Abstract
Background
Hypothermia has been reported to induce ventricular tachycardia and fibrillation (VT/VF) in patients with early repolarization (ER) pattern. This study examines the cellular mechanisms underlying VT/VF associated with hypothermia in an experimental model of ER syndrome (ERS) and examines the effectiveness of quinidine, cilostazol and milrinone to prevent hypothermia-induced arrhythmias.
Method and Results
Transmembrane action potentials (AP) were simultaneously recorded from 2 epicardial and 1 endocardial site of coronary-perfused canine left-ventricular wedge preparations, together with a pseudo-ECG. A combination of NS5806 (3–10 µM) and verapamil (1µM) was used to pharmacologically model the genetic mutations responsible for ERS. Acetylcholine (3µM) was used to simulate increased parasympathetic tone, which is known to promote ER. In control, lowering the temperature of the coronary perfusate to induce mild hypothermia (32°C-34°C) resulted in increased J wave area on the ECG and accentuated epicardial AP notch but no arrhythmic activity. In the setting of ER, hypothermia caused further accentuation of the epicardial AP notch, leading to loss of the AP dome at some sites but not others, thus creating the substrate for development of phase-2-reentry and VT/VF. Addition of the Ito antagonist quinidine (5 µM) or the phosphodiesterase III inhibitors cilostazol (10 µM) or milrinone (5 µM), diminished the ER manifestations and prevented the hypothermia-induced phase 2 reentry and VT/VF.
Conclusions
Hypothermia leads to VT/VF in the setting of ER by exaggerating repolarization abnormalities, leading to development of phase-2-reentry. Quinidine, cilostazol and milrinone suppress the hypothermia-induced VT/VF by reversing the repolarization abnormalities.
Keywords: ventricular tachycardia, ventricular fibrillation, phosphodiesterase inhibitor, J wave syndrome, early replarization syndrome
Introduction
An early repolarization (ER) pattern in the ECG is characterized by a J-point elevation with or without ST-segment elevation as well as notching or slurring of the terminal part of the QRS complex. ER is a relatively common ECG finding, long considered to be benign. Recent studies point to an association of ER with the development of ventricular tachycardia (VT) and fibrillation (VF) leading to sudden cardiac death.1–4 Early repolarization has been divided into three subtypes. Type 1 involves an ER pattern localized to the lateral leads. In Type 2 the ER pattern is present in the inferior and infero-lateral leads, and in Type 3 it is present globally in the inferior, lateral and anterior or right precordial leads. Individuals with Type 1 rarely develop VT/VF. Type 2 is associated with a higher level of risk and individuals displaying a Type 3 ER have the highest risk for developing life-threatening arrhythmias, including electrical storms.4
Hypothermia is known to contribute to the development of prominent J waves leading to VT/VF.4–6 Current guidelines recommend mild therapeutic hypothermia (TH) to prevent neurological damage following a cardiac arrest.7, 8 The extent to which hypothermia contributes to arrhythmogenesis and the mechanisms involved are not well defined. Recent reports point to an association between ER and the development of VT/VF in the setting of hypothermia.9,10 The present study was designed to test the hypothesis that ER, by causing outward shift in the balance of currents active during the early phases of the epicardial action potential (AP), exacerbates the response to hypothermia, thus leading to prominent J waves, phase 2 reentry and VT/VF. In addition, we examine the hypothesis that pharmacologic agents capable of producing an inward shift in the balance of current in the early phases of the epicardial AP, either by inhibiting transient outward current (Ito) (quinidine) or augmenting ICa (cilostazol and milrinone), can protect against hypothermia-induced VT/VF in the setting of ER.
Methods
Arterially Perfused Wedge of Canine Left Ventricle
We employed floating glass microelectrode techniques to simultaneously record APs from 2 epicardial (Epi) and 1 endocardial (Endo) site in coronary-perfused wedge preparations isolated from the inferior and lateral regions of the canine left ventricle (LV), together with pseudo-ECG recorded across the bath along the Endo-Epi axis. Detailed methods are provided in the online supplement. The Tyrode’s solution was warmed while passing through the heated coils to deliver the perfusate at 37°C. To simulate hypothermia, the solution was redirected to two coiled-perfusion lines in series immersed in beakers filled with water, before reaching the tissues. We lowered the temperature of the perfusate to 32°C.
Measurements of Action Potential Parameters
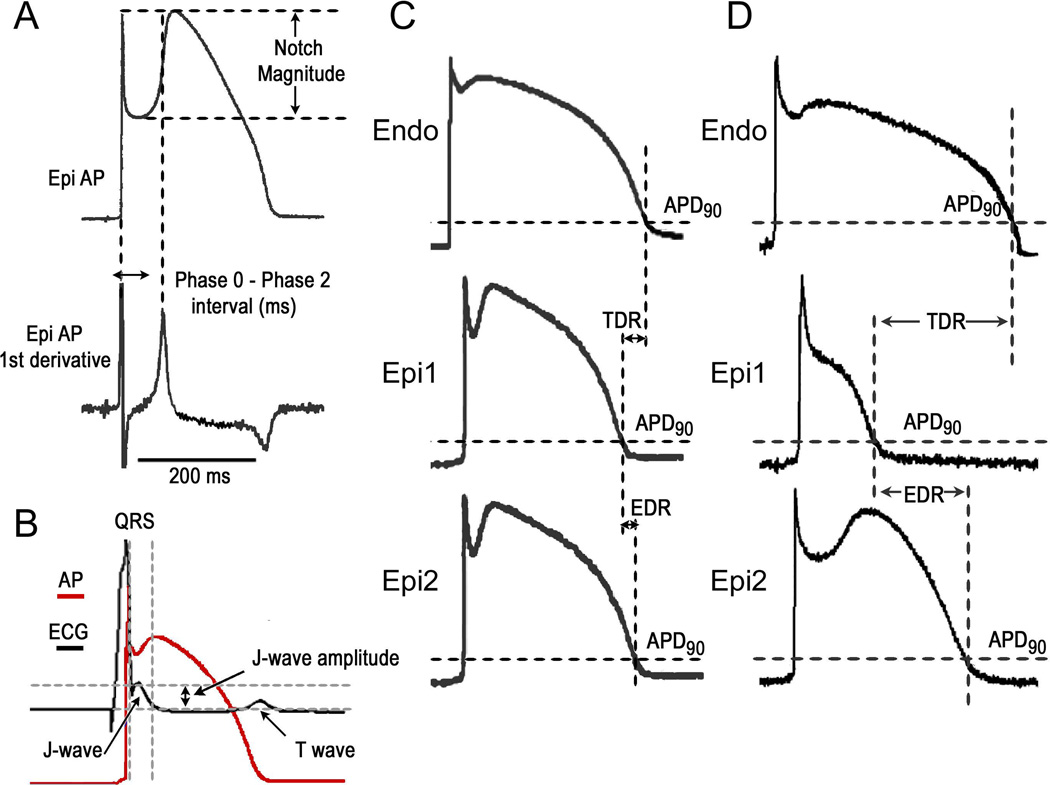
The Epi AP notch magnitude (NM) [phase 1 magnitude / phase 0 amplitude × 100], phase 0 to phase 2 interval; [time between the first 2 peaks of the derivative of the AP] as well as the notch index (NI); [NM × (Ph 0 to Ph 2 interval)] which approximates the area of the notch, were measured in AP recordings as previously described (Figure 1).11
Figure 1.
A-B. Method of quantitation of action potential (AP) and J wave parameters. C. Measurement of dispersion of AP repolarization. D. Measurement of dispersion of repolarization when the AP dome is lost at one epicardial (Epi) site but not the other. APD90 = action potential duration at 90% repolarization; EDR = epicardial dispersion of repolarization; Endo = endocardial; TDR = transmural dispersion of repolarization.
J Wave Area Calculations
The area of the J wave was calculated as follows: The start of J-wave was defined using derivative of the ECG signal. In case of clear separation it was set at the time when this derivative is zero which corresponds to the notch between R wave and J wave. When this separation was not clearly visible, this time was set at the moment when the negative derivative attains its maximal value (i.e. minimal rate of decline) after the maximal downslope of the R wave. The J wave area was expressed as mV × ms (Figure 1.).
Statistical Analysis
Results are presented as mean ± S.E.M. (standard error of the mean) throughout the manuscript. Statistical comparisons were made using Student’s t-test for Table 1 A-B. One way repeated measures analysis of variance were used for Table 1 C-E.; followed by pairwise comparisons corrected by Bonferroni method.
Table 1.
A. Effect of hypothermia on action potential duration at 90% of repolarization (APD90), maximum epicardial (Epi) dispersion of repolarization (EDR), transmural dispersion of repolarization (TDR), notch index, notch magnitude, notch duration and J wave area. B. Effect of hypothermia on these parameters in the setting of early repolarization. C. Effect of quinidine. D. Effect of cilostazol. E. Effect of milrinone.
|
Endo APD90 (ms) |
Epi1 APD90 (ms) |
Epi2 APD90 (ms) |
EDR (ms) |
TDR (ms) |
Notch Index* |
Notch magnitude (% of Ph0) |
Notchduration (ms) |
J wave area (mV × ms) |
|
|---|---|---|---|---|---|---|---|---|---|
| A | |||||||||
| Control (37°C) | 224.1±2.7 | 200.3±5.9 | 194.2±5.5 | 15.1±5.9 | 13.0±4.4 | 180.0±19.1 | 12.2±3.0 | 20.1±1.5 | 1.6±0.6 |
| Control (32°C) | 320.7±10.7b | 306.3±18.1b | 295.7±13.9b | 20.0±9.9 | 26.3±8.5 | 1918.3±393.3b | 39.0±3.7b | 47.1±5.9b | 10.8±3.8b |
| B | |||||||||
| Provocative agents† (37°C) | 234.5±4.8 | 209.1±4.7 | 212.2±3.9 | 5.6±1.7 | 11.5±2.7 | 1163.9±140.8 | 30.9±2.2 | 36.5±2.5 | 9.6±1.0 |
| Provocative agents† (32°C) | 353.0±18.2b | 154.5±14.2a | 298.9±18.4b | 140.0±17.4b | 181.9±22.2b | 4435.2±286.4b | 42.0±2.2a | 106.5±5.2b | 62.3±11.3b |
| C | |||||||||
| Control (37°C) | 225.9±2.7 | 195.3±5.2 | 197.2±7.0 | 10.9±2.6 | 15.3±5.7 | 250.5±92.2 | 12.3±4.3 | 20.5±1.9 | 1.6±0.9 |
| Provocative agents† (37°C) | 230.1±6.8 | 212.7±7.2 | 212.3±4.5 | 4.6±2.1 | 11.4±3.1 | 1107.5±201.2 | 28.3±3.4c | 38.4±3.0 | 11.1±2.4 |
| Provocative agents† (32°C) | 343.9±30.4b | 125.4±7.6b | 278.2±24.3a | 152.4±21.0b | 201.5±31.5b | 3823.4±217.7b | 41.7±3.9 | 94.6±9.9b | 75.4±22.2a |
| +Quinidine (5 µM) (32°C) | 369.9±15.1 | 352.0±16.3f | 349.6±16.4e | 4.1±1.5f | 21.5±11.0f | 1783.8±361.3f | 25.3±3.9e | 69.1±4.8e | 14.9±4.4e |
| D | |||||||||
| Control (37°C) | 213.2±4.9 | 192.7±4.2 | 196.2±4.2 | 6.6±2.1 | 13.5±5.0 | 208.5±46.7 | 10.8±2.6 | 19.3±1.1 | 1.5±0.5 |
| Provocative agents† (37°C) | 230.5±6.9 | 202.4±8.5 | 211.0±9.0 | 5.9±3.3 | 15.0±7.4 | 944.2±132.9 | 29.2±2.1d | 31.9±3.3 | 8.6±1.2 |
| Provocative agents† (32°C) | 345.1±26.0b | 159.6±12.9 | 285.2±24.6 | 119.4±34.4b | 157.8±33.4b | 6397.9±897.4b | 47.7±1.3b | 133.1±15.0b | 53.9±15.2b |
| +Cilostazol (10 µM) (32°C) | 336.9±34.5 | 323.5±32.9f | 328.4±32.3 | 8.6±3.5f | 14.9±11.1f | 2455.5±392.5f | 35.6±3.9f | 67.5±5.2f | 17.8±3.1e |
| E | |||||||||
| Control (37°C) | 215.9±9.9 | 192.7±4.6 | 198.5±4.1 | 3.9±1.3 | 9.7±1.6 | 230.0±87.9 | 12.3±3.9 | 17.5±1.0 | 2.0 ±0.5 |
| Provocative agents† (37°C) | 243.5±7.2 | 203.3±6.4 | 210.8±2.1 | 9.3±3.0 | 19.5±5.5 | 816.8±119.3 | 26.1±4.5c | 32.1±1.5 | 8.1±0.8 |
| Provocative agents† (32°C) | 362.0±35.8b | 171.9±29.6 | 353.5±22.3b | 175.3±21.9b | 170.9±51.6b | 5568.0±1022.7b | 49.6±2.1b | 109.8±16.2b | 56.3±14.4b |
| +Milrinone (5 µM) (32°C) | 371.2±14.9 | 341.6±22.5f | 345.4±16.7 | 16.8±2.9f | 18.2±5.5f | 2521.5±578.3f | 35.8±3.2f | 68.4±10.5e | 23.2±7.7 |
Notch index= Notch magnitude × (Ph 0 to Ph 2 interval) which approximates the area of the notch.
Provocative agents= NS5806 3-10 µM + Verapamil 1 µM + Acetylcholine 3 µM
EDR and TDR are measured in case of single stimulated beat with loss of the action potential dome at Epi2 site but not Epi1.
Results are mean ± S.E.M.
=p<0.05,
=p<0.01 32°Celsius vs. 37°Celsius,
=p<0.05,
=p<0.01 NS5806 3-10 µM + Verapamil 1 µM + Acetylcholine 3 µM (37°Celsius) vs. Control (37°Celsius),
=p<0.05,
=p<0.01 Quinidine 5 µM or Cilostazol 10 µM or Milrinone 5 µM (32°Celsius) vs. NS5806 3-10 µM + Verapamil 1 µM + Acetylcholine 3 µM (32°Celsius). Basic cycle length=1000 ms. n=7 for A; n=12 for B; n=5 for C; n=5 for D; n=5 for E. Endo = Endocardial.
Results
Effects of Hypothermia in Left Ventricular Wedge Preparation
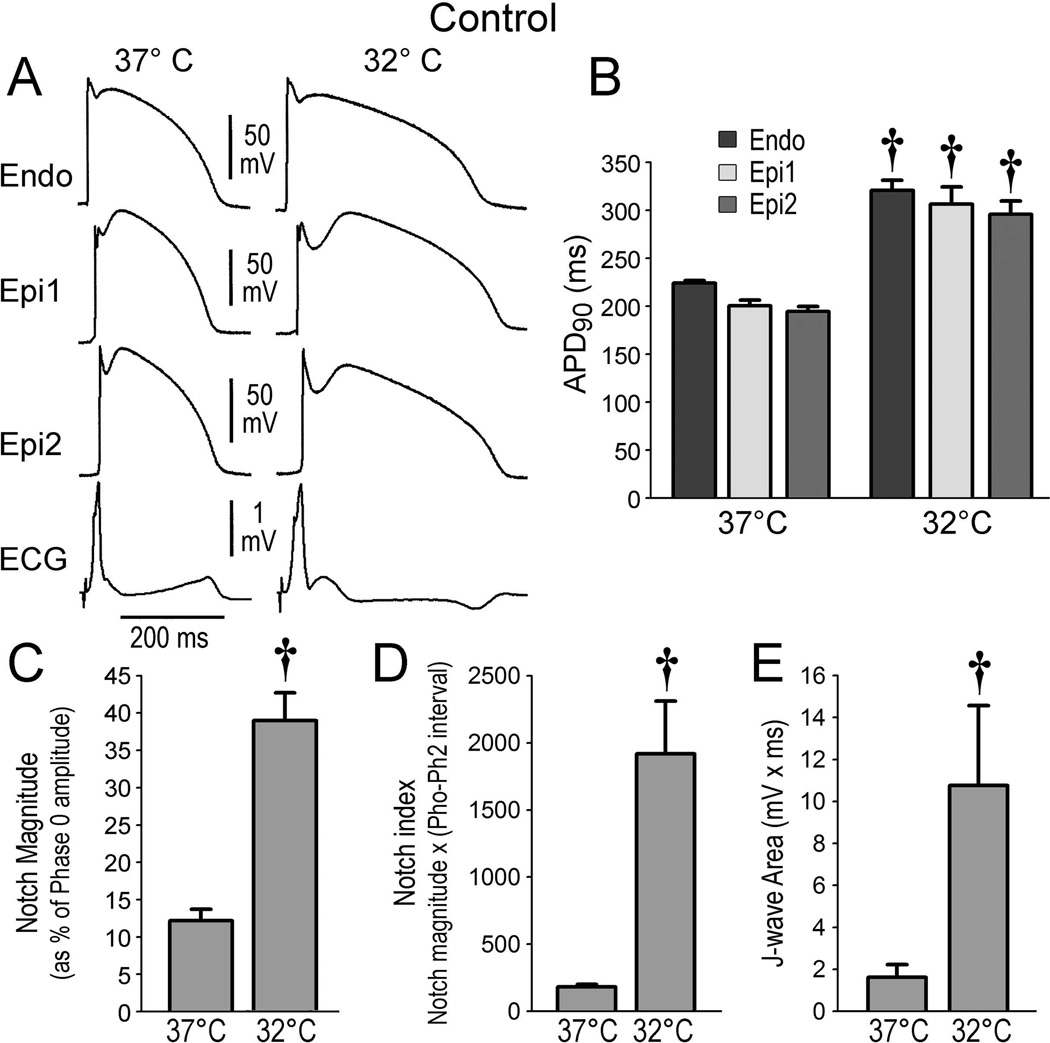
In an initial series of 7 experiments, we examined the effects of hypothermia in coronary-perfused canine LV wedge preparations. Lowering the temperature to 32–34°C caused prolongation of AP durations (APDs) and accentuation of the AP notch in the epicardium but not endocardium, thus augmenting the amplitude of the J wave (Figure 2, Table 1A). Under baseline condition, lowering the temperature to 32°C failed to cause loss of the Epi AP dome or to induce arrhythmic activities.
Figure 2.
Effect of lowering the temperature (from 37°C to 32°C) of the coronary perfusate in isolated canine left ventricular preparations under baseline conditions. A. Action potential traces simultaneously recorded from 2 epicardial and 1 endocardial sites together with a pseudo-ECG at 37°C and at 32°C. B-E. Composite data showing the effect of lowering coronary perfusate temperature to 32°C on action potential duration at 90% repolarization (APD90), notch magnitude, notch index and J wave area. Endo = endocardial; Epi = epicardial. All recordings were obtained at a basic cycle length of 1000 ms. Results are mean ± S.E.M. *p<0.05, †p<0.01 vs. 37°Celsius. n=7 for B, C, D and E.
Effects of Hypothermia in ER-Induced Left Ventricular Wedge Preparation
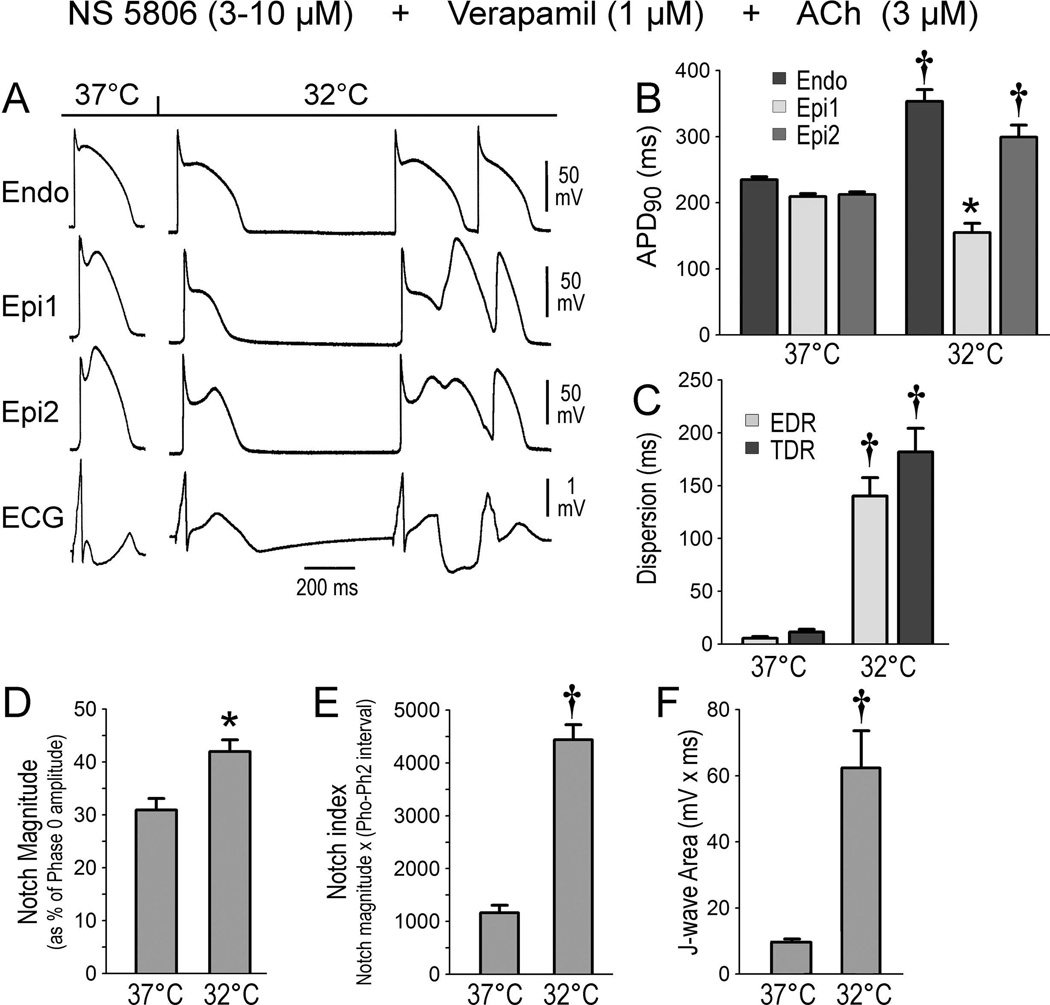
Because vagal influences are known to accentuate the electrocardiographic and arrhythmic manifestations of ER,12–14 in a second series of experiments, we induced an early repolarization phenotype using a combination of the Ito activator NS5806 (3–10 µM), the Ca2+ channel blocker verapamil (1 µM) and acetylcholine (ACh) (3 µM) added to the coronary perfusate. The combination accentuated the AP notch in epicardium but not endocardium, thus leading to augmentation of the electrocardiographic J wave but no arrhythmia (Figure 3A). Hypothermia caused a further increase of J wave area, NM and notch index, leading to all-or-none repolarization at the end of phase 1 of the Epi AP. Loss of the Epi AP dome at some sites but not others resulted in a prominent increase in epicardial dispersion of repolarization (EDR) and transmural dispersion of repolarization (TDR). The voltage gradient between the abbreviated Epi AP and the relatively normal Endo AP produced a prominent ST segment elevation. A prominent APD gradient developed between sites displaying a normal AP dome and adjacent sites where the dome was lost, thus creating a vulnerable window within epicardium as well as between epicardium and endocardium across the ventricular wall. Propagation of the dome from regions at which it was maintained to regions, at which it was lost, caused local re-excitation via a phase 2 reentry mechanism, leading to the development of closely coupled extrasystoles and polymorphic VT/VF (Figure 3).
Figure 3.
Arrhythmogenic effect of hypothermia in the setting of early repolarization (ER). A. All panels show traces recorded at 2 epicardial (Epi) and 1 endocardial (Endo) sites, together with a pseudo ECG. A. The first grouping was recorded 30 min after addition of NS5806 (10 µM), verapamil (1 µM) and acetylcholine (ACh) (3 µM) to the coronary perfusate at 37°C. The second grouping was recorded 10 min after lowering of the perfusate to 32°C; hypothermia in the setting of ER leads to the development of phase 2 reentry and polymorphic ventricular tachycardia. B-F. Composite data showing the effect of lowering coronary perfusate temperature to 32°C on action potential duration at 90% repolarization (APD90), notch magnitude, notch index and J wave area. EDR = epicardial dispersion of repolarization; TDR = transmural dispersion of repolarization. All recordings were obtained at a basic cycle length of 1000 ms. Results are mean ± S.E.M. *p<0.05, †p<0.01 vs. 37°C. n=12 for B, C, D, E and F.
Similar results were obtained in 12 experiments. Composite of the action potential and electrocardiographic parameters are presented in Figure 3 and Table 1B.
Effect of Quinidine, Cilostazol and Milrinone to Suppress and Prevent Hypothermia-Induced Arrhythmogenesis
Quinidine has been shown to restore transmural electrical homogeneity and abort arrhythmic activity in the J wave syndromes.2 In another series of 5 experiments we tested the hypothesis that quinidine could prevent the hypothermia-induced VT/VFdeveloping in the setting of ER owing to its effect to reduce the Ito. Ventricular tachycardia/ventricular fibrillation was first induced by exposure of the LV wedge to hypothermia + NS 5806 (3–10 µM) + verapamil (1µM) + ACh (3 µM). Temperature was then restored to 37°C, at which point the arrhythmia subsided. Quinidine (5 µM) was then added to the coronary perfusate and hypothermia was re-induced. Quinidine (5 µM) diminished the AP notch and J wave at 37°C and prevented loss of the Epi AP dome and development of the repolarization abnormalities, thus preventing the development of phase 2 reentry and VT/VF when temperature was reduced to 32°C (Figure 4). A similar effect of quinidine to prevent VT/VF was observed in 5 out of 5 preparations exposed to hypothermia (Figure 4, Table 1C). In 2 experiments, quinidine (5–10 µM) was added during the VT/VF episode at 32°C. In both cases the drug suppressed all arrhythmic activity and restored electrical homogeneity throughout the preparation.
Figure 4.
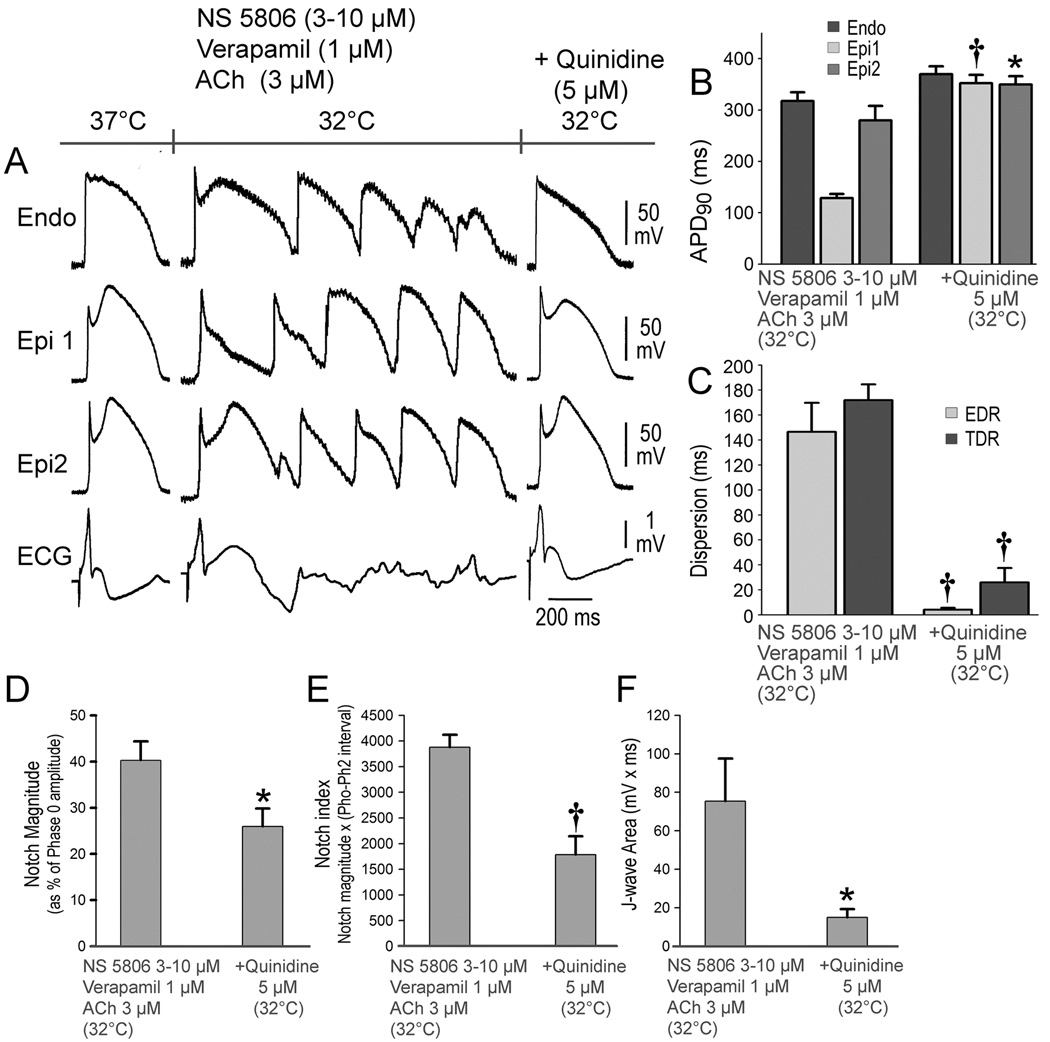
Quinidine prevents hypothermia-induced ventricular tachycardia and fibrillation (VT/VF) in the setting of early repolarization. A. The first grouping shows recordings from 2 epicardial (Epi) and 1 endocardial (Endo) sites, together with a pseudo-ECG after exposure of the left ventricular wedge to NS 5806 (3-10 µM) + verapamil (1µM) + acetylcholine (ACh) (3 µM). In the second grouping, the coronary perfusate temperature was lowered to 32°C, leading to development of VT/VF. Temperature was then restored to 37°C, at which point the arrhythmia subsided (not shown). The third grouping was recorded after addition of quinidine (5 µM) and re-induction of hypothermia. Quinidine (5 µM) diminished the action potential (AP) notch and J wave at 37°C and prevented loss of the Epi AP dome and development of the repolarization abnormalities, thus preventing the development of phase 2 reentry and VT/VF when temperature was reduced to 32°C. B-F. Composite data showing the effect of lowering coronary perfusate temperature to 32°C on AP duration at 90% repolarization (APD90), notch magnitude, notch index and J wave area in the presence and absence of quinidine. All recordings were obtained at a basic cycle length of 1000 ms. EDR = epicardial dispersion of repolarization; TDR = transmural dispersion of repolarization. Results are mean ± S.E.M. *p<0.05, †p<0.01 vs. NS 5806 + Verapamil + ACh combination. n=5 for B, C, D, E and F.
In a final series of experiments, we examined the effectiveness of the phosphodiesterase III inhibitors cilostazol and milrinone to prevent hypothermia-induced VT/VF in the setting of ER. These agents are known to augment ICa via their action to increase cAMP. Here again, we first demonstrated the ability of the combination of provocative agents and hypothermia (32°C) to accentuate ER, thus creating a large epicardial and transmural dispersion of repolarization giving rise to phase 2 reentry and VT/VF. We then restored temperature to 37°C, which reversed the repolarization abnormality and suppressed VT/VF. The addition of cilostazol (10 µM) or milrinone (5 µM) reduced the Epi AP notch at 37°C and prevented the repolarization abnormality as well as the development of phase 2 reentry and VT/VF when temperature was once again reduced to 32°C (Figures 5 and 6). Both agents prevented the hypothermia-induced increase in TDR, EDR; Epi AP notch magnitude, notch index, and J wave area on pseudo ECG. Cilostazol was successful in preventing the VT/VF in 5 out of 7 preparations. Similarly, milrinone (5 µM) prevented the hypothermia-induced VT/VF in 5 out of 7 preparations (Figures 5 and 6, Table 1D and E).
Figure 5.
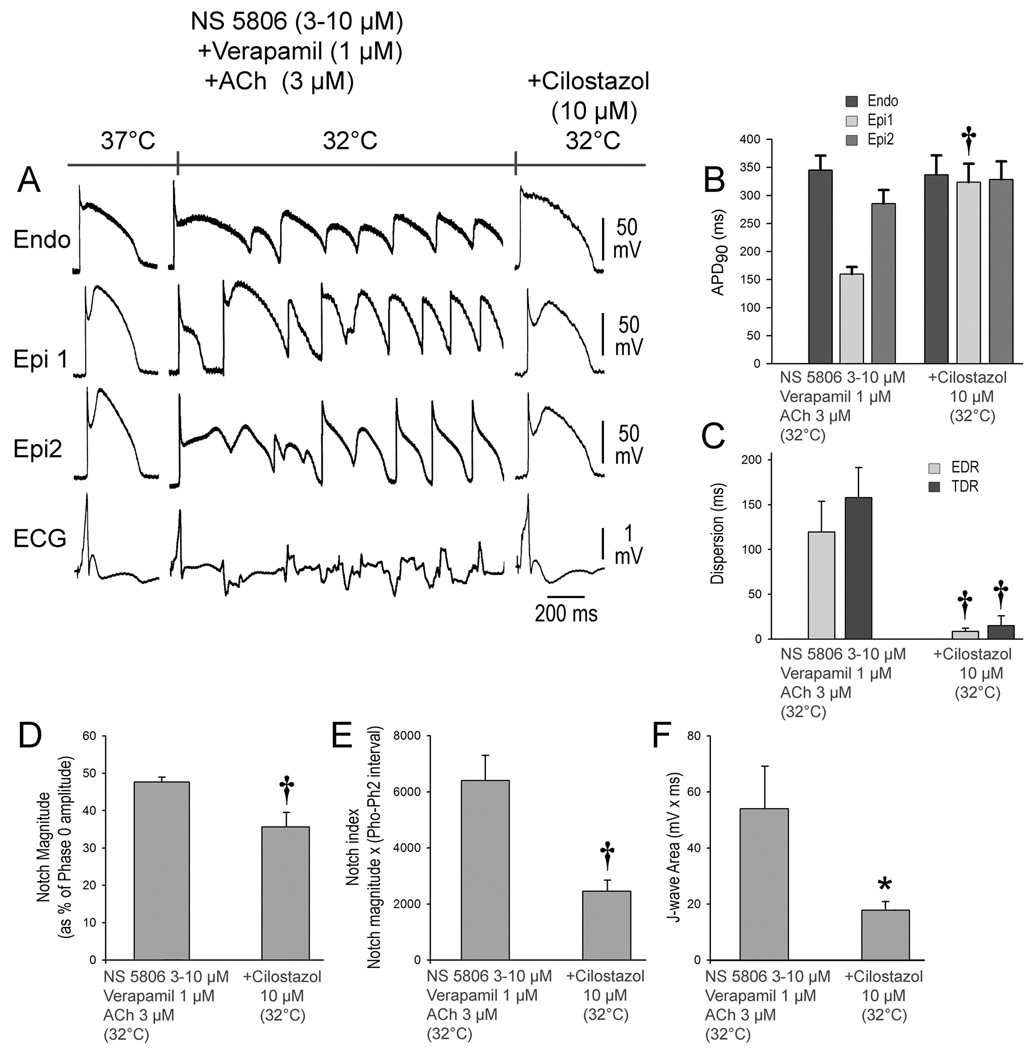
Cilostazol prevents hypothermia-induced ventricular tachycardia and ventricular fibrillation (VT/VF) in the setting of early repolarization. A. The first grouping shows recordings from 2 epicardial (Epi) and 1 endocardial (Endo) sites, together with a pseudo-ECG after exposure of the left ventricular wedge to NS 5806 (3-10 µM) + verapamil (1µM) + acetylcholine (ACh) (3 µM). In the second grouping, the coronary perfusate temperature was lowered to 32°C, leading to development of VT/VF. Temperature was then restored to 37°C, at which point the arrhythmia subsided (not shown). The third grouping was recorded after addition of cilostazol (10 µM) and re-induction of hypothermia. Cilostazol (10 µM) diminished the action potential (AP) notch and J wave at 37°C and prevented loss of the Epi AP dome and development of the repolarization abnormalities, thus preventing the development of phase 2 reentry and VT/VF in 5 of 7 preparations when temperature was reduced to 32°C. B-F. Composite data showing the effect of lowering coronary perfusate temperature to 32°C on AP duration at 90% repolarization (APD90), notch magnitude, notch index and J wave area in the presence and absence of cilostazol. EDR = epicardial dispersion of repolarization; TDR = transmural dispersion of repolarization. All recordings were obtained at a basic cycle length of 1000 ms. Results are mean ± S.E.M. *p<0.05, †p<0.01 vs. NS 5806 + Verapamil + ACh combination. n=5 for B, C, D, E and F.
Figure 6.
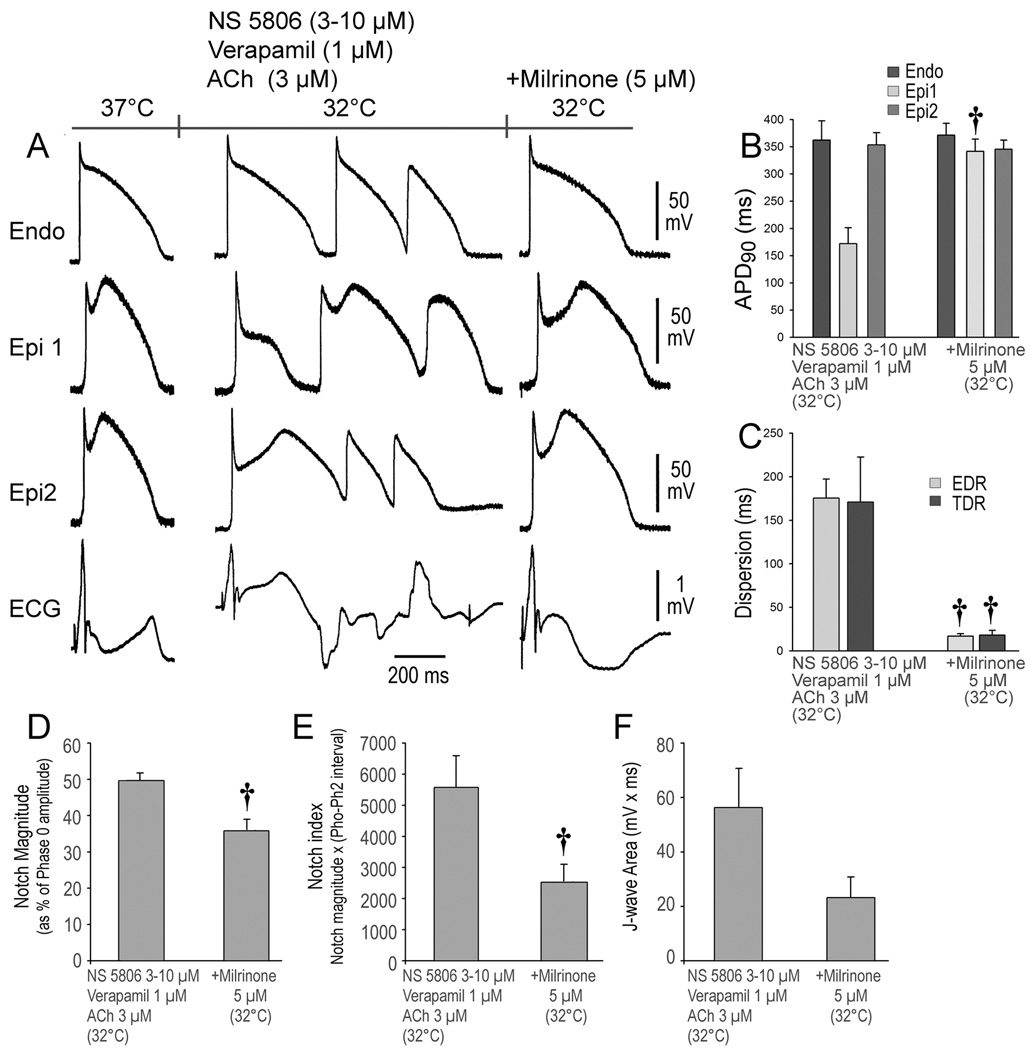
Milrinone prevents hypothermia-induced ventricular tachycardia and fibrillation (VT/VF) in the setting of early repolarization. A. The first grouping shows recordings from 2 epicardial (Epi) and 1 endocardial (Endo) sites, together with a pseudo-ECG after exposure of the left ventricular wedge to NS 5806 (3-10 µM) + verapamil (1µM) + Acetylcholine (ACh) (3 µM). The coronary perfusate temperature was lowered to 32°C, leading to development of VT/VF. Temperature was then restored to 37°C, at which point the arrhythmia subsided (not shown). The third grouping was recorded after addition of milrinone (5 µM) and re-induction of hypothermia. Milrinone (5 µM) diminished the action potential (AP) notch and J wave at 37°C and prevented loss of the Epi AP dome and development of the repolarization abnormalities, thus preventing the development of phase 2 reentry and VT/VF in 5 or 7 preparations when temperature was reduced to 32°C. B-E. Composite data showing the effect of lowering coronary perfusate temperature to 32°C on AP duration at 90% repolarization (APD90), notch magnitude, notch index and J wave area in the presence and absence of milrinone. EDR = epicardial dispersion of repolarization; TDR = transmural dispersion of repolarization. All recordings were obtained at a basic cycle length of 1000 ms. Results are mean ± S.E.M. *p<0.05, †p<0.01 vs. NS 5806 + Verapamil + ACh combination. n=5 for B, C, D, E and F.
Discussion
Therapeutic hypothermia is today widely used to prevent tissue injury secondary to cardiac arrest, ischemic stroke, traumatic brain and spinal cord injury, and neurogenic fever following brain trauma. Subjects are generally cooled to a target temperature of 32–34°C (90–93°F).15, 16 In cases of cardiac arrest, Holzer et al.17 reported that 55% of 137 patients in the hypothermia group experienced favorable outcomes, compared with only 39% in the group that received standard care following resuscitation. Among the adverse events encountered with TH is the development of cardiac arrhythmias.9, 10 Hypothermia’s adverse events increase in severity the lower a patient’s body temperature is reduced. The accepted medical standard is to not drop below 32°C (90°F).18
The factors that predispose to hypothermia-induced arrhythmogenesis and the mechanisms involved are poorly understood. Recent studies report an association between ER and the development of hypothermia-induced VT/VF.9, 10 The present study recapitulates this clinical phenomenon in an experimental model consisting of the canine LV coronary-perfused LV wedge preparation, provides a mechanistic understanding of the cellular basis for the development of VT/VF in the setting of ER and hypothermia, and identifies potential therapeutic agents that may be useful in suppressing or preventing the development of hypothermia-induced VT/VF.
Mechanism of Hypothermia-Induced arrhythmias in Patients with Early Repolarization Syndrome
The J wave syndrome, which includes both Brugada and early repolarization (ERS) syndromes, has been associated with genetic defects that cause a gain of function of Ito and a loss of function of ICa among many others.4 Both syndromes are also known to be aggravated by increased vagal tone.12, 13 In this study, we pharmacologically mimicked these genetic and autonomic factors to create ER using the Ito agonist NS5806 (3–10 µM), ICa antagonist verapamil (1 µM) and acetylcholine (3 µM). ACh contributes to the generation of the ERS phenotype by causing both indirect (via accentuated antagonism) and direct use-dependent inhibition of ICa.19 Litovsky and Antzelevitch demonstrated in 1990 the ability of ACh to accentuate thee epicardial AP notch and to cause loss of AP dome.19
Under baseline conditions, lowering temperature to 32°C caused the appearance of prominent J wave in the ECG due to accentuation of the Epi AP notch. In the absence of provocative agents designed to generate an ER pattern in the ECG, no arrhythmic activity was observed in any of the preparations. Following the generation of the ER phenotype, relatively mild hypothermia (32°C to 34°C) caused all-or-none repolarization, leading to loss of the AP dome at some epicardial sites but not others. This resulted in dispersion of AP repolarization both within epicardium and between epicardium and endocardium. Propagation of the AP dome, from sites at which the dome was maintained to sites at which it was lost, caused local re-excitation via a phase 2 re-entry mechanism, leading to the development of closely coupled extrasystoles and VT/VF.
Consistent with these observations is the demonstration by Nishida and co-workers that vagal nerve stimulation accentuates the hypothermia–induced spike and dome AP morphology in canine epicardium.20
Hypothermia is thought to accentuate the AP notch due to the difference in the Q10 for activation of Ito and ICa. The greater slowing of ICa activation leaves Ito less opposed, thus accentuating phase 1 repolarization and the AP notch. Thus an effective strategy to reduce the impact of hypothermia would be to either reduce Ito or augment ICa, thus producing an inward shift in the balance of current during the early phase of the AP. In three experimental series we tested this hypothesis by pretreating the preparations with either quinidine to block Ito or cilostazol and milrinone to augment ICa.
Potential Therapeutic Interventions
In support of our hypothesis, quinidine (5 µM) proved capable of both suppressing and preventing the hypothermia-induced VT/VF in all preparations in which the drug was tested. Cilostazol (10 µM) and milrinone (5 µM) were effective in preventing the hypothermia-induced VT/VF in most but not all preparations. These agents increase ICa secondary to an increase in cAMP. It is noteworthy that cilostazol was reported to be able to prevent VT/VF in a patient with ERS over an extended period of time.21 A beneficial effect of milrinone has as yet not been reported.
Conclusion
The present study demonstrates the effect of hypothermia to accentuate repolarization abnormalities within the left ventricular epicardium and that in the setting of ER, this effect of hypothermia is accentuated leading to the development of phase 2 reentry and VT/VF. We also provide support for the hypothesis that agents capable of producing an inward shift in the balance of current during the early phases of the AP can exert a protective and/or ameliorative effect. Quinidine by virtue of its Ito inhibition and cilostazol and milrinone, by virtue of their effects to augment ICa, are effective in partially reversing the hypothermia-induced repolarization abnormalities, thus restoring electrical homogeneity and abolishing the arrhythmogenic substrate.
Study Limitations
As with all data derived from experimental animal models, extrapolation of the data from in vitro models to the clinic must be done with great care.
Supplementary Material
Acknowledgments
We are grateful José Di Diego, MD for continuous support and personal guidance and to Serge Sicouri, MD for helpful discussions and support. We also gratefully acknowledge the technical assistance of Judy Hefferon, Robert Goodrow, and Rebecca Warren. We would like express our gratitude to Dr. Krisztina Boda for assistance with the statistical analysis.
Funding Sources: This study was supported by grant HL47678 from NHLBI (CA); NYSTEM grant #C026424 (CA); a postdoctoral fellowship grant of the Heart Rhythm Society (The Heart Rhythm Society Fellowship in Cardiac Pacing and Electrophysiology awarded to (IK); and the Masons of New York, Florida, Massachusetts, Connecticut, Maryland, and Rhode Island and Wisconsin.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol. 2000;33:299–309. doi: 10.1054/jelc.2000.18106. [DOI] [PubMed] [Google Scholar]
- 2.Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, De Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 3.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan GX, Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Levy S, Sbragia P. ECG repolarization syndrome abnormalities (J wave syndromes) and idiopathic ventricular fibrillation. J Interv Card Electrophysiol. 2011;32:181–186. doi: 10.1007/s10840-011-9597-6. [DOI] [PubMed] [Google Scholar]
- 7.2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support. Pediatrics. 2006;117:e989–e1004. doi: 10.1542/peds.2006-0219. [DOI] [PubMed] [Google Scholar]
- 8.Castren M, Silfvast T, Rubertsson S, Niskanen M, Valsson F, Wanscher M, Sunde K. Scandinavian clinical practice guidelines for therapeutic hypothermia and post-resuscitation care after cardiac arrest. Acta Anaesthesiol Scand. 2009;53:280–288. doi: 10.1111/j.1399-6576.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 9.Bastiaenen R, Hedley PL, Christiansen M, Behr ER. Therapeutic hypothermia and ventricular fibrillation storm in early repolarization syndrome. Heart Rhythm. 2010;7:832–834. doi: 10.1016/j.hrthm.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Federman NJ, Mechulan A, Klein GJ, Krahn AD. Ventricular fibrillation induced by spontaneous hypothermia in a patient with early repolarization syndrome. J Cardiovasc Electrophysiol. 2013;24:586–588. doi: 10.1111/jce.12030. [DOI] [PubMed] [Google Scholar]
- 11.Fish JM, Welchons DR, Kim YS, Lee SH, Ho WK, Antzelevitch C. Dimethyl lithospermate B, an extract of danshen, suppresses arrhythmogenesis associated with the Brugada syndrome. Circulation. 2006;113:1393–1400. doi: 10.1161/CIRCULATIONAHA.105.601690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross GJ. Early repolarization and ventricular fibrillation: vagally familiar? Heart Rhythm. 2010;7:653–654. doi: 10.1016/j.hrthm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Mizumaki K, Nishida K, Iwamoto J, Nakatani Y, Yamaguchi Y, Sakamoto T, Tsuneda T, Kataoka N, Inoue H. Vagal activity modulates spontaneous augmentation of J-wave elevation in patients with idiopathic ventricular fibrillation. Heart Rhythm. 2012;9:249–255. doi: 10.1016/j.hrthm.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 14.Gourraud JB, Le SS, Sacher F, Chatel S, Derval N, Portero V, Chavernac P, Sandoval JE, Mabo P, Redon R, Schott JJ, Le MH, Haissaguerre M, Probst V. Identification of large families in early repolarization syndrome. J Am Coll Cardiol. 2013;61:164–172. doi: 10.1016/j.jacc.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz BG, Kloner RA, Thomas JL, Bui Q, Mayeda GS, Burstein S, Hale SL, Economides C, French WJ. Therapeutic hypothermia for acute myocardial infarction and cardiac arrest. Am J Cardiol. 2012;110:461–466. doi: 10.1016/j.amjcard.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Rolfast CL, Lust EJ, de Cock CC. Electrocardiographic changes in therapeutic hypothermia. Crit Care. 2012;16:R100. doi: 10.1186/cc11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 18.Calver P, Braungardt T, Kupchik N, Jensen A, Cutler C. The big chill: improving the odds after cardiac arrest. RN. 2005;68:58–62. [PubMed] [Google Scholar]
- 19.Litovsky SH, Antzelevitch C. Differences in the electrophysiological response of canine ventricular subendocardium and subepicardium to acetylcholine and isoproterenol. A direct effect of acetylcholine in ventricular myocardium. Circ Res. 1990;67:615–627. doi: 10.1161/01.res.67.3.615. [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Fujiki A, Mizumaki K, Sakabe M, Sugao M, Tsuneda T, Inoue H. A canine model of Brugada syndrome using regional epicardial cooling of the right ventricular outflow tract. J Cardiovasc Electrophysiol. 2004;15:936–941. doi: 10.1046/j.1540-8167.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 21.Iguchi K, Noda T, Kamakura S, Shimizu W. Beneficial effects of cilostazol in a patient with recurrent ventricular fibrillation associated with early repolarization syndrome. Heart Rhythm. 2013;10:604–606. doi: 10.1016/j.hrthm.2012.11.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.