Abstract
Proteoglycan (PG)-induced arthritis (PGIA) is an autoimmune inflammatory disease controlled by multiple genes in the murine genome. BALB/c.DBA/2 congenic strains carrying four major PGIA chromosome loci were immunized, and positions of loci on chromosomes 3, 7, 8, and 19 (loci Pgia26, Pgia21, Pgia4, and Pgia12, respectively) were confirmed. Each congenic strain exhibited a different pattern of regulation of clinical and immunologic features of PGIA, and these features were significantly influenced by gender. Locus Pgia26 delayed PGIA onset in males and females, and the effect was associated with a lower rate of antigen-induced lymphocyte proliferation and lower production of IFN-γ, TNF-α, and IL-4. Pgia12 similarly delayed onset in males, but the effect was achieved via elevated proliferation of PG-specific lymphocytes and enhanced production of IFN-γ and IL-4. The effect of the Pgia21 locus was arthritis-suppressive in females but PGIA-permissive in congenic males. These opposite effects are attributed to 2-fold higher serum auto-antibody and IL-6 levels in males than in females. Our study supports the idea that each congenic strain represents a different immunologic subtype of PGIA, providing an explanation for the complex etiology and various clinical phenotypes of RA.
Keywords: proteoglycan-induced arthritis, congenic strains, inflammation, cytokines, autoimmunity, sex-effect
Introduction
Rheumatoid arthritis (RA) is a complex inflammatory disease affecting approximately 1% of the human population. Susceptible individuals carry specific gene combinations, including the major histocompatibility complex (MHC), and have been exposed to adverse environmental risk factors. Epidemiologic studies suggest a significant role for genetic risk factors, which have been shown to control 53–65% of disease incidence in different European populations, though heritability can reach 83% for male probands.1,2 Dissecting the genetic background of RA using genetic linkage analysis, followed by meta-analysis of genome scans of American and European Caucasians, determined the most common shared genetic risk factors to be within defined loci on human chromosomes 1, 2, 6, 8, 12, 16, and 18.3 Genetic maps of RA susceptibility provided by linkage analyses of different ethnic populations are not always matched, indicating the genetic heterogeneity of RA.4–11
Murine models for RA are thought to provide a less complicated system for mapping disease susceptibility genes than humans, since they are based on a less complex genetic background, and disease can be induced by known triggers, e.g., immunization with cartilage protein components and adjuvants, to provoke inflammation.12,13 Probably the most apparent indication of the value of murine models is the recent confirmation of the genomic interval flanked by complement component 5 (C5) and TNF receptor-associated factor 1 (TRAF1) encoding genes (TRAF1-C5 interval) as a genetic risk factor for RA,14 a linkage known for years for murine arthritis.15
Proteoglycan (PG)-induced arthritis (PGIA) is an autoimmune disease which can be induced in susceptible mouse strains by systemic immunization with human cartilage PG aggrecan.16,17 PGIA shares similarities to RA in joint histopathology and the progressive character of the disease. Like RA, PGIA is under the control of the major genetic permissive MHC locus.18 Additionally, both RA and PGIA are reliant on multiple non-MHC chromosome loci,19–21 and several Pgia quantitative trait loci (QTLs) are homologous to either RA or other human autoimmune disease loci.17,22
To find causative PGIA-susceptibility genes and genes controlling production of autoantibodies, pro- and anti-inflammatory cytokines, and lymphocyte responses during and prior to PGIA pathogenesis, we performed a genome-wide linkage analysis of crosses between BALB/c or C3H susceptible and several resistant (e.g., DBA/2) strains.19–23 We found that separate sets of genes control the incidence, severity, and onset of disease, although these clinical phenotypes might also be simultaneously affected by two loci/genes (composite QTL). Notably, we showed that gender is a major moderator of QTLs’ penetrance in PGIA.19 In the BALB/c x DBA/2 cross, where both parent strains carry identical H-2d alleles, thereby excluding the effect of MHC upon linkage analysis, we identified four major non-MHC PGIA loci on chromosomes 3, 7, 8 and 19. 17,19,22 According to the initial linkage analysis, major loci were Pgia26 on chromosome 3 (chr3) controlling the onset of arthritis in both females and males, Pgia21 locus on chr7 controlling incidence of the disease, Pgia4 on chr8 controlling PGIA severity in males, and Pgia12 on chr19 regulating severity in both males and females. 19 To confirm the positions and effects of these QTLs in a pure genetic background, we transferred these chromosome intervals of PGIA-resistant DBA/2 mice into the PGIA-susceptible BALB/c strain. We immunized and induced autoimmune arthritis in these congenic strains and compared disease-associated clinical and immunologic phenotypes.
In the present study, using a set of congenic mouse strains, we confirmed that multiple genes are involved in the control of PGIA. Our results with these four congenic strains validated the QTL positions and their genetic effects inferred from our initial linkage analyses. We demonstrated that, although the separation of arthritis-susceptibility genes in individual congenic strains finally leads to the same clinical phenotype and histopathology (PGIA), the immune responses underlying the disease are substantially different in the four congenic strains. Our study supports the idea that each congenic strain represents a different immunologic subtype of PGIA, providing an explanation for the complex etiology and various clinical phenotypes of RA.
Results
Initial genetic linkage analysis of (BALB/c x DBA/2)F2 hybrids
The major effect of the Pgia26 locus was found to be on disease onset in (BALB/c x DBA/2)F2 females (Table 1). F2 females carrying a DBA/2-type homozygous Pgia26 locus demonstrated almost a 7-times lower onset score (developing arthritis much later) than corresponding BALB/c-homozygous F2 females; therefore, the PGIA-suppressive allele originated from the DBA/2 strain (Table 1). The same chromosome locus did not show any significant effect on PGIA in F2 hybrid males.
Table 1.
Four major loci controlled PGIA in (BALB/c x DBA/2)F2 hybrids.
| QTL * | Chr | Mb | Gender | Suppressive allele | Mode of inheritance | Onset | Incidence | Severity |
|---|---|---|---|---|---|---|---|---|
| Pgia26 | 3 | 46.8 | F M |
DBA/2 -- |
recessive -- |
3.2 -- |
-- -- |
-- -- |
| Pgia21 | 7 | 47.0 | F M |
BALB/c BALB/c |
recessive recessive |
2.1 2.0 |
4.9 3.1 |
-- -- |
| Pgia4 | 8 | 9.3 | F M |
DBA/2 -- |
recessive -- |
-- -- |
-- -- |
3.0 -- |
| Pgia12 | 19 | 15.5 | F M |
-- DBA/2 |
-- co-dominant |
-- -- |
-- -- |
-- 3.4 |
Results of the initial linkage analysis19 are supplemented with loci sizes (Mb, million base pairs), mode of inheritance and the source genome of the arthritis-suppressive allele (suppressive allele). Only loci with LOD (logarithm of the odds ratio) scores over threshold 3.0 are presented for each clinical trait (onset, incidence, and severity) and separately for males (M) and females (F).
The Pgia21 locus on chr7 was the strongest QTL, controlling mainly disease susceptibility and, more weakly, disease onset in both males and females, but disease severity was not affected by genes within this locus (Table 1). DBA/2-type Pgia21-homozygous F2 mice were 2.5 times more susceptible to disease than BALB/c type littermates. According to this initial linkage analysis, chr7 of the DBA/2 strain harbored at least one recessive permissive allele for PGIA.
The Pgia4 locus on chr8 carried an arthritis-permissive allele of BALB/c origin, which controlled PGIA severity in males, but had no significant effect upon disease onset and incidence. Conversely, the Pgia12 locus on chr19 largely controlled PGIA severity in males (Table 1). The source of the disease-suppressive co-dominant allele was the DBA/2 strain.
Summarizing data from the genetic analysis of the BALB/c x DBA/2 cross, we have found that chr3, chr8 and chr19 of the PGIA-resistant DBA/2 strain carried co-dominant/recessive suppressive arthritis alleles, but only chr7 from DBA/2 contained a recessive arthritis-promoting gene. Altogether, these four major loci controlled a significant portion (~40%) of disease variance in this F2 cross.
Incidence, severity and onset of arthritis in DBA/2 QTL-specific congenic BALB/c strains
After the initial genetic linkage analysis and selection of QTLs, based primarily on their strength, we chose the above described four loci, which were of comparable statistical strength of linkage, ranging from 3.0 to 4.9 LOD score, with each locus occupying a 10–50 Mbp chromosome interval (Table 1). Locus Pgia26 on chr3 and locus Pgia21 on chr7 may comprise multiple loci within the initially identified genomic intervals, because the interval mapping curve shows a double-peaked shape.19 Based upon this consideration, we employed a conservative strategy for generating QTL-specific strains, transferring chromosome intervals that were larger than the putative target locus at a presumptive cut-off linkage level of LOD score 3.0 (Table 1). To confirm the chromosome positions of these QTLs, define their genetic effects on PGIA, and eventually identify causative genes within the loci, we generated congenic strains representing four major non-MHC PGIA loci located on chr3, chr7, chr8, and chr19. All four congenic strains were backcrossed for six generations using a genomic marker-assisted selection protocol.24
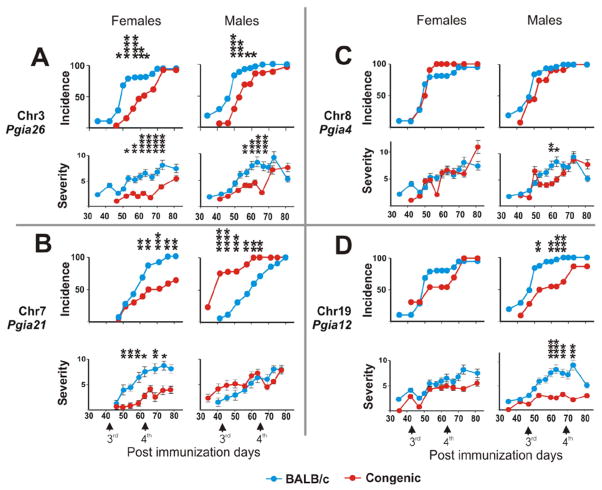
We have shown earlier that the genetic control of PGIA by a certain locus may be manifested by arthritis onset, severity, or susceptibility, and locus penetrance is often significantly affected by gender.18,19 Therefore, we analyzed the clinical and Immunologic phenotypes of PGIA separately in both males and females. The strongest disease-suppressing phenotype was found in BALB/c.DBA/2-Pgia26 congenic mice (BALB/c mice carrying Pgia26 from DBA/2 strain) (Fig. 1A). This Pgia26 locus on chr3 protected both males and females from arthritis. Consistent with initial linkage analysis, the major effect was found to be upon disease onset delaying arthritis by 10 days in congenic mice (Fig. 1A, Table 2). Consequently, for a period of almost four weeks PGIA incidence was 20–60% lower in this congenic strain than in age- and gender-matched wild-type BALB/c littermates, a combined group from intercrosses of all four congenic strains with parental BALB/c mice. The protective effect was evident after the third immunization from day 42 to day 70. Day-by-day pair-wise comparison of wild-type and congenic groups showed highly significant differences up to p < 0.0001 by Chi-square test (Fig. 1A, see incidence in females). Confirming the initial linkage analysis (Table 1), the effect of this QTL was much more substantial in females; however, it was also less pronounced but significant in congenic males. The protective effect of the Pgia26 locus was not absolute, and all mice eventually developed arthritis by the end of the observation period (day 81). The severity of disease in both males and females was also under the control of the Pgia26 locus (Fig. 1A), since joint swelling was considerably reduced (50% less, p < 0.0001) in congenic mice.
Figure 1.
Time course of arthritis incidence and severity during immunization period. (A) BALB/c.DBA/2-QTL congenic strains carrying Pgia26 locus of chr3 (n=31 females, n=38 males), (B) Pgia21 locus of chr7 (n=30 females, n=21 males), (C) Pgia4 locus of chr8 (n=31 females, n=19 males) and (D) Pgia12 locus of chr19 (n=33 females, n=25 males) are shown. Congenic strains carrying QTLs of DBA/2 origin (red circles) were compared to wild-type BALB/c combining littermates from all four congenic strain intercrosses with the parental BALB/c strain (blue circles) immunized either with PG and DDA (n=82 females and n=63 males for A, C and D) or with PG and CFA (n=24 females and n=16 males for B). Statistical significance for the day-by-day difference between congenic and wild-type combined mice was estimated with Chi-square test (for the disease incidence) and non-paired Student’s t-test (for severity; presented as mean ± SEM): * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Only stretches of significant differences containing at least one p<0.01 observation are shown. Vertical arrows on the bottom panels indicate third (day 42) and fourth (day 63) immunizations.
Table 2.
Major controlling PGIA chromosome loci and their clinical and immune effects in C.D2 congenic strains.
| QTL Mb * |
Gender | Effect | Onset | Incidence | Severity | Immune responses |
|---|---|---|---|---|---|---|
|
Pgia26 46.8 |
F | Protective | 10 days delay | n.s. | 50% lower | Suppressed lymphocyte responses |
| M | Protective | 10 days delay | n.s. | 50% lower | ||
|
| ||||||
|
Pgia21 47.8 |
F | Protective | n.s. | 40% less | 50–70% lower | Increased serum IgG1 and IL-6 Increased lymphocyte IL-4 and proliferation Suppressed lymphocyte TNF-α |
| M | Promoting | 6 days earlier | n.s. | n.s. | ||
|
| ||||||
|
Pgia4 37.6 |
F | n.s. | n.s. | n.s. | n.s. | Suppressed serum IgG1 |
| M | Protective (transient) | n.s. | n.s. | 40% lower (transient) | ||
|
| ||||||
|
Pgia12 45.4 |
F | n.s. | n.s. | n.s. | n.s. | Increased lymphocyte responses |
| M | Protective | 6 days delay | n.s. | 60% lower | ||
Sizes of the DBA/2-homozygous interval in congenic strains in Mb are shown below the locus name (QTL). Clinical and immune effects of the loci were gender-biased and are shown separately for females (F) and males (M). n.s., the genetic effect was statistically insignificant (p>0.01). All genetic effects were significant for at least p<0.01 between BALB/c and congenic mice for a period not shorter than 2 weeks, except for Pgia4, the effect of which was transient and lasted one week. Incidence was calculated at the end of immunization and observation period at day 81 and presents the mean value for all mice in the group. Severity was determined by visual scoring of paw/joint inflammation on day 62. Lymphocyte responses were measured in in vitro PG-stimulated spleen cultures (see “Materials and Methods”).
According to the initial genetic linkage analysis of F2 hybrids, the Pgia21 locus of DBA/2 chr7 origin carries arthritis-promoting genes (Table 1). Thus, congenic BALB/c.DBA/2-Pgia21 mice carry disease-promoting genes originating from both the BALB/c arthritis-susceptible genetic background and the transferred region of DBA/2 chr7. Therefore, we expected these congenic mice to develop PGIA faster and in a more severe form than the wild-type BALB/c strain. However, when the standard immunization protocol (PG emulsified in DDA adjuvant) was used, the PGIA susceptibility of congenic mice was statistically indistinguishable from that of wild-type BALB/c control mice (data not shown). To observe the putative PGIA-promoting effect of the locus, we immunized another group of these congenic mice with PG in complete Freund’s adjuvant (FCA) instead of in DDA, which was used in earlier studies,19 because PG injected with CFA induces slower PGIA progression than PG with DDA.25 Indeed, using the PG-Freund’s adjuvant immunization protocol, we detected highly significant differences between Pgia21-congenic and BALB/c wild-type littermates (Fig. 1B). Interestingly, the Pgia21 locus demonstrated opposite effects on arthritis phenotypes in congenic males versus congenic females. The Pgia21 locus was consistently disease-suppressive in females; only half of the congenic females developed joint inflammation by the end of the immunization period at day 81. Congenic males exhibited an opposite pattern of PGIA susceptibility (Fig. 1B). The majority (75%) of these males developed arthritis before the 4th immunization, and all males were arthritic by day 62. Disease severity followed approximately the same profile as PGIA incidence in congenic females, but severity in congenic males was indistinguishable from that of the wild-type cohort. Thus, the Pgia21 locus exhibited an arthritis-permissive effect in congenic males, whereas it was protective in congenic females (Table 2, Fig. 1).
Susceptibility to disease in BALB/c.DBA/2-Pgia4 congenic mice (chr8) was generally similar to that of the combined group of age-matched BALB/c-homozygous littermates (Fig. 1C). The DBA/2 Pgia4 chromosome interval demonstrated a weak and transient protective effect upon disease severity, which was detected only in congenic males and only for a short period of time (approximately 7 days) (Fig. 1C, see severity in males).
Congenic mice bearing the Pgia12 locus from chr19 of DBA/2 strain had significantly milder arthritis and lower disease penetrance than a combined group of wild-type BALB/c littermates (Fig. 1D). The Pgia12 locus exerted a noticeable suppressive effect upon disease onset, with arthritis starting at least one week later. Effects upon incidence and severity seemed to be secondary to the effect on onset, although the latter was significant only in congenic males.
As shown in Table 2, PGIA susceptibility (incidence) was under the primary control of chr7 in congenic females only. In other strains, congenic and wild-type BALB/c mice were similarly susceptible to PGIA. Effects upon incidence in Pgia26 (males and females), Pgia21 (males) and Pgia12 (males) were significant but transient, and should be considered secondary to onset regulation (Table 2). Severity of inflammation was under primary control of only the Pgia4 locus, although this result was transient and statistically borderline (Figure 1D).
Immune parameters in QTL-specific congenic strains
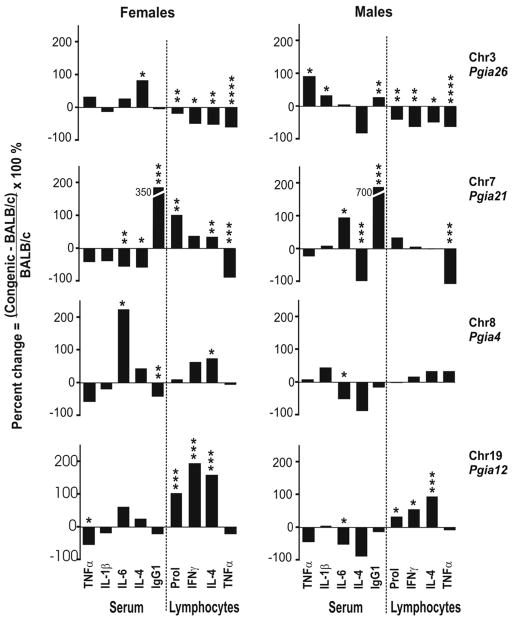
Systemic immunization of BALB/c mice with PG and adjuvant induces strong antigen-specific responses in both B and T cells, as demonstrated by antibody and cytokine production.17 Studies on disease transfer from arthritic to naïve syngeneic mice also underline the importance of cooperation between B and T lymphocytes.26,27 Our goal of measuring humoral and cellular immune responses in this set of congenic strains was two-fold. First, we wanted to determine the immune mechanisms of PGIA associated with each congenic strain. Second, we sought to identify an immune biomarker that could discriminate congenic from wild-type mice, and then use this biomarker to map the causative gene within a given chromosome interval. In mouse serum, we measured autoantibodies to mouse PG of both IgG1 and IgG2a isotypes (only the pre-dominant IgG1 isotype is presented in Fig. 2). We also measured TNF-α, IL-1β, IL-4 and IL-6 cytokine concentrations in the sera of congenic strains and wild-type BALB/c mice. Cellular responses were assayed in vitro using PG-stimulation of spleen lymphocytes. In vitro production of TNF-α, IFN-γ and IL-4 in response to PG-stimulation was measured, along with antigen-specific T cell proliferation (Fig. 2).
Figure 2.
Immune responses in QTL-specific congenic males and females. Variation of immune responses accompanied by similar clinical phenotype of PGIA supports the presence of different immunologic mechanisms of disease in each strain. Three groups of responses were measured in congenic mice and compared with wild-type BALB/c combined group of littermates: (i) serum cytokines TNF-α, IL-1β, IL-6 and IL-4, (ii) serum antibodies IgG1 to mouse PG (only these isotype autoantibodies are shown), (iii) antigen-specific in vitro lymphocyte responses: cell proliferation (Prol) and production of IFN-γ, IL-4 and TNF-α cytokines in 4-day old spleen cell cultures. Responses are presented as a percent change relative to BALB/c-type littermates. Statistical significance of the difference in responses was estimated with non-paired two-tailed Student’s t-test as shown: **** p < 0.0001; *** p < 0.001, ** p < 0.01, * p<0.05. Role of antibodies was most prominent in chr7 congenic strain, but equally arthritis suppressive loci in chr3 and chr19 congenic lines exhibited an opposite profile of lymphocyte proliferation and cytokine production.
Using the set of immune parameters described above, we have found that the pattern of immune responses is specific for each congenic strain (Fig. 2). In BALB/c.DBA/2-Pgia26 males and females (chr3), the strongest responses were found in antigen-stimulated lymphocytes. Interestingly, incubation of cells with PG suppressed lymphocyte proliferation and, most probably as a consequence of this, decreased production of TNF-α, IFN-γ and IL-4 cytokines by 50–70% (Fig. 2). The differences between Pgia26 congenic and wild-type males were also accompanied by higher production of TNF-α, IL-1β and IgG1 isotype autoantibodies in serum. All in vitro measured lymphocyte responses were approximately 50% lower than those in a combined group of wild-type BALB/c littermates (p < 0.01 – 0.0001). Serum antibody level was not significantly altered in this congenic strain, but serum IL-4 was elevated almost 2-fold compared to BALB/c females (Fig. 2). In brief, suppressed lymphocyte responses were the most characteristic immune aspect of the delayed and less severe arthritis in BALB/c.DBA/2-Pgia26 congenic mice (Table 2).
Clinically, the Pgia12 congenic males are similar to Pgia26 congenic mice, since both strains carry arthritis-suppressive genes of DBA/2 origin (Fig. 1, Table 2). Nonetheless, they differ immunologically. Chr19 congenic mice exhibited strong upregulation of lymphocyte proliferation, which is opposite to what we detected in Pgia26 congenic mice (Fig. 2). Serum biomarkers in chr19 congenic strain were the same as those in wild-type BALB/c mice (Fig. 2). In contrast, lymphocytes responded to PG stimulation with a 50–95% higher proliferation rate (stimulation index of 3.7 in females and 1.9 in males, p < 0.001), and, most likely due to this effect, IFN-γ and IL-4 productions were significantly higher: 90–195% increases were measured, compared to wild-type BALB/c lymphocytes. Congenic males revealed a pattern of immune response similar to that detected in congenic females, but less pronounced, which correlated with a significantly milder arthritis phenotype (Figs. 1D and 2). Observed clinical phenotypes associated with chromosome loci and measured immunologic parameters are summarized in Table 2.
The Pgia4 locus in chr8 did not show a strong association to either disease incidence or onset; only severity in males was under transient control of this locus during a short (approximately seven days) period (Fig. 1C). Correspondingly, immune parameters showed a pattern similar to that in the combined wild-type reference BALB/c group (Fig. 2). The strongest immunologic marker in this strain was the serum IL-6 level, which was elevated 3 fold in females, but slightly reduced in males when compared to BALB/c-type mice (Fig. 2). Surprisingly, chr8 congenic females demonstrated significant suppression of IgG1 type auto-antibody production (50% lower) and higher levels of IL-4 and IFN-γ production by PG-stimulated lymphocytes.
BALB/c.DBA/2-Pgia21 congenic mice (chr7) differed from BALB/c littermates in almost all measured immune parameters. The most striking difference was in the serum level of IgG1 type autoantibodies to mouse (self) PG, which was 3.5 times higher in congenic females and 7 times higher in congenic males than in wild-type BALB/c littermates (Fig. 2). The extremely high serum autoantibody level in males correlated with the presence of arthritis-promoting allele in these congenic males (Fig. 1B). Like the antibody level, IL-6 is also elevated. PGIA-suppressive IL-428 was correspondingly lower in carriers of the PGIA-promoting allele. Pgia21-congenic females, as opposed to males of the same strain, were less arthritic: the severity was lower in female mice. This may be due to the lower level of autoantibodies (350% higher than in BALB/c in females versus a 700% increase in males). The decreased autoantibody level may be associated with the decreased concentration of IL-6, a cytokine which has been shown to increase immunoglobulin production. In addition, IL-4, an anti-inflammatory cytokine, was significantly less in males than in females.
Discussion
Autoimmune arthritis in BALB/c mice, which can be induced by systemic immunization with PG, is a complex polygenic disease. As in RA, murine MHC plays a central role in the genetic and immune control of PGIA susceptibility, and H-2 complex locus on mouse chr17 is a major permissive genetic factor.18 However, direct comparison of the chromosome loci controlling collagen-induced arthritis (CIA) and PGIA in the same BALB/c x DBA/1 cross indicates that the role of murine MHC in PGIA is not as pervasive as in CIA, since the strength of genetic linkage between disease and H-2 complex was LOD 6.5 in PGIA versus LOD 22.0 in CIA.23 Because of the particular importance of non-MHC genetic component in PGIA, we focused on finding genes linked to murine arthritis in the cross of BALB/c with DBA/2, when both strains carry matching H-2d haplotype and, therefore, MHC influence upon linkage analysis is excluded.
We found a complex set of chromosome loci controlling arthritis in (BALB/c x DBA/2)F2 cross.17,19 Different sets of loci control disease incidence, onset time and severity, and the magnitude of effects is significantly affected by gender. Due to this overwhelming complexity, multiple approaches are needed to verify the biological effects of these QTLs and their genomic positions. To determine which chromosome loci should be selected for further congenic breeding, we took the two most important QTL features into consideration. The first QTL feature is the strength of the biological effect, which is reflected in the linkage LOD score. Regions on chr3, chr7, chr8 and chr19 were chosen for congenic transfer to BALB/c because they showed the strongest linkage with onset, susceptibility, or severity of PGIA.
The second criteria for QTL selection was the reproducibility of linkage detection in different crosses.19 For instance, Pgia26 overlapped with mCia2 locus controlling CIA in BALB/c x DBA/1 cross.23 Pgia4, Pgia21 and Pgia12 loci were also mapped in BALB/c x C3H/HeJCr and C3H/HeJCr x C57BL/6 F2 crosses.19,21 The Pgia4 locus also controlled lymphocyte responses and serum antibody and IL-1β levels. 19–21,23 The Pgia12 locus was linked to serum IL-6 levels in C3H/HeJCr x C57BL/6 and BALB/c x DBA/1 crosses.19,21,23
To explore the functional imporance of the selected chromosome loci in different arthritis models in mice and rats and in human joint diseases, we searched for genomic homology among all three mammalian genomes (Table 3). The Pgia26 locus on mouse chr3 was homologous to the genomic interval on rat chr2, which contains the Cia10 locus.29,30 This rat Cia10 locus regulates disease severity, pannus formation and joint damage.30 Pgia26 was also homologous to two human RA loci on chr1 and chr4 (Table 3). Detailed mapping of the human RA locus on chr4 is in progress.7,10 Murine Pgia26 corresponds to gout susceptibility31 in the human genome. This chromosome interval is also linked to C3b inactivator deficiency (due to alteration in the inhibitory complement component I)32 and to severe combined immunodeficiency due to mutation in the IL-2 encoding gene in humans.33 Another human region homologous to Pgia26 includes RA locus on chr1, harboring non-receptor protein tyrosine phosphatases, PTPN22/PTPN8 (Table 3). DNA polymorphisms in the PTPN22 encoding gene were associated with multiple human autoimmune diseases, including type 1 diabetes, Graves’ disease, systemic lupus erythematosus, juvenile idiopathic arthritis, autoimmune Addison’s disease, Hashimoto’s thyroiditis, and RA.34–40 Sequencing of the homologous gene in rats did not reveal significant polymorphisms, and gene expression was similar in parental strains.30 In the PGIA model, Ptpn22 is located within the proximal part of the Pgia26 locus.
Table 3.
Mouse-human-rat chromosome homology for four major murine PGIA loci.
|
QTL Chr |
Mouse | Rat | Human | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| QTL | Ref | chr | Mbp | QTLs | Ref | chr | Mbp | QTLs | Ref | |
|
Pgia26 Chr 3 |
mCia2 | 53,29 | 2 | 195–246 | Cia10 | 30,31 | 1 | 83–117 | Ptpn22 | 8,37,38 |
|
| ||||||||||
| 4 | 95–121 |
RA GOUT1 IL2, IF |
6,8 | |||||||
|
| ||||||||||
|
Pgia21 Chr 7 |
mCia7 | 43 | 1 | 85–141 | Cia2 | 44,54–56 | 15 | 20–25 | n.d. | |
| Eae6 | 15 | 78–100 | RA | 45 | ||||||
|
| ||||||||||
| Piax | 19 | 35–41 | n.d. | |||||||
| 19 | 54–57 | n.d. | ||||||||
|
| ||||||||||
|
Pgia4 Chr 8 |
n.d. | 16 | 39–64 | Pia14 | 46 | 8 | 8–17 | RA | 5,8 | |
| 8 | 29–31 | n.d. | ||||||||
|
| ||||||||||
|
Pgia12 Chr 19 |
n.d. | 1 | 205–242 | Eae7 | 44,47 | 9 | 0–6 | n.d. | ||
| 9 | 68–78 | OA | 48 | |||||||
|
| ||||||||||
| 10 | 53–54 | n.d. | ||||||||
| 10 | 90–95 | RA | 5,8 | |||||||
|
| ||||||||||
| 11 | 58–68 | OA | 49 | |||||||
Homology mapping was performed using MapView (http://www.ncbi.nlm.nih.gov/mapview/) and Rat Genome Database (http://rgd.mcw.edu/). Murine QTL positions and sizes were based on linkage analysis of (BALB/c x DBA/2)F2 hybrids (Fig. 2). Chromosome intervals shorter than 1 Mbp are not shown. Chr, murine chromosome assignment, Mbp, locus position in millions of base pairs, Ref, references; RA, rheumatoid arthritis; RAJ, systemic juvenile rheumatoid arthritis; OA, osteoarthritis; Ptpn22, non-receptor type 22 protein tyrosine phosphatase; GOUT1, gout susceptibility locus 1; IL2, severe combined immunodeficiency due to interleukin-2 (IL-2) deficiency; IF, C3b inactivator deficiency due to alteration in the inhibitory complement component i; BBS, Bardet-Biedl syndrome; n.d., no QTL was reported.
The major binary PGIA-permissive locus, besides H-2d, is Pgia21 on chr7. This locus, whose position and genetic effects were confirmed using a congenic approach in this study, overlaps with the major non-MHC QTL Cia2 in the rat genome (Table 3). Both Cia2 and Pgia21 are notably sex-biased loci that control arthritis susceptibility only in males.41 Interestingly, Pgia21 also overlaps with the mCia7 locus in the mouse genome, where it has been shown to control disease severity, but not early onset.42 Chromosome loci Eae6 and Piax, linked to experimental allergic encephalomyelitis and pristane-induced arthritis (PIA), murine models of multiple sclerosis and RA, respectively, overlapped with Pgia21 as well (reference43 and Table 3). A homologous human RA-linked QTL is located on chr15.44
The Pgia4 locus (chr8) corresponds to the Pia14 locus on rat chr1645 and a QTL for RA on human chr8 (Table 3).9 The Pgia12 locus on chr19 is clustered with suggestive Cia and Eae7 loci on the near telomeric part of rat chr1.41,43,46 In the human genome, this chromosome interval corresponds to RA locus on chr10. 9 Additionally, murine Pgia12 is clustered with human loci chr9 and chr11 that control cartilage degradation in osteoarthritis47,48 (Table 3).
Apparently each of the murine PGIA-linked loci is homologous in humans and rats with several syntenic loci, associated with either the pathology or the immunologic background of autoimmune or joint diseases. At the present level of genomic mapping, where each murine locus occupies up to 50 Mbp and contains several hundred genes, clustering with other disease-related loci appears to be significant; however, the overlap between loci might become weaker or disappear at higher resolution.
In the initial linkage analysis of the (BALB/c x DBA/2)F2 population, we found that alleles of BALB/c origin on chr3, chr8 and chr19 promote arthritis, but only one QTL on chr7 carries an arthritis-promoting allele from DBA/2 (Table 1). Immunization of congenic strains confirmed these observations for chr3 and chr19 congenic strains. For chr7 and chr8 the correlation with the initial linkage analysis was only partial. As expected, Pgia21 (chr7) congenic males revealed much earlier responses to PG immunization (Fig. 1, Table 2), although Pgia21-congenic females responded with lower disease susceptibility; thus, the Pgia21 locus exerted an opposite effect on males and females. We hypothesize that the complex genetic effects of this chromosome interval are due to the presence of at least two genes/loci within the region. In females, the first locus suppresses PGIA and the second one is silent. In males, the first locus is silent and the second promotes arthritis. This hypothesis of QTL heterogeneity is also supported by the different positions of QTL peaks in males and females in the initial linkage analysis of F2 hybrids.19 Further breeding of sub-congenic mice is needed to prove this hypothesis.
Congenic mice are powerful tools, since all effects are multiplied according to the number of mice used for immunization. Conversely, every mouse in an F2 population is unique, and its genetic background is complex. Gene-gene interactions in congenic mice are suppressed due to the “clean” and homogeneous genetic background. F2 mice, however, might represent any allele-allele combinations. These major differences in genetic background account for the discrepancies between linkage analysis and congenic strains. For example, we earlier detected interaction between chr3 and chr15 loci,49 implying that the Pgia26 locus needs another gene on a different chromosome for its correct penetrance. A phenomenon similar to gene-gene interaction was reported for a CIA locus in the same positions as chr15 and Pgia26. Cia30 locus on chr15 (corresponds to Pgia8) showed interaction with Cia5/Eae3 locus on chr3 (corresponds to Pgia26).50 Therefore, we can offer two possible reasons why Pgia26 appears to be a purely female-specific QTL in F2 hybrids, and now we have detected the control of the locus in males as well. The first reason is the different genetic structure of the BALB/c.DBA/2-Pgia26 congenic strain versus (BALB/c x DBA/2)F2 hybrid population. The second reason is the extreme sensitivity of the congenic approach, when the response of a single mouse is amplified in dozens of congenic animals.
We measured serum cytokines as well as T cell responses in this set of congenic mice. Interestingly, each of the four congenic strains revealed unique patterns of immune responses (Fig. 2). The most striking observation was the opposite T-cell responses in similarly less arthritis-susceptible chr3 and chr19 congenic mice. Despite the presence of PGIA-suppressive loci in both strains, arthritis in Pgia26 congenic mice (chr3) was associated with lower lymphocyte responses than in BALB/c mice. These reduced responses were also reflected in reduced cell proliferation rate and lower production of IL-4, IFN-γ and TNF-α (Fig. 2). Conversely, a similarly suppressive Pgia12 locus on chr19, demonstrated increased PG-stimulated lymphocyte proliferation and production of IFN-γ and IL-4. It is likely that the difference between Pgia26 and Pgia12 arthritis-suppressive effects was, at least in part, associated with TNF-α-producing sub-populations in Pgia26-congenic T lymphocytes.
Another congenic strain that also carries an arthritis-suppressive gene is Pgia21 congenic females (chr7). Remarkably, the immune profile of these mice is quite different from that in clinically similar chr3 and chr19 congenics (Fig. 2). Despite the fact that the Pgia21 locus seems to control the production of autoimmune IgG1 antibodies to mouse PG (350% higher than in wild-type BALB/c littermates), most likely another gene which is active in females, inhibits arthritis development. Comparison of chr7 females with chr7 congenic males might shed light on the identity of this gene located within the Pgia21 locus. In males, earlier arthritis development correlates with even more elevated levels of autoantibodies (700% higher than in wild-type BALB/c littermates). This antibody increase was supported by an increase of proinflammatory IL-6 and lower levels of PGIA-suppressive IL-4 in mouse serum (Fig. 2).
Immune responses in males and females of the same congenic group are generally well correlated; for example, there is lower T-cell proliferation and interleukin production in Pgia26; high auto-antibody and low production of TNF-α in Pgia21; and upregulation of proinflammatory cytokine production and lymphocyte proliferation in Pgia12. The detailed comparison of immune parameters in male and females of the same strain seems to be an important approach to dissecting the reason for the differences in arthritis progression and clinical traits. Thus, the greater responsiveness of female lymphocytes to PG-stimulation (proliferation rate, IFNγ, and IL-4) seems to be responsible for the PGIA-protective effect of the same locus in females versus males (Fig. 2). It seems that a stronger proliferative response of lymphocytes upon in vitro stimulation with antigen is inversely correlated with arthritis susceptibility (see Pgia21 and Pgia12 females). This may be due to different regulatory mechanisms of various T cell subsets in vitro and in vivo; this, however, requires additional studies.
Chr3, chr7, chr8, and chr19 QTLs in this set of congenic strains are represented with quite large chromosome intervals up to 48 Mb in size. Obviously, these intervals contain numerous genes, affecting mostly different immune parameters, and only one or two genes from the region is a causative (etiologic) factor of arthritis. Chr7 Pgia21 locus is a good example of such heterogeneity. Only because of the opposite effects upon PGIA (Fig. 1B) we are able to detect this multiplicity. In the case of several genes in the locus affecting the disease in the same direction we are unable to make any conclusion about homogeneity within the locus, and only sub-congenic strains will shed light upon locus structure. We believe that locus heterogeneity is probably a major reason for these multiple and often diverse immune responses. We conclude that at this phase of QTL mapping, all immune parameters should be considered in concerto. In other words, we have not yet found a single immune biomarker which would explain all effects and would lay the foundation for all observed (secondary) immune responses.
Experimental murine PG-induced arthritis is dependent on auto antibodies, T cells and a number of cytokines. In this study we measured only a few of the cytokines which were found to be important from the earlier studies. Obviously, when natural mutations within QTLs are considered, their effect is probably not as strong as in purely artificial gene-deficient models. Therefore, genes controlling bioactive protein expressions (chemokines, cytokines, growth factors), proteins such as those used as biologics for treatment of RA, are probably more important in disease-prone individuals than in the normal population.
Materials and Methods
Animal breeding
All animal experiments were approved by the Institutional Animal Care and Use Committee, Rush University Medical Center, Chicago, IL. For the present study, we have selected four major QTLs based on two criteria: (i) statistical strength of the linkage to one of the clinical features of the arthritis, and (ii) reproducibility of locus detection in different crosses. These four QTLs were on chr3 (Pgia26), chr7 (Pgia21), chr8 (Pgia4) and chr19 (Pgia12).19 PGIA-susceptible BALB/c female and PGIA-resistant DBA/2 male mice (National Cancer Institute, Kingston Colony, NY) were mated and the F1 males were backcrossed to parental BALB/c strain to obtain the N2 backcross generation (n=500). Animals were maintained in a pathogen-free environment. Marker-assisted selection protocol for speed congenic breeding was used to generate QTL-specific congenic strains.24 N2 males were genotyped with 230 genomic markers selected for detectable length polymorphism between the parental strains using information from the Mouse Genome Informatics database (http://www.informatics.jax.org). N2 males bearing DBA/2-type heterozygous QTLs on chr3, chr7, chr8, or chr19 and the highest number of BALB/c alleles on the rest of the genome were selected for the next backcross. Approximately 40 offspring males after each backcross were genotyped with seven QTL-specific markers, and additional markers were used for the genomic regions found to be heterozygous in the previous backcross. At backcross level N6, when the entire genome was BALB/c homozygous except for the selected QTLs, N6 males and females of the same genotype were intercrossed generating N6F1 population of BALB/c.DBA/2-QTL founders. We intercrossed these mice to generate the N6F2 population, which was immunized with PG to induce arthritis.
Antigen and immunization
Cartilage was obtained from osteoarthritic patients undergoing joint replacement surgery. The use of human cartilage for PG isolation was approved by the Institutional Review Board (Rush University Medical Center, Chicago, IL). PG was isolated as described elsewhere.51,52 Mice were immunized with human PG at 12 weeks of age using a standard immunization protocol.51,52 Briefly, emulsion containing 100 μg of PG protein and 2 mg of dimethyl-dioctadecyl ammonium bromide adjuvant (DDA) in 200 μl of PBS pH 7.4 was injected intraperitoneally on days 0, 21, 42 and 63. Alternatively, mice were intraperitoneally injected with emulsion of 100 μg of PG protein in 100 μl of Freund’s adjuvants (Difco, Detroit, MI).53 The first and fourth injections were given in Freund’s complete adjuvant (CFA), whereas the second and third boosters contained antigen in incomplete Freund’s adjuvant (IFA). Animal were immunized with either PG and DDA or PG and CFA/IFA, and comparative studies showed no differences between the two immunization protocols, except in chr7 congenic strain. The results of this congenic strain (chr7) are shown. Arthritis severity was determined using a visual scoring system based on the degree of swelling and redness of the front and hind paws.52,53
Animals were examined three times a week and inflammation was scored from 0 to 4 for each paw, thus resulting in a maximum cumulative arthritis score of 16 for each animal. 52,53 Severity score was recorded only for arthritic mice. The onset scores of arthritis (from 0 to 5) were proportionally distributed from days 32 to 81 giving the highest score (“5”) to the mouse having arthritis on day 32, and “0” indicating a non-arthritic animal on day 81. Mice were sacrificed 18 days (on day 81) after the fourth antigen-adjuvant injection. This was a long-term immunization protocol, because 98–100% of wild-type BALB/c mice (either littermates or age-matched controls) developed arthritis after the third PG injection using either DDA or CFA/IFA adjuvant.
Antibodies, lymphocyte responses and serum cytokine production
Serum levels of autoantibodies to mouse PG of IgG1 and IgG2a isotypes were measured by enzyme-linked immunosorbent assay (ELISA) as described previously.20,52 Serum cytokines TNF-α, IL-1β, IL-6 and IL-4 were determined using ELISA kits purchased from BD Bioscience (San Diego, CA) or R&D Systems (Minneapolis, MN) as described.23 Additional antigen-specific lymphocyte responses were measured in samples of spleen cells cultured in the presence of 25 μg/ml PG antigen. IL-1β, IL-4, IL-6, IL-10, IL-17, IFN-γ, and TNF-α productions were measured in cell culture supernatants on day 4 using capture ELISA from R&D Systems (Minneapolis, MN) as described. 20,23,52 In vitro T cell proliferation in spleen cell cultures in response to 25 μg/ml PG stimulation was assessed on day 5 by incorporation of [3H]-thymidine and was expressed as stimulation index, a ratio of incorporated [3H]-thymidine in PG-stimulated versus non-stimulated cultures.52 IL-2 production was measured by CTLL-2 assay.26,51,52 However, only those results are shown and discussed which showed correlations with the QTLs in the four congenic lines.
Statistical analysis
Statistical analysis was performed using SPSS statistical software (SPSS Inc., Chicago, IL). Since the incidence of the disease was a dichotomous variable, we used the Chi-square test to examine differences between populations. We used day-by-day comparison to evaluate the differences between a group of congenic mice and a combined group of BALB/c-homozygous littermates, which originated from intercrosses of four congenic strains with parental BALB/c strain. Two-sample non-paired Student’s t-test was employed for comparison of means of two groups, where the data showed normal distribution. Significance level was routinely set at p<0.05.
Acknowledgments
This work was supported by research grants P01 AR045652, R01 AR040310 of the National Institutes of Health (USA), the J.O. Galante, MD Endowment Chair (Rush University Medical Center, Chicago, IL), and The Grainger Foundation (Chicago, IL). Authors appreciate human cartilage samples received from surgeons of Midwest Orthopedics (Rush University Medical Center, Chicago, IL), and technical support of Dr. A. Nesterovitch and Dr. G. Hutas (Rush University Medical Center).
Abbreviations used in this paper
- Chr
chromosome
- CIA
collagen-induced arthritis
- DDA
dimethyldioctadecylammonium bromide
- LOD
logarithm of the odds ratio
- MHC
major histocompatibility complex
- PG
proteoglycan aggrecan
- PGIA
proteoglycan-induced arthritis
- QTL(s)
quantitative trait locus (loci)
- RA
rheumatoid arthritis
References
- 1.Lynn AH, Kwoh CK, Venglish CM, Aston CE, Chakravarti A. Genetic epidemiology of rheumatoid arthritis. Am J Hum Genet. 1995;57:150–159. [PMC free article] [PubMed] [Google Scholar]
- 2.MacGregor AJ, Snieder H, Rigby AS, Koskenvuo M, Kaprio J, Aho K, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 2000;43:30–37. doi: 10.1002/1529-0131(200001)43:1<30::AID-ANR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Choi S, Rho Y, Ji JD, Song GG, Lee Y. Genome scan meta-analysis of rheumatoid arthritis. Rheumatology (Oxford) 2006;45:166–170. doi: 10.1093/rheumatology/kei128. [DOI] [PubMed] [Google Scholar]
- 4.Cornelis F, Faure S, Martinez M, Prud’homme JF, Fritz P, Dib C, et al. New susceptibility locus for rheumatoid arthritis suggested by a genome-wide linkage study. Proc Natl Acad Sci U S A. 1998;95:10746–10750. doi: 10.1073/pnas.95.18.10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiozawa S, Hayashi S, Tsukamoto Y, Goko H, Kawasaki H, Wada T, et al. Identification of the gene loci that predispose to rheumatoid arthritis. Int Immunol. 1998;10:1891–1895. doi: 10.1093/intimm/10.12.1891. [DOI] [PubMed] [Google Scholar]
- 6.Barton A, Eyre S, Myerscough A, Brintnell B, Ward D, Ollier WE, et al. High resolution linkage and association mapping identifies a novel rheumatoid arthritis susceptibility locus homologous to one linked to two rat models of inflammatory arthritis. Hum Mol Genet. 2001;10:1901–1906. doi: 10.1093/hmg/10.18.1901. [DOI] [PubMed] [Google Scholar]
- 7.Jawaheer D, Seldin MF, Amos CI, Chen WV, Shigeta R, Monteiro J, et al. A genome-wide screen in multiplex rheumatoid arthritis families suggests genetic overlap with other autoimmune diseases. Am J Hum Genet. 2001;68:927–936. doi: 10.1086/319518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John S, Eyre S, Myerscough A, Barrett J, Silman A, Ollier W, et al. Linkage and association analysis of candidate genes in rheumatoid arthritis. J Rheumatol. 2001;28:1752–1755. [PubMed] [Google Scholar]
- 9.MacKay K, Eyre S, Myerscough A, Milicic A, Barton A, Laval S, et al. Whole-genome linkage analysis of rheumatoid arthritis susceptibility loci in 252 affected sibling pairs in the United Kingdom. Arthritis Rheum. 2002;46:632–639. doi: 10.1002/art.10147. [DOI] [PubMed] [Google Scholar]
- 10.Jawaheer D, Seldin MF, Amos CI, Chen WV, Shigeta R, Etzel C, et al. Screening the genome for rheumatoid arthritis susceptibility genes: A replication study and combined analysis of 512 multicase families. Arthritis Rheum. 2003;48:906–916. doi: 10.1002/art.10989. [DOI] [PubMed] [Google Scholar]
- 11.Osorio Y, Fortea J, Bukulmez H, Petit-Teixeira E, Michou L, Pierlot C, et al. Dense genome-wide linkage analysis of rheumatoid arthritis, including covariates. Arthritis Rheum. 2004;50:2757–2765. doi: 10.1002/art.20458. [DOI] [PubMed] [Google Scholar]
- 12.Cleland LG. Animal models of rheumatoid arthritis. Br J Rheumatol. 1996;35:1041–1042. doi: 10.1093/rheumatology/35.11.1041. [DOI] [PubMed] [Google Scholar]
- 13.Holmdahl R. Dissection of the genetic complexity of arthritis using animal models. J Autoimmun. 2003;21:99–103. doi: 10.1016/s0896-8411(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 14.Kurreeman FA, Padyukov L, Marques RB, Schrodi SJ, Seddighzadeh M, Stoeken-Rijsbergen G, et al. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS Med. 2007;4:e278. doi: 10.1371/journal.pmed.0040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Kristan J, Hao L, Lenkoski CS, Shen Y, Matis LA. A role for complement in antibody-mediated inflammation: C5- deficient DBA/1 mice are resistant to collagen-induced arthritis. J Immunol. 2000;164:4340–4347. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 16.Mikecz K, Glant TT, Poole AR. Immunity to cartilage proteoglycans in BALB/c mice with progressive polyarthritis and ankylosing spondylitis induced by injection of human cartilage proteoglycan. Arthritis Rheum. 1987;30:306–318. doi: 10.1002/art.1780300310. [DOI] [PubMed] [Google Scholar]
- 17.Glant TT, Finnegan A, Mikecz K. Proteoglycan-induced arthritis: immune regulation, cellular mechanisms and genetics. Crit Rev Immunol. 2003;23:199–250. doi: 10.1615/critrevimmunol.v23.i3.20. [DOI] [PubMed] [Google Scholar]
- 18.Adarichev VA, Bardos T, Christodoulou S, Phillips MT, Mikecz K, Glant TT. Major histocompatibility complex controls susceptibility and dominant inheritance, but not the severity of the disease in mouse models of rheumatoid arthritis. Immunogenetics. 2002;54:184–192. doi: 10.1007/s00251-002-0462-8. [DOI] [PubMed] [Google Scholar]
- 19.Adarichev VA, Nesterovitch AB, Bardos T, Biesczat D, Chandrasekaran R, Vermes C, et al. Sex effect on clinical and immunological quantitative trait loci in a murine model of rheumatoid arthritis. Arthritis Rheum. 2003;48:1708–1720. doi: 10.1002/art.11016. [DOI] [PubMed] [Google Scholar]
- 20.Otto JM, Cs-Szabó G, Gallagher J, Velins S, Mikecz K, Buzas EI, et al. Identification of multiple loci linked to inflammation and autoantibody production by a genome scan of a murine model of rheumatoid arthritis. Arthritis Rheum. 1999;42:2524–2531. doi: 10.1002/1529-0131(199912)42:12<2524::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Otto JM, Chandrasekaran R, Vermes C, Mikecz K, Finnegan A, Rickert SE, et al. A genome scan using a novel genetic cross identifies new susceptibility loci and traits in a mouse model of rheumatoid arthritis. J Immunol. 2000;165:5278–5286. doi: 10.4049/jimmunol.165.9.5278. [DOI] [PubMed] [Google Scholar]
- 22.Glant TT, Adarichev VA. Immunogenomics and Human Disease. John Wiley & Sons, Ltd; Chichester: 2006. Immunogenetics of experimentally induced arthritis; pp. 271–297. [Google Scholar]
- 23.Adarichev VA, Valdez JC, Bardos T, Finnegan A, Mikecz K, Glant TT. Combined autoimmune models of arthritis reveal shared and independent qualitative (binary) and quantitative trait loci. J Immunol. 2003;170:2283–2292. doi: 10.4049/jimmunol.170.5.2283. [DOI] [PubMed] [Google Scholar]
- 24.Markel P, Shu P, Ebeling C, Carlson GA, Nagle DL, Smutko JS, et al. Theoretical and empirical issues for marker-assisted breeding of congenic mouse strains. Nat Genet. 1997;17:280–284. doi: 10.1038/ng1197-280. [DOI] [PubMed] [Google Scholar]
- 25.Katz D, Lehrer S, Galan O, Lachmi B, Cohen S, Inbar I, et al. Unique immunomodulating properties of dimethyl dioctadecyl ammonium bromide (DDA) in experimental viral vaccines. Adv Exp Med Biol. 1996;397:115–125. doi: 10.1007/978-1-4899-1382-1_16. [DOI] [PubMed] [Google Scholar]
- 26.Bardos T, Mikecz K, Finnegan A, Zhang J, Glant TT. T and B cell recovery in arthritis adoptively transferred to SCID mice: Antigen-specific activation is required for restoration of autopathogenic CD4+ Th1 cells in a syngeneic system. J Immunol. 2002;168:6013–6021. doi: 10.4049/jimmunol.168.12.6013. [DOI] [PubMed] [Google Scholar]
- 27.Adarichev VA, Vermes C, Hanyecz A, Mikecz K, Bremer E, Glant TT. Gene expression profiling in murine autoimmune arthritis during the initiation and progression of joint inflammation. Arthritis Res Ther. 2005;7:R196–R207. doi: 10.1186/ar1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finnegan A, Mikecz K, Tao P, Glant TT. Proteoglycan (aggrecan)-induced arthritis in BALB/c mice is a Th1-type disease regulated by Th2 cytokines. J Immunol. 1999;163:5383–5390. [PubMed] [Google Scholar]
- 29.Gulko PS, Kawahito Y, Remmers EF, Reese VR, Wang JP, Dracheva SV, et al. Identification of a new non-major histocompatibility complex genetic locus on chromosome 2 that controls disease severity in collagen-induced arthritis in rats. Arthritis Rheum. 1998;41:2122–2131. doi: 10.1002/1529-0131(199812)41:12<2122::AID-ART7>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Brenner M, Meng HC, Yarlett NC, Griffiths MM, Remmers EF, Wilder RL, et al. The non-major histocompatibility complex quantitative trait locus Cia10 contains a major arthritis gene and regulates disease severity, pannus formation, and joint damage. Arthritis Rheum. 2005;52:322–332. doi: 10.1002/art.20782. [DOI] [PubMed] [Google Scholar]
- 31.Cheng LS, Chiang SL, Tu HP, Chang SJ, Wang TN, Ko AM, et al. Genomewide scan for gout in taiwanese aborigines reveals linkage to chromosome 4q25. Am J Hum Genet. 2004;75:498–503. doi: 10.1086/423429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abramson N, Alper CA, Lachmann PJ, Rosen FS, Jandl JH. Deficiency of C3 inactivator in man. J Immunol. 1971;107:19–27. [PubMed] [Google Scholar]
- 33.Weinberg K, Parkman R. Severe combined immunodeficiency due to a specific defect in the production of interleukin-2. N Engl J Med. 1990;322:1718–1723. doi: 10.1056/NEJM199006143222406. [DOI] [PubMed] [Google Scholar]
- 34.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 36.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 37.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 38.Viken MK, Amundsen SS, Kvien TK, Boberg KM, Gilboe IM, Lilleby V, et al. Association analysis of the 1858C>T polymorphism in the PTPN22 gene in juvenile idiopathic arthritis and other autoimmune diseases. Genes Immun. 2005;6:271–273. doi: 10.1038/sj.gene.6364178. [DOI] [PubMed] [Google Scholar]
- 39.Carlton VE, Hu X, Chokkalingam AP, Schrodi SJ, Brandon R, Alexander HC, et al. PTPN22 Genetic Variation: Evidence for Multiple Variants Associated with Rheumatoid Arthritis. Am J Hum Genet. 2005;77:567–581. doi: 10.1086/468189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, et al. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561–571. doi: 10.1086/429096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furuya T, Salstrom JL, McCall-Vining S, Cannon GW, Joe B, Remmers EF, et al. Genetic dissection of a rat model for rheumatoid arthritis: significant gender influences on autosomal modifier loci. Hum Mol Genet. 2000;9:2241–2250. doi: 10.1093/oxfordjournals.hmg.a018915. [DOI] [PubMed] [Google Scholar]
- 42.Yang H-T, Jirholt J, Svensson L, Sundvall M, Jansson L, Pettersson U, et al. Identification of genes controlling collagen-induced arthritis in mice: striking homology with susceptibility loci previously identified in the rat. J Immunol. 1999;163:2916–2921. [PubMed] [Google Scholar]
- 43.Bergsteinsdottir K, Yang H-T, Pettersson U, Holmdahl R. Evidence for common autoimmune disease genes controlling onset, severity, and chronicity based on experimental models for multiple sclerosis and rheumatoid arthritis. J Immunol. 2000;164:1564–1568. doi: 10.4049/jimmunol.164.3.1564. [DOI] [PubMed] [Google Scholar]
- 44.Wise CA, Bennett LB, Pascual V, Gillum JD, Bowcock AM. Localization of a gene for familial recurrent arthritis. Arthritis Rheum. 2000;43:2041–2045. doi: 10.1002/1529-0131(200009)43:9<2041::AID-ANR15>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 45.Lu S, Nordquist N, Holmberg J, Olofsson P, Pettersson U, Holmdahl R. Both common and unique susceptibility genes in different rat strains with pristane-induced arthritis. Eur J Hum Genet. 2002;10:475–483. doi: 10.1038/sj.ejhg.5200832. [DOI] [PubMed] [Google Scholar]
- 46.Teuscher C, Butterfield RJ, Ma RZ, Zachary JF, Doerge RW, Blankenhorn EP. Sequence polymorphisms in chemokines Scya1 (TCA-3), Scya2 (monocyte chemoattractant protein (MCP)-1), and Scya12 (MCP-5) are candidates for eae7, a locus controlling susceptibility to monophasic remitting/nonrelapsing experimental allergic encephalomyelitis. J Immunol. 1999;163:2262–2266. [PubMed] [Google Scholar]
- 47.Chapman K, Mustafa Z, Irven C, Carr AJ, Clipsham K, Smith A, et al. Osteoarthritis-susceptibility locus on chromosome 11q, detected by linkage. Am J Hum Genet. 1999;65:167–174. doi: 10.1086/302465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37:138–144. doi: 10.1038/ng1496. [DOI] [PubMed] [Google Scholar]
- 49.Adarichev VA, Nesterovitch AB, Biesczat DC, Glant TT. Genome scan for arthritis genes revealed significant chromosome locus-locus interation in mouse proteoglycan-induced arthritis. Arthritis & Rheumatism. 2003;48:S229. [Google Scholar]
- 50.Karlsson J, Johannesson M, Lindvall T, Wernhoff P, Holmdahl R, Andersson A. Genetic interactions in Eae2 control collagen-induced arthritis and the CD4+/CD8+ T cell ratio. J Immunol. 2005;174:533–541. doi: 10.4049/jimmunol.174.1.533. [DOI] [PubMed] [Google Scholar]
- 51.Hanyecz A, Berlo SE, Szanto S, Broeren CPM, Mikecz K, Glant TT. Achievement of a synergistic adjuvant effect on arthritis induction by activation of innate immunity and forcing the immune response toward the Th1 phenotype. Arthritis Rheum. 2004;50:1665–1676. doi: 10.1002/art.20180. [DOI] [PubMed] [Google Scholar]
- 52.Glant TT, Mikecz K. Proteoglycan aggrecan-induced arthritis. A murine autoimmune model of rheumatoid arthritis. Methods Mol Med. 2004;102:313–338. doi: 10.1385/1-59259-805-6:313. [DOI] [PubMed] [Google Scholar]
- 53.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987;30:201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]