Summary
Abatacept (cytotoxic T-lymphocyte–associated antigen 4–immunoglobulin fusion protein [CTLA-4–Ig]) is a costimulatory inhibitor that targets B7-1 (CD80). The present report describes five patients who had focal segmental glomerulosclerosis (FSGS) (four with recurrent FSGS after transplantation and one with primary FSGS) and proteinuria with B7-1 immunostaining of podocytes in kidney-biopsy specimens. Abatacept induced partial or complete remissions of proteinuria in these patients, suggesting that B7-1 may be a useful biomarker for the treatment of some glomerulopathies. Our data indicate that abatacept may stabilize β1-integrin activation in podocytes and reduce proteinuria in patients with B7-1–positive glomerular disease.
The renal glomeruli are highly specialized structures that ensure selective ultrafiltration of plasma, by which most proteins are retained in the blood.1 The glomerular filtration barrier consists of the glomerular capillary endothelium, the glomerular basement membrane, and specialized cells, the podocytes, that serve as a final barrier to urinary loss of plasma proteins.1 Disrupted podocyte function damages the kidney filtration mechanism, resulting in proteinuria and, in some circumstances, the nephrotic syndrome.1 Proteinuria is common to a heterogeneous group of kidney diseases, including minimal-change disease, FSGS, membranous nephropathy, and diabetic nephropathy, all of which affect millions of persons worldwide and often result in end-stage renal disease (ESRD).1 In particular, primary FSGS as well as recurrent FSGS after kidney transplantation remain largely untreatable, leading to ESRD and, after transplantation, to allograft loss.2
Abatacept (CTLA-4–Ig) is an inhibitor of the T-cell costimulatory molecule B7-1 (CD80).3 B7-1 is induced in podocytes in various animal models of proteinuria.4 Podocyte B7-1 expression is not evident in normal human kidney podocytes but is found in patients with certain glomerular diseases. Because we observed B7-1 immunostaining in 13 of 21 randomly selected biopsy specimens of native kidneys from patients with proteinuric kidney disease, including primary FSGS, we deduced that B7-1 had been induced during the disease. We also observed B7-1 staining in every biopsy specimen from patients with recurrent FSGS that we examined. We treated five patients with abatacept3; nephrotic-range proteinuria resolved in all four patients with rituximab-resistant recurrent FSGS and in one patient with glucocorticoid-resistant primary FSGS.
Methods
Cases of FSGS
Clinical features of the cases of FSGS are summarized in Table 1, with details presented in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Table 1.
Characteristics of Five Patients with Focal Segmental Glomerulosclerosis (FSGS).
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age and sex | 28-yr-old woman | 19-yr-old woman | 14-yr-old boy | 7-yr-old boy | 27-yr-old woman |
| Kidney donor | Living related donor; earlier transplant from a living related donor failed owing to recurrent FSGS | Cadaveric donor; earlier transplant from a living related donor failed owing to recurrent FSGS | Living related donor | Cadaveric donor | No donor (native kidney) |
| Induction immunosuppression | Daclizumab (1 mg/kg, two doses), antithymocyte globulin (1 mg/kg, five doses), rituximab (375 mg/m2, one dose) | Daclizumab (1 mg/kg, two doses), antithymocyte globulin (1 mg/kg, five doses), rituximab (375 mg/m2, one dose) | Antithymocyte globulin (1 mg/kg, five doses), basiliximab (10 mg/kg, two doses), rituximab (375 mg/m2, one dose) | Antithymocyte globulin (1 mg/kg, five doses), basiliximab (10 mg/kg, two doses), rituximab (375 mg/m2, one dose) | |
| Maintenance immunosuppression | Tacrolimus (target serum level, 5–7 ng/ml), mycophenolate mofetil (500 mg twice daily), glucocorticoids | Tacrolimus (target serum level, 5–7 ng/ml), mycophenolate mofetil (500 mg twice daily), glucocorticoids | Tacrolimus (target serum level, 5–7 ng/ml), mycophenolate mofetil (125–250 mg twice daily), glucocorticoids | Tacrolimus (target serum level, 5–7 ng/ml), mycophenolate mofetil (125–250 mg twice daily), glucocorticoids | |
| Treatment for FSGS before abatacept therapy | Plasmapheresis | Plasmapheresis | Plasmapheresis | Plasmapheresis | Prednisone, cyclosporine, tacrolimus |
| Abatacept therapy | Single dose (10 mg/kg) | Single dose (10 mg/kg) | Two doses (10 mg/kg) | Two doses (10 mg/kg) | 10 mg/kg on days 1, 15, and 30 and monthly thereafter |
| Most recent laboratory test results* | 48-mo follow-up (February 2013): serum albumin, 3.4 g/dl; serum creatinine, 1.3 mg/dl; urinary protein-to-creatinine ratio, 0.50 | 36-mo follow-up (February 2013): serum albumin, 3.8 g/dl; serum creatinine, 0.7 mg/dl; urinary protein-to-creatinine ratio, 0.41 | 12-mo follow-up (February 2013): serum albumin, 4.0 g/dl; serum creatinine, 0.9 mg/dl; urinary protein-to-creatinine ratio, 0.08 | 10-mo follow-up (March 2013): serum albumin, 4.3 g/dl; serum creatinine, 0.3 mg/dl; urinary protein-to-creatinine ratio, 0.05 | 12-mo follow-up (October 2013): serum albumin, 3.8 g/dl; serum creatinine, 0.4 mg/dl; urinary protein-to-creatinine ratio, 0.50 |
To convert values for creatinine to micromoles per liter, multiply by 88.4. A urinary protein-to-creatinine ratio of less than 0.15 is considered normal.
In Vitro Studies
Cell Motility
Increased podocyte migration in vitro is a surrogate marker of proteinuria in vivo.5 To determine whether abatacept can act directly on podocytes, we performed cell-migration assays with podocytes stably expressing B7-1 or a truncated B7-1 construct — that is, a construct lacking the cytoplasmic tail (B7-1Δtail) (Fig. S4D in the Supplementary Appendix).
β1-Integrin Activation and Cell Spreading
We investigated the effect of B7-1 and its inhibitor, abatacept, on β1-integrin activation in podocytes6,7 by means of confocal microscopy or fluorescence-activated cell sorting, as detailed in the Supplementary Appendix. We used phorbol myristate acetate–induced spreading of nonadherent K562 cells8 as an independent, functional test of the effects of B7-1 and abatacept on β1-integrin activation.6,7
B7-1 Detection in Kidney-Biopsy Specimens
B7-1 immunostaining was performed on frozen kidney-biopsy sections with the use of goat antihuman B7-1 (CD80) antibody (R&D Systems). For details, see the Supplementary Appendix.
Results
Patients
Four patients with rituximab-resistant recurrent FSGS after transplantation (Patients 1 through 4) (Table 1, and Fig. S1 in the Supplementary Appendix) and one patient with glucocorticoid-resistant primary FSGS (Patient 5) (Table 1, and Fig. S2 in the Supplementary Appendix), with B7-1 staining of podocytes in kidney-biopsy specimens, were treated with abatacept.3 Abatacept treatment for these five patients was consistent with institutional policies (at the University of Miami and Massachusetts General Hospital; abatacept has been approved by the Food and Drug Administration for the treatment of rheumatoid arthritis). The decision to treat patients with abatacept was based on the results of detailed mechanistic in vitro studies of podocytes.
In Vitro Studies
Podocyte Migration
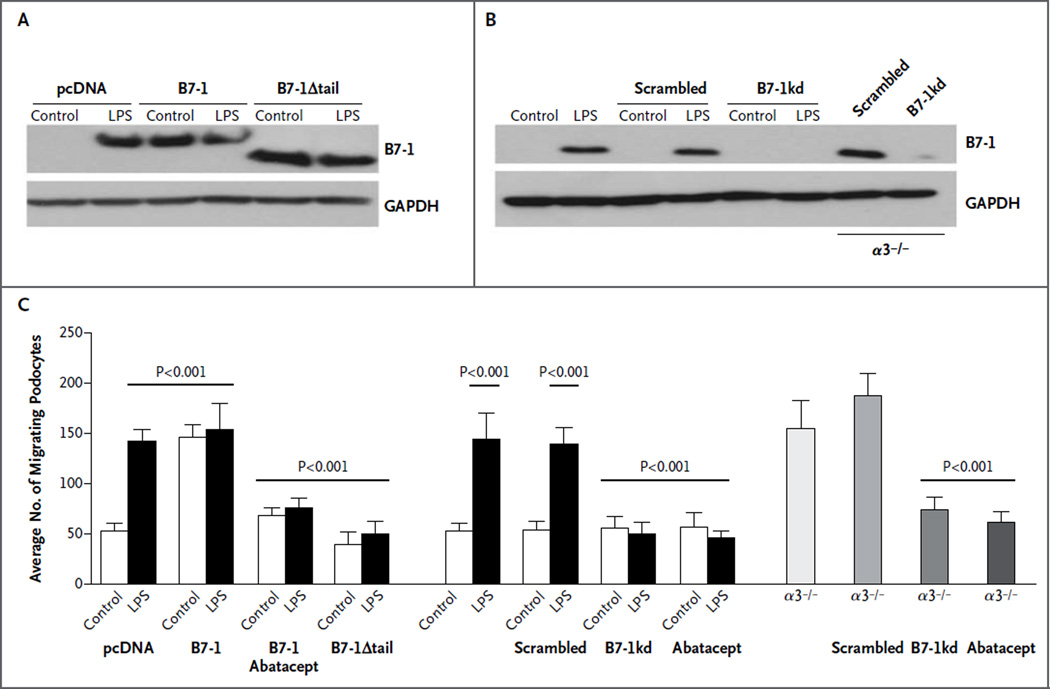
The addition of lipopolysaccharide to culture medium induced B7-1 protein expression in podocytes (Fig. 1A). Constitutive B7-1 protein expression was found in α3 integrin–knockout (α3−/−) podocytes (Fig. 1B). Abatacept blocked lipopolysaccharide-induced or B7-1–induced podocyte migration (Fig. S3A in the Supplementary Appendix). B7-1 gene silencing or expression of the truncated construct (B7-1Δtail) also suppressed podocyte migration (Fig. S3A and S3B in the Supplementary Appendix). Abatacept treatment or B7-1 gene silencing reversed α3−/− podocyte motility to baseline levels (Fig. S3C in the Supplementary Appendix). Figure 1C shows the quantified effects of abatacept on podocyte migration.
Figure 1. Blockade of Disease-Associated Podocyte Migration by Abatacept.
Panel A shows Western blot analysis of B7-1 protein in podocytes with stable transfection of vector control (pcDNA), B7-1, or B7-1Δtail before the addition of lipopolysaccharide (LPS), which was the control condition, and after the addition of LPS. Panel B shows B7-1 protein in normal podocytes, non-silencing short hairpin RNA (shRNA)–expressing podocytes (scrambled), B7-1 knockdown podocytes (B7-1kd), scrambled shRNA-expressing α3−/− podocytes (α3−/−/scrambled), and B7-1 knockdown α3−/− podocytes (α3−/−/B7-1kd). In the analyses shown in Panels A and B, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. Panel C shows the results of quantitative analysis of podocyte migration. P values were calculated with the use of Bonferroni’s multiple-comparison test; see the Supplementary Appendix for details. The T bars indicate standard deviations.
Biochemical Analysis of B7-1 Binding to β1 Integrin
Because abatacept inhibited migration of α3−/− podocytes, we hypothesized that B7-1 modulates integrin function. Endogenous coimmunoprecipitation studies showed that β1 interacted with α5 and αV integrins in normal and α3−/− podocytes (Fig. S4A in the Supplementary Appendix), suggesting that α5 and αV are the α-chain partners of β1 in the absence of α3. We observed colocalization of B7-1 with vinculin in focal contacts (cell–matrix adhesions that contain integrins) of α3−/− podocytes (Fig. S4B in the Supplementary Appendix) and observed a specific interaction between endogenous B7-1 and β1 integrin in α3−/− podocytes (Fig. S4C in the Supplementary Appendix). Domain mapping and pull-down studies with recombinant purified proteins (Fig. S4D, S4E, and S4F in the Supplementary Appendix) revealed a direct interaction between the cytoplasmic tails of B7-1 and β1, suggesting that this interaction is central to the effect of B7-1 on β1-integrin function. To test whether the observed interaction was specific for B7-1 and β1, we conducted a series of coimmunoprecipitation studies of transfected HEK293 cells. Binding of talin, which is known to interact with β integrins,7 to β1 or β3 integrin served as a positive control. In addition to β1, B7-1 also interacted with β3 integrin (Fig. S5A in the Supplementary Appendix) through direct binding between the two proteins (Fig. S5B in the Supplementary Appendix). In contrast, B7-2 (CD86),9 which is not expressed in podocytes,4 did not interact with β1 or β3 integrin (Fig. S5A in the Supplementary Appendix).
Analysis of β1-Integrin Activation in Podocytes
The addition of lipopolysaccharide to culture medium or B7-1 overexpression caused a near-complete loss of β1-integrin activation6,7 without affecting total β1 integrin levels (Fig. S6A in the Supplementary Appendix). Abatacept restored β1-integrin activation in B7-1–expressing podocytes, even in the presence of lipopolysaccharide (Fig. S6A in the Supplementary Appendix). Over-expression of the B7-1Δtail construct or gene silencing of B7-1 preserved β1-integrin activation in the presence of lipopolysaccharide (Fig. S6A and S6B in the Supplementary Appendix). Like abatacept treatment, B7-1 gene silencing also restored β1 activation in α3−/− podocytes (Fig. S6C in the Supplementary Appendix). In independent flow-cytometric experiments, we confirmed that B7-1 blocks β1-integrin activation in podocytes (Fig. S6D in the Supplementary Appendix).
Analysis of Integrin-Dependent Cell Spreading
B7-1 suppressed α5β1 integrin–mediated spreading of K562 cells, which was restored by abatacept (Fig. S7A, S7B, and S7C in the Supplementary Appendix). In contrast, B7-1 did not affect β3 integrin–dependent cell spreading (Fig. S7D, S7E, and S7F in the Supplementary Appendix).
Molecular Basis of B7-1 Effects on β1-Integrin Activation
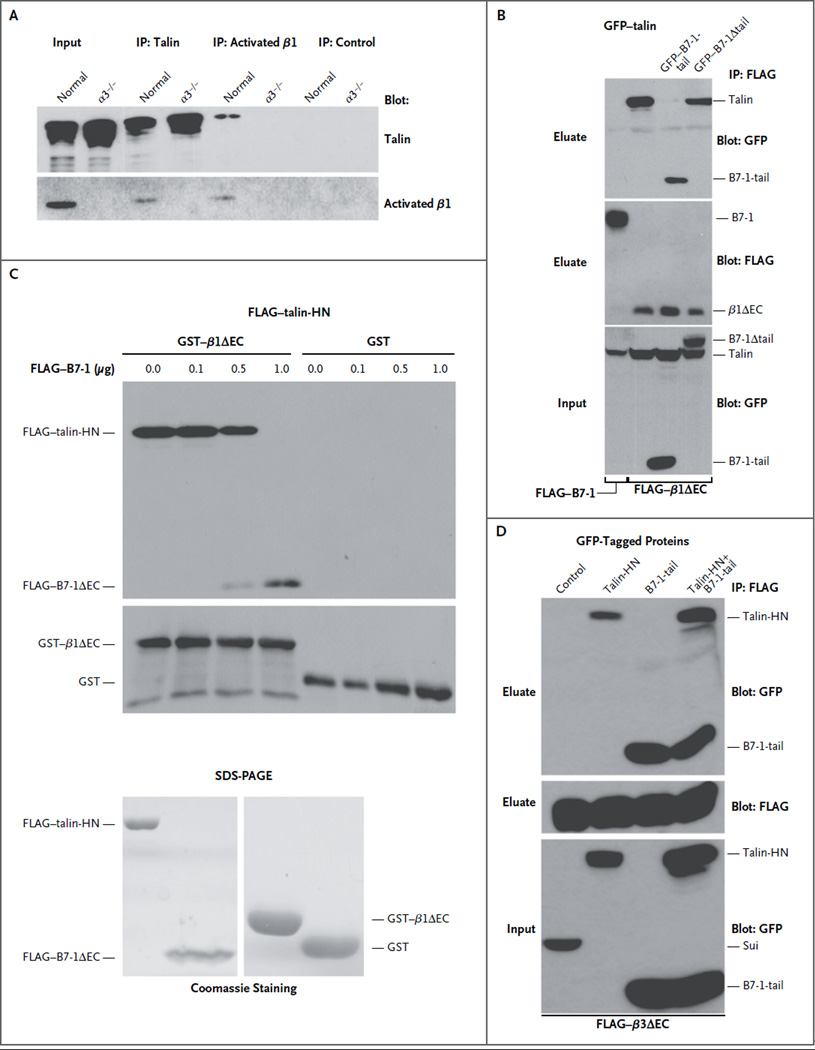
We investigated the mechanism of B7-1 disruption of β1-integrin activation. The cytoplasmic protein talin is known to bind to cytoplasmic β-integrin tails, disrupting the salt bridge between the α and β subunits10 and resulting in integrin activation.6 Because B7-1 can also bind to β1 integrin, we hypothesized that B7-1 may block β1-integrin activation by competing with talin for β1-integrin binding. In endogenous coimmunoprecipitation studies, we confirmed an interaction between talin and β1 integrin in normal podocytes but not in α3−/− podocytes. We also performed coimmunoprecipitation studies of HEK293 cells after they were cotransfected with various talin, β1-integrin, and B7-1 constructs. These experiments revealed that the cytoplasmic fragment of B7-1 (B7-1-tail) bound to the cytoplasmic tail of β1 integrin lacking the extracellular domain (β1ΔEC) at the expense of talin (Fig. 2B). In contrast, B7-1 lacking its cytoplasmic tail (B7-1Δtail) did not disrupt β1 binding to talin (Fig. 2B).
Figure 2. Disruption of the Binding of Talin to β1 Integrin, but Not to β3 Integrin, by B7-1.
As shown in Panel A, endogenous talin coprecipitated with activated β1 integrin in normal podocytes but not in α3−/− podocytes. Immunoprecipitation (IP) with anti–green fluorescent protein (GFP) antibody served as a negative control. Input refers to protein extracts that served as starting material from which endogenous proteins were immunoprecipitated. As shown in Panel B, FLAG–B7-1 did not bind to talin (GFP–talin-HN, left lane); HN denotes head N-terminal domain. GFP–B7-1-tail but not GFP–B7-1Δ tail blocked the interaction of talin (GFP–talin-HN) with β1 integrin (FLAG–β1ΔEC) in cotransfected HEK293 cells. Instead, B7-1-tail coprecipitated with β1 integrin. As shown at the top of Panel C, immobilized β1 integrin (GST–β1ΔEC) but not the GST control bound directly to purified talin (FLAG–talin-HN). In the presence of increasing amounts of FLAG–B7-1ΔEC, the binding of talin-HN to GST–β1ΔEC was gradually lost, whereas the binding of B7-1 to β1 integrin could be detected. As shown at the bottom of Panel C, Coomassie-stained sodium dodecyl sulfate–polyacrylamide-gel electrophoresis (SDS-PAGE) analysis showed the purity of recombinant proteins. As shown in Panel D, co-expression of GFP–B7-1-tail did not block the interaction of talin (GFP–talin-HN) with β3 integrin (FLAG–β3ΔEC) in triple-transfected HEK293 cells. Instead, both GFP–B7-1-tail and GFP–talin-HN coprecipitated with FLAG–β3ΔEC. No binding was found with the fusion protein GFP–sui (negative control).
As the ultimate test of whether B7-1 competes with talin for β1 binding, we conducted in vitro reconstitution studies with purified recombinant proteins (Fig. 2C). In the absence of B7-1 (FLAG–B7-1), talin (FLAG–talin-HN) bound to purified β1 (GST–β1ΔEC) but not to the GST control (Fig. 2C). In contrast, the addition of B7-1 (FLAG–B7-1ΔEC) led to the binding of B7-1 to purified β1 integrin (GST–β1ΔEC) at the expense of talin (FLAG–talin-HN) in a concentration-dependent fashion (Fig. 2C). We confirmed that B7-1 specifically competes with talin for binding to β1 integrin but not to β3 integrin (Fig. 2D), in line with our observations in cell-spreading assays (Fig. S7 in the Supplementary Appendix). Taken together, these data indicated that B7-1 mediates podocyte injury and protein-uria by disrupting the binding of talin to β1 integrin but not to β3 integrin (Fig. S8A in the Supplementary Appendix) and that this disruption can be blocked by administering abatacept (Fig. S8B in the Supplementary Appendix).
B7-1 Immunostaining in Human Kidney-Biopsy Specimens
To test whether podocyte B7-1 might serve as a biomarker for some proteinuric kidney diseases, we examined its expression in biopsy specimens of native kidneys from patients with various glomerular diseases and in biopsy specimens of renal allografts. Immunostaining results according to diagnosis, patient sex and age, time since transplantation (if applicable), and protein level are shown in Tables S1 and S2 in the Supplementary Appendix; representative images are shown in Figure S9 in the Supplementary Appendix. In biopsy specimens without pathologic glomerular features (Fig. S9A and Table S1 in the Supplementary Appendix) and in all biopsy specimens of renal allografts from patients without recurrent proteinuria (Fig. S9B and Table S2 in the Supplementary Appendix), only weak arteriolar immunostaining was observed. Three of five biopsy specimens from patients with a diagnosis of minimal-change disease showed granular staining for B7-1 along peripheral capillary walls, indicating a podocyte distribution (Fig. S9C and S9D and Table S1 in the Supplementary Appendix). B7-1 was absent in specimens from four of five patients with secondary FSGS, while weak focal podocyte immunostaining was found in a specimen from one patient (Table S1 in the Supplementary Appendix). In contrast, specimens from two of the three patients with primary FSGS in this series had diffuse and strong linear podocyte B7-1 staining (Fig. S9E and S9F and Table S1 in the Supplementary Appendix), which was similar to the staining in the specimen from Patient 5 (Fig. S2B in the Supplementary Appendix). In keeping with previous results,4 specimens from all three patients with lupus nephritis had moderate-to-strong granular staining in podocytes or mesangium (Table S1 in the Supplementary Appendix). Specimens from patients with IgA nephropathy showed weak mesangial staining but no podocyte B7-1 staining (Fig. S9G and Table S1 in the Supplementary Appendix). The strongest B7-1 staining was seen in specimens from patients with membranous nephropathy, both PLA2R-positive and PLA2R-negative11 (Fig. S9H and Table S1 in the Supplementary Appendix). Among allograft-biopsy specimens, B7-1 staining was seen in the specimen from the one patient with recurrent FSGS in this series (Table S2 in the Supplementary Appendix), which was similar to the findings in the specimens from Patients 1 and 3 (Fig. S1E). Eleven allograft-biopsy specimens, all obtained from patients who did not have recurrent FSGS, were negative for B7-1 (Table S2 in the Supplementary Appendix).
Discussion
To date, therapy for FSGS and associated kidney disorders has been nonspecific, often ineffective, and fraught with side effects. Abatacept, a co-stimulatory inhibitor that targets B7-1, is currently approved for the treatment of rheumatoid arthritis and has been used to treat other autoimmune diseases.3,12–14 Using abatacept, we successfully induced a partial or complete remission in five patients with primary or recurrent FSGS. Our clinical and in vitro data, taken together, indicate that podocyte B7-1 induction in primary and recurrent FSGS offers a rationale for using abatacept to treat a subgroup of patients with proteinuric kidney diseases.
The analysis of a series of 22 randomly selected biopsy specimens of native human kidneys identified a subpopulation of patients with minimal-change disease or primary FSGS who had B7-1 immunostaining of podocytes. In contrast, immunostaining for B7-1 was negative in 4 of 5 biopsy specimens obtained from patients with secondary FSGS, despite substantial podocyte injury. Furthermore, B7-1 immunostaining of podocytes was observed in the allograft specimen from a patient with recurrent FSGS, whereas the allograft specimens from all the other patients were negative. We speculate that B7-1 immunostaining of kidney-biopsy specimens might identify a subgroup of patients with proteinuric kidney diseases who would benefit from treatment with abatacept.
Mechanistically, B7-1 promotes disease-associated podocyte migration through inactivation of β1 integrin, which is reversed by abatacept. Whereas in T cells B7-1 acts by binding to CD28, CTLA-4, or PD-L1 through its extracellular domains,9,15 in podocytes the cytoplasmic tail of B7-1 is necessary and sufficient to block β1-integrin activation, by competing with talin for β1-integrin binding. Our results thus indicate that protection of β1-integrin activation in podocytes is the putative mechanism underlying the antiprotein-uric action of abatacept.
Patient 5, who had relapsing nephropathy, received abatacept in conjunction with glucocorticoids alone rather than with a full post-transplantation immunosuppressive regimen. Despite modest doses of glucocorticoids, abatacept induced a remission of the nephrotic syndrome in this patient for the first time in more than 1 year. In this patient (and the others treated), for whom therapeutic options were limited, abatacept appeared to induce clinical remission.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (DK57683 and DK062472, to Dr. Mundel; DK093783, to Dr. Weins; DK068253 and HL109582, to Dr. Gupta; DK0903616, to Drs. Fornoni and Burke; and DK090316, DK083511, and DK093746, to Dr. Greka), the Boston Area Diabetes Endocrinology Research Center and the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK057521, to Dr. Greka), Deutsche Forschungsgemeinschaft (HA 6334/1-1, to Dr. Hakroush), and the NephCure Foundation and Transplant Foundation of South Florida (to Drs. Fornoni and Burke), and by an American Society of Nephrology Gottschalk Career Development Award and a NephCure Young Investigator Award (to Dr. Greka).
We thank Terri Woo for technical assistance; Reinhard Fässler, Martinsried, Germany, for β1 integrin complementary DNA (cDNA); Shimon Sakaguchi, Kyoto, Japan, for B7-2 (CD86) cDNA; Anna Huttenlocher, Madison, WI, for green fluorescent protein–talin cDNA; and our colleagues at the University of Miami (Carolyn Abitbol, Jayanthi Chandar, Wacharee Seeherunvong, Gaston Zilleruelo, Adela Mattiazzi, Giselle Guerra, Warren Kupin, and David Roth) for help with patient care.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 3.Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353:1114–1123. doi: 10.1056/NEJMoa050524. [Erratum, N Engl J Med 2005;353:2311.] [DOI] [PubMed] [Google Scholar]
- 4.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagida-Asanuma E, Asanuma K, Kim K, et al. Synaptopodin protects against proteinuria by disrupting Cdc42: IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Gylling A, Alonso JL, et al. The beta-tail domain (betaTD) regulates physiologic ligand binding to integrin CD11b/CD18. Blood. 2007;109:3513–3520. doi: 10.1182/blood-2005-11-056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 10.Wegener KL, Partridge AW, Han J, et al. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoi AY, Littlejohn GO. Abatacept in the treatment of lupus. Expert Opin Biol Ther. 2012;12:1399–1406. doi: 10.1517/14712598.2012.713934. [DOI] [PubMed] [Google Scholar]
- 13.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 14.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.