Abstract
Two populations of cells within the hypothalamus exert opposite actions on food intake: proopiomelanocortin (POMC) neurons decrease it, while neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons increase it. 17β-Estradiol (E2) is a potent anorexigenic hormone that exerts both genomic and non-genomic, rapid actions on these metabolic neurons. This review focuses on the rapid membrane effects of E2 in both POMC and NPY/AgRP neurons and how these combined effects mediate the anorexigenic effects of this steroid.
Keywords: 17β-estradiol, NPY/AgRP, POMC, energy homeostasis
I. E2 feedback and control of non-reproductive homeostatic functions
Besides its quintessential role in the feedback control of the reproductive axis, E2 modulates a number of hypothalamic-regulated autonomic functions, most notably energy homeostasis and temperature. E2 signaling via ERα is a critical component in the hypothalamic regulation of energy balance [1]. In rodents, hypo-estrogenic states are clearly associated with decreased activity and an increase in body weight [2,3,4,5,6,7,8,9,10]. In humans, a loss-of-function mutation in ERα has a clear metabolic phenotype in man with expression of type 2 diabetes, hyperinsulinemia and obesity [11]. However, global reinstatement of an ERα that is lacking the ERE targeting domain is sufficient for “rescuing” the metabolic deficits in mice [12]. These findings suggest an important role for non-ERE mediated E2 signaling. Moreover, brain-specific knockout of ERα causes hyperphagia and hypometabolism [13,14], and selective knockdown of ERα in proopiomelanocortin (POMC) neurons appears to recapitulate the hyperphagic phenotype in female mice [14]. However, there are at least two caveats that impact the interpretation of gene deletion experiments. Firstly, ERα is a transcription factor affecting the expression of hundreds of genes important for cell signaling, and many of these genes are essential for mER initiated responses that contribute to POMC excitability and hence control of energy homeostasis [15,16]. Secondly, POMC-Cre (mice utilized in [14]) is also expressed in progenitor neurons that are destined to become neuropeptide Y (NPY) neurons and perhaps other hypothalamic and extrahypothalamic neurons [17,18]. Therefore, ERα knockout spreads well beyond the single neuron phenotype as originally proposed. Hence, one must be cautious in interpreting ERα knockout (global or targeted) experiments.
Experiments dating back three decades determined that the anorectic effects of E2 in rodents are mediated through CNS sites of action since direct injections of E2 into the paraventricular nucleus of the hypothalamus or arcuate/ventromedial nucleus are effective to reduce food intake, body weight and increase wheel running activity in females [19,20,3]. This is due, in part, to the actions of E2 on arcuate POMC and NPY neurons [21], which optogenetic and ablation studies have definitively proven essential in suppressing or enhancing food intake, respectively [22,23,24]. A number of experiments have shown that E2 up-regulates the expression of the peptide β-endorphin in POMC neurons in ovariectomized female guinea pigs and increases the mRNA expression of POMC in both mice and guinea pigs [25,26,27,28]. Furthermore, there is a decrease in hypothalamic POMC mRNA levels in postmenopausal women [29]. In addition, E2 reverses the ovariectomy-induced increase in arcuate NPY protein and mRNA expression in rodents [30,31,27]. Therefore, it appears that the arcuate nucleus and particularly POMC and NPY neurons are major targets for the anorectic actions of estrogen, which underscores their importance in the control of energy homeostasis. In addition, POMC neurons are critical for the regulation of feeding behavior and are also involved in the rewarding aspects of food intake [32,33].
II. Discovery of a Gq-mER signaling pathway in POMC neurons—the “yin”
Over the past two decades, substantial evidence has been generated in the support of a novel Gαq-coupled membrane ER (Gαq-mER). Intracellular sharp electrode and whole cell patch recording from guinea pig and mouse hypothalamic slices have been used to characterize this Gαq-mER [34,35,8]. These hypothalamic slice studies established that E2 acts rapidly and stereospecifically within physiologically-relevant concentrations (EC50 = 7.5 nM) to significantly reduce the potency of μ-opioid and GABAB agonists (i.e., desensitize) to activate an G protein-coupled inwardly rectifying K+ channels (GIRKS) in POMC neurons [34,35]. In these anorexigenic neurons, estrogenic desensitization of μ-opioid and GABAB receptors is mimicked either by stimulation of adenylyl cyclase with forskolin or by direct protein kinase A (PKA) activation with the non-hydrolyzable cAMP analog Sp-cAMP, in a concentration-dependent manner [34,35]. Furthermore, the selective PKA antagonists KT5720 and Rp-cAMP block the effects of E2. As one would predict from the extensive literature on desensitization of GPCRs [36], PKA is downstream in a signaling cascade that is initiated by a Gαq-coupled membrane ER that is linked to activation of phospholipase C (PLC)-protein kinase C (PKC)-PKA [35,8]. E2 does not alter the affinity of the μ-opioid and GABAB receptors for their respective receptors [37]. Furthermore, the ER antagonists ICI 164,384 and ICI 182,780 block the actions of E2 with subnanomolar affinity (Ki = 0.5 nA) that is similar to Ki for antagonism of ERα [38,34]. These pharmacological findings clearly argue for a novel G-protein-coupled membrane receptor with high selectivity for E2.
About a decade ago a diphenylacrylamide compound, STX, that does not bind ERα or ERβ [35,8] was developed to selectively target the Gαq-mER and its downstream signaling cascade --phospholipase Cβ-protein kinase Cδ-protein kinase A pathway-- that mediates μ-opioid and GABAB desensitization in hypothalamic neurons. The design arose out of studies in which we found that E2 stereospecifically (17α-estradiol is not active) activates the Gαq-mER signaling pathway (see above), and these actions were blocked by the ER antagonist ICI 182,780 [34,35,8]. The results from these physiological and pharmacological experiments led to the design of STX, which is structurally similar to 4-OH tamoxifen, to target the Gαq-mER signaling pathway [35]. Most importantly, both STX and E2 activated this Gαq signaling pathway in POMC neurons from mice lacking both ERα and ERβ and in GPR30-knockout mice [8,39]. However, definitive characterization (i.e., cloning) of this novel Gαq-mER is currently a work in progress.
III. Discovery of a Gq-mER signaling pathway in NPY/AgRP neurons—the “yang”
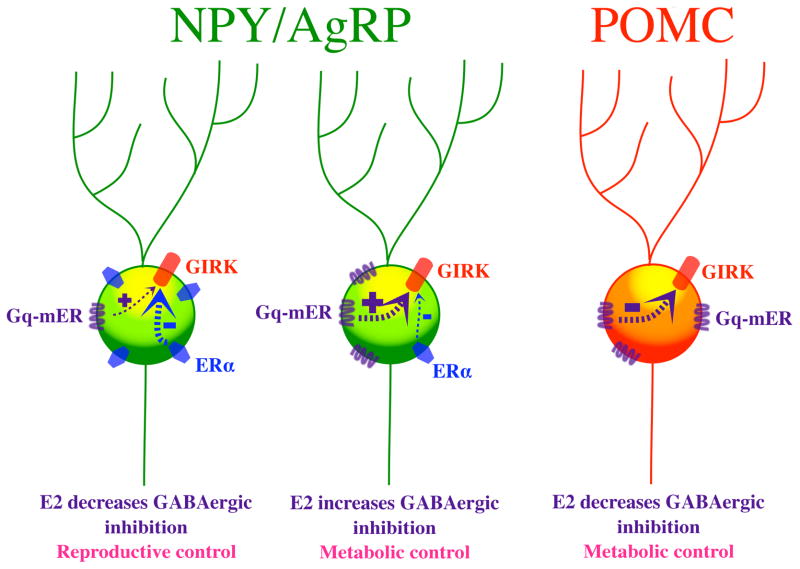
We have recently shown that a functionally complementary Gq-mER pathway is present in NPY/AgRP neurons [40]. In contrast to POMC cells, the Gq-mER ligand STX rapidly enhances the ability of GABAB receptors to activate GIRKS, and co-administering the estrogen receptor antagonist ICI 182,780 inhibits such effects. Interestingly, NPY/AgRP cells also apparently possess a rapid ERα signaling component [40]: activating ERα with PPT in NPY neurons rapidly causes effects opposite to those of the Gq-mER, suppressing the ability of GABAB receptors to activate GIRKS. In gonadectomized mice, administering the “nonselective” E2 either enhances or suppresses GABAB-mediated currents in different NPY/AgRP neurons [40]. Co-administering PI3 Kinase inhibitor, specifically a selective inhibitor of p110beta, results in E2 only enhancing the GABAB mediated response. Therefore, ERα appears to be coupled via PI3K to inhibition of the GABAB response in NPY/AgRP neurons. Thus, one hypothesis is that the effects of E2 depend on the relative expression of ERα vs. Gq-mER in these orexigenic cells (Figure 1). When Gq-mER expression predominates, the NPY/AgRP cell may serve a metabolic function, while those cells expressing relatively more ERα help regulate reproduction.
Figure 1.
Schematic representation showing how E2 may inhibit or excite NPYAgRP and POMC neurons in the hypothalamus. Abbreviations: Gq-mER, Gq-coupled membrane estrogen receptor; GIRK, G protein-coupled inwardly rectifying potassium channel; ERα, estrogen receptor α; NPY/AgRP, neuropeptide Y/Agouti-related peptide; POMC, proopiomelanocortin
IV. Gq-mER and control of energy homeostasis
The ability of STX to mimic the selective actions of E2 on POMC and NPY/AgRP neuronal activity bolstered our hypothesis that the Gαq-mER may have a role in the control of energy homeostasis. Indeed, in translational animal experiments we have demonstrated that peripheral administration of STX mimics the effects of E2 in controlling energy homeostasis [8,28,41]. As predicted from the complementary effects of STX on membrane excitability in POMC and NPY/AgRP neurons, both E2 and STX reduce food intake and, subsequently, the post-ovariectomy body weight gain. STX and E2 inhibit food intake in ovariectomized guinea pigs by reducing meal frequency, and there is a subsequent reduction in abdominal fat accumulation [41]. In support of a hypothalamic site of action, treatment with STX, similar to E2, induces new gene transcription in the arcuate nucleus [28]. Many of the regulated genes in the arcuate nucleus are involved in the control of neuronal excitability (e.g., Cav3.1) and intracellular signaling in hypothalamic neurons [28]. For example, the PI3 kinase regulatory subunits are regulated by E2 and STX: PI3 kinase p55 mRNA is increased by E2 treatment [42] and PI3 kinase p85α mRNA is upregulated by STX [28]. Therefore, Gαq-mER appears to function in the estrogenic control of energy homeostasis in the arcuate nucleus.
V. Gq-mER and regulation of core body temperature
Another critical homeostatic function modulated by circulating estrogens is the maintenance of core body temperature (Tc). In fact, hot flashes affect 75–85% of perimenopausal and postmenopausal women [43]. Hot flashes are characterized by periods of sweating and peripheral vasodilation and are often associated with increased environmental temperature [44]. Therefore, a hot flash can be defined as an exaggerated heat dissipation response initiated by the preoptic temperature sensitive neurons. Although the mechanism behind this response is not known, repeated observations have found that the majority of hot flashes are preceded by elevation in Tc independent of peripheral vasoconstriction or elevated metabolic rate [45,46]. Therefore, it has been postulated that elevated Tc may serve as one trigger of menopausal hot flashes [47,44]. In general, there is compelling experimental evidence that the incidence of hot flashes in hypo-estrogenic females is decreased by E2 treatment [48,49,47]. The estrogenic reduction in the naloxone-induced rise in tail skin temperature of morphine-dependent ovariectomized rats has been the industry standard for a hot flash model [50,51,52]. The morphine-dependence, naloxone-precipitated withdrawal rat model has a number of drawbacks because of the adverse autonomic reactions independent of an elevation in core and skin temperature. But the model does highlight the involvement of CNS opioid effects (e.g., from POMC neurons) directly or indirectly on preoptic temperature sensitive neurons [53].
We established a guinea pig “hot flash” model based on the hypothesis that the expression of vasomotor symptoms in menopausal women is due to the reduced thermo-neutral zone in core body temperature [46]. In the ovariectomized female guinea pig, both E2 and STX significantly reduce Tc compared to animals receiving vehicle injections [41]. The exact cellular target for E2 and specifically the Gαq-mER modulation of Tc has not been identified. However, one potential cellular mechanism is the direct action of E2 via the Gαq-mER on thermosensitive (GABAergic) neurons in the medial preoptic area of the hypothalamus [54,55,56]. Previous studies in ovariectomized female guinea pigs have shown that medial preoptic GABAergic neurons respond to acute E2 treatment via the Gαq-mER signaling pathway to attenuate GABAB inhibitory input, leading to increased GABA neuronal activity [57]. Moreover, activation of medial preoptic GABA neurons are responsible for evoking the vasomotor responses in rodents [58] underlying heat dissipation responses (i.e., vasodilatation, sweating) seen in women experiencing hot flashes.
Importantly, the coupling of Gαq-mER to downstream signaling pathways is similar to the serotonin 5HT2A/2C receptors, which when activated, lower Tc and are implicated in thermoregulation dysfunction caused by ovariectomy [59,60]. Selective serotonin reuptake inhibitors elevate endogenous serotonin levels and are therefore efficacious for treating hot flashes [61] and can significantly attenuate the effects of ovariectomy on thermoregulation in rodents [62]. Moreover, both the Gαq-mER and 5HT2C receptors increase POMC neuronal excitability [63]. These similarities imply that serotonin via its Gαq-coupled receptor and E2 via Gαq-mER have similar cellular targets in the hypothalamic neurons that regulate Tc and energy homeostasis.
VI. Summary
It is obvious from the plethora of studies using membrane-delimited E2 ligands and the Gq-mER selective ligand STX that “genomic” actions of E2 in the brain do not require the direct nuclear targeting of estrogen receptors (ERα and ERβ). Signals that are initiated by E2 at the plasma membrane can trigger multiple intracellular signaling cascades including activation of MAPK, PI3K, and PKC pathways [64,65,66,67,68] that result in the phosphorylation of hundreds of proteins that ultimate can affect cell excitability and gene transcription. E2 can bind to the novel Gαq-mER to upregulate cAMP in POMC neurons by increasing adenylyl cyclase activity within minutes [34]. Cyclic-AMP activates PKA, which in turn phosphorylates K+ channels [69] and/or calcium channels to [70] to alter their activity. In addition, PKA can phosphorylate CREB to elicit new gene transcription [71,72,73,74]. Alternately, the Gq-mER in NPY/AgRP neurons elicits rapd effects on K+ channels opposite to those seen in POMC cells (Figure 1). Therefore, not only does membrane excitability change within a matter of seconds, but gene transcription can be activated within a relatively short time course (within tens of minutes) in neurons independent of classic estrogen receptors (ERα and ERβ) interacting with EREs. Thus, we need to elucidate the role of membrane estrogen receptors not only in hypothalamic functions but also in higher cortical functions. By developing selective non-steroidal agonists for targeting mERs we will be able to treat menopausal symptoms without the concern for the deleterious reproductive and cardiovascular effects of estrogens.
Highlights.
17β-estradiol (E2) rapidly controls membrane excitability through non-genomic mechanisms in hypothalamic neurons that control feeding.
E2 decreases GABAergic inhibition on POMC cells, which are anorexigenic, via a Gq-coupled membrane estrogen receptor (Gq-mER).
E2 decreases or increases GABAergic inhibition of orexigenic NPY/AgRP neurons depending on whether it binds to ERα or Gq-mER, respectively.
The Gq-mER also plays a role in thermoregulation in mammals.
Acknowledgments
The authors thank current and former members of their laboratories who contributed to the work described herein, especially Drs. Jian Qiu, Troy A. Roepke and Chunguang Zhang and Martha A. Bosch. The authors also acknowledge the technical support of Ms. Marina V. Rulevskaya and Mr. M. Rick Rollins.
Grants
Research reported in this publication was supported by National Institute of Health R01 grants NS 38809 (MJK), NS 43330 (OKR), DK 68098 (MJK & OKR). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–7. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 2.Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–49. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- 3.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–8. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- 4.Czaja JA. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav. 1984;33:553–8. doi: 10.1016/0031-9384(84)90370-6. [DOI] [PubMed] [Google Scholar]
- 5.McCaffrey TA, Czaja JA. Diverse effects of estradiol-17 beta: concurrent suppression of appetite, blood pressure and vascular reactivity in conscious, unrestrained animals. Physiol Behav. 1989;45:649–57. doi: 10.1016/0031-9384(89)90086-3. [DOI] [PubMed] [Google Scholar]
- 6.Jones MEE, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97:12735–40. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–71. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 8.Qiu J, Bosch MA, Tobias SC, Krust A, Graham S, Murphy S, et al. A G protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–55. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 10.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–8. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 11.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–61. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 12.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, et al. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. Journal of Clinical Investigation. 2011;121:604–12. doi: 10.1172/JCI41702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, et al. Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–6. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Nedugadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct Hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–65. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari C, et al. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endo. 2003;17:2070–83. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 16.Malyala A, Pattee P, Nagalla SR, Kelly MJ, Rønnekleiv OK. Suppression subtractive hybridization and microarray identification of estrogen regulated hypothalamic genes. Neurochem Res. 2004;29:1189–200. doi: 10.1023/b:nere.0000023606.13670.1d. [DOI] [PubMed] [Google Scholar]
- 17.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature Med. 2010;16:403–5. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endo. 2012;153:1–13. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colvin GB, Sawyer CH. Induction of running activity by intracerebral implants of estrogen in overiectomized rats. Neuroendo. 1969;4:309–20. doi: 10.1159/000121762. [DOI] [PubMed] [Google Scholar]
- 20.Ahdieh HB, Wade GN. Effects of hysterectomy on sexual receptivity, food intake, running wheel activity, and hypothalamic estrogen and progestin receptors in rats. J Comp Physiol Psychol. 1982;96:886–92. [PubMed] [Google Scholar]
- 21.Roepke TA. Oestrogen modulates hypothalmic control of energy homeostasis through multiple mechanisms. J Neuroendocrinol. 2009;21:141–50. doi: 10.1111/j.1365-2826.2008.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–5. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 23.Wu Q, Whiddon BB, Palmiter RD. Ablation of neurons expressing agouti-related protein, but not melanin concentrating hormone, in leptin-deficient mice restores metabolic function and fertility. Proc Natl Acad Sci USA. 2012;109:3155–60. doi: 10.1073/pnas.1120501109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–5. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton JE, Loose MD, Kelly MJ, Rønnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- 26.Bethea CL, Hess DL, Widmann AA, Henningfeld JM. Effects of progesterone on prolactin, hypothalamic beta-endorphin, hypothalamic substance P, and midbrain serotonin in guinea pigs. Neuroendo. 1995;61:695–703. doi: 10.1159/000126897. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinology. 2007;19:426–31. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 28.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endo. 2008;149:6113–24. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel TW, Rance NE. Proopiomelanocortin gene expression is decreased in the infundibular nucleus of postmenopausal women. Mol Brain Res. 1999;69:202–8. doi: 10.1016/s0169-328x(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 30.Crowley WR, Tessel RE, O’Donohue TL, Adler BA, Kalra SP. Effects of ovarian hormones on the concentrations of immunoreactive neuropeptide Y in discrete brain regions of the female rat: correlation with serum luteinizing hormone (LH) and median eminence LH-releasing hormone. Endocrinology. 1985;117:1151–5. doi: 10.1210/endo-117-3-1151. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Withdrawal of estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett. 1996;204:81–4. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- 32.Hayward MD, Pintar JE, Low MJ. Selective reward deficit in mice lacking β-endorphin and enkephalin. J Neurosci. 2002;22:8251–8. doi: 10.1523/JNEUROSCI.22-18-08251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, et al. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endo. 2003;144:1753–60. doi: 10.1210/en.2002-221096. [DOI] [PubMed] [Google Scholar]
- 34.Lagrange AH, Rønnekleiv OK, Kelly MJ. Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol Pharmacol. 1997;51:605–12. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- 35.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Rønnekleiv OK, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham MJ, Fang Y, Selley DE, Kelly MJ. μ-opioid agonist-stimulated [35S]GTPgammaS binding in guinea pig hypothalamus: Effects of estrogen. Brain Res. 1998;791:341–6. doi: 10.1016/s0006-8993(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 38.Weatherill PJ, Wilson APM, Nicholson RI, Davies P, Wakeling AE. Interaction of the antioestrogen ICI 164,384 with the oestrogen receptor. J Ster Bioc Mol Biol. 1988;30:263–6. doi: 10.1016/0022-4731(88)90103-3. [DOI] [PubMed] [Google Scholar]
- 39.Qiu J, Rønnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein coupled estrogen membrane receptor. Steroids. 2008;73:985–91. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: Role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab. 2013;305:E632–E640. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlová-Wuttke D, et al. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endo. 2010;151:4926–37. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malyala A, Zhang C, Bryant D, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 43.Moline ML, Broch L, Zak R, Gross V. Sleep in women across the life cycle from adulthood through menopause. Sleep Med Rev. 2003;7:155–77. doi: 10.1053/smrv.2001.0228. [DOI] [PubMed] [Google Scholar]
- 44.Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106. doi: 10.1016/j.ajog.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 45.Freedman RR, Norton D, Woodward S, Cornelissen G. Core body temperature and circadian rhythm of hot flashes in meopausal women. J Clin Endo Metab. 1995;80:2354–8. doi: 10.1210/jcem.80.8.7629229. [DOI] [PubMed] [Google Scholar]
- 46.Freedman RR. Hot flashes: behavorial treatments, mechanisms, and relation to sleep. American Journal of Medicine. 2005;118:1245–305. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 47.Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77:487–90. doi: 10.1016/s0015-0282(01)03009-6. [DOI] [PubMed] [Google Scholar]
- 48.Tankersley CG, Nicholas WC, Deaver DR, Mikita D, Kenney WL. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol. 1992;73:1238–45. doi: 10.1152/jappl.1992.73.4.1238. [DOI] [PubMed] [Google Scholar]
- 49.Brooks EM, Morgan AL, Pierzga JM, Wladkowski SL, O’Gorman JT, Derr JA, et al. Chronic hormone replacement therapy alters thermoregulatory and vasomotor fuction in postmenopausal women. J Appl Physiol. 1997;97:477–84. doi: 10.1152/jappl.1997.83.2.477. [DOI] [PubMed] [Google Scholar]
- 50.Simpkins JW, Katovich MJ, Song C. Similarites between morphine withdrawal in the rat and the menopausal hot flush. Life Sci. 1983;32:1957–66. doi: 10.1016/0024-3205(83)90047-4. [DOI] [PubMed] [Google Scholar]
- 51.Merchenthaler I, Funkhouser JM, Carver JM, Lundeen SG, Ghosh K, Winneker RC. The effect of estrogens and antiestrogens in a rat model for hot flush. Maturitas. 1998;30:307–16. doi: 10.1016/s0378-5122(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 52.Komm BS, Kharode YP, Bodine PVN, Harris HA, Miller CP, Lyttle CR. Bazedoxifene Acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinolgy. 2005;146:3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J Neurosci. 2009;29:11954–64. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffin JD, Saper CB, Boulant JA. Synaptic and morphological characteristics of temperature-sensitive and -insensitive rat hypothalamic neurones. J Physiol. 2001;537.2:521–35. doi: 10.1111/j.1469-7793.2001.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–10. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boulant JA. Neuronal basis of Hammel’s model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–54. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- 57.Wagner EJ, Rønnekleiv OK, Bosch MA, Kelly MJ. Estrogen biphasically modifies hypothalamic GABAergic function concomitantly with negative and positive control of luteinizing hormone release. J Neurosci. 2001;21:2085–93. doi: 10.1523/JNEUROSCI.21-06-02085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA. 2010;107:8848–53. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berendsen HHG, Weekers AHJ, Kloosterboer HJ. Effect of tibolone and raloxifene on the tail temperature of oestrogen-deficient rats. Eur J Pharmacol. 2001;419:47–54. doi: 10.1016/s0014-2999(01)00966-9. [DOI] [PubMed] [Google Scholar]
- 60.Sipe K, Leventhal L, Burroughs K, Cosmi S, Johnston GH, Deecher DC. Serotonin 2A receptors modulate tail-skin temperature in two rodent models of estrogen deficiency-related thermoregulatory dysfunction. Brain Res. 2004;1028:191–202. doi: 10.1016/j.brainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 61.Stearns V, Ullmer L, Lopez JF, Smith Y, Isaacs C, Hayes DF. Hot flushes. Lancet. 2002;360:1851–61. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 62.Deecher DC, Alfinito PD, Leventhal L, Cosmi S, Johnston GH, Merchanthaler I, et al. Alleviation of thermoregulatory dysfunction with the new serotonin and norepinephrine reuptake inhibitor desvenlafaxine succinate in ovariectomized rodent models. Endocrinology. 2007;148:1376–83. doi: 10.1210/en.2006-1163. [DOI] [PubMed] [Google Scholar]
- 63.Qiu J, Xue C, Bosch MA, Murphy JG, Fan W, Rønnekleiv OK, et al. Serotonin 5HT2c receptor signaling in hypothalamic POMC neurons: role in energy homeostasis in females. Mol Pharm. 2007;72:885–96. doi: 10.1124/mol.107.038083. [DOI] [PubMed] [Google Scholar]
- 64.Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–3. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- 65.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci U S A. 2001;98:13391–5. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cato ACB, Nestl A, Mink S. Rapid actions of steroid receptors in cellular signaling pathways. Science’s STKE 2002. 2002:re9–re21. doi: 10.1126/stke.2002.138.re9. [DOI] [PubMed] [Google Scholar]
- 67.Yang S-H, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 68.Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Current Opinion in Neurobiology. 2003;13:354–65. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang C, Kelly MJ, Rønnekleiv OK. 17β-estradiol rapidly increases adenosine 5′-triphosphate-sensitive potassium channel activity in gonadotropin-releasing hormone neurons via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–84. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–62. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou Y, Watters JJ, Dorsa DM. Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endo. 1996;137:2163–6. doi: 10.1210/endo.137.5.8612562. [DOI] [PubMed] [Google Scholar]
- 72.Gu G, Rojo AA, Zee MC, Yu J, Simerly RB. Hormonal regulation of CREB phosphorylation in the anteroventral periventricular nucleus. J Neurosci. 1996;16:3035–44. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watters JJ, Dorsa DM. Transcriptional effects of estrogen on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci. 1998;18:6672–80. doi: 10.1523/JNEUROSCI.18-17-06672.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abrahám IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–61. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]