Abstract
Aims
The well-known limitations of vitamin K antagonists (VKA) led to development of new oral anticoagulants (NOAC) in non-valvular atrial fibrillation (NVAF). The aim of this meta-analysis was to determine the consistency of treatment effects of NOAC irrespective of age, comorbidities, or prior VKA exposure.
Methods and Results
All randomized, controlled phase III trials comparing NOAC to VKA up to October 2012 were eligible provided their results (stroke/systemic embolism (SSE) and major bleeding (MB)) were reported according to age (≤ or >75 years), renal function, CHADS2 score, presence of diabetes mellitus or heart failure, prior VKA use or previous cerebrovascular events. Interactions were considered significant at p <0.05. Three studies (50,578 patients) were included, respectively evaluating apixaban, rivaroxaban, and dabigatran versus warfarin. A trend towards interaction with heart failure (p = 0.08) was observed with respect to SSE reduction, this being greater in patients not presenting heart failure (RR = 0.76 [0.67–0.86]) than in those with heart failure (RR = 0.90 [0.78–1.04]); Significant interaction (p = 0.01) with CHADS2 score was observed, NOAC achieving a greater reduction in bleeding risk in patients with a score of 0–1 (RR 0.67 CI 0.57–0.79) than in those with a score ≥2 (RR 0.85 CI 0.74–0.98). Comparison of MB in patients with (RR 0.97 CI 0.79–1.18) and without (RR 0.76 CI 0.65–0.88) diabetes mellitus showed a similar trend (p = 0.06). No other interactions were found. All subgroups derived benefit from NOA in terms of SSE or MB reduction.
Conclusions
NOAC appeared to be more effective and safer than VKA in reducing SSE or MB irrespective of patient comorbidities. Thromboembolism risk, evaluated by CHADS2 score and, to a lesser extent, diabetes mellitus modified the treatment effects of NOAC without complete loss of benefit with respect to MB reduction.
Introduction
Non-valvular atrial fibrillation (NVAF) is a major cause of ischemic stroke and systemic embolism and is consequently characterized by increased mortality and morbidity and higher costs of medical care [1], [2]. Vitamin K antagonists (VKA), principally warfarin, have proved to be highly effective in preventing thromboembolic events in patients with paroxysmal, persistent, or permanent NVAF [3]. In 29 randomized trials involving more than 28,000 patients, pooled according to meta-analytic methods, adjusted-dose warfarin reduced the risk of stroke by 64% compared to the control and by 37% compared to aspirin, but at the cost of an increased risk of bleeding [3]. Furthermore, warfarin was associated with a 26% reduction in all-cause mortality, compared to no anticoagulation therapy, in randomized, controlled trials in patients with NVAF [3].
New oral anticoagulants (NOAC), directly inhibiting thrombin or factor Xa, have recently been developed. Their wide therapeutic windows allow the use of fixed doses without any need for laboratory monitoring [4], [5]. These new drugs could potentially overcome the well-known limitations of VKA, such as slow onset of action, need for regular blood sampling to monitor the international normalized ratio (INR), narrow therapeutic windows, marked inter-individual variations in drug metabolism, and multiple drug-drug and drug-food interactions, all of which lead to an increased risk of bleeding [6], [7], [8]. NOAC are associated with a reduced risk of stroke and systemic embolism as well as major bleeding, especially intracranial bleeding [9], [10], [11].
However, certain characteristics of patients with NVAF may modify the treatment effects of NOAC [12]. Post hoc analyses of trial data suggest that VKA-naïve patients have a different response when first treated with warfarin compared to those previously exposed to VKA, manifested by an increase in major bleeding [13], [14], [15]. Moreover, an age >75 years, comorbidities such as congestive heart failure, hypertension, diabetes mellitus, and previous stroke or transient ischemic attack, independently predict thromboembolism and are included in the CHADS2 score, the most reliably validated index for discriminating patients at higher risk of stroke [16]. Several of these factors, namely advanced age, previous stroke and hypertension, are also associated with a risk of bleeding, as assessed by the HAS-BLED score [17], [18]. These comorbidities consequently affect the incidence of thromboembolic or bleeding events, or both, and may modify the benefits and harms of NOAC. Apart from the interrelationship between risk factors for stroke and bleeding, and the issue of VKA status (prior exposure or no prior exposure), the interpretation of subgroup analyses corresponding to these comorbidities is hampered in published trials by the small number of outcome events within each subgroup and the lack of power to detect interactions. At the same time, the multiple interaction tests performed in each trial engendered a risk of type 1 error, i.e. a false positive conclusion in favor of superiority of the treatment investigated over the comparator. The aim of the present meta-analysis was to evaluate the consistency of the reductions in stroke and bleeding risks in patients with NVAF irrespective of their comorbidities and VKA status.
Methods
Inclusion criteria
The meta-analysis was performed according to a prospectively developed protocol (available from the corresponding author on request), which pre-specified the research objective, search strategy, study eligibility criteria, and methods of data extraction and statistical analysis. All subgroup variables were defined before the analyses.
Studies were eligible for inclusion in the present meta-analysis if they were randomized, controlled trials conducted in patients with NVAF and reported results according to CHADS2 score, age, presence of heart failure and diabetes mellitus, estimated glomerular filtration rate, prior exposure to VKA, and previous stroke or transient ischemic attack (TIA). Patients in the control group had to have received VKA and patients in the treated group had to have received an oral Factor Xa or thrombin inhibitor. Double-blind and open-label trial designs with or without blinded outcome evaluation were eligible.
Data sources and searches
Medline (PubMed) and Embase were searched up to October 2012 using sensitive methods and employing the key words rivaroxaban, apixaban, betrixaban, edoxaban (DU-176b), eribaxaban, ximelagatran, dabigatran, LY 517717, darexaban (YM150), letaxaban, AZD0837, TTP889, RB006, MCC977 and TAK442 [19], [20]. Search terms included combinations of free text and medical subject headings (MeSH or Emtree). The complete search strategies may be requested from the authors. The references cited by the studies, reviews and meta-analyses retrieved by searching PubMed and Embase were also examined. Unpublished and ongoing trials were sought in clinical trial registers, including those of the National Institute of Health, the National Research Register, Current Controlled Trials, Meta-Embol and Trials Central. We also searched the Internet using the keywords listed above, including websites dedicated to the dissemination of clinical trial results, such as TheHeart.org, and the websites of the European Medicines Agency and the US Food and Drug Administration.
Unpublished studies were included in the meta-analysis if their design had been previously published in detail and patient characteristics, follow-up and the main results had been presented at international congresses. No restrictions concerning non-English language or small population size were applied. All qualifying studies were assessed for adequate blinding of randomization, completeness of follow-up, and objectivity of the outcome assessment. Phase II trials and studies with short-term follow-ups (<12 weeks) were excluded.
Outcomes
The primary efficacy endpoint was the composite of stroke and systemic embolism. The primary safety endpoint was major bleeding (including both intracranial and extracranial bleeding), as defined by International Society on Thrombosis and Haemostasis [21].
Data extraction
Studies were selected and data extracted by two reviewers (JCL and CC) independently. The risk of bias was assessed by the Cochrane Collaboration's tool. The hazard ratio (or the relative risk) and its confidence interval were extracted for all subgroups and directly included in the pooled results [22]. Data regarding inclusion criteria, events by subgroup and treatment were abstracted for each individual study or post hoc analysis. The results obtained on the intention-to-treat population were used for the main efficacy analyses. The risk of bias was assessed by the Cochrane Collaboration's tool [23]. Disagreements were resolved by a third reviewer. If a trial compared two NOAC treatments to the reference treatment (VKA), the number of patients in the reference arm was divided by two so that each patient was included in the meta-analysis only once.
Statistical analysis
The relative risks (RR) or hazard ratios were weighted by the inverse of their variance and combined using the logarithm of RR method according to fixed-effect and random-effect models by R [24], [25]. Interaction was systematically tested for all subgroups and was considered as significant at p <0.05. The statistical heterogeneity between studies was assessed using Cochran's χ2 and I2 tests with a threshold of 0.10 [26]. In the event of heterogeneity, the results were pooled according to a random-effect model. Results were presented graphically, including the RR and corresponding 95% confidence intervals (95% CI).
Results
Literature search and study selection
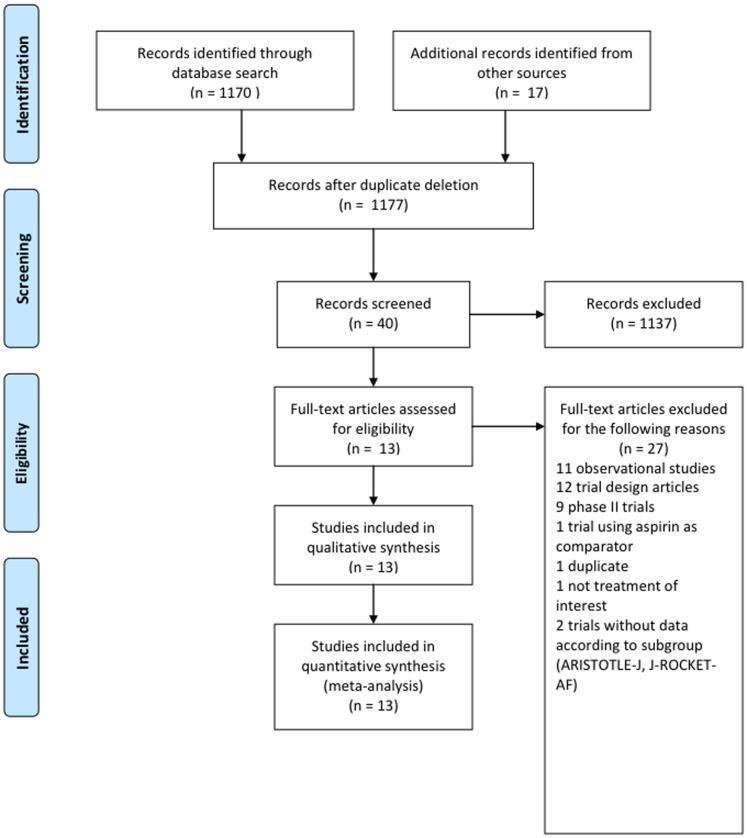
We identified 1170 references through electronic searches and 17 references by manual searches and contact with experts (Figure 1). Among these, three studies (including 50,578 patients) were eligible for analysis [9], [10], [11], the results of which were reported in 11 publications in peer-reviewed journals, one international congress abstract, and one Food and Drug Administration (FDA) report [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]. Patient characteristics, study designs and methodological features are shown in Table 1. Patient inclusion criteria were based on various combinations of the known risk factors for thromboembolism included in the CHADS2 score and consequently the proportion of patients with a CHADS2 score <2 differed greatly from study to study, ranging from 0 to 34%. The proportion of VKA-naïve patients varied from 37% to 50%. The risk of bias according to the Cochrane Collaboration's tool mainly reflected the high quality of the trials included (Table 2). One prospective, randomized, open trial with a blind evaluation was included (Tables 1 and 2). Randomization was performed according to a computer-generated and centralized interactive voice-response system in all trials. One study stratified patients according to their prior VKA exposure and site of enrollment. In view of the few studies included, the source of heterogeneity was not explored.
Figure 1. Flow chart for trial selection.
Table 1. Characteristics of included studies.
| Trial, year | Design | NOAC class | Mean age (years) Men (%) | VKA- naïve patients | Patients with CHADS2 score <2 (%) | ITT | Mean follow-up (months) | TTR (%) |
| RE-LY, 2009 | Phase III PROBE | Thrombin inhibitor | 71.64% | 50%* | 32% | All outcomes | 24.0 | 64 |
| ROCKET, 2011 | Phase III Double-Blind | Anti- factor Xa† | 73.60% | 37%** | 0% | Stroke and SE | 23.2 | 55 |
| ARISTOTLE, 2011 | Phase III Double-Blind | Anti- factor Xa† | 70.65% | 43*** | 34% | All outcomes | 21.6 | 62 |
ITT: intention-to-treat analysis; PROBE: prospective, randomized, open trial with a blind evaluation; SE, systemic embolism; TTR: time during which the INR was in the therapeutic range. † Doses were reduced in patients with renal failure. VKA-naïve was defined as * <63 and ***<31 days of lifetime VKA exposure, respectively, ** not defined in the trial protocol
Table 2. Assessment of the risk of bias according to the Cochrane Collaboration's tool.
| RE-LY, 2009 | + | + | − | + | + | + |
| ROCKET, 2011 | + | + | + | + | + | + |
| ARISTOTLE, 2011 | + | + | + | + | + | + |
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
+: Low risk of bias; −: high risk of bias
Direct thrombin inhibitors were assessed in one study and direct factor Xa inhibitors in two studies (Table 1). Two trials used a reduced dose of the NOAC in patients with renal failure (apixaban 2.5 mg bid and rivaroxaban 15 mg, respectively) [9], [10], [37]. All studies used adjusted-dose warfarin (target INR, 2.0 to 3.0) as the comparator. The proportion of time during which the INR was in the therapeutic range ranged from 55% to 64% (Table 1). Renal function (GFR) was estimated by the Cockcroft-Gault method in all studies. The data according to subgroup are presented in the Table 3.
Table 3. Results of ARISTOTLE, RE-LY, and ROCKET-AF trials according to subgroup.
| Trial Name, Year | Group | Outcome | Heart failure (Yes/No) | Previous stroke-TIA (Yes/No) | CrCl (<50 mL/min 50–80 mL/min >80 mL/min) | CHADS2 score | Age (>75 years/<75 years) | Diabetes mellitus (Yes/No) | Prior VKA useNaïve VKA patients |
| RELY, 2009 | D 110 mg bid | SSE | HR 0.99 (0.69–1.42) HR 0.86 (0.67–1.09) | 55/1195 128/4819 | HR 0.89 (0.61–1.31) HR 0.91 (0.68–1.20) HR 0.83 (0.52–1.32) | 0–1 42/19582 59/2088 >2 82/1968 | HR 0.88 (0.66–1.17) HR 0.93 (0.70–1.22) | HR 0.74 (0.51–1.08) HR 0.97 (0.76–1.23) | 94/3011 89/3004 |
| MB | HR 0.83 (0.64–1.09) HR 0.79 (0.67–0.94) | 65/1195 277/4819 | 120/1151 154/2714 57/1899 | 0–1 74/1958 2 121/2088 >2 147/1968 | 204/2349 138/3666 | 96/1177 246/4837 | 166/3011 176/3004 | ||
| D 150 mg bid | SSE | HR 0.75 (0.51–1.10) HR 0.61 (0.47–0.79) | 51/1233 83/4843 | HR 0.47 (0.30–0.74) HR 0.65 (0.47–0.88) HR 0.71 (0.44–1.15) | 0–1 26/1958 2 35/2137 >2 73/1981 | HR 0.67 (0.49–0.90) HR 0.63 (0.46–0.86) | HR 0.62 (0.42–0.91) HR 0.66 (0.51–0.88) | 73/3049 61/3026 | |
| MB | HR 0.79 (0.60–1.03) HR 0.99 (0.84–1.16) | 102/1233 297/4843 | 123/1188 182/2777 80/1882 | 0–1 84/1958 2 127/2137 >2 188/1981 | 246/2466 153/3610 | 117/1124 282/4952 | 209/3049 190/3026 | ||
| VKA | SSE | - | 65/1195 137/4827 | - | 0–1 40/1859 2 60/2230 >2 102/1933 | - | - | 105/2929 97/3093 | |
| MB | - | 97/1195 324/4827 | 112/1081 206/2806 94/1887 | 0–1 105/1859 2 144/2230 >2 172/1933 | 206/2423 215/3599 | 102/1195 319/4827 | 216/2929 205/3093 | ||
| R 15 or 20 mg od | SSE | 160/4438 109/2642 | 187/3892 82/3189 | 77/1490 126/3298 65/2285 | 2 30/924 >2 239/6156 | 125/3082 144/3999 | 95/2851 174/4230 | 168/4413 101/2668 | |
| ROCKET, 2010 | MB | 106/4428 83/2632 | 136/3881 53/3180 | 50/1485 91/3290 47/2278 | 2 21/922 >2 168/6138 | 82/3073 107/3988 | 70/2842 119/4219 | 114/4401 75/2660 | |
| VKA | SSE | 172/4413 134/2676 | 190/3875 116/3215 | 86/1459 151/3400 68/2222 | 2 36/933 >2 270/6155 | 154/3082 152/4008 | 114/2796 192/4294 | 175/4440 131/2650 | |
| MB | 141/4409102/2672 | 151/386992/3213 | 60/1456 128/3396 54/2221 | 2 24/931>2 219/6149 | 124/3077119/4005 | 94/2793149/4289 | 140/4437103/2645 | ||
| A 2.5 or 5 mg bid | SSE | 70/3235 142/5885 | 73/1748 139/7372 | 54/1502 87/3817 70/3761 | 0–1 44/3100 2 74/3262 >2 94/2758 | 79/2850 133/6270 | 57/2284 155/6836 | 102/5208 110/3912 | |
| ARISTOTLE, 2011 | MB | 87/3235 240/5885 | 77/1748 250/7372 | 73/1502 157/3817 96/3761 | 0–1 76/3100 2 125/3262 >2 126/2758 | 151/2850 176/6270 | 112/2284 215/6836 | 185/5208 142/3912 | |
| VKA | SSE | 79/3216 186/5865 | 98/1790 167/7291 | 69/1515 116/3770 79/3757 | 0–1 51/3083 2 82/3254 >2 132/2744 | 109/2828 156/6253 | 75/2263 190/6818 | 138/5193 127/3888 | |
| MB | 137/3216 325/5865 | 106/1790 356/7291 | 142/1515 199/3770 119/3757 | 0–1 126/3083 2 163/3254 >2 173/2744 | 224/2828 238/6253 | 114/2263 348/6818 | 274/5193 188/3888 |
A: apixaban; Bid: twice daily; D: dabigatran; HR: hazard ratio; MB: major bleeding; NR: not reported; od: once daily; R: rivaroxaban; SE: systemic embolism; SSE: stroke and/or systemic embolism; TIA: transient ischemic attack; VKA: vitamin K antagonist.
Stroke/systemic embolism and major bleeding risk reduction
Age and renal insufficiency
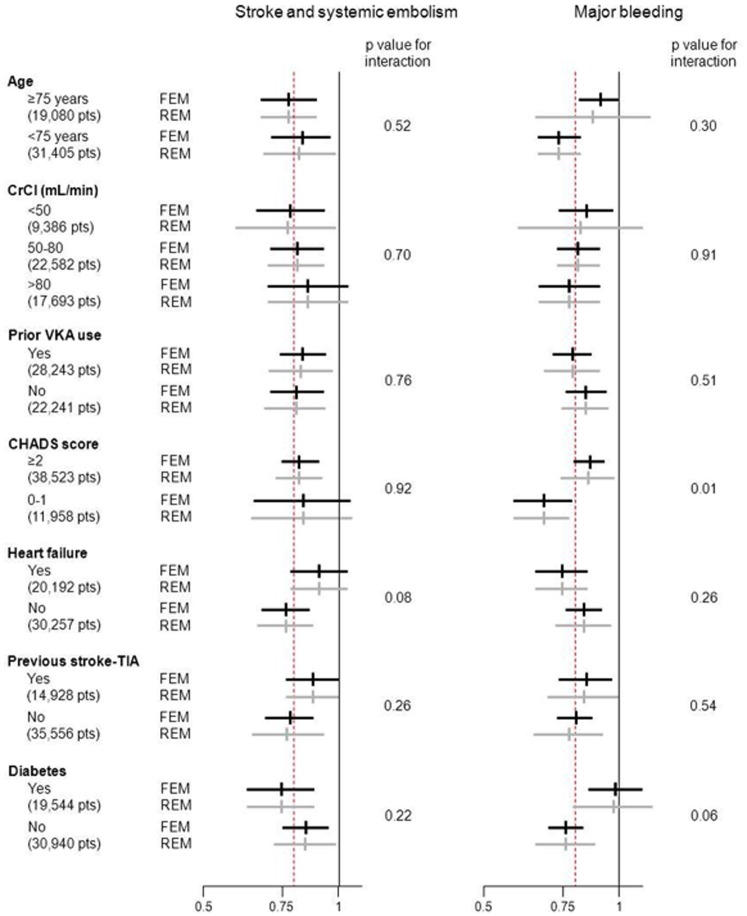
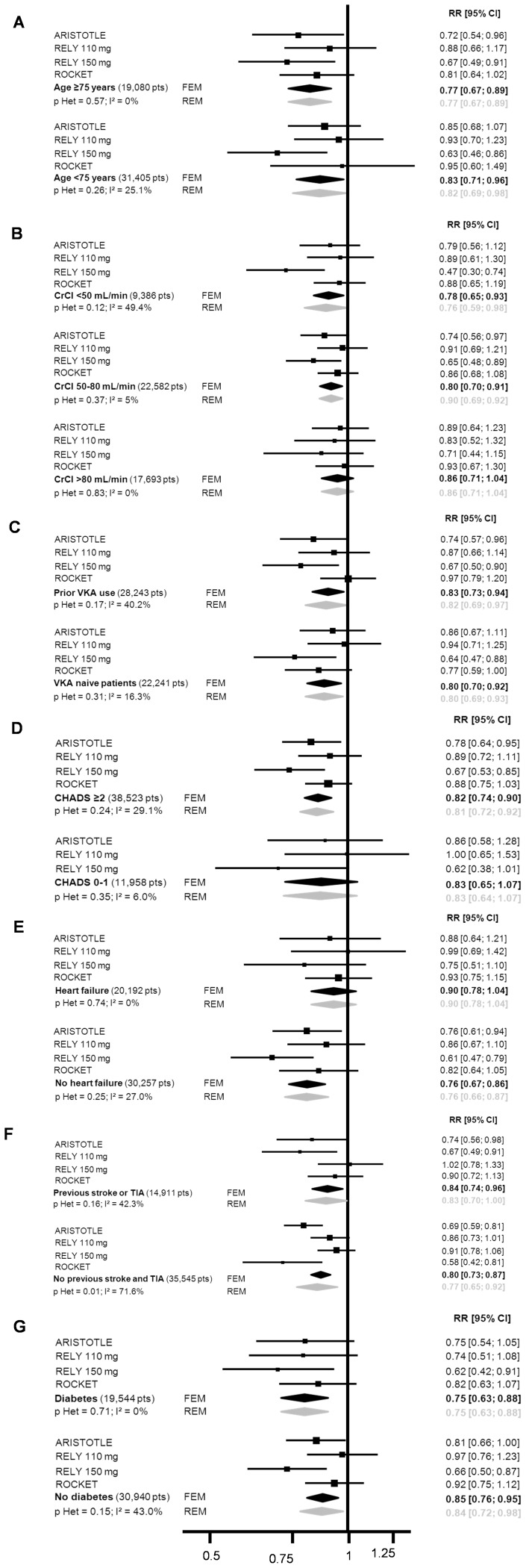
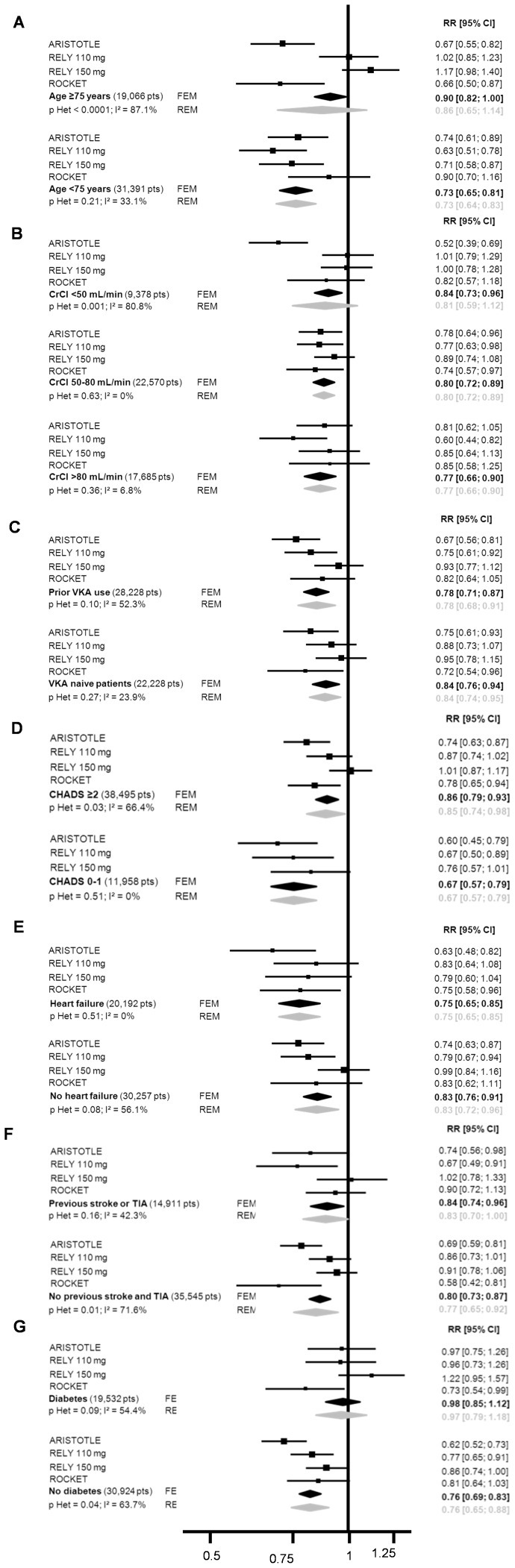
Treatment benefit with regard to SSE risk reduction favored NOAC compared to VKA in both patients aged over 75 years and younger patients (Figures 2 and 3). The benefit remained in favor of NOAC with regard to major bleeding, even though the reduction in risk was lower in elderly patients (RR = 0.86 [0.65–1.14]) than in younger patients (RR = 0.73 [0.64–0.83], p interaction = 0.30) (Figures 2 and 4). Similar results were observed in patients with normal renal function and those with moderate or severe renal impairment, the reductions in SSE and MB being similar in the two subgroups. No interaction was found between these two subgroups.
Figure 2. Relative risk of stroke and systemic embolism and major bleeding reduction according to age, comorbidities and VKA status.
Figure 3. Detailed forest plot of stroke and systemic embolism according to (A) age, (B) renal function, (C) prior VKA exposure, (D) CHADS2 score, (E) heart failure, (F) prior stroke or transient ischemic attack, (G) diabetes mellitus.
Figure 4. Detailed forest plot of major bleeding according to (A) age, (B) renal function, (C) prior VKA exposure, (D) CHADS2 score, (E) heart failure, (F) prior stroke or transient ischemic attack, (G) diabetes mellitus.
Previous exposure to VKA
NOAC were superior to VKA irrespective of subgroup, reductions in SSE (Figure S2 and S3) and MB (Figures 2 and 4) being seen in both VKA-naïve patients (RR = 0.80 [0.70–0.92] and RR = 0.84 [0.76–0.94], respectively) and those previously exposed to VKA (RR = 0.83 [0.73–0.94] and RR = 0.78 [0.68–0.91], respectively), with no interaction.
CHADS2 score
The treatment effect of NOAC in terms of SSE reduction was similar (p = 0.92) irrespective of the CHADS2 score (Figures 2 and 3). The risk of thromboembolism, as defined by the CHADS2 score, significantly modified the effect of NOAC on MB reduction (p interaction = 0.01), a greater effect being evident in patients with CHADS2 scores of 0–1 (RR = 0.67 [0.57–0.79]) than in those with CHADS2 scores ≥2 (RR = 0.85 [0.74–0.98]) (Figures 2 and 4).
Comorbidities included in the CHADS2 score: heart failure, prior stroke/transient ischemic attack, and diabetes mellitus
A trend towards interaction with heart failure (p = 0.08) was observed with respect to SSE reduction, this being greater in patients not presenting heart failure (RR = 0.76 [0.67–0.86]) than in those with heart failure (RR = 0.90 [0.78–1.04]) (Figures 2 and 3). As regards MB, the test for interaction showed a non-significant trend (p = 0.06) towards a difference between patients with (RR = 0.97 [0.79–1.18]) and without (RR = 0.76 [0.65–0.88]) diabetes mellitus (Figures 2 and 4). No interaction was detected for any other comorbidity considered.
Discussion
The goal of this study was to assess the consistency of the benefit-risk balance of NOAC in patients with NVAF irrespective of their characteristics. Overall, our meta-analysis showed a similar treatment effect of NOAC in almost all the subgroups encountered in clinical practice, with no qualitative interaction in terms of SSE or MB reduction, i.e. no reversal of treatment effect leading to an increase of events with NOAC compared to warfarin. However, there was a significant quantitative interaction, expressed by a difference in magnitude of the treatment effect according to subgroup, the effect of NOAC with regard to MB reduction being smaller in patients with a high risk of SSE (CHADS2 score ≥2). There was also a strong trend towards interaction with diabetes mellitus in patients with a CHADS2 score ≥2. It is conceivable that co-prescription of antiplatelet drugs, more frequent in patients with a CHADS2 score ≥2 or diabetes mellitus, might explain an increased incidence of bleeding events but post hoc analysis of the RE-LY trial did not indicate an interaction with co-administration of clopidogrel or aspirin in terms of MB [38]. Some authors have questioned the repercussions of the variable proportion of patients with a high CHADS2 score across phase III trials [39], [40]. In particular, the population included in the ROCKET-AF trial differed from those of ARISTOTLE and RE-LY in that it comprised a higher proportion of patients with comorbidities. In addition, heart failure may modify the benefit of NOACs with respect to SEE reduction, but the magnitude of the interaction did not permit to draw firm conclusions. Our results tend to corroborate this concern and call for careful interpretation of indirect comparisons of the results of trials assessing NOAC [40].
In published trials, the safety and efficacy profiles of NOAC were not worse than those of VKA, irrespective of patient age and prior exposure to VKA [15], [32]. Moreover, all subgroups derived a significant benefit from these new drugs in terms of reductions in MB and/or SSE. NOAC reduced major bleeding in all subgroups at risk of this iatrogenic event, such as those aged ≥75 years, those having experienced a stroke in the past, those with a high CHADS2 score and those presenting renal impairment [18], [31]. The two subgroups at greatest risk of NOAC accumulation, i.e. elderly patients and those with renal failure, both showed a higher incidence of bleeding and thromboembolic events [41]. However, both these subgroups nevertheless derived benefit from NOAC in terms of diminished SSE risk, with no signal indicating an increase in MB, except in the case of dabigatran 150 mg, which was associated with a trend towards an increased risk of MB compared to VKA. In addition, comparison of both dabigratran doses with warfarin revealed a significant statistical interaction between treatment and risk of major bleeds in elderly patients [34]. In patients with renal failure, subgroup analysis showed a heterogeneity of treatment effect, related to a relative increase in bleeding events with dabigatran compared to rivaroxaban and apixaban. We postulated that the percentage renal clearances of 80%, 33% and 25% respectively, in the three treatment groups, might have led to an increased bleeding risk with dabigatran due to drug accumulation. Overall, the reduction in the rate of SSE observed with NOAC versus VKA was similar in patients at increased risk of thromboembolism events, such as those having experienced a prior TIA/stroke, those presenting diabetes mellitus or heart failure, and those aged ≥75 years [42]. The results were same whether or not the patients had previously been exposed to VKA.
Our study suffers from several limitations. First, it comprised a meta-analysis of subgroups. However, most of these subgroups were well defined and included in the stratification scheme for randomization in each study. As discussed above, it is likely that the the studies included in this meta-analysis were not powered to reach significance for many outcomes in subgroups such as those comprising patients with a CHADS2 score <2, those having previously experienced a TIA or stroke or those with a GFR<50 mL/min, due to the small population sizes. Second, we found significant heterogeneity for eight subgroups, but unfortunately, could not analyze its possible causes in view of the small number of trials included. Heterogeneity was mainly observed with respect to MB, a composite outcome encompassing both intracranial and extracranial bleeding. Whereas the disparity between the effect of NOAC and that of VKA followed the same trend in all trials with respect to SSE, the results for MB diverged, the risk of gastrointestinal bleeding being greater with rivaroxaban and with dabigatran at 150 mg than with VKA [9], [11], [43]. Besides the interaction between CHADS2 score and treatment effect, the intrinsic pharmacodynamic properties of the different drugs, e.g. their extent of renal excretion, might explain such differences in the reduction of extracranial bleeding. Finally, we could not exclude inflation of the type 1 error due to the multiple tests performed. For this reason, we choose a conservative threshold of significance (p <0.05) to limit the risk of false positive results despite the lack of power of the interaction test [44].
In conclusion, NOAC appear to be more effective and safer than VKA in reducing SSE or MB irrespective of patient comorbidities. The risk of thromboembolism, as evaluated by the CHADS2 score, and to a lesser extent the presence of diabetes mellitus and heart failure, modified the treatment effect of NOA without complete loss of benefit in terms of MB reduction. Other comorbidities, especially moderate renal impairment or prior VKA use, were not associated with significant differences in treatment effect with regard to either bleeding or ischemic risk reduction. Overall, these new drugs were beneficial for all patient subgroups in the absence of any contraindication.
Supporting Information
(DOC)
Acknowledgments
We are grateful to Paula Harry (MediBridge SA, France) for her valuable help in the preparation of this manuscript. Steering committee of the Meta-Embol Group: S. Laporte (chair), P. Mismetti, P. Zufferey, Saint-Etienne; F. Couturaud, Brest; M. Cucherat, F. Gueyffier, A. Leizorovicz, Lyon; G. Meyer, C.M. Samama, G. Steg, Paris. Members of the Meta-Embol Group: P. Albaladejo, Grenoble; L. Bertoletti, C. Chapelle, P. Garnier, C. Labruyère, N Mottet, Saint-Etienne; P. Girard, E. Marret, F. Parent, N. Rosencher, Paris; J.C. Lega, P. Nony, Lyon; D. Wahl, Nancy.
Funding Statement
This work formed part of the META EMBOL project, supported by the Programme Hospitalier de Recherche Clinique 2008, Ministà ¨re de la Santé, France. This work formed part of the META EMBOL project, supported by the Programme Hospitalier de Recherche Clinique 2008, Ministà ¨re de la Santé, France. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dulli DA, Stanko H, Levine RL (2003) Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology 22: 118–123. [DOI] [PubMed] [Google Scholar]
- 2. Slot KB, Berge E, Dorman P, Lewis S, Dennis M, et al. (2008) Impact of functional status at six months on long term survival in patients with ischaemic stroke: prospective cohort studies. BMJ 336: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 4. Rupprecht HJ, Blank R (2010) Clinical pharmacology of direct and indirect factor Xa inhibitors. Drugs 70: 2153–2170. [DOI] [PubMed] [Google Scholar]
- 5. Garcia D, Libby E, Crowther MA (2010) The new oral anticoagulants. Blood 115: 15–20. [DOI] [PubMed] [Google Scholar]
- 6. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, et al. (2006) ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol 48: 854–906. [DOI] [PubMed] [Google Scholar]
- 7. Singer DE, Albers GW, Dalen JE, Fang MC, Go AS, et al. (2008) Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133: 546S–592S. [DOI] [PubMed] [Google Scholar]
- 8. Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, et al. (2003) Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 349: 1019–1026. [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883–891. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, et al. (2011) Apixaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 365: 981–992. [DOI] [PubMed] [Google Scholar]
- 11. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, et al. (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 12. Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, et al. (2011) Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 123: 2363–2372. [DOI] [PubMed] [Google Scholar]
- 13. Olsson SB (2003) Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet 362: 1691–1698. [DOI] [PubMed] [Google Scholar]
- 14. Albers GW, Diener HC, Frison L, Grind M, Nevinson M, et al. (2005) Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA 293: 690–698. [DOI] [PubMed] [Google Scholar]
- 15. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, et al. (2006) Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 367: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 16. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, et al. (2001) Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285: 2864–2870. [DOI] [PubMed] [Google Scholar]
- 17. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 31: 2369–2429. [DOI] [PubMed] [Google Scholar]
- 18. Lip GY, Frison L, Halperin JL, Lane DA (2011) Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J Am Coll Cardiol 57: 173–180. [DOI] [PubMed] [Google Scholar]
- 19. Wong SS, Wilczynski NL, Haynes RB (2006) Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 94: 41–47. [PMC free article] [PubMed] [Google Scholar]
- 20. Haynes RB, McKibbon KA, Wilczynski NL, Walter SD, Werre SR (2005) Optimal search strategies for retrieving scientifically strong studies of treatment from Medline: analytical survey. BMJ 330: 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulman S, Kearon C (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3: 692–694. [DOI] [PubMed] [Google Scholar]
- 22. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 25.Team RC (2012) R: A Language and Environment for Statistical Computing. http://www.r-project.org. Accessed 2014 January 12.
- 26. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 27. Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, et al. (2012) Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 11: 503–511. [DOI] [PubMed] [Google Scholar]
- 28. Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, et al. (2012) Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 159: 331–339. [DOI] [PubMed] [Google Scholar]
- 29. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, et al. (2012) Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 33: 2821–2830. [DOI] [PubMed] [Google Scholar]
- 30.Darius H, Clemens A, Healey JS, Avezum A, Rangadham N, et al.. (2012) Comparison of dabigatran versus warfarin in diabetic patients with atrial fibrillation: results from the RE-LY Trial. American Heart Association's Scientific Sessions. [DOI] [PubMed]
- 31.Oldgren J, Alings M, Darius H, Diener HC, Eikelboom J, et al. (2011) Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med 155: : 660–667, W204. [DOI] [PubMed] [Google Scholar]
- 32. Ezekowitz MD, Wallentin L, Connolly SJ, Parekh A, Chernick MR, et al. (2010) Dabigatran and warfarin in vitamin K antagonist-naive and -experienced cohorts with atrial fibrillation. Circulation 122: 2246–2253. [DOI] [PubMed] [Google Scholar]
- 33. Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, et al. (2010) Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 9: 1157–1163. [DOI] [PubMed] [Google Scholar]
- 34.Food and Drug Administration website. Available: http://wwwfdagov/downloads/advisorycom- mittees/committeesmeetingmaterials/drugs/cardiovascularandrenal-drugsadvisorycommittee/ucm226009pdf. Accessed 2013 Jul 2.
- 35. Fox KA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, et al. (2011) Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J 32: 2387–2394. [DOI] [PubMed] [Google Scholar]
- 36. Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, et al. (2012) Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 11: 315–322. [DOI] [PubMed] [Google Scholar]
- 37. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, et al. (2012) Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation - the J-ROCKET AF study. Circ J 76: 2104–2111. [DOI] [PubMed] [Google Scholar]
- 38. Dans AL, Connolly SJ, Wallentin L, Yang S, Nakamya J, et al. (2013) Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation 127: 634–640. [DOI] [PubMed] [Google Scholar]
- 39. Ahrens I, Lip GY, Peter K (2011) What do the RE-LY, AVERROES and ROCKET-AF trials tell us for stroke prevention in atrial fibrillation? Thromb Haemost 105: 574–578. [DOI] [PubMed] [Google Scholar]
- 40. Harenberg J, Marx S, Wehling M (2012) Head-to-head or indirect comparisons of the novel oral anticoagulants in atrial fibrillation: what's next? Thromb Haemost 108: 407–409. [DOI] [PubMed] [Google Scholar]
- 41. Eikelboom JW, Connolly SJ, Gao P, Paolasso E, De Caterina R, et al. (2012) Stroke risk and efficacy of apixaban in atrial fibrillation patients with moderate chronic kidney disease. J Stroke Cerebrovasc Dis 21: 429–435. [DOI] [PubMed] [Google Scholar]
- 42. Lip GY, Frison L, Halperin JL, Lane DA (2010) Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 41: 2731–2738. [DOI] [PubMed] [Google Scholar]
- 43.Holster IL, Valkhoff VE, Kuipers EJ, Tjwa ET (2013) New Oral Anticoagulants Increase Risk for Gastrointestinal Bleeding - A Systematic Review and Meta-Analysis. Gastroenterology. [DOI] [PubMed]
- 44. Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)