Abstract
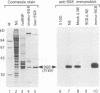
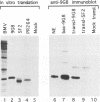
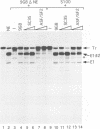
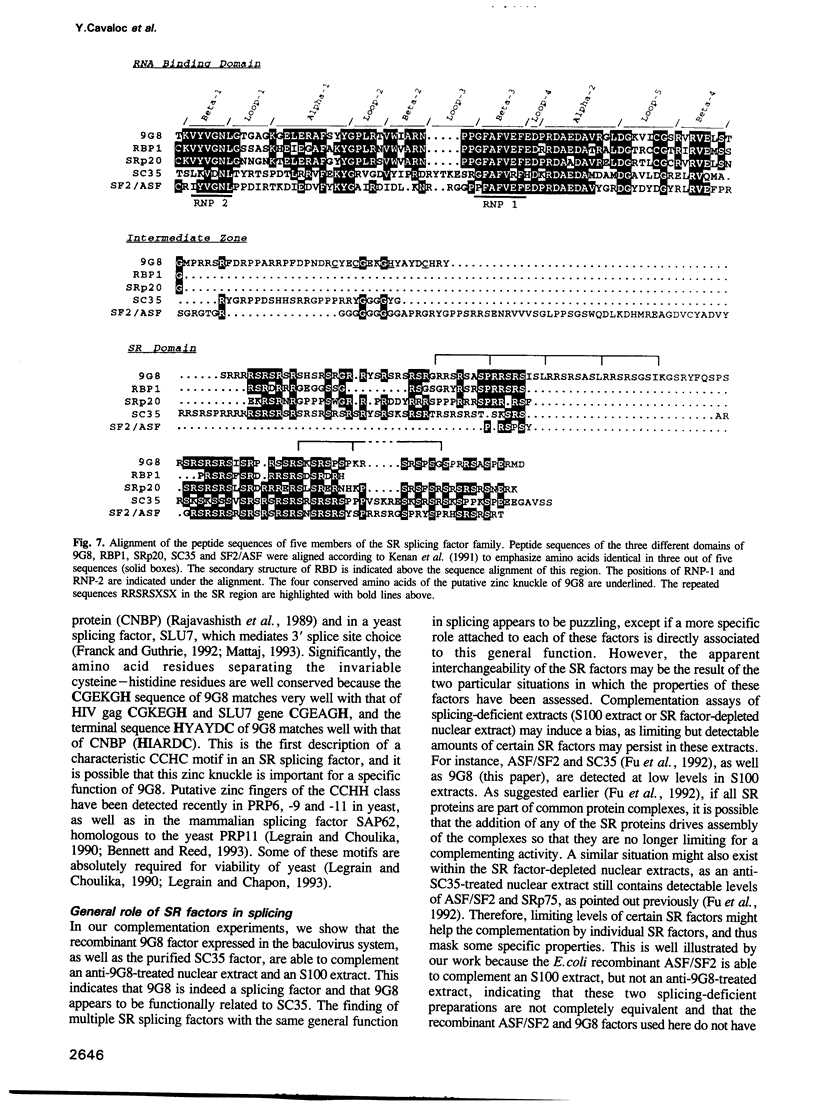
By adopting a monoclonal antibody approach, we have identified a novel splicing factor of 35 kDa which we have termed 9G8. The isolation and characterization of cDNA clones indicate that 9G8 is a novel member of the serine/arginine (SR) splicing factor family because it includes an N-terminal RNA binding domain (RBD) and a C-terminal SR domain. The RNA binding domain of 9G8 is highly homologous to those of the SRp20 and RBP1 factors (79-71% identity), but the homology is less pronounced in the cases of SF2/ASF and SC35/PR264 (45-37% identity). Compared with the other SR splicing factors, 9G8 presents some specific sequence features because it contains an RRSRSXSX consensus sequence repeated six times in the SR domain, and a CCHC motif in its median region, similar to the zinc knuckle found in the SLU7 splicing factor in yeast. Complete immunodepletion of 9G8 from a nuclear extract, which is accompanied by a substantial depletion of other SR factors, results in a loss of splicing activity. We show that a recombinant 9G8 protein, expressed using a baculovirus vector and excluding other SR factors, rescues the splicing activity of a 9G8-depleted nuclear extract and an S100 cytoplasmic fraction. This indicates that 9G8 plays a crucial role in splicing, similar to that of the other SR splicing factors. This similarity was confirmed by the fact that purified human SC35 also rescues the 9G8-depleted extract. The identification of the 9G8 factor enlarges the essential family of SR splicing factors, whose members have also been proposed to play key roles in alternative splicing.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ast G., Goldblatt D., Offen D., Sperling J., Sperling R. A novel splicing factor is an integral component of 200S large nuclear ribonucleoprotein (InRNP) particles. EMBO J. 1991 Feb;10(2):425–432. doi: 10.1002/j.1460-2075.1991.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayane M., Preuss U., Köhler G., Nielsen P. J. A differentially expressed murine RNA encoding a protein with similarities to two types of nucleic acid binding motifs. Nucleic Acids Res. 1991 Mar 25;19(6):1273–1278. doi: 10.1093/nar/19.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens S. E., Galisson F., Legrain P., Lührmann R. Evidence that the 60-kDa protein of 17S U2 small nuclear ribonucleoprotein is immunologically and functionally related to the yeast PRP9 splicing factor and is required for the efficient formation of prespliceosomes. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8229–8233. doi: 10.1073/pnas.90.17.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Michaud S., Kingston J., Reed R. Protein components specifically associated with prespliceosome and spliceosome complexes. Genes Dev. 1992 Oct;6(10):1986–2000. doi: 10.1101/gad.6.10.1986. [DOI] [PubMed] [Google Scholar]
- Bennett M., Reed R. Correspondence between a mammalian spliceosome component and an essential yeast splicing factor. Science. 1993 Oct 1;262(5130):105–108. doi: 10.1126/science.8211113. [DOI] [PubMed] [Google Scholar]
- Bentley R. C., Keene J. D. Recognition of U1 and U2 small nuclear RNAs can be altered by a 5-amino-acid segment in the U2 small nuclear ribonucleoprotein particle (snRNP) B" protein and through interactions with U2 snRNP-A' protein. Mol Cell Biol. 1991 Apr;11(4):1829–1839. doi: 10.1128/mcb.11.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosi R., Gröning K., Behrens S. E., Lührmann R., Krämer A. Interaction of mammalian splicing factor SF3a with U2 snRNP and relation of its 60-kD subunit to yeast PRP9. Science. 1993 Oct 1;262(5130):102–105. doi: 10.1126/science.8211112. [DOI] [PubMed] [Google Scholar]
- Brosi R., Hauri H. P., Krämer A. Separation of splicing factor SF3 into two components and purification of SF3a activity. J Biol Chem. 1993 Aug 15;268(23):17640–17646. [PubMed] [Google Scholar]
- Cáceres J. F., Krainer A. R. Functional analysis of pre-mRNA splicing factor SF2/ASF structural domains. EMBO J. 1993 Dec;12(12):4715–4726. doi: 10.1002/j.1460-2075.1993.tb06160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Ireland D. C., Smith R. A., Mayeda A., Krainer A. R. Pathways for selection of 5' splice sites by U1 snRNPs and SF2/ASF. EMBO J. 1993 Sep;12(9):3607–3617. doi: 10.1002/j.1460-2075.1993.tb06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D., Guthrie C. An essential splicing factor, SLU7, mediates 3' splice site choice in yeast. Genes Dev. 1992 Nov;6(11):2112–2124. doi: 10.1101/gad.6.11.2112. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Isolation of a complementary DNA that encodes the mammalian splicing factor SC35. Science. 1992 Apr 24;256(5056):535–538. doi: 10.1126/science.1373910. [DOI] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. The 35-kDa mammalian splicing factor SC35 mediates specific interactions between U1 and U2 small nuclear ribonucleoprotein particles at the 3' splice site. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1725–1729. doi: 10.1073/pnas.89.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Mayeda A., Maniatis T., Krainer A. R. General splicing factors SF2 and SC35 have equivalent activities in vitro, and both affect alternative 5' and 3' splice site selection. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11224–11228. doi: 10.1073/pnas.89.23.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D. Specific commitment of different pre-mRNAs to splicing by single SR proteins. Nature. 1993 Sep 2;365(6441):82–85. doi: 10.1038/365082a0. [DOI] [PubMed] [Google Scholar]
- Gattoni R., Chebli K., Himmelspach M., Stévenin J. Modulation of alternative splicing of adenoviral E1A transcripts: factors involved in the early-to-late transition. Genes Dev. 1991 Oct;5(10):1847–1858. doi: 10.1101/gad.5.10.1847. [DOI] [PubMed] [Google Scholar]
- Ge H., Manley J. L. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990 Jul 13;62(1):25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- Ge H., Zuo P., Manley J. L. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991 Jul 26;66(2):373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Gentz R., Chen C. H., Rosen C. A. Bioassay for trans-activation using purified human immunodeficiency virus tat-encoded protein: trans-activation requires mRNA synthesis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):821–824. doi: 10.1073/pnas.86.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Berg J. M. A retroviral Cys-Xaa2-Cys-Xaa4-His-Xaa4-Cys peptide binds metal ions: spectroscopic studies and a proposed three-dimensional structure. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4047–4051. doi: 10.1073/pnas.86.11.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Biochemical mechanisms of constitutive and regulated pre-mRNA splicing. Annu Rev Cell Biol. 1991;7:559–599. doi: 10.1146/annurev.cb.07.110191.003015. [DOI] [PubMed] [Google Scholar]
- Guthrie C. Messenger RNA splicing in yeast: clues to why the spliceosome is a ribonucleoprotein. Science. 1991 Jul 12;253(5016):157–163. doi: 10.1126/science.1853200. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Zuo P., Manley J. L., Baker B. S. The Drosophila RNA-binding protein RBP1 is localized to transcriptionally active sites of chromosomes and shows a functional similarity to human splicing factor ASF/SF2. Genes Dev. 1992 Dec;6(12B):2569–2579. doi: 10.1101/gad.6.12b.2569. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. Purification and characterization of pre-mRNA splicing factor SF2 from HeLa cells. Genes Dev. 1990 Jul;4(7):1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. The essential pre-mRNA splicing factor SF2 influences 5' splice site selection by activating proximal sites. Cell. 1990 Jul 13;62(1):35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Mayeda A., Kozak D., Binns G. Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators. Cell. 1991 Jul 26;66(2):383–394. doi: 10.1016/0092-8674(91)90627-b. [DOI] [PubMed] [Google Scholar]
- Krämer A., Utans U. Three protein factors (SF1, SF3 and U2AF) function in pre-splicing complex formation in addition to snRNPs. EMBO J. 1991 Jun;10(6):1503–1509. doi: 10.1002/j.1460-2075.1991.tb07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Legrain P., Chapon C. Interaction between PRP11 and SPP91 yeast splicing factors and characterization of a PRP9-PRP11-SPP91 complex. Science. 1993 Oct 1;262(5130):108–110. doi: 10.1126/science.8211114. [DOI] [PubMed] [Google Scholar]
- Legrain P., Choulika A. The molecular characterization of PRP6 and PRP9 yeast genes reveals a new cysteine/histidine motif common to several splicing factors. EMBO J. 1990 Sep;9(9):2775–2781. doi: 10.1002/j.1460-2075.1990.tb07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Bingham P. M. Arginine/serine-rich domains of the su(wa) and tra RNA processing regulators target proteins to a subnuclear compartment implicated in splicing. Cell. 1991 Oct 18;67(2):335–342. doi: 10.1016/0092-8674(91)90185-2. [DOI] [PubMed] [Google Scholar]
- Lutz Y., Jacob M., Fuchs J. P. The distribution of two hnRNP-associated proteins defined by a monoclonal antibody is altered in heat-shocked HeLa cells. Exp Cell Res. 1988 Mar;175(1):109–124. doi: 10.1016/0014-4827(88)90259-5. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W. RNA recognition: a family matter? Cell. 1993 Jun 4;73(5):837–840. doi: 10.1016/0092-8674(93)90265-r. [DOI] [PubMed] [Google Scholar]
- Mayeda A., Zahler A. M., Krainer A. R., Roth M. B. Two members of a conserved family of nuclear phosphoproteins are involved in pre-mRNA splicing. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1301–1304. doi: 10.1073/pnas.89.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M. The role of small nuclear RNAs in RNA splicing. Curr Opin Cell Biol. 1993 Jun;5(3):448–454. doi: 10.1016/0955-0674(93)90010-n. [DOI] [PubMed] [Google Scholar]
- Patton J. G., Porro E. B., Galceran J., Tempst P., Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993 Mar;7(3):393–406. doi: 10.1101/gad.7.3.393. [DOI] [PubMed] [Google Scholar]
- Perez A., Kastner P., Sethi S., Lutz Y., Reibel C., Chambon P. PMLRAR homodimers: distinct DNA binding properties and heteromeric interactions with RXR. EMBO J. 1993 Aug;12(8):3171–3182. doi: 10.1002/j.1460-2075.1993.tb05986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Taylor A. K., Andalibi A., Svenson K. L., Lusis A. J. Identification of a zinc finger protein that binds to the sterol regulatory element. Science. 1989 Aug 11;245(4918):640–643. doi: 10.1126/science.2562787. [DOI] [PubMed] [Google Scholar]
- Roth M. B., Zahler A. M., Stolk J. A. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol. 1991 Nov;115(3):587–596. doi: 10.1083/jcb.115.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby S. W., Abelson J. Pre-mRNA splicing in yeast. Trends Genet. 1991 Mar;7(3):79–85. doi: 10.1016/0168-9525(91)90276-V. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Zamore P. D., Green M. R. A factor, U2AF, is required for U2 snRNP binding and splicing complex assembly. Cell. 1988 Jan 29;52(2):207–219. doi: 10.1016/0092-8674(88)90509-0. [DOI] [PubMed] [Google Scholar]
- Scherly D., Boelens W., Dathan N. A., van Venrooij W. J., Mattaj I. W. Major determinants of the specificity of interaction between small nuclear ribonucleoproteins U1A and U2B'' and their cognate RNAs. Nature. 1990 Jun 7;345(6275):502–506. doi: 10.1038/345502a0. [DOI] [PubMed] [Google Scholar]
- Schmitt P., Gattoni R., Keohavong P., Stévenin J. Alternative splicing of E1A transcripts of adenovirus requires appropriate ionic conditions in vitro. Cell. 1987 Jul 3;50(1):31–39. doi: 10.1016/0092-8674(87)90659-3. [DOI] [PubMed] [Google Scholar]
- Stévenin J., Gattoni R., Keohavong P., Jacob M. Mild nuclease treatment as a probe for a non-random distribution of adenovirus-specific RNA sequences and of cellular RNA in nuclear ribonucleoprotein fibrils. J Mol Biol. 1982 Mar 5;155(3):185–205. doi: 10.1016/0022-2836(82)90001-8. [DOI] [PubMed] [Google Scholar]
- Tazi J., Kornstädt U., Rossi F., Jeanteur P., Cathala G., Brunel C., Lührmann R. Thiophosphorylation of U1-70K protein inhibits pre-mRNA splicing. Nature. 1993 May 20;363(6426):283–286. doi: 10.1038/363283a0. [DOI] [PubMed] [Google Scholar]
- Tian M., Maniatis T. A splicing enhancer complex controls alternative splicing of doublesex pre-mRNA. Cell. 1993 Jul 16;74(1):105–114. doi: 10.1016/0092-8674(93)90298-5. [DOI] [PubMed] [Google Scholar]
- Vellard M., Soret J., Sureau A., Perbal B. A novel type of RNA-binding protein is potentially encoded by the opposite strand of the trans-spliced c-myb coding exon. C R Acad Sci III. 1991;313(13):591–597. [PubMed] [Google Scholar]
- Vellard M., Sureau A., Soret J., Martinerie C., Perbal B. A potential splicing factor is encoded by the opposite strand of the trans-spliced c-myb exon. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2511–2515. doi: 10.1073/pnas.89.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Maniatis T. Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 1993 Dec 17;75(6):1061–1070. doi: 10.1016/0092-8674(93)90316-i. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Lane W. S., Stolk J. A., Roth M. B. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev. 1992 May;6(5):837–847. doi: 10.1101/gad.6.5.837. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Neugebauer K. M., Lane W. S., Roth M. B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993 Apr 9;260(5105):219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]
- Zamore P. D., Patton J. G., Green M. R. Cloning and domain structure of the mammalian splicing factor U2AF. Nature. 1992 Feb 13;355(6361):609–614. doi: 10.1038/355609a0. [DOI] [PubMed] [Google Scholar]
- Zillmann M., Zapp M. L., Berget S. M. Gel electrophoretic isolation of splicing complexes containing U1 small nuclear ribonucleoprotein particles. Mol Cell Biol. 1988 Feb;8(2):814–821. doi: 10.1128/mcb.8.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo P., Manley J. L. Functional domains of the human splicing factor ASF/SF2. EMBO J. 1993 Dec;12(12):4727–4737. doi: 10.1002/j.1460-2075.1993.tb06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]