. 1990 Jun 29;61(7):1157–1160. doi: 10.1016/0092-8674(90)90674-4. [DOI] [PubMed] [Google Scholar]
- Albertson D. G., Thomson J. N. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976 Aug 10;275(938):299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- Bader B. L., Magin T. M., Hatzfeld M., Franke W. W. Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail-less intermediate filament protein. EMBO J. 1986 Aug;5(8):1865–1875. doi: 10.1002/j.1460-2075.1986.tb04438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barstead R. J., Waterston R. H. Vinculin is essential for muscle function in the nematode. J Cell Biol. 1991 Aug;114(4):715–724. doi: 10.1083/jcb.114.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik E., Osborn M., Weber K. Intermediate filaments in muscle and epithelial cells of nematodes. J Cell Biol. 1986 Jun;102(6):2033–2041. doi: 10.1083/jcb.102.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik E., Osborn M., Weber K. Intermediate filaments in non-neuronal cells of invertebrates: isolation and biochemical characterization of intermediate filaments from the esophageal epithelium of the mollusc Helix pomatia. J Cell Biol. 1985 Aug;101(2):427–440. doi: 10.1083/jcb.101.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik E., Weber K. Intermediate filaments in the giant muscle cells of the nematode Ascaris lumbricoides; abundance and three-dimensional complexity of arrangements. Eur J Cell Biol. 1988 Feb;45(2):291–301. [PubMed] [Google Scholar]
- Bektesh S., Van Doren K., Hirsh D. Presence of the Caenorhabditis elegans spliced leader on different mRNAs and in different genera of nematodes. Genes Dev. 1988 Oct;2(10):1277–1283. doi: 10.1101/gad.2.10.1277. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Thomas J. Cis and trans mRNA splicing in C. elegans. Trends Genet. 1988 Nov;4(11):305–308. doi: 10.1016/0168-9525(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Coulson A., Kozono Y., Lutterbach B., Shownkeen R., Sulston J., Waterston R. YACs and the C. elegans genome. Bioessays. 1991 Aug;13(8):413–417. doi: 10.1002/bies.950130809. [DOI] [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand J., Zimmerman L. B., McKay R. D., Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992 Oct;103(Pt 2):589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- Dibb N. J., Newman A. J. Evidence that introns arose at proto-splice sites. EMBO J. 1989 Jul;8(7):2015–2021. doi: 10.1002/j.1460-2075.1989.tb03609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodemont H., Riemer D., Weber K. Structure of an invertebrate gene encoding cytoplasmic intermediate filament (IF) proteins: implications for the origin and the diversification of IF proteins. EMBO J. 1990 Dec;9(12):4083–4094. doi: 10.1002/j.1460-2075.1990.tb07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring V., Stick R. Gene structure of nuclear lamin LIII of Xenopus laevis; a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J. 1990 Dec;9(12):4073–4081. doi: 10.1002/j.1460-2075.1990.tb07629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C. Information content of Caenorhabditis elegans splice site sequences varies with intron length. Nucleic Acids Res. 1990 Mar 25;18(6):1509–1512. doi: 10.1093/nar/18.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R., Waterston R. H. Muscle cell attachment in Caenorhabditis elegans. J Cell Biol. 1991 Aug;114(3):465–479. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Gounari F., Merdes A., Quinlan R., Hess J., FitzGerald P. G., Ouzounis C. A., Georgatos S. D. Bovine filensin possesses primary and secondary structure similarity to intermediate filament proteins. J Cell Biol. 1993 May;121(4):847–853. doi: 10.1083/jcb.121.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. Y., Hirsh D. A second trans-spliced RNA leader sequence in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8640–8644. doi: 10.1073/pnas.86.22.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991 Nov;115(4):887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S., Franke W. W. Organization and sequence of the human gene encoding cytokeratin 8. Gene. 1990 Feb 14;86(2):241–249. doi: 10.1016/0378-1119(90)90285-y. [DOI] [PubMed] [Google Scholar]
- Krieg T. M., Schafer M. P., Cheng C. K., Filpula D., Flaherty P., Steinert P. M., Roop D. R. Organization of a type I keratin gene. Evidence for evolution of intermediate filaments from a common ancestral gene. J Biol Chem. 1985 May 25;260(10):5867–5870. [PubMed] [Google Scholar]
- Lane E. B., Rugg E. L., Navsaria H., Leigh I. M., Heagerty A. H., Ishida-Yamamoto A., Eady R. A. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992 Mar 19;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Lees J. F., Shneidman P. S., Skuntz S. F., Carden M. J., Lazzarini R. A. The structure and organization of the human heavy neurofilament subunit (NF-H) and the gene encoding it. EMBO J. 1988 Jul;7(7):1947–1955. doi: 10.1002/j.1460-2075.1988.tb03032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn C., Spence A. M., Stern B. D., Zhou Y., Su M. W., Hedgecock E. M., Culotti J. G. UNC-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell. 1992 Oct 16;71(2):289–299. doi: 10.1016/0092-8674(92)90357-i. [DOI] [PubMed] [Google Scholar]
- Marchuk D., McCrohon S., Fuchs E. Remarkable conservation of structure among intermediate filament genes. Cell. 1984 Dec;39(3 Pt 2):491–498. doi: 10.1016/0092-8674(84)90456-2. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Osman M., Paz M., Landesman Y., Fainsod A., Gruenbaum Y. Molecular analysis of the Drosophila nuclear lamin gene. Genomics. 1990 Oct;8(2):217–224. doi: 10.1016/0888-7543(90)90274-x. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Magnesium precipitation of ribonucleoprotein complexes. Expedient techniques for the isolation of undergraded polysomes and messenger ribonucleic acid. Biochemistry. 1974 Aug 13;13(17):3606–3615. doi: 10.1021/bi00714a032. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Mirsky R., Raff M. C., Thorpe R., Dowding A. J., Anderton B. H. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981 Dec;27(3 Pt 2):419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Quax W., Egberts W. V., Hendriks W., Quax-Jeuken Y., Bloemendal H. The structure of the vimentin gene. Cell. 1983 Nov;35(1):215–223. doi: 10.1016/0092-8674(83)90224-6. [DOI] [PubMed] [Google Scholar]
- Remington S. G. Chicken filensin: a lens fiber cell protein that exhibits sequence similarity to intermediate filament proteins. J Cell Sci. 1993 Aug;105(Pt 4):1057–1068. doi: 10.1242/jcs.105.4.1057. [DOI] [PubMed] [Google Scholar]
- Riemer D., Dodemont H., Weber K. A nuclear lamin of the nematode Caenorhabditis elegans with unusual structural features; cDNA cloning and gene organization. Eur J Cell Biol. 1993 Dec;62(2):214–223. [PubMed] [Google Scholar]
- Riemer D., Dodemont H., Weber K. Analysis of the cDNA and gene encoding a cytoplasmic intermediate filament (IF) protein from the cephalochordate Branchiostoma lanceolatum; implications for the evolution of the IF protein family. Eur J Cell Biol. 1992 Jun;58(1):128–135. [PubMed] [Google Scholar]
- Riemer D., Dodemont H., Weber K. Cloning of the non-neuronal intermediate filament protein of the gastropod Aplysia californica; identification of an amino acid residue essential for the IFA epitope. Eur J Cell Biol. 1991 Dec;56(2):351–357. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Obliquely striated muscle. 3. Contraction mechanism of Ascaris body muscle. J Cell Biol. 1967 Jul;34(1):15–33. doi: 10.1083/jcb.34.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothnagel J. A., Dominey A. M., Dempsey L. D., Longley M. A., Greenhalgh D. A., Gagne T. A., Huber M., Frenk E., Hohl D., Roop D. R. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992 Aug 21;257(5073):1128–1130. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieth J., Brooke G., Kuersten S., Lea K., Blumenthal T. Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 to downstream coding regions. Cell. 1993 May 7;73(3):521–532. doi: 10.1016/0092-8674(93)90139-h. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Sulston J., Du Z., Thomas K., Wilson R., Hillier L., Staden R., Halloran N., Green P., Thierry-Mieg J., Qiu L. The C. elegans genome sequencing project: a beginning. Nature. 1992 Mar 5;356(6364):37–41. doi: 10.1038/356037a0. [DOI] [PubMed] [Google Scholar]
- Szaro B. G., Pant H. C., Way J., Battey J. Squid low molecular weight neurofilament proteins are a novel class of neurofilament protein. A nuclear lamin-like core and multiple distinct proteins formed by alternative RNA processing. J Biol Chem. 1991 Aug 15;266(23):15035–15041. [PubMed] [Google Scholar]
- Tomarev S. I., Zinovieva R. D., Piatigorsky J. Primary structure and lens-specific expression of genes for an intermediate filament protein and a beta-tubulin in cephalopods. Biochim Biophys Acta. 1993 Nov 16;1216(2):245–254. doi: 10.1016/0167-4781(93)90151-3. [DOI] [PubMed] [Google Scholar]
- Tyner A. L., Eichman M. J., Fuchs E. The sequence of a type II keratin gene expressed in human skin: conservation of structure among all intermediate filament genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4683–4687. doi: 10.1073/pnas.82.14.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Burke D. J., Sulston J. E., Coulson A. R., Albertson D. G., Ammons D., Klass M., Hogan E. Genomic organization of major sperm protein genes and pseudogenes in the nematode Caenorhabditis elegans. J Mol Biol. 1988 Jan 5;199(1):1–13. doi: 10.1016/0022-2836(88)90374-9. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]
- Way J., Hellmich M. R., Jaffe H., Szaro B., Pant H. C., Gainer H., Battey J. A high-molecular-weight squid neurofilament protein contains a lamin-like rod domain and a tail domain with Lys-Ser-Pro repeats. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6963–6967. doi: 10.1073/pnas.89.15.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Dodemont H., Kossmagk-Stephan K. Amino acid sequences and homopolymer-forming ability of the intermediate filament proteins from an invertebrate epithelium. EMBO J. 1988 Oct;7(10):2995–3001. doi: 10.1002/j.1460-2075.1988.tb03162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Plessmann U., Ulrich W. Cytoplasmic intermediate filament proteins of invertebrates are closer to nuclear lamins than are vertebrate intermediate filament proteins; sequence characterization of two muscle proteins of a nematode. EMBO J. 1989 Nov;8(11):3221–3227. doi: 10.1002/j.1460-2075.1989.tb08481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zewe M., Höger T. H., Fink T., Lichter P., Krohne G., Franke W. W. Gene structure and chromosomal localization of the murine lamin B2 gene. Eur J Cell Biol. 1991 Dec;56(2):342–350. [PubMed] [Google Scholar]