Abstract
Stiffening of conduit arteries is a risk factor for cardiovascular morbidity. Aortic wall stiffening increases pulsatile hemodynamic forces that are detrimental to the microcirculation in highly perfused organs such as the heart, brain and kidney. Arterial stiffness is associated with hypertension but presumed to be due to an adaptive response to increased hemodynamic load. In contrast, a recent clinical study found that stiffness precedes and may contribute to the development of hypertension, although the mechanisms contributing to hypertension are unknown. Here we report that in a diet-induced model of obesity, arterial stiffness, measured in vivo, develops within one month of the initiation of the diet and precedes the development of hypertension by five months. Diet-induced obese mice recapitulate the metabolic syndrome and are characterized by inflammation in visceral fat and aorta. Normalization of the metabolic state by weight loss returned arterial stiffness and blood pressure to normal. Our findings support the hypothesis that arterial stiffness is a cause, rather than a consequence of hypertension.
Keywords: Arterial stiffness, hypertension, obesity, pulse wave velocity, inflammation
Introduction
The aorta and its major branches stiffen with age 1 and in obesity 2 independently of atherosclerosis 3. In recent epidemiological and clinical studies, arterial stiffness emerged as an independent predictor of cardiovascular events, even after adjusting for risk factors such as age, sex, body mass index (BMI) and blood pressure 4.
Hypertension is associated with arterial stiffness 5, 6 and conventional thinking suggests that hypertension stimulates aortic remodeling and stiffening, in addition to vascular smooth muscle cell hypertrophy 7, as an adaptive process to increase wall-to-lumen width ratio in response to long-term changes in hemodynamic forces 8-10. However, a large longitudinal study of the general population recently established that arterial stiffness precedes an increase in systolic blood pressure and newly diagnosed hypertension 11. In contrast, initial blood pressure was not independently predictive of subsequent aortic stiffening measured in the same individuals 4-10 years later. Although stiffness-induced hemodynamic changes have been implicated in the development of hypertension 12, 13, lack of animal models for studying the relationship between arterial stiffness and hypertension has hampered the discovery of mechanisms and has led to a recent effort by the National Heart, Lung and Blood Institute of the National Institutes of Health to establish such models 14.
Because of the rising prevalence of obesity-associated type-2 diabetes and the cardiovascular complications thereof 15-17, we sought to determine if arterial stiffness occurs in a mouse model of diet-induced obesity. Arterial stiffness is increased in obese and diabetic individuals, even at a young age (10-24 years) 18, and in a genetic model of obesity (ob/ob mice) 19. Conversely, weight loss in overweight and obese individuals is associated with a reduction in arterial stiffness 20. Understanding the mechanisms underlying the development and regression of arterial stiffness might lead to additional options for prevention and treatment of hypertension and associated complications.
The goals of this study were to determine the temporal relationship between the development of arterial stiffness and hypertension and their potential reversal by return to normal diet, as well as the mechanisms thereof.
Methods
A detailed description of methods can be found in online supplements at http://hyper.ahajournals.org.
Results
High fat, high sucrose (HFHS) diet recapitulates human metabolic syndrome in mice
HFHS feeding significantly increased body weight (BW) (Fig. S1A) and fat mass compared to ND by 2 months, while lean mass remained unchanged over time (Fig. S1B). Also within 2 months mice became glucose intolerant (Fig. S1C) and insulin resistant (Fig. S1D). In addition to obesity and glucose intolerance, HFHS-fed mice developed chronic inflammation, as evidenced by substantial infiltration of activated macrophages in visceral fat surrounding the kidneys (Fig. S2A). Microalbuminuria, an indicator of kidney damage, was significantly increased after 4 months of HFHS diet (Fig. S2B).
Arterial stiffness is increased by HFHS diet and precedes systolic hypertension
Pulse wave velocity (PWV), an index of arterial stiffness measured by ultrasonography, was significantly increased by 2.4-fold after 1 month of HFHS and remained elevated up to 8 months compared to ND (Fig. 1A). PWV was similarly elevated when assessed from the aortic arch to abdominal aorta as it was in the abdominal aortic segment (6.3 ± 1.3 vs 3.4 ± 0.6 mm/ms in ND and 5.7 ± 0.9 vs 2.8 ± 1.0 mm/ms in ND, respectively; p < 0.05). The increase in PWV was confirmed by invasive hemodynamic measurements both at baseline and after phenylephrine (Fig. 1B and Table S1).
Figure 1. Arterial stiffness precedes hypertension in diet-induced obese mice.
Pulse wave velocity (PWV, mm/ms), an index of arterial stiffness measured by Doppler echocardiography, mean ± SD, n=4-10 each group (A) and invasively with high-fidelity pressure catheters, mean ± SEM, n=8 each group (B) is increased in HFHS-fed mice within 2 months. Mean arterial pressure was modulated by intravenous infusion of phenylephrine (0.1 μg/g BW). *, p<0.05 vs ND or ND-baseline; #, p<0.05 vs HFHS-baseline; $, p<0.05 vs ND-phenylephrine. Mice develop systolic hypertension after 6 months of HFHS (C) compared to ND (D). SBP, systolic blood pressure. *, p<0.05 vs baseline (time 0) or ND.
In order to address the temporal relationship between arterial stiffness and hypertension, young mice (8 weeks old) were implanted with radiotelemetry pressure transducers and followed after starting HFHS for up to one year. The first statistically significant increase in systolic (SBP, Fig.1C) and mean arterial pressure (MAP, Fig. S3A) was observed after 6 months while diastolic blood pressure was not significantly different (DBP, Fig. S3B). Pulse pressure (PP), an indirect index of arterial stiffness, gradually increased and reached statistical significance at 6 months (from baseline 26.4 ± 2.0 to 37.8 ± 2.2 mmHg; n=4-6; Fig. S3C). SBP (Fig. 1D), MAP (Fig. S3D) and PP (Fig. S3F) were also significantly and consistently elevated in HFHS- (n=11) compared to ND- (n=9) fed mice when blood pressure transducers were implanted between 5 and 6 months of diet and recorded for 1 month thereafter.
Spectral analysis of high frequency telemetry recordings, performed to assess whether obesity-associated activation of the sympathetic nervous system 21 could contribute to the hypertension observed after 6 month of HFHS diet, showed increased sympathetic modulation of blood pressure and heart rate (HR) in mice fed HFHS diet for 8 months compared to ND (Table S2). This was accompanied by significantly increased plasma norepinephrine in mice fed HFHS for 8 months compared to ND but not for 2 months (Table S3). Consistent with HFHS-associated sympathetic activation, HR was slightly but significantly elevated in HFHS-fed mice (Fig. S3G) compared to ND (Fig. S3H) and this was reduced to normal values when obese mice were reversed to normal diet (Fig. S3I).
Early vascular changes in HFHS-fed mice
Within 2 months of diet when the increase in PWV due to HFHS diet was fully developed, aortas from HFHS-mice had impaired relaxation to acetylcholine (Fig. 2A) indicating reduced endothelial nitric oxide (NO) function. In addition, the activity of tissue transglutaminase-2, a NO-sensitive enzyme that contributes to arterial stiffening in aged mice and rats by increasing extracellular matrix cross-linking 22, was significantly increased in aortic lysates from HFHS-fed mice, consistent with reduced NO bioavailability (Fig. 2B). Inflammation associated with obesity was evidenced at 2 months by ∼3-fold increases in aortic mRNA expression of TNFα, MCP-1 and MIP1 (Fig. 2C). There was no significant increase in collagen content in aortas from obese mice compared to ND (Fig. S4).
Figure 2. Impaired NO bioavailability and aortic inflammation in diet-induced obese mice.
(A) Vasorelaxation to acetylcholine (1×10−9-1×10−5 mol/L) in aortas from mice fed ND or HFHS diet. n=5 each group; *, p<0.05. (B) Transglutaminase-2 (TG-2) activity, an index of NO bioavailability, measured on aortic lysates from ND- and HFHS-fed mice. Dot blot intensity quantitation expressed graphically as % normal diet. n=4 each group. *, p<0.05. (C) Inflammatory cytokines TNFα, MCP-1 and MIP1α mRNA were significantly upregulated in aortic extracts from HFHS-fed mice (n=8) compared to ND (n=8). Data are expressed as fold change over ND. *, p<0.05.
Arterial stiffness and hypertension are reversed by return to normal diet
To determine whether arterial stiffness in obese mice is reversible, mice fed HFHS diet for 5 months were reverted to ND (henceforth referred to as HFHS/ND vs ND/ND control mice that were on ND for the entire duration). In just 2 weeks, obese mice lost 12.5 % of BW, and returned to BW of ND-fed mice within 2 months of diet reversal (Fig. 3A, left). A 50% loss in fat mass occurred with no significant change in lean mass and was accompanied by normalization of hyperinsulinemia (Fig. 3B) indicating amelioration of metabolic state.
Figure 3. Reversal to normal diet reduces arterial stiffness and hypertension in HFHS-fed mice.
(A) Body weights (BW, g) and PWV (mm/ms) in HFHS-fed mice decreased to control values after reversal to ND. n=8 each group; *, p<0.05 vs ND-baseline; #, p<0.05 vs HFHS-baseline. (B) Reversal to ND rapidly reduced fat mass (g, left y axis) and plasma insulin levels (μg/L, right y axis) in obese mice. n=4-14 in each group. (C) Systolic (SBP) and mean arterial (MAP) pressures of obese mice were significantly decreased after reversal to ND while diastolic pressure (DBP) did not change (n=6). *, p< 0.05 vs baseline. Baseline indicates 5 months on diet (ND or HFHS) and Reversal indicates 4 months of ND after 5 months of HFHS or ND.
Similarly, PWV was reduced to control values within 2 months of diet reversal (Fig. 3A, right). At this time point, SBP and MAP were significantly reduced to values not significantly different from those in control mice (Fig. 3C).
In vitro aortic stiffness, aortic inflammation and oxidant stress are reduced after return to ND
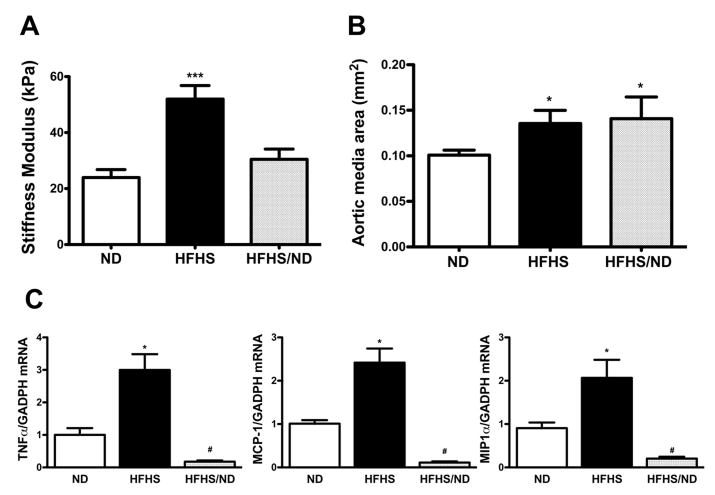
The force required for a fixed indentation of the basement membrane of endothelium-denuded aorta showed that HFHS significantly increased stiffness by 2-fold (52 ± 4.8, n=7 vs 24 ± 2.8 kPa in ND, n=10). This returned to normal levels after diet reversal in HFHS/ND (30.5 ± 3.7 kPa, n=8)(Fig. 4A).
Figure 4. In vitro aortic stiffness and medial area are increased in diet-induced obesity.
(A) Stiffness modulus (kPa) on aortic rings subjected to 0.1 μm indentation was increased in HFHS-fed mice and reduced to normal values after reversal to ND (HFHS/ND). n=7-10 each group; ***, p<0.0005 vs ND. (B) Medial area of thoracic aortas from HFHS-fed mice was significantly increased compared to ND and was not affected by reversal to ND; n=4-8; *, p<0.05 vs ND. (C) HFHS-induced inflammatory cytokines TNFα, MCP-1 and MIP1α mRNA in aortic extracts were reduced to normal levels after reversal to ND (n=8 each group). Data are expressed as fold change over ND. *, p<0.05 vs ND; #, p<0.05 vs HFHS.
HFHS diet stimulated aortic hypertrophy compared to ND (medial area: 0.135 ± 0.014 in HFHS vs 0.100 ± 0.005 mm2 in ND; p < 0.05). Although this might have contributed to aortic stiffness, medial area remained unchanged after reversal to ND (0.14 ± 0.02 mm2, n=8; Fig. 4B).
HFHS-induced upregulation of inflammatory genes in aortas was normalized after reversal to ND (Fig. 4C). In addition, immuno-staining of aortic sections with a specific anti-N-ε-(γ-glutamyl)-lysine antibody, indicative of TG-2 activity, revealed reduced extracellular matrix cross-links after diet reversal (Fig. 5A) despite there was no change in TG-2 expression between different diet groups (Fig. S5). Aortic immuno-staining with a specific anti-sulphonylated sarco/endoplasmic reticulum Ca++-ATPase (SERCA), showed that HFHS increased SERCA oxidation, used as index of oxidant stress 23-25, compared to ND, and this was decreased by reversal to ND (Fig. 5B).
Figure 5. Aortic extracellular matrix crosslinks and oxidant stress are induced by HFHS and reduced after diet reversal.
Representative pictures of (A) extracellular matrix N-ε-(γ-glutamyl)-lysine crosslinks (10X magnification), indicative of TG-2 activity, and (B) SERCA sulphonylated at cysteine 674 (OxSERCA) (40X magnification), used as index of oxidants, in aortas from ND-, HFHS-fed mice and after reversal to ND (HFHS/ND). n=8-16; *, p<0.05 vs ND; #, p<0.05 vs HFHS. Graphs indicate immunostaining intensities (expressed in millions of pixels). For a color version, see Figure S6 in supplemental material.
Discussion
Here we report for the first time that, in an animal model of diet-induced obesity, arterial stiffness develops rapidly and precedes the onset of hypertension. In mice fed a diet rich in fat and sucrose, PWV, measured both by Doppler ultrasonography and invasively, was elevated coincident with increased fat mass, glucose intolerance and hyperinsulinemia but preceded the gradual onset of kidney damage, manifested by albuminuria, cardiac diastolic dysfunction 26 and hypertension. In accordance with increased PWV, we found evidence of increased material stiffness of the aortic intimal extracellular matrix, measured in vitro by atomic force microscopy, in response to HFHS feeding that was normalized by reversal to normal diet. Extracellular membrane stiffening of the intima can increase endothelial permeability, potentially contributing to the development of atherosclerosis 27. Material stiffness of the aortic extracellular matrix 28 or vascular cells 29 have recently emerged as novel biomechanical paradigms of aging-associated arterial stiffness.
Tachycardia-associated sympathetic hyperactivity is found in obese hypertensive individuals and is explained, in part, by impaired baroreflex 21. Similar to obese individuals, we found sympathetic activation and tachycardia in HFHS-fed mice that could, in part, contribute to hypertension in these mice. Notably, aortic stiffening could impair the activation of carotid and aortic baroreceptors since artery distensibility is the main stimulus for a baroreflex. Although the causal link between arterial stiffness and the development of hypertension is lacking partially due to the inherent functional relationship between arterial wall stiffness and distending pressure 30, our findings support the hypothesis that arterial stiffness contributes to later hypertension in our model of diet-induced obesity. Interestingly, normalization of metabolic state achieved by weight loss after reversing obese mice to normal diet was associated with a rapid reduction in HFHS-induced arterial stiffness and systolic hypertension suggesting that normalization of metabolic state may contribute to amelioration of abnormal vascular function.
HFHS-fed mice recapitulate many aspects of the metabolic syndrome including insulin resistance, increased plasma triglycerides and hypertension 31, 32 making it an excellent model to study obesity-linked cardiovascular complications. Pro-inflammatory cytokines TNFα, MCP-1 and MIP1α were upregulated by ∼3-fold in aortas from HFHS-fed mice and returned to control levels after reversing obese mice to normal diet. Inflammation and reduced vascular NO bioavailability are known important determinants of cardiovascular diseases, including arterial stiffness, particularly in settings of aging and diabetes 33, 34. In rheumatoid arthritis patients, TNFα is associated with an increase in carotid-femoral PWV and L-arginine/ADMA ratio, indicative of reduced cellular NO-producing capacity. Thus, TNFα may contribute to aortic stiffness, at least in part, by regulating NO bioavailability in settings of chronic inflammation35. Aortas of HFHS-fed mice had both increased expression of TNFα and impaired aortic relaxation to acetylcholine indicative of reduced endothelial NO production, as early as two months on HFHS diet. The reduced NO bioavailability was also evident by an increase in tissue transglutaminase 2 (TG-2) activity in aortic lysates from HFHS-fed mice (Fig. 2B) that was decreased after reversal to ND (Fig. 5A). TG-2 is ubiquitously expressed in vascular cells and catalyzes a transamidation reaction to form strong N- ε -(γ-glutamyl)-lysine bonds between extracellular matrix proteins. TG-2 activity is inhibited by S-nitrosylation such that in settings of low NO bioavailability, it translocates to the extracellular space and becomes more active in forming matrix cross-links associated with arterial stiffness 22. Moreover, vascular extracellular matrix cross-links known as advanced glycosylation end products, could form non-enzymatically in settings of chronic hyperglycemia 36 possibly contributing to arterial stiffness in HFHS-fed mice.
In addition to decreased NO bioavailability, we found increased sulphonylated SERCA, a marker of oxidant stress 24, 25, 37 in aortas from obese mice compared to ND or mice reversed to ND. Oxidative post-translational modifications, such as sulphonylation of SERCA at cysteine 674, prevent NO-mediated SERCA-dependent Ca++ uptake into the sarco/endoplasmic reticulum and, therefore, impair vascular smooth muscle cell relaxation 38. Decreased SERCA activity and endothelial NO in settings of HFHS-induced oxidant stress could therefore contribute to HFHS-induced arterial stiffness, at least in part, by increasing vascular smooth muscle tone. Taken together, our findings indicate that inflammation and decreased NO function could play a causative role in aortic and extracellular matrix remodeling and tone in obesity-induced arterial stiffness.
In conclusion, we showed for the first time that in an animal model of diet-induced obesity arterial stiffness precedes hypertension. Multiple mechanisms involving inflammatory mediators, matrix cross-linking and decreased NO bioactivity may contribute to vascular remodeling and stiffness in advance of hypertension that may be, at least in part, a result of arterial stiffness causing undue stress on resistance vessels. The finding that reversal to normal diet improves vascular function in addition to the metabolic state indicates that arterial stiffness can be a novel target for early pharmacological or life-style interventions to prevent hypertension and associated complications in settings of obesity.
Perspectives
Arterial stiffness is an independent risk factor for cardiovascular events. Hypertension is associated with arterial stiffness although their temporal relations remain controversial. Here we report that in diet-induced obesity arterial stiffness precedes the onset of hypertension. Weight loss improves not only the diet-induced metabolic impairment in obese mice, but also arterial stiffness and hypertension. Considering the epidemic incidence of obesity in the United States, mainly due to excessive consumption of fat- and sucrose-rich diets, arterial stiffness could represent a novel therapeutic target to prevent obesity-associated cardiovascular complications, including hypertension.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
-
WHAT IS NEW?
Arterial stiffness is increased in a diet-induced model of obesity and precedes hypertension.
Arterial stiffness and high blood pressure are reversed to normal when the metabolic state of obese mice is normalized by weight loss.
-
WHAT IS RELEVANT?
Arterial stiffness could represent a novel therapeutic target to prevent obesity-associated cardiovascular complications, including hypertension.
-
SUMMARY
Whether arterial stiffness could develop in advance of hypertension is unknown. Here we report that, in a model of diet-induced obesity, arterial stiffness precedes the onset of hypertension. Normalization of metabolic state by weight loss in obese mice returned arterial stiffness and high blood pressure to normal. Arterial stiffness could be a novel therapeutic target to prevent cardiovascular complications, including hypertension, in settings of obesity.
Acknowledgments
We would like to thank Pratibha Chauhan and Xiuyun Hou for their technical assistance.
SOURCES OF FUNDING1.: This work was supported by NHLBI R01 grants HL105287 to RAC and HL097296 to CRK and was partially supported by the Evans Center for Interdisciplinary Biomedical Research ARC on “Molecular, Biomechanical and Genetic mechanisms of Arterial Stiffness” at Boston University (http://www.bumc.bu.edu/evanscenteribr/).
Footnotes
Conflict of interest/Disclosure: GM is the owner of Cardiovascular Engineering, Inc., a biomedical device manufacturer from which some instrumentation, used to conduct this study, was purchased.
References
- 1.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 2.Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension. 2001;38:429–433. doi: 10.1161/01.hyp.38.3.429. [DOI] [PubMed] [Google Scholar]
- 3.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: A clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltran A, McVeigh G, Morgan D, Glasser SP, Neutel JM, Weber M, Finkelstein SM, Cohn JN. Arterial compliance abnormalities in isolated systolic hypertension. Am J Hypertens. 2001;14:1007–1011. doi: 10.1016/s0895-7061(01)02160-4. [DOI] [PubMed] [Google Scholar]
- 6.Gedikli O, Kiris A, Ozturk S, Baltaci D, Karaman K, Durmus I, Baykan M, Celik S. Effects of prehypertension on arterial stiffness and wave reflections. Clin Exp Hypertens. 2010;32:84–89. doi: 10.3109/10641960902993103. [DOI] [PubMed] [Google Scholar]
- 7.Owens GK. Control of hypertrophic versus hyperplastic growth of vascular smooth muscle cells. Am J Physiol. 1989;257:H1755–1765. doi: 10.1152/ajpheart.1989.257.6.H1755. [DOI] [PubMed] [Google Scholar]
- 8.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 9.Boutouyrie P, Bussy C, Lacolley P, Girerd X, Laloux B, Laurent S. Association between local pulse pressure, mean blood pressure, and large-artery remodeling. Circulation. 1999;100:1387–1393. doi: 10.1161/01.cir.100.13.1387. [DOI] [PubMed] [Google Scholar]
- 10.Safar ME, O'Rourke MF. Handbook of hypertension: Arterial stiffness in hypertension. Edinburg, Scotland: Elservier; 2006. [Google Scholar]
- 11.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, Spurgeon HP, Ferrucci L, Lakatta EG. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the baltimore longitudinal study of aging. J Am Coll Cardiol. 2008;51:1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yambe M, Tomiyama H, Yamada J, Koji Y, Motobe K, Shiina K, Yamamoto Y, Yamashina A. Arterial stiffness and progression to hypertension in japanese male subjects with high normal blood pressure. J Hypertens. 2007;25:87–93. doi: 10.1097/01.hjh.0000254375.73241.e2. [DOI] [PubMed] [Google Scholar]
- 14.Http://grants.Nih.Gov/grants/guide/rfa-files/rfa-hl-10-027.Html
- 15.Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D'Agostino RB., Sr Trends in the incidence of type 2 diabetes mellitus from the 1970s to the 1990s: The framingham heart study. Circulation. 2006;113:2914–2918. doi: 10.1161/CIRCULATIONAHA.106.613828. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Arch Intern Med. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 17.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, et al. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sikka G, Yang R, Reid S, Benjo A, Koitabashi N, Camara A, Baraban E, O'Donnell CP, Berkowitz DE, Barouch LA. Leptin is essential in maintaining normal vascular compliance independent of body weight. Int J Obes (Lond) 2010;34:203–206. doi: 10.1038/ijo.2009.208. [DOI] [PubMed] [Google Scholar]
- 20.Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension. 2010;55:855–861. doi: 10.1161/HYPERTENSIONAHA.109.147850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Dell'Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36:538–542. doi: 10.1161/01.hyp.36.4.538. [DOI] [PubMed] [Google Scholar]
- 22.Santhanam L, Tuday EC, Webb AK, et al. Decreased s-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 23.Tong X, Evangelista A, Cohen RA. Targeting the redox regulation of serca in vascular physiology and disease. Curr Opin Pharmacol. 2010;10:133–138. doi: 10.1016/j.coph.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong X, Hou X, Jourd'heuil D, Weisbrod RM, Cohen RA. Upregulation of nox4 by tgf{beta}1 oxidizes serca and inhibits no in arterial smooth muscle of the prediabetic zucker rat. Circ Res. 2010;107:975–983. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lancel S, Qin F, Lennon SL, Zhang J, Tong X, Mazzini MJ, Kang YJ, Siwik DA, Cohen RA, Colucci WS. Oxidative posttranslational modifications mediate decreased serca activity and myocyte dysfunction in galphaq-overexpressing mice. Circ Res. 2010;107:228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin F, Siwik DA, Luptak I, Hou X, Wang L, Higuchi A, Weisbrod RM, Ouchi N, Tu VH, Calamaras TD, Miller EJ, Verbeuren TJ, Walsh K, Cohen RA, Colucci WS. The polyphenols resveratrol and s17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. S1751–1756. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sazonova OV, Lee KL, Isenberg BC, Rich CB, Nugent MA, Wong JY. Cell-cell interactions mediate the response of vascular smooth muscle cells to substrate stiffness. Biophys J. 2011;101:622–630. doi: 10.1016/j.bpj.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension. 1990;15:339–347. doi: 10.1161/01.hyp.15.4.339. [DOI] [PubMed] [Google Scholar]
- 31.Gallou-Kabani C, Vige A, Gross MS, Rabes JP, Boileau C, Larue-Achagiotis C, Tome D, Jais JP, Junien C. C57bl/6j and a/j mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring) 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- 32.Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Differential effects of fat and sucrose on the development of obesity and diabetes in c57bl/6j and a/j mice. Metabolism. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53:258–261. doi: 10.3349/ymj.2012.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angel K, Provan SA, Mowinckel P, Seljeflot I, Kvien TK, Atar D. The l-arginine/asymmetric dimethylarginine ratio is improved by anti-tumor necrosis factor-alpha therapy in inflammatory arthropathies. Associations with aortic stiffness. Atherosclerosis. 2012;225:160–165. doi: 10.1016/j.atherosclerosis.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 36.Brownlee M, Cerami A, Vlassara H. Advanced products of nonenzymatic glycosylation and the pathogenesis of diabetic vascular disease. Diabetes Metab Rev. 1988;4:437–451. doi: 10.1002/dmr.5610040503. [DOI] [PubMed] [Google Scholar]
- 37.Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes serca cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-glutathiolation by peroxynitrite activates serca during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.