Abstract
A photo-reactive diazirine derivative was attached to the 2-thiocytidine residue at position 32 of tRNA(Arg)I from Escherichia coli. This modified tRNA was bound under suitable conditions to the A, P or E site of E.coli ribosomes. After photo-activation of the diazirine label, the sites of cross-linking to 16S rRNA were identified by our standard procedures. Each of the three tRNA binding sites showed a characteristic pattern of cross-linking. From tRNA at the A site, a major cross-link was observed to position 1378 of the 16S RNA, and a minor one to position 936. From the P site, there were major cross-links to positions 693 and to 957 and/or 966, as well as a minor cross-link to position 1338. The E site bound tRNA showed major cross-links to position 693 (identical to that from the P site) and to positions 1376/1378 (similar, but not identical, to the cross-link observed from the A site). Immunological analysis of the concomitantly cross-linked ribosomal proteins indicated that S7 was the major target of cross-linking from all three tRNA sites, with S11 as a minor product. The results are discussed in terms of the overall topography of the decoding region of the 30S ribosomal subunit.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhangu R., Wollenzien P. The mRNA binding track in the Escherichia coli ribosome for mRNAs of different sequences. Biochemistry. 1992 Jun 30;31(25):5937–5944. doi: 10.1021/bi00140a033. [DOI] [PubMed] [Google Scholar]
- Bochkariov D. E., Kogon A. A. Application of 3-[3-(3-(trifluoromethyl)diazirin-3-yl)phenyl]-2,3- dihydroxypropionic acid, carbene-generating, cleavable cross-linking reagent for photoaffinity labeling. Anal Biochem. 1992 Jul;204(1):90–95. doi: 10.1016/0003-2697(92)90144-v. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Mitchell P., Osswald M., Stade K., Bochkariov D. Clustering of modified nucleotides at the functional center of bacterial ribosomal RNA. FASEB J. 1993 Jan;7(1):161–167. doi: 10.1096/fasebj.7.1.8422963. [DOI] [PubMed] [Google Scholar]
- Brimacombe R. RNA-protein interactions in the Escherichia coli ribosome. Biochimie. 1991 Jul-Aug;73(7-8):927–936. doi: 10.1016/0300-9084(91)90134-m. [DOI] [PubMed] [Google Scholar]
- Brunner J., Semenza G. Selective labeling of the hydrophobic core of membranes with 3-(trifluoromethyl)-3-(m-[125I]iodophenyl)diazirine, a carbene-generating reagent. Biochemistry. 1981 Dec 8;20(25):7174–7182. doi: 10.1021/bi00528a019. [DOI] [PubMed] [Google Scholar]
- Brunner J., Senn H., Richards F. M. 3-Trifluoromethyl-3-phenyldiazirine. A new carbene generating group for photolabeling reagents. J Biol Chem. 1980 Apr 25;255(8):3313–3318. [PubMed] [Google Scholar]
- Chen J. K., Franke L. A., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of tRNA1Arg to the 30S ribosomal subunit using aryl azide reagents attached to the anticodon loop. Biochemistry. 1985 Aug 27;24(18):4777–4784. doi: 10.1021/bi00339a011. [DOI] [PubMed] [Google Scholar]
- Ciesiolka J., Gornicki P., Ofengand J. Identification of the site of cross-linking in 16S rRNA of an aromatic azide photoaffinity probe attached to the 5'-anticodon base of A site bound tRNA. Biochemistry. 1985 Aug 27;24(18):4931–4938. doi: 10.1021/bi00339a031. [DOI] [PubMed] [Google Scholar]
- Dolder M., Michel H., Sigrist H. 3-(Trifluoromethyl)-3-(m-isothiocyanophenyl)diazirine: synthesis and chemical characterization of a heterobifunctional carbene-generating crosslinking reagent. J Protein Chem. 1990 Aug;9(4):407–415. doi: 10.1007/BF01024616. [DOI] [PubMed] [Google Scholar]
- Dontsova O., Dokudovskaya S., Kopylov A., Bogdanov A., Rinke-Appel J., Jünke N., Brimacombe R. Three widely separated positions in the 16S RNA lie in or close to the ribosomal decoding region; a site-directed cross-linking study with mRNA analogues. EMBO J. 1992 Aug;11(8):3105–3116. doi: 10.1002/j.1460-2075.1992.tb05383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnirke A., Geigenmüller U., Rheinberger H. J., Nierhaus L. H. The allosteric three-site model for the ribosomal elongation cycle. Analysis with a heteropolymeric mRNA. J Biol Chem. 1989 May 5;264(13):7291–7301. [PubMed] [Google Scholar]
- Gulle H., Hoppe E., Osswald M., Greuer B., Brimacombe R., Stöffler G. RNA-protein cross-linking in Escherichia coli 50S ribosomal subunits; determination of sites on 23S RNA that are cross-linked to proteins L2, L4, L24 and L27 by treatment with 2-iminothiolane. Nucleic Acids Res. 1988 Feb 11;16(3):815–832. doi: 10.1093/nar/16.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hixson S. H., Hixson S. S. P-Azidophenacyl bromide, a versatile photolabile bifunctional reagent. Reaction with glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Sep 23;14(19):4251–4254. doi: 10.1021/bi00690a016. [DOI] [PubMed] [Google Scholar]
- Kruse T. A., Clark B. F. The effect of specific structural modification on the biological activity of E. coli arginine tRNA. Nucleic Acids Res. 1978 Mar;5(3):879–892. doi: 10.1093/nar/5.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Stade K., Osswald M., Brimacombe R. Site-directed cross-linking studies on the E. coli tRNA-ribosome complex: determination of sites labelled with an aromatic azide attached to the variable loop or aminoacyl group of tRNA. Nucleic Acids Res. 1993 Feb 25;21(4):887–896. doi: 10.1093/nar/21.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Binding of tRNA to the ribosomal A and P sites protects two distinct sets of nucleotides in 16 S rRNA. J Mol Biol. 1990 Jan 5;211(1):135–145. doi: 10.1016/0022-2836(90)90016-F. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Nolan J. M., Burke D. H., Pace N. R. Circularly permuted tRNAs as specific photoaffinity probes of ribonuclease P RNA structure. Science. 1993 Aug 6;261(5122):762–765. doi: 10.1126/science.7688143. [DOI] [PubMed] [Google Scholar]
- Ofengand J., Delaney P., Bierbaum J. Photo-induced cross-linking of 4Srd and Cyd residues in Escherichia coli tRNA and its use as a conformational probe. Methods Enzymol. 1974;29:673–684. doi: 10.1016/0076-6879(74)29059-1. [DOI] [PubMed] [Google Scholar]
- Podkowiński J., Górnicki P. Ribosomal proteins S7 and L1 are located close to the decoding site of E. coli ribosome--affinity labeling studies with modified tRNAs carrying photoreactive probes attached adjacent to the 3'-end of the anticodon. Nucleic Acids Res. 1989 Nov 11;17(21):8767–8782. doi: 10.1093/nar/17.21.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinberger H. J., Geigenmüller U., Wedde M., Nierhaus K. H. Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol. 1988;164:658–670. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- Rinke-Appel J., Jünke N., Brimacombe R., Dukudovskaya S., Dontsova O., Bogdanov A. Site-directed cross-linking of mRNA analogues to 16S ribosomal RNA; a complete scan of cross-links from all positions between '+1' and '+16' on the mRNA, downstream from the decoding site. Nucleic Acids Res. 1993 Jun 25;21(12):2853–2859. doi: 10.1093/nar/21.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
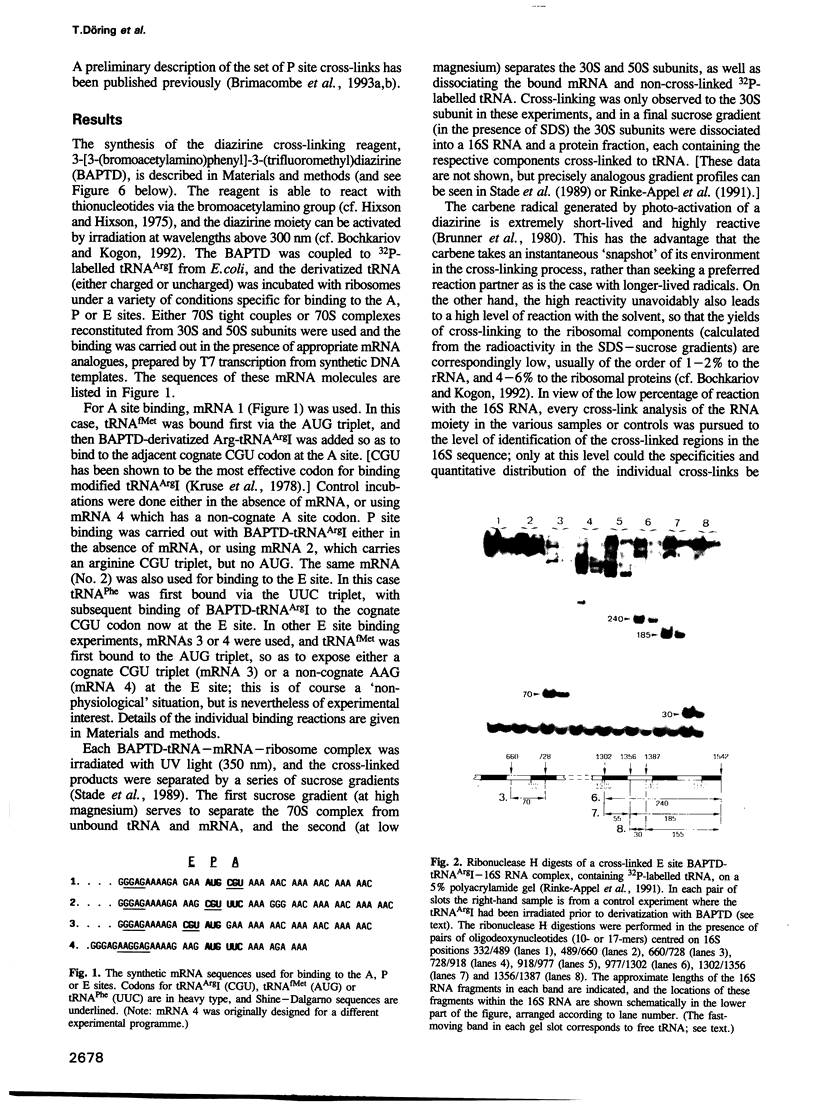
- Rinke-Appel J., Jünke N., Stade K., Brimacombe R. The path of mRNA through the Escherichia coli ribosome; site-directed cross-linking of mRNA analogues carrying a photo-reactive label at various points 3' to the decoding site. EMBO J. 1991 Aug;10(8):2195–2202. doi: 10.1002/j.1460-2075.1991.tb07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling-Bartetzko S., Franceschi F., Sternbach H., Nierhaus K. H. Apparent association constants of tRNAs for the ribosomal A, P, and E sites. J Biol Chem. 1992 Mar 5;267(7):4693–4702. [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner G., Kuechler E., Barta A. Photo-affinity labelling at the peptidyl transferase centre reveals two different positions for the A- and P-sites in domain V of 23S rRNA. EMBO J. 1988 Dec 1;7(12):3949–3955. doi: 10.1002/j.1460-2075.1988.tb03281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvers L. A., Kopylov A. M., Wower J., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of the anticodon loop of yeast tRNA(Phe) to 30S-subunit protein S7 at the ribosomal A and P sites. Biochimie. 1992 Apr;74(4):381–389. doi: 10.1016/0300-9084(92)90116-v. [DOI] [PubMed] [Google Scholar]
- Tate W., Greuer B., Brimacombe R. Codon recognition in polypeptide chain termination: site directed crosslinking of termination codon to Escherichia coli release factor 2. Nucleic Acids Res. 1990 Nov 25;18(22):6537–6544. doi: 10.1093/nar/18.22.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Malloy T. A., 4th, Hixson S. S., Zimmermann R. A. Probing tRNA binding sites on the Escherichia coli 30 S ribosomal subunit with photoreactive analogs of the anticodon arm. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):38–44. doi: 10.1016/0167-4781(90)90138-r. [DOI] [PubMed] [Google Scholar]
- Wower J., Scheffer P., Sylvers L. A., Wintermeyer W., Zimmermann R. A. Topography of the E site on the Escherichia coli ribosome. EMBO J. 1993 Feb;12(2):617–623. doi: 10.1002/j.1460-2075.1993.tb05694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]






