Abstract
Aging of the hematological system causes anemia, reduced immunity, and increased incidence of hematological malignancies. Hematopoietic stem cells (HSCs) play a crucial role in this process as their functions decline during aging. Sirtuins are a family of protein lysine modifying enzymes that have diverse roles in regulating metabolism, genome stability, cell proliferation, and survival, and have been implicated in mammalian aging and longevity. Here we provide an updated overview of sirtuins in aging research; particularly, how increased activity of SIRT1, SIRT3, or SIRT6 improves several aging parameters, and may possibly increase lifespan in mice. We review the literature on how sirtuins may play a role in HSC aging and hematological malignancies, and how key signaling pathways of HSCs may be affected by sirtuins. Among them, SIRT1 plays a critical role in chronic myelogenous leukemia, an age-dependent malignancy, and inhibition of SIRT1 sensitizes leukemic stem cells to tyrosine kinase inhibitor treatment and blocks acquisition of resistant oncogene mutations. In-depth understanding of sirtuins in HSC aging and malignancy may help design novel strategies to deter hematological aging and improve treatment of hematological malignancies.
Keywords: Sir2, sirtuin, aging, hematopoietic stem cells, leukemia, lymphoma
I. INTRODUCTION
Mammalian aging is a multiorgan process characterized by the progressive decline in cellular integrity and physiological functions, leading to eventual organismal death. Conditions that increase in prevalence with aging include cancer, obesity, type II diabetes, cardiovascular disease, dementia, arthritis, and osteoarthritis. Throughout history, humans have sought “miracles” to delay aging and extend lifespan. Research in aging has accelerated significantly in the past few decades, with the identification of several key genetic determinants of aging.1 However, at present, caloric restriction (CR) remains the only consistent experimental means to extend lifespan in model organisms.2 It is found that the sirtuin family of genes may underlie the CR effect,2, 3 a hypothesis that has generated enormous scientific interest. As cancer is a leading age-dependent disease,4 sirtuins are undoubtedly involved in some aspect of tumorigenesis regulation. Extensive summaries of sirtuin functions and their roles in cancer have been covered in several recent reviews.5–8 In this article, we will present an updated overview of sirtuins in aging research, followed by a more focused review on their roles in hematological system aging and hematological malignancies. The hematological system undergoes constant somatic tissue regeneration to replenish blood cells, a process that is distinct from such postmitotic tissues as the nervous system and the heart, where somatic tissue regeneration not related to wound healing is rare.9 However, hematopoietic stem cell-mediated tissue regenerative capacity is not devoid of age-dependent alterations, and thus it merits special attention in this review.
II. THE SAGA OF SIRTUINS IN AGING
Yeast silent information regulator 2 (Sir2) is the founding member of the sirtuin family.10 Initial studies revealed that Sir2 is required for lifespan extension upon CR and activation of Sir2 or its orthologs promotes longevity in yeast, worms, and flies.11–13 More recently, these studies have been questioned with respect to the accuracy of the models employed. Burnett et al.14 casted doubt on the role of Sir2 on lifespan extension in the model organisms used previously. Herranz et al.15 using transgenic mice overexpressing mammalian ortholog of Sir2 also failed to show lifespan extension. However, more complex functions are being illustrated in mammalian sirtuins and their effects on certain aging-related phenotypes have been observed.
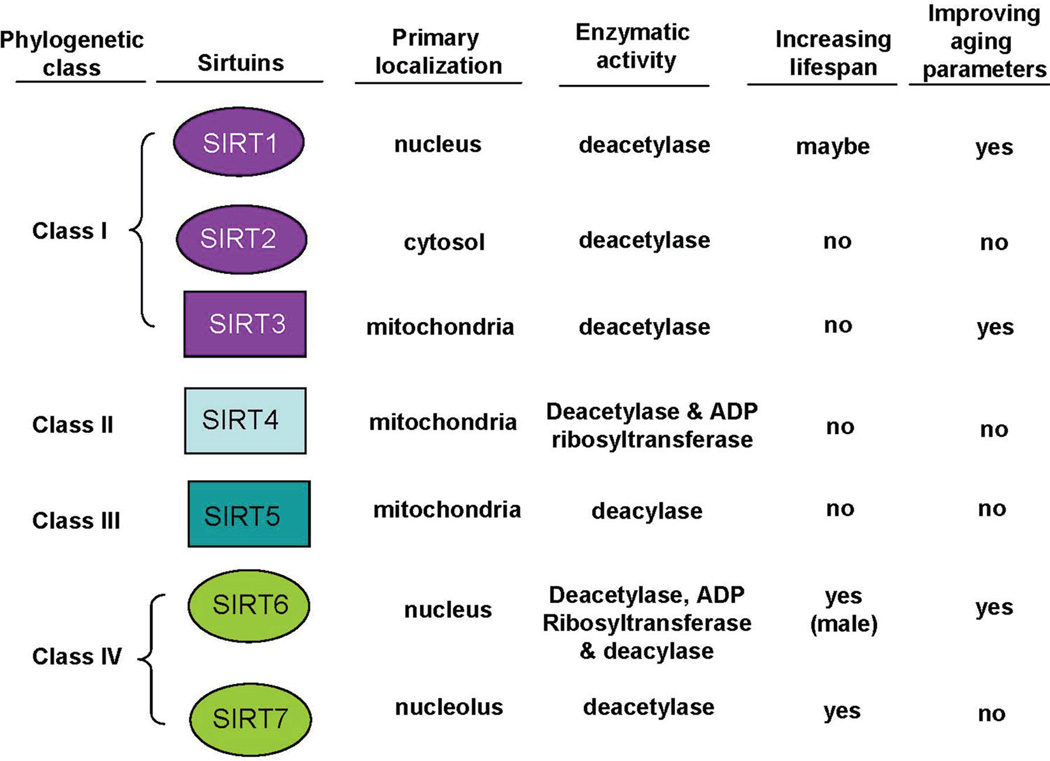
Mammalian sirtuins consist of seven family members, SIRT1–7. SIRT1–3 are protein deacetylases: SIRT1 is primarily a nuclear deacetylase but it can also shuttle to the cytosol and has a wide range of cellular substrates, SIRT2 is primarily a cytosolic protein, and SIRT3 is a major mitochondrial deacetylase.5, 6 SIRT4 is a mitochondrial mono-ADP ribosyltransferase and deacetylase, and SIRT5 is a mitochondrial protein lysine deacylase (for demalonylation and desuccinylation). SIRT6 is a nuclear deacetylase and mono-ADP ribosyl-transferase, whereas SIRT7 is a nuclear deacetylase with high abundance within the nucleolus. Most recently, a new type of enzymatic activity of SIRT6 was identified, namely removal of long-chain fatty acyl groups from protein lysines.16 Phylogenetically, SIRT1–3 are class I enzymes, SIRT4 class II, SIRT5 class III, and SIRT6 and SIRT7 class IV (Fig. 1). Class I and IV sirtuins are eukaryote-specific enzymes whereas class II and III sirtuins have homologs in bacteria.17
Figure 1.
Overview of sirtuins’ activities and functions in aging and mammalian lifespan. “No” denotes no effect or not yet been shown.
A. SIRT1 and Aging
SIRT1 is the largest mammalian sirtuin and shares the highest homology with yeast Sir2. SIRT1 has a wide range of downstream targets that are involved in an array of biological functions, including cell proliferation, survival, stress response, metabolism, energy homeostasis, and the circadian clock.5–7 SIRT1 is extensively studied with regard to aging in both transgenic and gene knockout mouse models. Lifespan changes with SIRT1 transgenic mouse models have been limited; however, changes in some parameters that are associated with aging are consistently noted. Constitutive, yet moderate, overexpression of SIRT1 in mice leads to improved metabolic profiles compared to control mice. For example, these mice show enhanced glucose tolerance, reduced food intake, reduced adipose inflammation, and fatty liver compared to controls, and SIRT1 overexpressing mice are protected from high-fat diet-induced tumors.15, 18, 19 Using a different strategy of SIRT1 overexpression, Guarente’s group showed that mice with SIRT1 knockin alleles have reduced total body mass, improved serum cardiovascular markers, and improved glucose and insulin sensitivity.20 Interestingly, two groups have recently shown that brain-specific SIRT1 overexpression reduces signs of aging-associated deficiencies and in one transgenic line, both male and female mice can live longer.21, 22 Specific overexpression of SIRT1 in β cells of the pancreas increases insulin secretion and glucose tolerance, parameters that are beneficial against development of diabetes.23 By contrast, deletion of SIRT1 in the central nervous system, specifically in the hypothalamus, results in diet-induced obesity.24 Liver-specific SIRT1 ablation leads to steatosis and inflammation, and the mice gain more weight compared to littermate controls.25 Taken together, data from mouse genetics confirm that SIRT1 is protective against inflammation, dyslipidemia, and insulin resistance, common features of metabolic syndromes and cardiovascular diseases. Despite these findings, a 3-year study showed that whole-body SIRT1 overexpression fails to protect mice from age-dependent spontaneous development of lymphoma, a leading cancer in the mice.15 Similarly, SIRT1 knockout mice do not exhibit increased incidence of spontaneous tumors over the aging course, as reviewed previously.26
B. SIRT2 and Aging
There is scant evidence for a protective role of SIRT2 against aging phenotypes. SIRT2 knockout mice are cancer prone and therefore have shorter lifespan compared to control mice.27 SIRT2 knockout mice present with gross chromosomal abnormalities, due to defects in mitosis. SIRT2 deletion in the brain had no effect on neurodegeneration or cholesterol metabolism, as was observed in lower model organisms of Huntington’s disease.28, 29
C. SIRT3 and Aging
There is evidence that SIRT3 may be a putative mediator of mammalian longevity. Single nucleotide polymorphisms (SNPs) have been identified within the human SIRT3 gene and are near-exclusively harbored in individuals greater than 90 years old.30 A second SNP, resulting in a SIRT3 point mutation, reduces enzymatic efficiency and has been associated with metabolic disorders.31 Another SNP was identified that can either increase or decrease longevity depending on genotypes.32 Thus far, SIRT3 is the only sirtuin with SNPs that are linked to human longevity.
Genetic studies reveal that SIRT3 is a nutrient sensor that controls metabolism and redox states and regulates animal health. SIRT3−/− mice show symptoms reminiscent of human metabolic syndromes, such as insulin resistance, obesity, and hepatic steatosis when fed a high-fat diet.31 Hirschey et al.33 identified long-chain acyl coenzyme A dehydrogenase (LCAD) as a SIRT3 deacetylation target. LCAD is involved in fatty acid β-oxidation, and deacetylation by SIRT3 activates LCAD. During the fasting state, energy is provided through catabolic oxidation of lipid, proteins/amino acids, and other large energy stores. Therefore, in SIRT3 knockout animals, fatty acids accumulate even under fasting conditions and show lower ATP concentrations.33 In addition, SIRT3 deacetylates glutamate dehydrogenase (GDH), which converts the amino acid glutamate to α-ketoglutarate.34 α-ketoglutarate can then be funneled into the Krebs cycle to produce energy. Furthermore, SIRT3 can deacetylate several components of the electron transport chain for efficient aerobic ATP production.35 SIRT3 knockdown in muscle cells leads to decline of mitochondrial respiration.36 The net outcome of SIRT3-mediated metabolism is the efficient energy production from fatty acid oxidation, protein catabolism and aerobic respiration.
Mitochondrial respiration accounts for about 90% of intracellular ROS production.37 ROS has long been considered a major cause of aging due to its damaging effects on cellular macromolecules.38 SIRT3 plays a central role in stimulating antioxidant systems to counteract ROS. SIRT3 deacetylates and activates superoxide dismutase 2 (SOD2), a major mitochondrial scavenger of superoxides.39, 40 Another antioxidant mechanism orchestrated by SIRT3 involves its deacetylation and activation of isocitrate deydrogenase2 (IDH2), a component of the Krebs cycle.41 The reaction of IDH2 produces NADPH that is used to recycle oxidized glutathione, one of the cell’s major antioxidants. Someya et al.41 showed that CR prevents hearing loss in aged animals, which is dependent on SIRT3-IDH2 mediated antioxidant production. Further, SIRT3 can form a complex with FOXO to transcribe the catalase and SOD2 antioxidant genes.42 Consistent with the above observations, SIRT3 knockout cells produce elevated levels of ROS. Age-dependent cardiac hypertrophy can lead to heart failure, and SIRT3 has been shown to counteract the pathogenesis in part by promoting antioxidant production.43 SIRT3 knockout female mice succumb to age-dependent spontaneous mammary gland tumors.44 However, firm evidence of SIRT3 with respect to mouse lifespan extension has not yet emerged.
D. SIRT4 and Aging
SIRT4 is a mitochondrial ADP-ribosyltransferase that modifies GDH and inhibits its activity,45 opposing the effect of SIRT3. Inhibiting GDH by SIRT4 in islet cells blocks the release of insulin in response to amino acids and CR. Supporting the previous work on SIRT4, Laurent et al.46 revealed that SIRT4 targets PPARγ and blocks its transcriptional role in fatty acid oxidation in the liver. SIRT4 also plays a role in lipid homeostasis by inhibiting the activity of malonyl CoA decarboxylase (MCD).47 In this regard, SIRT4 is a protein deacetylase that deacetylates MCD and blocks its role in catabolism of fatty acids. Recently, it has been shown that SIRT4 inhibits tumorigenesis by suppressing glutamine anaplerosis, and SIRT4 knockout mice develop spontaneous lung tumors.48, 49 However, premature aging phenotypes have not been reported in SIRT4 knockout mice.
E. SIRT5 and Aging
Mitochondrial SIRT5 is involved in the urea cycle by deacetylating and activating carbamoyl phosphate synthase 1 (CPS1).50 However, the deacetylation activity of SIRT5 is very weak. Recently, SIRT5 has been shown to possess stronger demalonylase and desuccinylase activity against CPS1 and other mitochondrial targets.51–53 SIRT5 knockout mice exhibit urea cycle defect and hyperammonemia after fasting,50 but precise roles of SIRT5 in aging and cancer remain to be elucidated.
F. SIRT6 and Aging
There is evidence that SIRT6 plays a significant role in aging. SIRT6-deficient mice die at around 4 weeks postnatally, exhibiting progeroid phenotypes, hypoglycemia, increased glucose uptake, cardiac hypertrophy and heart failure, hypersensitivity to DNA damage, and genomic instability.54 SIRT6 deacetylates histone H3 at lysine 9 (H3K9), particularly at telomeric regions.55 Loss of SIRT6 results in aneuploidy and end-to-end attachment of chromosomes. This, in turn, triggers a DNA damage response and premature senescence. In addition to protecting telomeres, SIRT6 seems to actively promote DNA damage repair.56–58 Furthermore, SIRT6 inhibits NF-κB transactivation of various pro-inflammatory genes.59 SIRT6 complexes with RelA at target gene promoters and deacetylates H3K9 to block transcription at these sites. Remarkably, when SIRT6 knockout mice are crossed with RelA+/− mice, many of the progeroid symptoms are inhibited. Consistent with these observations, male mice overexpressing SIRT6 live longer than control mice.60 SIRT6 overexpressing mice also show better serum lipid profiles, less visceral fat accumulation, better glucose sensitivity, and are protected against diet-induced obesity. Conversely, SIRT6 specific ablation in the central nervous system of mice causes age-associated obesity.61 Further highlighting a positive role for SIRT6 in warding off aging-associated morbidities, Sundaresan et al.62 showed that SIRT6 protects mice from cardiac failure by inhibiting the IGF-1/Akt pathway at the chromatin level. In sum, SIRT6 promotes healthy lifespan and longevity through both chromatin regulation and deacetylation of nonhistone proteins.
G. SIRT7 and Aging
SIRT7 is highly enriched in the nucleolus, where it regulates RNA polymerase I transcription of ribosomal DNA.63 Recently, SIRT7 was shown to selectively deacetylate histone H3K18, and regulate transcription of a subset of nuclear genes and maintain the transformed phenotypes of cancer cells.64 Although there is only limited information on the role of SIRT7 in aging, homozygous SIRT7 knockout mice show signs of premature aging, including kyphosis and loss of subcutaneous fat, and enhanced inflammatory cardiomyopathy as well as enhanced cardiomyocyte apoptosis.65 In addition, these mice die by approximately 10 months of age. SIRT7 null cells undergo apoptosis due to hyperacetylation and hyperactivation of p53, but the precise mechanisms of SIRT7 deficiency in premature aging remain to be determined.
III. AGING OF THE HEMATOLOGICAL SYSTEM AND AGE-DEPENDENT HEMATOLOGICAL MALIGNANCIES
Hematopoiesis is a tightly regulated system that constructs the strong immune firewall and oxygen supply carrier of the body. The hierarchy of hematopoietic system includes series of fully differentiated cell types including myeloid cells, lymphoid cells, and red blood cells, along with their progenitors. All these cell types are derived from hematopoietic stem cells (HSCs) that possess the self-renewal capability and multilineage potential. Since many terminally differentiated blood cells have a relatively short lifespan, the hematopoietic system undergoes continuous regeneration from HSCs to replenish blood cells. However, even HSCs themselves are not exempt from aging, and the function of HSCs declines with age. Aging of the hematological system leads to several pathophysiological conditions in the elderly, including onset of anemia, decline of immune competence, and increased incidence of hematological malignancies.66–68
HSC aging plays a central role in the hematological system aging. Aging HSCs exhibit increased cell cycle entry and expansion of the HSC compartment along with a skewed differentiation program favoring the myeloid lineage.69, 70 Aging HSCs display an intrinsic transcriptome change,71 and the skewing of lineage potential is attributed to clonal expansion of myeloid-biased HSCs during aging.72–74 Aging of HSCs is distinct from other somatic tissues in that global DNA methylation is increased in aged HSCs, in contrast to its decrease in most other somatic tissues.75 Spontaneous DNA damage is accumulated in aged HSCs,76 and genome maintenance machineries including non-homologous end joining (NHEJ) repair are required for maintaining HSC functions during aging.76, 77 In addition to DNA damage, genomic integrity, epigenetic alteration, and telomere attrition, such factors as mitochondrial dysfunction, cell senescence pathways and altered intercellular communication may also contribute to HSC aging.78, 79 Yet it remains to be determined how these factors may interplay and how HSC aging is precisely regulated. From a practical view, it remains to be determined how aging HSCs may be rejuvenated.
Hematological malignancy incidence surges with age. Chronic myelogenous leukemia (CML) has a median age of onset at 50–60, and chronic lymphocytic leukemia has a medium age of 65, and they rarely occur in children.80, 81 The incidence of acute myeloid leukemia (AML) also increases dramatically in the elderly.67 Moreover, AML in the elderly has more complex cytogenetic profile, and is more resistant to chemotherapy and with much lower survival rate than AML in pediatric patients.67, 82–84 The incidence of lymphomas also increases exponentially in the elderly, and the age is one of the most significant contributing factors for lymphomagenesis.85 The top categories of non-Hodgkin lymphomas, i.e., follicular lymphoma, diffuse large B-cell lymphoma (germinal center B-cell-like and activated B cell-like), mucosa-associated lymphoid tissue lymphoma, and mantle cell lymphoma, which account for about three quarters of all lymphomas, have the median age of 55–66.
IV. SIRTUINS IN HEMATOLOGICAL AGING AND MALIGNANCY
This field is still in its early stage, and there are only a limited number of papers describing sirtuins (SIRT1, 3, 6, and 7) in hematological aging and malignancy. However, some relevant information is beginning to emerge, as will be discussed below.
A. SIRT1
SIRT1 was reported to be dispensable for the function of adult hematopoietic stem cells because SIRT1 knockout adult mice exhibit normal hematopoiesis, normal HSC number, and normal reconstitution capability at a young age.86–88 In the initial SIRT1 knockout study, the percentages of lineage cells labeled by Gr-1/Mac-1 (myeloid cells), B220 (B cells), and CD3e (T cells) are also normal.89 However, during aging, SIRT1 knockout mice show decreased hematocrit and elevated leukocytosis.86 Although SIRT1 deficiency induces developmental abnormalities and causes increased mortality at a young age, there is no significant difference in the lifespan of SIRT1 knockout mice compared to wild-type littermates after 7 months.86 At present, there is no published evidence of lifespan extension effect of SIRT1 overexpression on the hematopoietic system under normal conditions. SIRT1 was reported to regulate T cell immune tolerance through NF-κB and AP-1 pathways.90 SIRT1 null mice show hyperactive immune response, which leads to higher potential of inflammation and autoimmune-disease-like phenotypes.90 However, such autoimmune disease phenotypes may not be derived autonomously from hematopoietic cells, because lethally irradiated wild-type mice receiving such SIRT1−/− donor bone marrow do not exhibit aberrant immune phenotypes (unpublished observation). It is noted that SIRT1−/− mice frequently harbor ocular development defects,89 reducing eyelid opening and increasing ocular discharge, a condition that could trigger facial chronic inflammation. Some older SIRT1−/− mice also develop penile prolapse that could also increase inflammatory response (unpublished observation).
SIRT1 is critical for DNA damage repair, and ablation of SIRT1 causes hypersensitivity to toxic agents and severe stress, which is exemplified by the bone marrow reconstitution defect in SIRT1-deficient mice when challenged with irradiation.89 SIRT1 was shown to redistribute on chromatin to promote DNA damage repair and maintain genomic stability in response to oxidative stress, which may contribute to the prolonged survival of p53+/− mice treated with alleged SIRT1 activators.91 However, an increased leukemia/lymphoma incidence is noticed by the putative SIRT1 activator treatment in the study.91 In a more recent study, Singh et al.92 showed that hematopoietic stem/progenitor cells expand in conditional SIRT1 knockout mice with Cre expression induced by tamoxifen treatment. Such an effect has not been observed in other SIRT1−/− mice,86–88 and is dependent on the tamoxifen, which causes significant bone marrow toxicity and reduces bone marrow cellularity by half.92
In another line of SIRT1 conditional mice constitutively expressing vav-iCre, loss of SIRT1 in combination with tamoxifen treatment results in impairment of recipient mouse survival in the serial transplantation study.92 However, this is complicated by the unexpected finding that mice receiving vav-iCre control mouse bone marrow are very short-lived during the third round of transplantation.92 This defect contrasts with reports showing that bone marrow from young C57BL/6 mice can be transplanted for 4 rounds without noticeable change,93, 94 suggesting the likely impact of iCre, which is an improved Cre with humanized codons to reduce gene silencing in animals and thus its expression significantly increases in the hematological system.95, 96 Moreover, Cre itself is a bacterial protein, and its robust expression can cause chromosome rearrangement, 97 and according to the Jax Mice webpage, homozygous vav-iCre mice are not viable. Thus, it is possible that robust expression of Cre, using the vav-iCre transgenic line, may produce bacterial protein shock to HSCs, which may further compromise their functions when stress-response protein SIRT1 is removed. More moderate conditions are perhaps required in order to assess roles of SIRT1 in HSC aging in the future.
On the other hand, SIRT1 is activated in multiple hematopoietic malignant diseases including CML87, AML,98 and lymphoma.99 In fact, SIRT1 is thus far the only sirtuin that has been examined in great detail with respect to hematological malignancy. Activation of SIRT1 by BCR-ABL significantly contributes to malignant transformation of HSCs and CML disease progression.87 SIRT1 inhibition sensitizes CML leukemic stem cells to the treatment by tyrosine kinase inhibitor imatinib.100 By using an acquired BCR-ABL mutagenesis model,101 SIRT1 was shown to promote the acquisition of oncogene mutations in CML cells for drug resistance by altering DNA damage repair machineries and stimulating error-prone NHEJ repair.102 It has also been recognized that SIRT1 may be involved in multiple pathways that promote cancer drug resistance, including decreasing drug penetration, conferring proliferation and anti-apoptotic advantages to cancer cells, facilitating acquired resistance through genetic mutations, and acquiring cancer stem cell properties.26 Thus, targeting SIRT1 may be clinically promising for killing cancer cells and eliminating CML stem cells. Besides CML, SIRT1 is also overexpressed in AML98 and overexpression of SIRT1 is associated with poor prognosis of diffuse large B-cell lymphoma;99 but the precise roles of SIRT1 in those hematological malignancies are not yet clear. In view of the effects of SIRT1 in both the normal and malignant hematopoietic system, it is possible that SIRT1 manipulation may result in distinct effects, depending on the specific context. As aging is intimately linked with tumorigenesis, further studies on the multiple roles of SIRT1 in the events of malignant transformation during aging seem to be promising areas for future investigation.
B. SIRT3
SIRT3 regulates the global acetylation landscape of mitochondrial proteins and oxidative stress. SIRT3 knockout mice are healthy and morphologically normal.103 According to the murine study from Chen’s group,104 SIRT3 is highly expressed in young HSCs and it is suppressed with aging. During youth, SIRT3 deficiency has no effect on the HSC pool and hematopoietic homeostasis. However, in old age, under the stress of serial bone marrow transplantation, SIRT3 deficiency decreases the HSC pool and compromises HSC self-renewal capacity. SIRT3 overexpression rescues the functional defects of aged HSCs. Mechanistically, SIRT3 loss leads to reduced SOD2 activity and increased oxidative stress in hematopoietic stem/progenitor cells during serial transplantation, which may drive stem cells out of quiescence and reduce their survival.104
C. SIRT6
SIRT6-deficient mice develop a progeroid degenerative syndrome accompanying with severe reduction of CD4+CD8+ T cells in thymus, B cell progenitors in bone marrow, and lymphocytes in spleen.54 However, recipient mice transplanted with SIRT6 knockout bone marrow showed normal hematopoietic functions, indicating that SIRT6 may play a role in the hematopoietic system through a non-autonomous manner. Noticeably, SIRT6 deficiency leads to altered signaling of circulating IGF1, imbalanced glucose metabolism, and heart hypertrophy, and inhibition of IGF1 signaling corrects hypertrophy of SIRT6-deficient heart.62
D. SIRT7
SIRT7 knockout mice have a shortened lifespan and suffer from degenerative heart hypertrophy accompanied by an increased number of granulocytes and T lymphocytes, together with elevated levels of the IL-12 and IL-13 inflammatory cytokines.65 SIRT7 deficiency causes acetylation and hyperactivation of p53 in cardiomyocytes, increasing apoptosis and decreasing cell stress resistance. Inflammatory infiltration of heart further accentuates the hypertrophy phenotype.65 However, it is not clear whether the role of SIRT7 in the hematopoietic system is a direct or indirect effect, and whether SIRT7 may also regulate p53 in HSCs. Intriguingly, SIRT7 mRNA level decreases during HSC aging, together with SIRT2 and SIRT3.105 Therefore, it would be interesting to further explore SIRT7 in the hematopoietic system aging in the future.
V. SIRTUIN REGULATION OF SIGNALING PATHWAYS IN HEMATOPOIETIC STEM CELLS
Many sirtuins are involved in HSC aging-related signaling pathways, including DNA repair pathways, p53, NF-κB, FOXO, and mTOR. In general, sirtuins play positive roles in the maintenance of genome stability in normal cells by promoting DNA damage repair and increasing cell survival. Interestingly, however, error-prone NHEJ is the major repair pathway in HSCs,106 and aging cells turn out to have low repair fidelity, similar to cancer cells.107 Hyperactive NHEJ repair stimulated by sirtuins in aging HSCs may introduce mutations into the aging HSC genome well before oncogenic transformation takes place. These mutations may ultimately cooperate with or accelerate malignant transformation. Meanwhile, certain “senescence invasion” advantages conferred by sirtuins may compromise the tumor-suppression gatekeepers, and eventually lead to elevated malignancy risk. This would affect the HSC aging process that could be exemplified by the sirtuins involved in p53, NF-κB, FOXO, and mTOR pathways as follows.
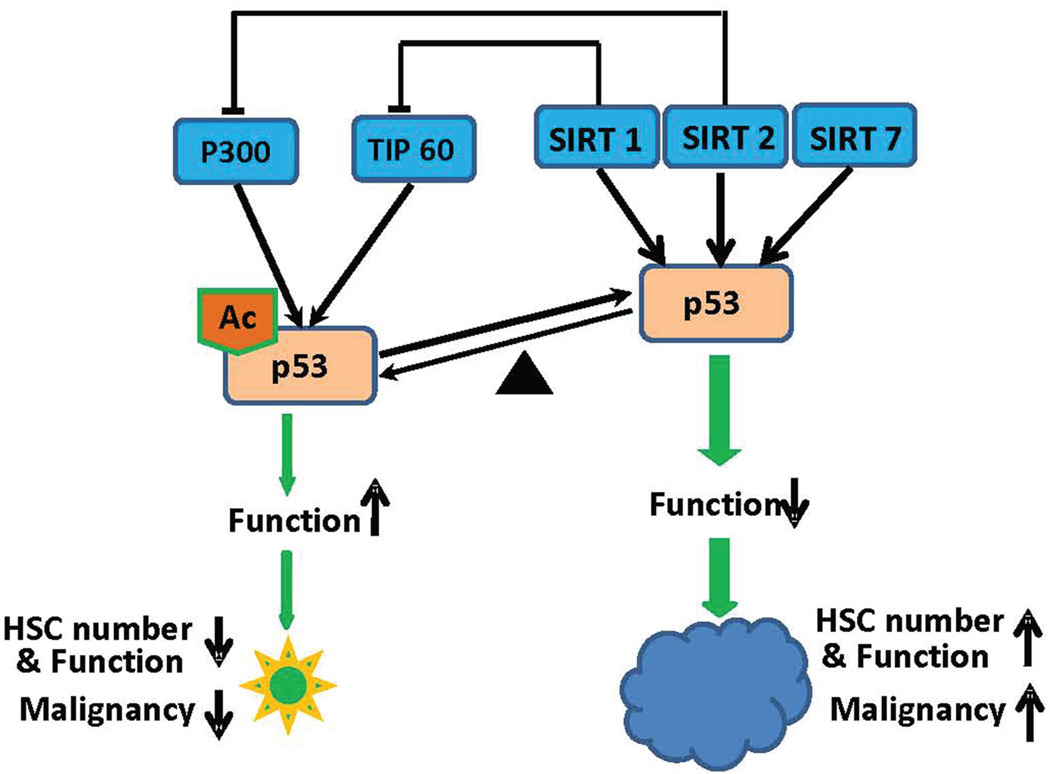
Several sirtuin family genes have been shown to regulate p53 function directly or indirectly (Fig. 2). SIRT1 and SIRT7 can directly deacetylate p53 and reduce the activity of p53.65, 108, 109 SIRT1 and SIRT2 also inhibit autoacetylation of acetyltransferases TIP60 or p300 to indirectly reduce the acetylation level of p53.110, 111 As a key tumor suppressor gene, p53 was shown to ensure the maintenance of genomic integrity in mature cells112 and stem cells.113 On the other hand, p53 plays an interesting role in the hematopoietic system. p53 null mice show increased HSC frequency and function in young animals.114 However, during aging reduced p53 in p53+/− mice is associated with more proliferating HSCs and enhanced hematopoiesis capability compared to that in wild-type mice.115 Although p53 is associated with cell cycle and cell death regulation,116 the elevated HSC function is accompanied by the increased tumor-related mortality.114 The enhanced p53 function also leads to HSC depletion, premature aging, and shortened lifespan accompanied with lower cancer incidence.115, 117 A similar trade-off between tissue renewal (aging) and tumor suppression has also been shown in humans who carry TP53-inhibiting polymorphisms.118 Therefore, the compromised p53 activity by elevated sirtuin proteins could be a risk factor for increasing opportunistic tumorigenesis and the appropriately enhanced p53 activity might benefit healthy aging free from malignancy (Fig. 2).
Figure 2.
Schematic illustration of sirtuins, p53, and their regulation of hematopoietic stem cells. Sirtuins (SIRT1, 2, 7) suppress the acetylation of p53 directly by deacetylating Ac-p53 or indirectly by inhibiting acetyltransferases TIP60 and p300. As acetylation of p53 activates its function, suppression of p53 by sirtuins may lead to increased HSC number and function, accompanied by increased tumor incidence. In contrast, hyperacetylated and activated p53 decreases HSC number and function, but may reduce the chance of malignant transformation of HSCs.
NF-κB pathway is another pathway that is regulated by sirtuin proteins. SRT1 was shown to physically interact with the RelA/P65 subunit of NF-κB and suppress the transcription of downstream target genes. SIRT6 also interacts with RelA and deacetylates histone H3 lysine 9 (H3K9) at NF-κB target gene promoters to attenuate the NF-κB signaling.59 NF-κB pathway is a key player in controlling the host immune-inflammation system.119 Persistently elevated NF-κB is associated with inflammatory diseases, including rheumatoid arthritis, and contributes to carcinogenesis and cancer resistance.120 However, NF-κB activity is also essential for lymphocyte (both B and T cells) survival, activation, and normal immune responses. As T and B cells play pivotal roles in tumor surveillance,121 the appropriate NF-κB activity level is critical to activate lymphocytes that can function to eliminate the nascent tumor initiating cells. Although NF-κB activation has been considered as a hallmark and mediator of aging122 and NF-κB inhibition leads to delayed onset of age-related symptoms and pathologies,123 there is no report showing that NF-κB inhibition can extend longevity. Nevertheless, it should be noted that compromised NF-κB signaling in normal hematopoietic cells caused by elevated sirtuins (SIRT1 and SIRT6) may cause immune non-responsiveness, and increase spontaneous oncogenesis.
The IGF/mTOR signaling pathway contributes to HSC aging by inducing HSC senescence. mTORC1 activity exhibits an age-dependent increase in mice. Constitutive mTORC1 activation through deletion of TSC1 (a component of the mTOR inhibitory complex upstream of mTORC1) leads to elevated expression levels of p16, p19, and p21, which results in HSC depletion.124 Meanwhile, treatment with the mTOR inhibitor, rapamycin, preserves the HSC pool to a level matching that in young mice.124 This accords with the observation that mTORC1 is involved in controlling HSC quiescence.125 Consistent with this finding, PTEN deletion induces PI3K pathway hyperactivation through mTORC1, leading to HSC hyperproliferation and eventual exhaustion, and it can be rescued by rapamycin treatment.126 However, it remains a concern that mTOR inhibitor treatment compromises the tumor suppressor response,126 which may lead to unknown consequences. Linking to this pathway, SIRT1 has been shown to negatively regulate mTOR signaling127 and is required for the effects of rapamycin on cells senescence.128 Such interaction may indicate that SIRT1 and mTOR could form a self-restricted balance to ensure the normal physiological cell proliferation and self-renewal function in healthy HSCs.
FOXO transcription factors are also critical for maintaining hematopoietic stem cell homeostasis and integrity through regulation of HSC response to physiological oxidative stress, quiescence, and survival.129 Deficiency of FOXO1, FOXO3, and FOXO4 in HSC leads to remarkable reduction of HSC number and function.130 On the other hand, FOXOs are considered as tumor suppressors and their inactivation is frequently found in cancer and malignant stem cells in which the self-renewal capability is restored by other events.129 Activity of FOXO can be regulated by acetylation levels. Sirtuins interact with and deacetylate FOXOs to regulate their functions. For example, SIRT1 deacetylates FOXO3a and increases its ability to induce cell cycle arrest and resistance to oxidative stress but inhibits the ability to induce cell apoptosis.131, 132 SIRT1-mediated deacetylation of FOXO1 was reported to regulate FOXO1 nuclear localization and activity, impacting the selection of its transcriptional programs.133, 134 In the context of sirtuin-FOXO connection, the precise roles of sirtuin modification of FOXOs in HSC maintenance remain to be delineated.
VI. FUTURE PROSPECTS
Understanding roles of sirtuins in hematological aging and malignancy may have significant impact on caring for the aging population. Given the oncogenic role of SIRT1 in CML, targeting SIRT1 can be exploited to improve treatment of the disease. This strategy may also be useful for other types of hematological malignancies. It is predicted that revealing HSC aging mechanisms could one day help devise strategies to reverse HSC aging and improve management and care of various hematological diseases in the elderly. HSCs can be easily harvested, and bone marrow transplantation is currently routinely performed. It may be feasible to rejuvenate aging HSCs specifically in vitro followed by transplantation back to the patients. By doing so, aging HSCs will be specifically rejuvenated to improve hematological performance that includes increased output of red blood cells and lymphoid cells, increased immune competence, and reduced risk of developing hematological disorders such as anemia and malignancies. If sirtuins are proven to be central to HSC aging, sirtuin modulation could be used to achieve such a goal. Future research on the full spectrum of sirtuin interactions with HSC is clearly essential for addressing the complex relationship between aging and hematological cancers.
ACKNOWLEDGMENTS
The authors would like to acknowledge the research support from the National Cancer Institute of the National Institutes of Health under award number R01 CA143421, and the State of California Tobacco Related Disease Research Program (TRDRP) award 20XT-0121 to W.Y.C. The contents are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
ABBREVIATIONS
- ADP
adenine diphosphate
- AML
acute myeloid leukemia
- ATP
adenine triphosphate
- CML
chronic myelogenous leukemia
- CPS1
carbamoyl phosphate synthase 1
- CR
caloric restriction
- FOXO
forkhead box protein O class
- GDH
glutamate dehydrogenase
- HSC
hematopoietic stem cell
- IDH2
isocitrate deydrogenase 2
- LCAD
long-chain acyl coenzyme A dehydrogenase
- NHEJ
non-homologous end joining
- ROS
reactive oxygen species
- Sir2
silent information regulator 2
- SNP
single nucleotide polymorphism
- SOD2
superoxide dismutase 2
- IGF
insulin-related growth factor
- mTOR
mammalian target of rapamycin
- PTEN
phosphatase and tensin homolog
REFERENCES
- 1.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 2.Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17(3):313–321. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- 3.Guarente L. Sirtuins in aging and disease. Cold Spring Harbor Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 4.DePinho RA. The age of cancer. Nature. 2000;408(6809):248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 5.Yuan H, Su L, Chen WY. The emerging and diverse roles of sirtuins in cancer: a clinical perspective. OncoTargets Ther. 2013;6:1399–1416. doi: 10.2147/OTT.S37750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth M, Chen WY. Sorting out functions of sirtuins in cancer. Oncogene. 2013 doi: 10.1038/onc.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radical Biol Med. 2013;56:133–171. doi: 10.1016/j.freeradbiomed.2012.10.525. [DOI] [PubMed] [Google Scholar]
- 9.Mullenix PS, Huddleston SJ, Stojadinovic A, Trachiotis GD, Alexander EP. A new heart: Somatic stem cells and myocardial regeneration. J Surg Oncolo. 2012;105(5):475–480. doi: 10.1002/jso.23014. [DOI] [PubMed] [Google Scholar]
- 10.Shore D, Squire M, Nasmyth KA. Characterization of 2 genes required for the position-effect control of yeast mating-type genes. EMBO J. 1984;3(12):2817–2823. doi: 10.1002/j.1460-2075.1984.tb02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 12.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477(7365) doi: 10.1038/nature10296. 482-U136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, Hang HC, Hao Q, Lin H. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496(7443):110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000;273(2):793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- 18.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105(28):9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8(4):333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo JY, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6(6):759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 21.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153(7):1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucosestimulated insulin secretion in mice. Cell Metab. 2005;2(2):105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo XM, Li XL. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metabolism. 2009;9(4):327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Chen W. Emerging roles of SIRT1 in cancer drug resistance. Genes Cancer. 2013;4(3–4):82–90. doi: 10.1177/1947601912473826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HS, Vassilopoulos A, Wang RH, Lahusen T, Xiao Z, Xu XL, Li CL, Veenstra TD, Li B, Yu HT, Ji JF, Wang XW, Park SH, Cha YI, Gius D, Deng CX. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20(4):487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobrowska A, Donmez G, Weiss A, Guarente L, Bates G. SIRT2 Ablation has no effect on tubulin acetylation in brain, cholesterol biosynthesis or the progression of Huntington’s Disease phenotypes in vivo. PloS One. 2012;7(4):e34805. doi: 10.1371/journal.pone.0034805. [Epub 2012 Apr 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang ZM, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010;107(17):7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benedictis G. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85(2):258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44(2):177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De Benedictis G. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38(10):1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 33.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Alt F, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285) doi: 10.1038/nature08778. 121-U137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlicker C, Gertz M, Papatheodorou P, Kachholz B, Becker CFW, Steegborn C. Substrates and regulation mechanisms for the human mitochondrial Sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382(3):790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 35.Ahn BH, Kim HS, Song SW, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jing EX, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108(35):14608–14613. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 39.Tao RD, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang HY, Kim HS, Flynn CR, Hill S, McDonald WH, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu XL, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Someya S, Yu W, Hallows WC, Xu JZ, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim HS, Mishra M, Sun L, Nguyen P, Ahn BH, Leclerc J, Deng CX, Spitz DR, Gius D. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int J Biol Sci. 2008;4(5):291–299. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sadoshima J. Sirt3 Targets mPTP and prevents aging in the heart. Aging-US. 2011;3(1):12–13. doi: 10.18632/aging.100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, van der Meer R, Nguyen P, Savage J, Owens KM, Vassilopoulos A, Ozden O, Park SH, Singh KK, Abdulkadir SA, Spitz DR, Deng CX, Gius D. SIRT3 is a mitochondrialocalized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17(1):41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, Wolberger C, Prolla TA, Weindruch R, Alt FW, Guarente L. SIRT4 inhibits glutamate dehydrogehase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 46.Laurent G, de Boer VC, Finley LW, Sweeney M, Lu H, Schug TT, Cen Y, Jeong SM, Li X, Sauve AA, Haigis MC. SIRT4 represses PPARalpha activity to suppress hepatic fat oxidation. Mol Cell Biol. 2013 Nov;33(22):4552–4561. doi: 10.1128/MCB.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, Lovitch SB, Sharpe AH, Kurland IJ, Steegborn C, Gygi SP, Muoio DM, Ruderman NB, Haigis MC. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013;50(5):686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, Xu X, Li C, Wang RH, Lee J, Csibi A, Cerione R, Blenis J, Clish CB, Kimmelman A, Deng CX, Haigis MC. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell. 2013;23(4):450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T, Henske EP, Haigis MC, Cantley LC, Stephanopoulos G, Yu J, Blenis J. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153(4):840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137(3):560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du JT, Zhou YY, Su XY, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, He B, Chen W, Zhang S, Cerione RA, Auwerx J, Hao Q, Lin HN. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park J, Chen Y, Tishkoff DX, Peng C, Tan MJ, Dai LZ, Xie ZY, Zhang Y, Zwaans BMM, Skinner ME, Lombard DB, Zhao YM. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50(6):919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng C, Lu ZK, Xie ZY, Cheng ZY, Chen Y, Tan MJ, Luo H, Zhang Y, He W, Yang K, Zwaans BMM, Tishkoff D, Ho L, Lombard D, He TC, Dai JB, Verdin E, Ye Y, Zhao YM. the first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10(12) doi: 10.1074/mcp.M111.012658. M111 012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu PF, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 55.Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TLA, Barrett JC, Chang HY, Bohr VA, Ried T, Gozani O, Chua KF. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186) doi: 10.1038/nature06736. 492-U416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaidi A, Weinert BT, Choudhary C, Jackson SP. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science. 2010;329(5997):1348–1353. doi: 10.1126/science.1192049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Toiber D, Erdel F, Bouazoune K, Silberman DM, Zhong L, Mulligan P, Sebastian C, Cosentino C, Martinez-Pastor B, Giacosa S, D’Urso A, Naar AM, Kingston R, Rippe K, Mostoslavsky R. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol Cell. 2013;51(4):454–468. doi: 10.1016/j.molcel.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCord RA, Michishita E, Hong T, Berber E, Boxer LD, Kusumoto R, Guan SH, Shi XB, Gozani O, Burlingame AL, Bohr VA, Chua KF. SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging-US. 2009;1(1):109–121. doi: 10.18632/aging.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawahara TLA, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KCL, Boxer LD, Chang HY, Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappa B-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483(7388):218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 61.Schwer B, Schumacher B, Lombard DB, Xiao CY, Kurtev MV, Gao J, Schneider JI, Chai H, Bronson RT, Tsai LH, Deng CX, Alt FW. Neural sirtuin 6 (Sirt6) ablation attenuates somatic growth and causes obesity. Proc Natl Acad Sci U S A. 2010;107(50):21790–21794. doi: 10.1073/pnas.1016306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med. 2012;18(11):1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ford E, Voit R, Liszt G, Magin C, GrumMt I, Guarente L. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 2006;20(9):1075–1080. doi: 10.1101/gad.1399706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barber MF, Michishita-Kioi E, Xi YX, Tasselli L, Kioi M, Moqtaderi Z, Tennen RI, Paredes S, Young NL, Chen KF, Struhl K, Garcia BA, Gozani O, Li W, Chua KF. SIRT7 links H3K18 deacetylation to maintenance of oncogenic transformation. Nature. 2012;487(7405):114–118. doi: 10.1038/nature11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, Braun T, Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102(6):703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 66.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 67.Lichtman MA, Rowe JM. The relationship of patient age to the pathobiology of the clonal myeloid diseases. Semin Oncol. 2004;31(2):185–197. doi: 10.1053/j.seminoncol.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 68.Beghe C, Wilson A, Ershler WB. Prevalence and outcomes of anemia in geriatrics: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):3S–10S. doi: 10.1016/j.amjmed.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 69.Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2(9):1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 70.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108(50):20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rossi DJ, Bryder D, Zahn JM, Ahlenius H, Sonu R, Wagers AJ, Weissman IL. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102(26):9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6(3):265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107(12):5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, Rossi DJ. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12(4):413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 76.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 77.Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447(7145):686–690. doi: 10.1038/nature05875. [DOI] [PubMed] [Google Scholar]
- 78.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12(2):152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120(7):2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaffer AL, 3rd, Young RM, Staudt LM. Pathogenesis of human B cell lymphomas. Annu Rev Immunol. 2012;30:565–610. doi: 10.1146/annurev-immunol-020711-075027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frohling S, Schlenk RF, Kayser S, Morhardt M, Benner A, Dohner K, Dohner H, German-Austrian AMLSG. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: results from AMLSG trial AML HD98-B. Blood. 2006;108(10):3280–3388. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 83.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH. Medical Research Council Adult Leukemia Working P. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–1320. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 84.Hiddemann W, Kern W, Schoch C, Fonatsch C, Heinecke A, Wormann B, Buchner T. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17(11):3569–3576. doi: 10.1200/JCO.1999.17.11.3569. [DOI] [PubMed] [Google Scholar]
- 85.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 86.Leko V, Varnum-Finney B, Li H, Gu Y, Flowers D, Nourigat C, Bernstein ID, Bedalov A. SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood. 2012;119(8):1856–1860. doi: 10.1182/blood-2011-09-377077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan H, Wang Z, Li L, Zhang H, Modi H, Horne D, Bhatia R, Chen WY. Activation of stress response gene SIRT1 by BCR-ABL promotes leukemogenesis. Blood. 2012;119(8):1904–1914. doi: 10.1182/blood-2011-06-361691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narala SR, Allsopp RC, Wells TB, Zhang G, Prasad P, Coussens MJ, Rossi DJ, Weissman IL, Vaziri H. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell. 2008;19(3):1210–1219. doi: 10.1091/mbc.E07-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100(19):10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang J, Lee SM, Shannon S, Gao B, Chen W, Chen A, Divekar R, McBurney MW, Braley-Mullen H, Zaghouani H, Fang D. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119(10):3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oberdoerffer P, Michan S, McVay M, Mostoslavsky R, Vann J, Park SK, Hartlerode A, Stegmuller J, Hafner A, Loerch P, Wright SM, Mills KD, Bonni A, Yankner BA, Scully R, Prolla TA, Alt FW, Sinclair DA. SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell. 2008;135(5):907–918. doi: 10.1016/j.cell.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh SK, Williams CA, Klarmann K, Burkett SS, Keller JR, Oberdoerffer P. Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. J Exp Med. 2013;210(5):987–1001. doi: 10.1084/jem.20121608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102(2):517–520. doi: 10.1182/blood-2002-07-2334. [DOI] [PubMed] [Google Scholar]
- 94.Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453(7198):1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E, Stewart AF, Seeburg PH, Sprengel R. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32(1):19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- 96.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33(2):314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 97.Schmidt EE, Taylor DS, Prigge JR, Barnett S, Capecchi MR. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci U S A. 2000;97(25):13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bradbury CA, Khanim FL, Hayden R, Bunce CM, White DA, Drayson MT, Craddock C, Turner BM. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia. 2005;19(10):1751–1759. doi: 10.1038/sj.leu.2403910. [DOI] [PubMed] [Google Scholar]
- 99.Jang KY, Hwang SH, Kwon KS, Kim KR, Choi HN, Lee NR, Kwak JY, Park BH, Park HS, Chung MJ, Kang MJ, Lee DG, Kim HS, Shim H, Moon WS. SIRT1 expression is associated with poor prognosis of diffuse large B-cell lymphoma. Am J Surg Pathol. 2008;32(10):1523–1531. doi: 10.1097/PAS.0b013e31816b6478. [DOI] [PubMed] [Google Scholar]
- 100.Li L, Wang L, Wang Z, Ho Y, McDonald T, Holyoake TL, Chen W, Bhatia R. Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21(2):266–281. doi: 10.1016/j.ccr.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan H, Wang Z, Gao C, Chen W, Huang Q, Yee JK, Bhatia R, Chen WY. BCR-ABL gene expression is required for its mutations in a novel KCL-22 cell culture model for acquired resistance of chronic myelogenous leukemia. J Biol Chem. 2010;285(7):5085–5096. doi: 10.1074/jbc.M109.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang Z, Yuan H, Roth M, Stark JM, Bhatia R, Chen WY. SIRT1 deacetylase promotes acquisition of genetic mutations for drug resistance in CML cells. Oncogene. 2013;32(5):589–598. doi: 10.1038/onc.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3(2):319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegue E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7(2):174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seluanov A, Mittelman D, Pereira-Smith OM, Wilson JH, Gorbunova V. DNA end joining becomes less efficient and more error-prone during cellular senescence. Proc Natl Acad Sci U S A. 2004;101(20):7624–7629. doi: 10.1073/pnas.0400726101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 109.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107(2):137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 110.Black JC, Mosley A, Kitada T, Washburn M, Carey M. The SIRT2 deacetylase regulates autoacetylation of p300. Mol Cell. 2008;32(3):449–455. doi: 10.1016/j.molcel.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J, Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J Biol Chem. 2010;285(15):11458–11464. doi: 10.1074/jbc.M109.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Albrechtsen N, Dornreiter I, Grosse F, Kim E, Wiesmuller L, Deppert W. Maintenance of genomic integrity by p53: complementary roles for activated and non-activated p53. Oncogene. 1999;18(53):7706–7717. doi: 10.1038/sj.onc.1202952. [DOI] [PubMed] [Google Scholar]
- 113.Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol. 2003;31(6):521–527. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- 115.Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109(4):1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Elf SE, Miyata Y, Sashida G, Liu Y, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, Reva B, Koff A, Nimer SD. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4(1):37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park SH, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 118.van Heemst D, Mooijaart SP, Beekman M, Schreuder J, de Craen AJ, Brandt BW, Slagboom PE, Westendorp RG. Long Life study g. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol. 2005;40(1–2):11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 119.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 120.Dolcet X, Llobet D, Pallares J, Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446(5):475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- 121.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salminen A, Kaarniranta K. NF-kappaB signaling in the aging process. J Clin Immunol. 2009;29(4):397–405. doi: 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 123.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in aging and disease. Aging Dis. 2011;2(6):449–465. [PMC free article] [PubMed] [Google Scholar]
- 124.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2(98):ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8(7):1003–1006. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee JY, Nakada D, Yilmaz OH, Tothova Z, Joseph NM, Lim MS, Gilliland DG, Morrison SJ. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7(5):593–605. doi: 10.1016/j.stem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5(2):e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang S, Cai G, Fu B, Feng Z, Ding R, Bai X, Liu W, Zhuo L, Sun L, Liu F, Chen X. SIRT1 is required for the effects of rapamycin on high glucose-inducing mesangial cells senescence. Mech Ageing Dev. 2012;133(6):387–400. doi: 10.1016/j.mad.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 129.Tothova Z, Gilliland DG. FoxO transcription factors and stem cell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1(2):140–152. doi: 10.1016/j.stem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 130.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 131.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116(4):551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 132.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 133.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280(21):20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 134.Yang Y, Hou H, Haller EM, Nicosia SV, Bai W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24(5):1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]