Abstract
Radioimmunotherapy (RIT) for relapsed indolent non-Hodgkin lymphoma produces overall response rates (ORR) of 80% with mostly partial remissions. Synthetic CpG oligonucleotides change the phenotype of malignant B-cells, are immunostimulatory, and can produce responses when injected intratumorally and combined with conventional radiation. In this phase I trial we tested systemic administration of both CpG and RIT. Eligible patients had biopsy-proven previously treated CD20+ B-cell NHL and met criteria for RIT. Patients received rituximab 250 mg/m2 days 1,8, and 15; 111In-ibritumomab tiuxetan days 1, 8; CpG 7909 days 6, 13, 20, 27; and 0.4 mCi/kg of 90Y-ibritumomab tiuxetan day 15. The doses of CpG 7909 tested were 0.08, 0.16, 0.32 (six patients each) and 0.48 mg/kg (12 patients) IV over 2 hours without dose limiting toxicity. The ORR was 93% (28/30) with 63% (19/30) complete remission (CR); median progression free survival of 42.7 months (95% CI 18-NR); and median duration of response (DR) of 35 months (4.6-76+). Correlative studies demonstrated a decrease in IL10 and TNFα, and an increase in IL1β, in response to therapy. CpG 7909 at a dose of 0.48 mg/kg is safe with standard RIT and produces a high CR rate and long DR; these results warrant confirmation.
Keywords: lymphoma, radioimmunotherapy, rituximab, ibritumomab tiuxetan, CpG 7909
Introduction
Radioimmunoconjugates utilize the selectivity of a monoclonal antibody to target radiation to tumor cells using radionuclides with high-energy delivered over a short path length to avoid damage to the surrounding normal tissues. In patients with relapsed NHL, single doses of RIT have an ORR of 80% with 30% CR, a median time to progression (TTP) of 1-1.5 years with 20% of patients achieving long-term remissions [1-3]. Similar to chemotherapy results in indolent NHL, the best predictor of a long-term response to RIT is the achievement of a CR [1, 3]. Based on these results, the US FDA has approved 90Y-ibritumomab tiuxetan (Zevalin®, Spectrum Pharmaceuticals) for the treatment of relapsed low grade and follicular B-cell NHL and as consolidation after induction chemotherapy; 131I-tositumomab (Bexxar®, GlaxoSmithKline) is approved for relapsed low grade, follicular, and transformed NHL.
Several strategies are being tested to improve RIT results. These include moving RIT upfront as a single agent [4], consolidation after chemotherapy [5, 6], adding a radiosensitizer such as the porphyrin derivative motexafin gadolinium [7], and combining RIT with stem cell support where the myelosuppression is overcome with the use of autologous stem cells using standard [8, 9] and dose-escalated [10-14] approaches. Each of these has shown promise in early phase clinical trials.
CpG 7909 is a 24 mer oligodeoxynucleotide (ODN) synthesized on a nuclease-resistant phosphorothioate backbone that contains four CG sequences and is designed to activate the immune system through the Toll-like receptor 9 (TLR 9). CpG 7909 has direct effects on human malignant B cells and plasmacytic dendritic cells [15, 16]. It can increase CD20 expression on malignant B cells, induce a type 1 T helper cell (TH1) adaptive immune response, and be safely administered to patients with B cell malignancies as a single-agent [17, 18] or in combination with rituximab [19]. Early phase clinical evaluation indicates CpG 7909 can enhance the development of an immune response to B cell lymphoma following exposure to radiation [20]. Thus, combining CpG 7909 with RIT could enhance clinical response to RIT by either increasing delivery of radiation to the cancer cell due to enhanced expression of the target antigen, or facilitating development of an active anti-lymphoma immune response.
In this study, we combined intravenous (IV) CpG 7909 with standard dose RIT using 90Y-ibritumomab tiuxetan. We selected the IV rather than the subcutaneous (SC) route that has been used in prior studies [17, 18] because the IV CpG 7909 has virtually no side effects and is more likely to reach the tumor and induce direct effects on the B cell lymphoma that express the TLR9 receptor [17, 18]. The goal was to determine the maximum tolerated doses (MTD) of IV CpG 7909 that can be delivered in four doses (two prior and two after RIT) for patients with relapsed NHL. Secondary goals were to learn the ORR, DR, and TTP with this regimen. Translational research goals were to study the effects of CpG 7909 on 90Y-ibritumomab tiuxetan biodistribution, learn the effect of CpG 7909 in vitro on gene expression, and determine if CpG 7909 produced detectable immunological effects in the blood in this treatment setting.
Patients and Methods
Patient Eligibility
Trial LS0382 (NCT00438880) was conducted by the University of Iowa/Mayo Clinic Lymphoma SPORE and was approved by the Institutional Review Boards of each site. Patients ≥18 years with biopsy-proven relapsed/refractory CD20+ B-cell NHL of the following types were eligible: small lymphocytic (SLL), lymphoplasmacytoid, follicular grades 1-3, marginal zone, and diffuse large. Patients were required to have measurable disease (≥1 lesion ≥2 cm); <25% of cellular marrow involvement with NHL, no evidence of myelodysplasia; absolute neutrophil count (ANC) ≥1.5 × 10(9)/L; platelet count ≥150 × 10(9)/L; a total lymphocyte count <5 × 10(9)/L for patients with SLL; serum creatinine and bilirubin <2mg/dL; and an ECOG performance status of 0, 1, or 2. All patients provided written, informed consent prior to study entry.
Patients with central nervous system NHL, NHL in association with HIV infection, prior transplant or RIT, external beam radiation to >25% of active marrow, cytology positive pleural effusion, serious non-malignant disease, other active malignancy, known human anti-mouse or anti-chimeric antibody, severe skin rash with prior rituximab, and patients on corticosteroids for NHL were ineligible.
Study Treatment
This phase I trial tested four dose levels of CpG 7909 combined with a standard dose of 90Y-ibritumomab tiuxetan RIT. The patients received four doses of CpG 7909 - two before and two after the RIT (Supporting Information Figure S1); rituximab 250 mg/m2 on days 1, 8, and 15; 111Indium ibritumomab tiuxetan 5 mCi on day 1 and 10 mCi day 8; 0.4 mCi/kg (maximum 32 mCi) 90Y-ibritumomab tiuxetan day 15; and CpG 7909 IV days 6, 13, 20, and 27. There were three doses of rituximab and two doses of 111Indium ibritumomab tiuxetan to allow study of the effects of the CpG 7909 on tumor and normal organ ibritumomab dosimetry. Each dose level of CpG 7909 was to enroll six patients with 12 at the MTD. Six patients were to be treated at each dose level and observed for at least 10 weeks after treatment or until blood counts had nadired and the ANC recovered to ≥1.5 × 10(9)/L and the platelet count was ≥50 × 10(9)/L. CpG 7909 was supplied by Pfizer (New York, New York) and diluted in physiologic saline into clear glass containers and delivered IV over 2 hours as an outpatient.
Tumor and normal organ dosimetry was performed after each dose of 111In-ibritumomab tiuxetan as previously described [21, 22]. In brief, images were obtained within 1 hour, at 2-3 hours, and at 4-6 hours post-injection. Additional images were obtained on day 2, day 4 or 5 and day 6 or 7. The gamma camera imaging was whole body at each time point. On day 3 or 4 SPECT tomographic imaging was performed. At each imaging time point, 5 mL of EDTA whole blood was collected and counted in a gamma well counter. The images were reviewed for altered biodistribution defined as diffuse increased uptake in normal lungs, kidney uptake with greater intensity than the liver, or intense areas of uptake throughout the normal bowel.
Hematologic DLT criteria used for the study were grade 4 toxicity for ANC (<0.5 × 109/L) or platelets (<10 × 109/L) for ≥14 days or grade 3 toxicity for ANC (≥0.5 - <1.0 × 109/L) or platelets (≥10 - <50 × 109/L) for ≥28 days. Non-hematologic DLT criteria were any grade 3 solid organ toxicity not explainable by another obvious cause. Infusion reactions to rituximab were not considered as a DLT.
Measurable lesions were assessed by computed tomography (CT) at baseline, and weeks 4 and 12 following RIT. After week 12, patients entered observation and were followed without any further therapy every 3 months for one year and then went to event monitoring where they were followed every 6 months for long-term toxicity, relapse, and survival.
Statistical Design
Six patients were enrolled in each cohort. If one or less of the six patients experienced DLT the next cohort were to be treated at the next higher dose level. If DLT was seen in two or more patients at a given dose level then the MTD was exceeded and the next lower dose would be considered the MTD. Twelve patients were to be treated at the MTD or highest dose of CpG 7909. Tumor responses were determined from CT scan measurements per the International Workshop Criteria of 1999.[23] DR was the time from date of initial response until date of last disease assessment. TTP was the time from date of study registration until date of progression. Patients still responding at time of last follow up were censored. Overall survival (OS) was the time from date of study registration until date of death due to any cause. Patients alive at last follow up were censored at date last known alive. PFS was the time from date of study registration until date of progression or death due to any cause.
Translational Research
Patients provided permission for blood sampling for serum and blood mononuclear cells pre-treatment and at days 6, 8 and 15 pre-RIT (Supporting Information Figure S1) and were repeated at weeks 4, 8 and 12 post-RIT for lymphocyte subsets and serum cytokines. NK and ADCC assays were performed as previously described.[17] In brief, blood lymphocytes were harvested from whole blood by Ficoll Hypaque (Sigma-Aldrich, St. Louis, MO) separation and used as effectors in a standard 51Cr release assay. For measuring NK activity, K562 were used as targets and cytotoxicity assed after four hours incubation. Enhanced NK activity was determined by the addition of IL-2 (100 units/ml) to the fresh NK cell assay. For ADCC, which is antibody-dependent but species non-specific, two target cell lines were used: ZKBV (an NK cell resistant human B lymphoblastoid cell line) and CL27A (an NK resistant murine lymphoma cell line). ADCC was determined by the addition of a mouse polyclonal anti-CL27A antibody to CL27A, or by the addition of a monoclonal anti-CD20 antibody (Rituximab; Genentech Inc., South San Francisco, CA) to ZKBV cells prior to combination of effector and target cells. Lysis of CL27a and ZKBV cells with and without antibody was measured after four hours incubation.
Total T (CD3), T-cell subsets (CD4 and CD8), B cells (CD19), myeloid dendritic cells (HLA-DR+CD11c+), and plasmacytoid dendritic cells (HLADR+CD123+) were quantified at each time point.
The following serum cytokines were measured by ELISA (Luminex, Austin, TX): interleukin (IL)-1β, IL-2, IL-5, IL-6, IL-10, IL-12, IL-15, TNF-α, and interferonγ. The natural-log-transformed cytokine measurements for all patient cohorts were combined and modeled simultaneously as a quadratic function of CpG 7909 dose using linear regression. Dose-specific estimates of baseline fold changes (ratio of follow-up to pre-treatment) and statistical tests for significant changes are reported based on the fitted regression models. Fold change values <1 indicate decreases in cytokine levels, and values >1 indicate increases.
Gene expression profiling (GEP) was performed on malignant B-cells before and after CpG 7909 treatment in vitro to explore the potential effects of CpG 7909 on gene expression. Fresh malignant lymphoma cell suspensions [24] were obtained from consenting patients through the Lymphoma SPORE Biospecimen Core; placed in nutrient media and divided into two fractions: control (no CpG) and treatment with CpG 7909 5 μg/ml for 72 hours [25]. The cells were then separated into B-cell (CD19+) and non-B-cell (T-cells, NK-cells, DCs, and macrophages) fractions by use of a magnetic bead B-cell isolation kit (Miltenyi Biotech, Auburn, CA). Total RNA was isolated using Qiagen's Rnaeasy Total RNA Isolation Kit. cDNA microarray analyses using the HG-U133 A 2.0 Affymetrix genechip arrays (Affymetrix, Inc., Santa Clara, CA) were conducted in the Mayo Cancer Center Microarray Core.
Results
Determination of the Maximum Tolerated Dose of CpG 7909
Thirty patients (Table I) were enrolled and completed the therapy as designed. Comparison of tumor and normal organ dosimetry results before and after CpG 7909 showed no detectable alteration in the tumor or normal organ biodistribution of 111In-ibritumomab tiuxetan in any of the 30 patients. No patient failed 111In-ibritumomab imaging and all proceeded to 90Y-ibritumomab tiuxetan RIT. Two patients (6.7%) met hematologic DLT criteria - one each at dose levels 2 (0.16 mg/kg) and 4 (0.48 mg/kg); therefore, MTD criteria were not met for any dose level. Six additional patients were treated at the 0.48 mg/kg dose without additional problems and thus 0.48 mg/kg is the recommended dose for phase II.
Table 1. Characteristics of the 30 patients treated with CpG 7909 and 90Y-ibritumomab tiuxetan.
| Patient Characteristics | Total (N=30) |
|---|---|
| Age (years) | |
| Median (Range) | 60.5 (36 - 80) |
|
| |
| Gender | |
| Female | 13 (43%) |
|
| |
| Performance Score | |
| 0 | 25 (83%) |
| 1 | 5 (17%) |
|
| |
| Lymphoma Type | |
| Extranodal marginal zone B-cell lymphoma | 3 (10%) |
| Follicular lymphoma-I | 12 (40%) |
| Follicular lymphoma-II | 10 (33.3%) |
| Follicular lymphoma, NOS | 1 (3.3%) |
| Diffuse large B-cell lymphoma | 3 (10%) |
| Lymphoplasmacytoid | 1 (3.3%) |
|
| |
| Clinical Stage at Time of Treatment | |
| I | 5 (16.7%) |
| II | 3 (10%) |
| III | 3 (10%) |
| IV | 19 (63.3%) |
|
| |
| Number of Prior Regimens | |
| 1 vs. 2 vs. 3 or more | 15:9:6 |
| Median (range) | 1.5 (1-6) |
|
| |
| Prior Therapy | |
| Radiation therapy | 10 (33%) |
| Immunotherapy (rituximab) | 17 (57%) |
| Anthracycline | 11 (37%) |
| Alkylating agent | 20 (67%) |
|
| |
| Extra Nodal Site Involvement | |
| Yes | 22 (73.3%) |
|
| |
| Number of Extranodal Sites | |
| Median (Range) | 4 (1-8) |
|
| |
| Current Disease Status | |
| First relapse | 15 (50%) |
| Second relapse | 9 (30%) |
| Third relapse | 2 (6.7%) |
| Relapse NOS | 3 (10%) |
| Refractory | 1 (3.3%) |
|
| |
| International Prognostic Index | |
| 0 | 4 (13.3%) |
| 1 | 8 (26.7%) |
| 2 | 16 (53.3%) |
| 3 | 2 (6.7%) |
|
| |
| Bone Marrow Involvement | 12 (40.0%) |
Tumor Response, Progression, and Survival
The ORR to this combination of four doses of CpG 7909, three doses of rituximab, and one dose of 0.4 mCi/kg 90Y-ibritumomab tiuxetan was 93% (28/30) with 63% (19/30) CR/Cru and 30% (9/30) PR (Supporting Information Table SII). The ORR was 96% (26/27) in the patients with relapsed indolent NHL and 67% (2/3) for relapsed aggressive NHL. The median DR was 48.3 months (range, 4.6-80+ months). The median TTP for all 30 patients was 42.7 months (95% CI 17.8-NR) (Supporting Information Table SII); median PFS of 42.7 months (95% CI 17.8-NR) (Figure 2); and the median OS has not yet been reached. At a median follow-up of 67.8 months (range, 46-82+ months), 93% (28/30) of patients remain alive with both patient deaths due to lymphoma. As of August 2012, 43% (13/30) of patients remain in continuous remission (Figure 3) without therapy.
Figure 2.
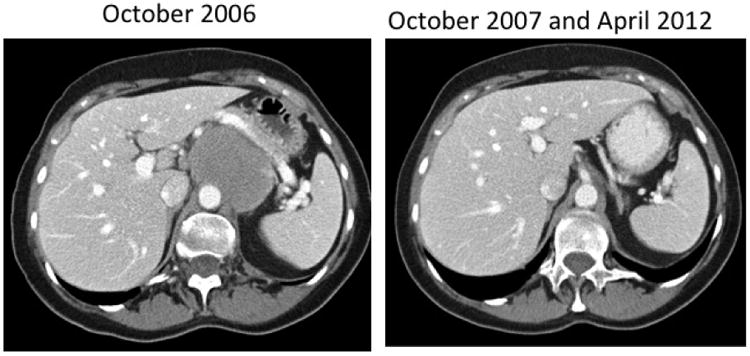
Computed tomography scan of a 70 year old woman with relapsed follicular grade I NHL after three prior chemotherapy regimens. The patient received 0.48 mg/kg (level 4) CpG7909 in 2006 and remains in unmaintained remission in 2012.
Figure 3.
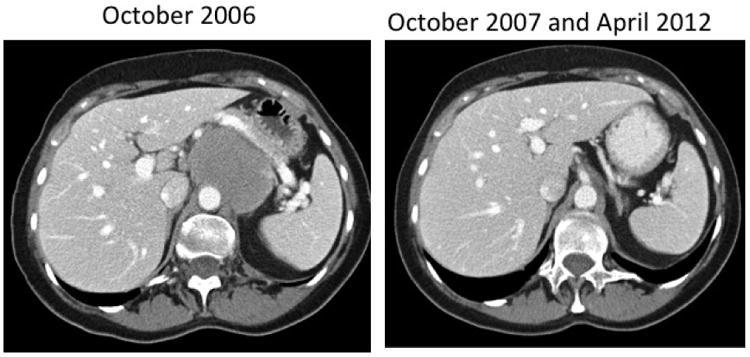
Computed tomography scan of a 70 year old woman with relapsed follicular grade I NHL after three prior chemotherapy regimens. The patient received 0.48 mg/kg (level 4) CpG7909 in 2006 and remains in unmaintained remission in 2012.
Adverse Events
The main toxicity was reversible myelosuppression (Supporting Information Table SIII) with 77% and 63% of patients developing grade III/IV neutropenia or thrombocytopenia, respectively. Only one patient developed febrile neutropenia and there were no hemorrhages. One patient had grade 3 pain over her bulky neck lymphadenopathy after infusion of CpG 7909. This patient completed therapy and obtained a durable CR. To date there have been no cases of myelodysplastic syndrome.
Effects on Lymphocyte Subsets
The rituximab and RIT produced effective B-cell depletion by day 6 and blood B-cells remained undetectable through week 12. Total T-cells and T-cell subsets decreased to a nadir by week 4 and then recovered by week 12. These changes were similar between CpG 7909 dose groups and are consistent with the kinetics of myelosuppression and B-cell depletion that occurs with rituximab and RIT. Because of this highly effective blood B-cell depletion, we were unable to assess changes in costimulatory (CD40, CD80, CD86, CD54) molecule expression on B-cells and to assess effects on myeloid and plasmacytoid DCs. However, when we focused on only the pre-treatment through day 15 (the day of RIT) there was an increase in the both myeloid and plasmacytoid DCs in the blood; there was no detectable difference between CpG 7909 dose levels. Measurement of NK cell killing and ADCC demonstrated considerable variability between subjects with no statistically significant differences between cohorts (data not shown).
Effects of Treatment on Serum Cytokines
Serial serum cytokine measurements were evaluated at key time points that evaluated the effect of CpG before and after RIT. The results of cytokines that underwent change are expressed as fold-change differences compared to the baseline pre-treatment values (Table IV). There were statistically significant increases in IFNγ, IL12, and IL1β and decreases in IL10 and TNFα following therapy (Table IV). Changes across dose levels tended to show more change at the higher doses of CpG with some variability.
Table IV.
Fold-change in serum cytokine values from baseline to week 12 following all treatment. Day 15 results reflect two doses each of CpG and rituximab. Week 12 values are after all 4 CpG doses, 3 doses of rituximab, 1 dose of 90Y-ibritumomab tiuxetan and recovery of myelosuppression.
| Cytokine | Sample Day | CpG Dose Level (mg/kg) | |||
|---|---|---|---|---|---|
| 0.08 | 0.16 | 0.32 | 0.48 | ||
| IFNγ | Day 15 | 1.16 | 1.02 | 1.02 | 1.45 |
| IFNγ | Week 12 | 0.74 | 1.10 | 1.58** | 1.31 |
| IL10 | Day 15 | 0.77 | 0.85 | 0.86 | 0.68 |
| IL10 | Week 12 | 0.27* | 0.50* | 0.49 | 0.09* |
| IL12 | Day 15 | 1.04 | 1.33 | 1.69* | 1.54 |
| IL12 | Week 12 | 0.67* | 0.90 | 1.65* | 2.99 |
| IL15 | Day 15 | 1.22 | 1.16 | 1.23 | 1.61 |
| IL15 | Week 8 | 7.10** | 1.16 | 0.29** | 1.35 |
| IL1β | Day 15 | 1.03 | 1.77** | 1.63 | 0.31* |
| IL1β | Week 12 | 1.00 | 2.41 | 4.67** | 2.08 |
| IL2 | Day 15 | 0.84 | 0.96 | 1.15 | 1.21 |
| IL2 | Week 12 | 0.58 | 0.81 | 1.05 | 0.79 |
| IL5 | Day 15 | 0.98 | 1.00 | 0.97 | 0.85** |
| IL5 | Week 12 | 1.05 | 1.42* | 1.40* | 0.61 |
| IL6 | Day 15 | 0.98 | 0.90 | 0.87 | 0.97 |
| IL6 | Week 12 | 1.03 | 4.70* | 10.07** | 1.02 |
| TNFα | Day 15 | 0.91 | 0.94 | 1.00 | 1.06 |
| TNFα | Week 12 | 0.60 | 1.15 | 0.99 | 0.12** |
Abbreviations
p<0.05;
p<0.01
Effect of CpG 7909 on Gene Expression in Primary Lymphoma Cells
Fresh lymphoma biopsy samples from 5 separate patients not from the clinical trial were used for GEP before and after incubation in vitro with CpG 7909. Three samples were aggressive (DLBCL) and two were indolent (small lymphocytic lymphoma; marginal zone lymphoma). The GEP results were pooled and results expressed as fold change in gene expression in lymphoma B cells. Only genes with p-values <0.05 were considered. Supporting Information Figure S4 depicts the top genes in lymphoma B-cells that responded to CpG 7909 with changes in gene expression. For example, we observed a marked downregulation in the signaling lymphocyte activation molecule SLAMF8 with CpG 7909 (p=0.01). SLAMF8, also known as CD353, is typically expressed in B-cells and monocytes.[26] In contrast, the expression of the IL-21R gene was markedly increased with CpG 7909 (p=0.009). Ligand activation of IL-21R leads to proliferative effects on a variety of blood cells including B-cells and is being tested in cancer patients [27].
Discussion
This study demonstrates the safety and efficacy of the addition of four doses of IV CpG 7909 to a standard dose of RIT for relapsed NHL. The tumor and normal organ dosimetry were a safety measure for this phase I study. We did not observe any adverse effects of CpG 7909 on ibritumomab tumor or normal organ biodistribution. The rationale for administering CpG 7909 prior to RIT is the in vitro observation that CpG 7909 as a single-agent can increase expression of CD20 by the malignant B cells, thus raising the hypothesis that such therapy could enhance delivery of radiation to the lymphoma [25]. Despite this hypothesis, imaging studies before and after CpG 7909 showed no meaningful effect on tumor or normal organ biodistribution of ibritumomab tiuxetan with the CpG 7909. CD20 upregulation on tumor cells could have occurred, but at a level of detection below what could be detected by gamma camera imaging. Although we could not prove in vivo that CpG 7909 was enhancing ibritumomab uptake in the tumor, we also found no evidence of an adverse change in biodistribution. In a recently completed clinical study in chronic lymphocytic leukemia, IV CpG 7909 at 0.45 mg/kg had no effect on CD20 expression while higher doses (1.05 mg/kg) did increase expression of CD20.[18] Thus, the dose of CpG 7909 we used could have been too low to impact CD20 expression and biodistribution of ibritumomab tiuxetan.
The rationale for the post-RIT doses of CpG 7909 were based on the known immunostimulatory properties of CpG 7909 including induction of increased expression of costimulatory molecules by both malignant B cells and plasmacytic dendritic cells [15, 28, 29]. Brody et al [20] injected CpG 7909 into indolent NHL tumors before and after external beam radiation therapy. Fifteen patients were treated and 27% had a response in the non-irradiated, non-CpG injected node providing evidence that a systemic immune response had been generated. The two doses of CpG 7909 after RIT were designed to stimulate immunity after the RIT had induced death of lymphoma cells and antigen release. Serum measurements demonstrated a statistically significant decrease in IL10 and TNFα, and an increase in IL1β. CpG 7909 can induce IL-10 secretion in vitro. We saw the opposite most likely because the three doses of rituximab and one dose of RIT essentially depleted all B-cells. This is actually reassuring because we have recently demonstrated that increased IL-10 in B-cell NHL is associated with B-cell lymphoma [30] is an adverse prognostic factor for progression,[31, 32] and activates JAK/STAT pathway signaling in lymphoma cells [32]. It is difficult to reach any firm conclusions on the relative roles of CpG 7909 and RIT in the observed cytokine changes, as such changes have not been assessed following RIT alone. However, the CpG 7909 dose response suggests CpG 7909 played a role.
The fact that CpG 7909 has been found to be non-myelosuppressive in prior clinical trials made CpG 7909 an ideal agent to combine with RIT. Four doses of 0.48 mg/kg CpG 7909 were found to be safe in our studies as well as others and is the dose we recommend IV for future studies [17, 19]. Although future studies could test the 1.05 mg/kg IV dose found to be safe in CLL [18] it would require further phase I study since in the CLL study only one dose was given and there was no RIT.
The ORR in this study was very high at 93%. This ORR is higher than previous RIT studies in relapsed patients that typically produced ORRs in the 80% range [2, 33-36]. Even more impressive than the ORR is the durability of the responses observed in this study. Most studies of single-agent RIT in relapsed patients have demonstrated a median TTP of 1-1.5 years; with CpG 7909/RIT the median TTP was 40.5 months (95% CI 17.8-NR) and 43% patients have not yet relapsed. This latter figure is also much higher than the 20% found in previous reviews of RIT [3, 37].
There are several possible reasons for these encouraging results. It is possible that this was selection bias. For example, we required a platelet count ≥150 × 10(9)/L to ensure that all patients received 0.4 mCI/kg (maximum of 32 mCi) and this may have selected for a healthier patient population. We acknowledge that a randomized trial of CpG 7909/RIT vs RIT alone would be necessary to definitively prove the benefit of CpG 7909. Secondly, the addition of the CpG 7909 with its dual effects on the tumor and the immune system could indeed have induced development of an active anti-lymphoma response that contributed to the improved DR. Thirdly, this regimen included three doses of rituximab instead of the two that are given with the conventional ibritumomab tiuxetan regimen, and lastly there were rituximab-naïve patients in this study. On the other hand, we did not provide rituximab maintenance and thus these TTP and DR results are solely the results of this regimen.
We conclude that CpG 7909 can be added safely to RIT. It does not impact on biodistribution of the radionuclide, but does result in changes in cytokines that are consistent with development of a more robust anti-lymphoma immune response. Most importantly, the high CR rate and long DR observed with this combination compared to standard RIT warrant confirmation in additional trials.
Supplementary Material
Figure 1.
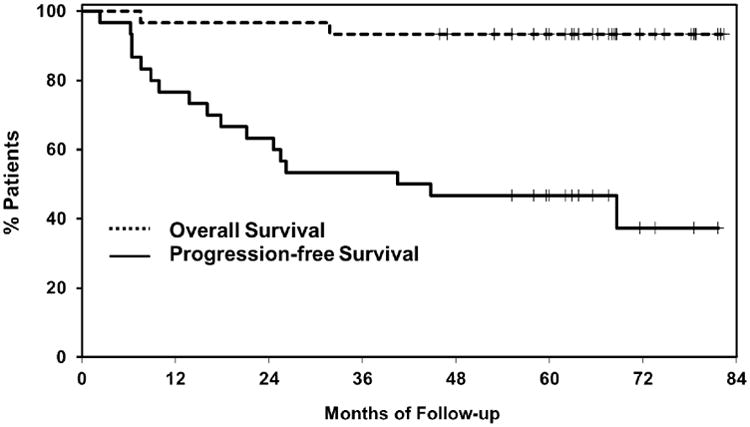
Overall and progression-free survival of the 30 patients treated with CpG7909 and radioimmunotherapy.
Acknowledgments
Supported in part by University of Iowa/Mayo Clinic SPORE CA97274, the Predolin Foundation, and R01CA127433
Footnotes
Conflict of Interest: The conduct of the study was supported by Spectrum Pharmaceuticals and Pfizer. The authors received research support but no other support.
NCT Number: NCT00438880
References
- 1.Witzig TE, Molina A, Gordon LI, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 2.Witzig TE, Fishkin P, Gordon LI, et al. Treatment recommendations for radioimmunotherapy in follicular lymphoma: a consensus conference report. Leuk Lymphoma. 2011;52:1188–1199. doi: 10.3109/10428194.2011.570396. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RI, Kaminski MS, Wahl RL, et al. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin's lymphomas. J Clin Oncol. 2005;23:7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 4.Scholz CW, Pinto A, Linkesch W, et al. 90Yttrium-Ibritumomab-Tiuxetan as First-Line Treatment for Follicular Lymphoma: 30 Months of Follow-Up Data From an International Multicenter Phase II Clinical Trial. J Clin Oncol. 2013;31:308–313. doi: 10.1200/JCO.2011.41.1553. [DOI] [PubMed] [Google Scholar]
- 5.Morschhauser F, Radford J, Van Hoof A, et al. Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J Clin Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 6.Press OW, Unger JM, Rimsza LM, et al. Phase III Randomized Intergroup Trial of CHOP Plus Rituximab Compared With CHOP Chemotherapy Plus 131Iodine-Tositumomab for Previously Untreated Follicular Non-Hodgkin Lymphoma: SWOG S0016. J Clin Oncol. 2013;31:314–320. doi: 10.1200/JCO.2012.42.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evens AM, Spies WG, Helenowski IB, et al. The novel expanded porphyrin, motexafin gadolinium, combined with [90Y]ibritumomab tiuxetan for relapsed/refractory non-Hodgkin's lymphoma: preclinical findings and results of a phase I trial. Clin Cancer Res. 2009;15:6462–6471. doi: 10.1158/1078-0432.CCR-09-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krishnan A, Nademanee A, Fung HC, et al. Phase II trial of a transplantation regimen of yttrium-90 ibritumomab tiuxetan and high-dose chemotherapy in patients with non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:90–95. doi: 10.1200/JCO.2007.11.9248. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan A, Palmer JM, Tsai NC, et al. Matched-Cohort Analysis of Autologous Hematopoietic Cell Transplantation with Radioimmunotherapy versus Total Body Irradiation-Based Conditioning for Poor-Risk Diffuse Large Cell Lymphoma. Biol Blood Marrow Transplant. 2012;18:441–450. doi: 10.1016/j.bbmt.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winter JN, Inwards DJ, Spies S, et al. Yttrium-90 ibritumomab tiuxetan doses calculated to deliver up to 15 Gy to critical organs may be safely combined with high-dose BEAM and autologous transplantation in relapsed or refractory B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:1653–1659. doi: 10.1200/JCO.2008.19.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopal AK, Gooley TA, Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood. 2003;102:2351–2357. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 12.Gopal AK, Rajendran JG, Gooley TA, et al. High-dose [131I]tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults > or = 60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25:1396–1402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
- 13.Gopal AK, Rajendran JG, Petersdorf SH, et al. High-dose chemo-radioimmunotherapy with autologous stem cell support for relapsed mantle cell lymphoma. Blood. 2002;99:3158–3162. doi: 10.1182/blood.v99.9.3158. [DOI] [PubMed] [Google Scholar]
- 14.Rajendran JG, Gopal AK, Fisher DR, et al. Myeloablative 131I-tositumomab radioimmunotherapy in treating non-Hodgkin's lymphoma: comparison of dosimetry based on whole-body retention and dose to critical organ receiving the highest dose. J Nucl Med. 2008;49:837–844. doi: 10.2967/jnumed.107.043190. [DOI] [PubMed] [Google Scholar]
- 15.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–1194. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg AM. CpG Still Rocks! Update on an Accidental Drug. Nucleic Acid Ther. 2012 doi: 10.1089/nat.2012.0340. [DOI] [PubMed] [Google Scholar]
- 17.Link BK, Ballas ZK, Weisdorf D, et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J Immunother. 2006;29:558–568. doi: 10.1097/01.cji.0000211304.60126.8f. [DOI] [PubMed] [Google Scholar]
- 18.Zent CS, Smith BJ, Ballas ZK, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:211–217. doi: 10.3109/10428194.2011.608451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard JP, Link BK, Emmanouilides C, et al. Phase I trial of toll-like receptor 9 agonist PF-3512676 with and following rituximab in patients with recurrent indolent and aggressive non Hodgkin's lymphoma. Clin Cancer Res. 2007;13:6168–6174. doi: 10.1158/1078-0432.CCR-07-0815. [DOI] [PubMed] [Google Scholar]
- 20.Brody JD, Ai WZ, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner HN, Jr, Wiseman GA, Marcus CS, et al. Administration guidelines for radioimmunotherapy of non-Hodgkin's lymphoma with (90)Y-labeled anti-CD20 monoclonal antibody. J Nucl Med. 2002;43:267–272. [PubMed] [Google Scholar]
- 22.Wiseman GA, White CA, Stabin M, et al. Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin's lymphoma. Eur J Nucl Med. 2000;27:766–777. doi: 10.1007/s002590000276. [DOI] [PubMed] [Google Scholar]
- 23.Cheson B, Horning S, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphoma. Journal of Clinical Oncology. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 24.Witzig TE, Gonchoroff NJ, Greipp PR, et al. Rapid S-phase determination of non-Hodgkin's lymphomas with the use of an immunofluorescence bromodeoxyuridine labeling index procedure. American Journal of Clinical Pathology. 1989;91:298–301. doi: 10.1093/ajcp/91.3.298. [DOI] [PubMed] [Google Scholar]
- 25.Jahrsdorfer B, Hartmann G, Racila E, et al. CpG DNA increases primary malignant B cell expression of costimulatory molecules and target antigens. J Leukoc Biol. 2001;69:81–88. [PubMed] [Google Scholar]
- 26.Matesanz-Isabel J, Sintes J, Llinas L, et al. New B-cell CD molecules. Immunol Lett. 2011;134:104–112. doi: 10.1016/j.imlet.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Steele N, Anthony A, Saunders M, et al. A phase 1 trial of recombinant human IL-21 in combination with cetuximab in patients with metastatic colorectal cancer. Br J Cancer. 2012;106:793–798. doi: 10.1038/bjc.2011.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann G, Weiner GJ, Krieg AM. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charbonneau B, Maurer MJ, Ansell SM, et al. Pretreatment circulating serum cytokines associated with follicular and diffuse large B-cell lymphoma: a clinic-based case-control study. Cytokine. 2012;60:882–889. doi: 10.1016/j.cyto.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansell SM, Maurer MJ, Ziesmer SC, et al. Elevated pretreatment serum levels of interferon-inducible protein-10 (CXCL10) predict disease relapse and prognosis in diffuse large B-cell lymphoma patients. Am J Hematol. 2012;87:865–869. doi: 10.1002/ajh.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta M, Han JJ, Stenson M, et al. Elevated serum IL-10 levels in diffuse large B-cell lymphoma: a mechanism of aberrant JAK2 activation. Blood. 2012;119:2844–2853. doi: 10.1182/blood-2011-10-388538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witzig TE, Flinn IW, Gordon LI, et al. Treatment With Ibritumomab Tiuxetan Radioimmunotherapy in Patients With Rituximab-Refractory Follicular Non-Hodgkin's Lymphoma. J Clin Oncol. 2002;20:3262–3269. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized Controlled Trial of 90Y-Labeled Ibritumomab Tiuxetan (Zevalin') Radioimmunotherapy versus Rituximab Immunotherapy for Patients with Relapsed or Refractory Low-Grade, Follicular, or Transformed B-cell Non-Hodgkin's Lymphoma. Jounal of Clinical Oncology. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 35.Horning SJ, Younes A, Jain V, et al. Efficacy and Safety of Tositumomab and Iodine-131 Tositumomab (Bexxar) in B-Cell Lymphoma, Progressive After Rituximab. J Clin Oncol. 2005;23:712–719. doi: 10.1200/JCO.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 37.Wiseman GA, Witzig TE. Yttrium-90 (90Y) ibritumomab tiuxetan (Zevalin) induces long-term durable responses in patients with relapsed or refractory B-Cell non-Hodgkin's lymphoma. Cancer Biother Radiopharm. 2005;20:185–188. doi: 10.1089/cbr.2005.20.185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


