Summary
Background
Millions of people worldwide are chronically exposed to arsenic through drinking water, including 35–77 million people in Bangladesh. The association between arsenic exposure and mortality rate has not been prospectively investigated by use of individual-level data. We therefore prospectively assessed whether chronic and recent changes in arsenic exposure are associated with all-cause and chronic-disease mortalities in a Bangladeshi population.
Methods
In the prospective cohort Health Effects of Arsenic Longitudinal Study (HEALS), trained physicians unaware of arsenic exposure interviewed in person and clinically assessed 11 746 population-based participants (aged 18–75 years) from Araihazar, Bangladesh. Participants were recruited from October, 2000, to May, 2002, and followed-up biennially. Data for mortality rates were available throughout February, 2009. We used Cox proportional hazards model to estimate hazard ratios (HRs) of mortality, with adjustment for potential confounders, at different doses of arsenic exposure.
Findings
407 deaths were ascertained between October, 2000, and February, 2009. Multivariate adjusted HRs for all-cause mortality in a comparison of arsenic at concentrations of 10·1–50·0 μg/L, 50·1–150·0 μg/L, and 150·1–864·0 μg/L with at least 10·0 μg/L in well water were 1·34 (95% CI 0·99–1·82), 1·09 (0·81–1·47), and 1·68 (1·26–2·23), respectively. Results were similar with daily arsenic dose and total arsenic concentration in urine. Recent change in exposure, measurement of total arsenic concentrations in urine repeated biennially, did not have much effect on the mortality rate.
Interpretation
Chronic arsenic exposure through drinking water was associated with an increase in the mortality rate. Follow-up data from this cohort will be used to assess the long-term effects of arsenic exposure and how they might be affected by changes in exposure. However, solutions and resources are urgently needed to mitigate the resulting health effects of arsenic exposure.
Funding
US National Institutes of Health.
Introduction
Exposure to arsenic through groundwater has been a major public health problem in the USA, Taiwan, Mexico, Mongolia, Argentina, India, Chile, and Bangladesh. WHO described the arsenic crisis in Bangladesh as “the largest mass poisoning of a population in history”.1 An estimated 35–77 million people in Bangladesh have been chronically exposed to increased concentrations of arsenic through drinking water, beginning in the 1970s when about 10 million hand-pumped wells were installed to provide pathogen-free groundwater for the prevention of waterborne diseases.2,3 However, the natural contamination of the groundwater with arsenic in these wells was not realised until the 1990s.
Exposure to arsenic in drinking water has been associated with several cancers; toxic effects on the liver, skin, kidney, cardiovascular system, and lung; and fatal poisoning.4–10 Dose-dependent associations have been shown between arsenic levels in well water and cancers of the bladder, kidney, skin, and lung.6,8,9,10 Dose-response associations between arsenic exposure and peripheral vascular disease have also been reported.11–13
Increased mortality rates from chronic diseases in arsenic-exposed populations have been reported in studies done in the USA, Chile, Argentina, Taiwan, and Bangladesh.4,7,11,14–18 These studies were restricted, however, to group-level data and were retrospective in design. Such limitations—individual-level inferences based on aggregate data and error in exposure measurement—do not resolve doubts about the association between mortality rates and arsenic exposure.
The Health Effects of Arsenic Longitudinal Study (HEALS)19 provides a valuable opportunity for us to investigate the association between arsenic exposure and mortality rates using a prospective design and repeated individual-level assessment of arsenic exposure. In this study, we use data from the HEALS cohort to assess the risk of all-cause and chronic-disease mortalities in relation to chronic arsenic exposure at the individual level through well water and repeated measurements of total arsenic concentrations in urine. We also assess the effect of changes in 2–4-year arsenic exposure on risk of all-cause mortality.
Methods
Study area and population
We designed the HEALS study19 to investigate health outcomes associated with chronic arsenic exposure from groundwater in a sample of adults in Araihazar, Bangladesh. Between October, 2000, and May, 2002, we sampled married individuals (an eligibility criterion to keep loss to follow-up to a minimum) aged 18–75 years, and residing in the study area for at least 5 years. All 5966 wells in the study area were tested for the presence of arsenic in the water. Trained study physicians, unaware of the arsenic concentrations in the well water used by the participants, did in-person interviews and clinical assessments, and collected urine and blood samples from participants in their homes using structured protocols. Active follow-up of the cohort was done from September, 2002, to May, 2004 (follow-up 1), June, 2004, to August, 2006 (follow-up 2), and January, 2007, to February, 2009 (follow-up 3), through in-person visits by use of the same procedures developed for the baseline interview.
Assessment of mortality rate
From 2000 to 2009, vital status was assessed at every follow-up interview visit at home. We used a verbal autopsy questionnaire, validated by the International Centre for Diarrhoeal Disease Research, Bangladesh, in a Bangladeshi population, to investigate and assign the cause of death in our study participants.20 A trained physician—unaware of the arsenic concentration the deceased participant was exposed to—interviewed the informant in person to complete the verbal autopsy questionnaire (including questions about the deceased individual’s history of chronic illnesses and symptoms to ascertain the cause of death). Verbal autopsies were reviewed by a group of expert physicians, and a cause of death was assigned and coded by use of WHO’s tenth revision of the International Classification of Diseases (ICD-10).
Follow-up time was calculated as the number of days between baseline interview and date of death or, if alive, date of the last interview or report of being alive; such participants were censored. Deaths from chronic diseases were defined by exclusion of deaths not known to be related to arsenic exposure (n=82; ICD-10 codes A00-B99, O00-O99, R00-R99, S00-T99, and V01-Y98).
Assessment of arsenic exposure
At baseline, participants identified the well they used as their primary source of drinking water, from which we then assigned an arsenic concentration that they were exposed to. Arsenic concentrations in well water were measured by use of graphite furnace atomic absorption spectrometry, with a detection limit of 5·0 μg/L. Samples below the limit of detection were subsequently reanalysed by use of inductively coupled plasma-mass spectrometry, with a detection limit of 0·1 μg/L.21 Arsenic dose (μg per day) was calculated as
To incorporate information about the duration of arsenic exposure, we calculated a cumulative arsenic index for all known wells as
We did a sensitivity analysis to investigate the effect of using the cumulative arsenic index relative to simple arsenic dose per day.
Total arsenic concentration in urine was measured by use of graphite furnace atomic absorption spectrometry, with a detection limit of 2·0 μg/L,22 and that of creatinine in urine was measured with a colourimetric diagnostics kit (Sigma, St Louis, MO, USA). The total arsenic concentration in urine was then divided by the concentration of creatinine in the urine to obtain a creatinine-adjusted total arsenic concentration in the urine expressed as μg/g creatinine.23
Arsenic cutoff points in well water for the first and second quartiles were adjusted to correspond to WHO’s guideline for arsenic in drinking water (≤10 μg/L) and the national standard for arsenic in drinking water in Bangladesh (≤50 μg/L). Total arsenic concentration in urine and arsenic dose per day were quartiled according to the baseline distribution of the cohort.
Relevant covariates
Covariate data, based on a priori causal knowledge, was derived from the baseline interview. Sociodemographic factors included sex, age (years), and years of education. Smoking status was classified as current, former, and never. The study physician measured the height, weight, and systolic blood pressure at the baseline interview.
Statistical analyses
The Cox proportional hazards model was used to estimate hazard ratios (HRs) and their 95% CIs for evaluating the associations between quartiles of arsenic exposure and all-cause mortality or, in separate models, mortality associated with chronic disease. Insufficient power precluded subset analyses of cause-specific deaths. Separate models were fitted for each arsenic exposure (arsenic concentration in well water, arsenic dose per day, and total arsenic con centration in urine). All models were initially adjusted for age (years) and sex, and further adjusted in multivariate analyses for the potential confounders: body-mass index (BMI; kg/m2), systolic blood pressure (mm Hg), education (years), and smoking status (former or never, current or never). Observations with missing data for one or more confounders were excluded from the analysis. With a Cox proportional hazards model stratified according to quartile of arsenic exposure in well water, and adjusted for age, sex, smoking status, BMI, systolic blood pressure, and education, we plotted cumulative hazard functions for every quartile of arsenic exposure in well water. Since several participants in our study sample drank from the same well (one to 14 participants per well), we accounted for this clustering in our analysis using robust SEs for the proportional hazards model,24 analogous to using generalised estimating equations in other regression models.25
With repeated measurements of total arsenic concentration in urine for the cohort, assessed every 2 years from all participants, we also evaluated mortality rate in relation to recent changes in exposure to arsenic. The median total arsenic concentration in urine at baseline (199 μg/g) was used to dichotomise the baseline, first, and second follow-up exposures. In the model that consisted of exposure patterns at baseline and follow-up 1, mortality rates after follow-up 1 were modelled (n=268). In the model that consisted of exposure patterns at baseline and follow-up 2, mortality rates after follow-up 2 were modelled (n=158). Models were adjusted for all previously mentioned potential confounders.
We calculated the attributable proportion of mortality to arsenic exposure using a regression approach to account for possible confounding.26 With this approach, attributable proportion is computed as
in which pj is the proportion of all cases (deaths) that are within the jth exposure stratum, HRj is the multivariate adjusted HR of the jth stratum compared with the reference stratum (ie, HR0=1).
Statistical analyses were done with SAS (version 9.2), including the procedure PHREG for survival analyses.
Role of the funding source
The study sponsors had no role in the design, data gathering, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Results
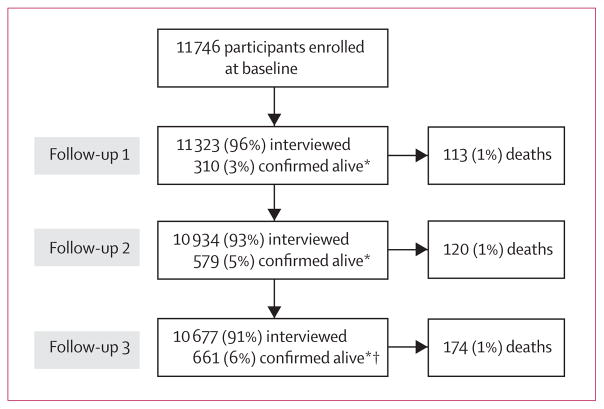
From 12 050 residents who met our eligibility criteria from an enumerated total of 65 876 in the study area, 11 746 (97% response rate) men and women (4801 married couples and 2144 married individuals whose spouses did not participate) were enrolled in the HEALS cohort. The mean follow-up time was 6·5 years (77 155 total person-years). 407 deaths were ascertained between October, 2000, and February, 2009 (figure 1). Date of death was ascertained by relatives (n=403) or neighbours (n=3) of deceased participants. We were unable to ascertain the relationship status of one informant. In three cases, we could not ascertain the causes of death. Causes of death were related to some infectious and parasitic diseases (ICD-10 code A00-B99; n=29); neoplasms (C00-D48; n=66); endocrine, nutritional, and metabolic diseases (E00-E90; n=4); diseases of the nervous system (G00-G99; n=4), circulatory system (I00-I99; n=176), respiratory system (J00-J99; n=35), digestive system (K00-K93; n=27), musculoskeletal system and connective tissue (M00-M99; n=1), and genitourinary system (N00-N99; n=9); pregnancy, childbirth, and puerperium (O00-O99; n=10); symptoms, signs, and abnormal clinical and laboratory findings, not otherwise classified (R00-R99; n=30); injury, poisoning, and some other consequences of external causes (S00-T99; n=2); and external causes of morbidity and mortality (V01-Y98; n=11).
Figure 1. Study profile.
*Not available at the time of interview, but were confirmed to be alive by a close relative or neighbour. †One participant could not be confirmed as being alive and was censored on the date of the interview for follow-up 2.
Table 1 shows the distribution of demographic, clinical, and exposure characteristics of the baseline cohort and deceased individuals. Participants who died were more likely to be male, have no formal education, be a former or current smoker, be 50 years or older, have a low BMI (<18·5 kg/m2), and have high systolic blood pressure (≥140 mm Hg). Pearson correlation coefficients for the arsenic exposures were 0·67–0·97, with the strongest correlation between arsenic concentration in well water and arsenic dose per day.
Table 1.
Selected characteristics of participants in relation to vital status
| Arsenic (μg/L) in well water | Baseline cohort (n=11746) | Deaths (n=407) | Crude death rate (per 1000 person-years) | |
|---|---|---|---|---|
|
Sex
| ||||
| Male | 102·2 (115·7) | 5042 (43%) | 298 (73%) | 9·0 |
| Female | 101·2 (114·9) | 6704 (57%) | 109 (27%) | 2·5 |
|
| ||||
|
Age (years)
| ||||
| 18–30 | 101·3 (116·4) | 3653 (31%) | 27 (7%) | 1·1 |
| 31–40 | 100·7 (112·7) | 4186 (36%) | 71 (17%) | 2·6 |
| 41–50 | 102·9 (116·0) | 2730 (23%) | 127 (31%) | 7·1 |
| 51–60 | 102·5 (119·8) | 1072 (9%) | 150 (37%) | 22·3 |
| 61–75 | 107·6 (110·7) | 104 (0·9%) | 32 (8%) | 54·9 |
|
| ||||
|
Body-mass index (kg/m2)
| ||||
| <18·5 | 107·1 (119·6) | 4555 (39%) | 217 (53%) | 7·3 |
| 18·5–24·9 | 99·9 (114·1) | 6107 (52%) | 156 (38%) | 3·9 |
| ≥25·0 | 85·2 (101·8) | 805 (7%) | 22 (5%) | 4·1 |
|
| ||||
|
Education (years)
| ||||
| 0 | 101·2 (112·2) | 5237 (45%) | 215 (53%) | 6·3 |
| 1–5 | 105·2 (119·8) | 3470 (30%) | 103 (25%) | 4·5 |
| 6–16 | 98·2 (115·1) | 3033 (26%) | 89 (22%) | 4·4 |
|
| ||||
|
Systolic blood pressure (mm Hg)
| ||||
| <140 | 101·9 (115·9) | 10 542 (90%) | 298 (73%) | 4·3 |
| ≥140 | 98·3 (110·4) | 945 (8%) | 98 (24%) | 16·2 |
|
| ||||
|
Cigarette smoking status
| ||||
| Never smoked | 101·9 (116·0) | 7568 (64%) | 117 (29%) | 2·3 |
| Ex-smoker | 110·0 (123·1) | 778 (7%) | 82 (20%) | 16·6 |
| Smoker | 99·1 (111·7) | 3395 (29%) | 207 (51%) | 9·3 |
|
| ||||
|
Arsenic (μg/L) in well water
| ||||
| 0·1–10·0 | 3·2 (2·9) | 2743 (23%) | 77 (19%) | 4·2 |
| 10·1–50·0 | 28·5 (11·4) | 2511 (21%) | 94 (23%) | 5·8 |
| 50·1–150·0 | 94·5 (29·4) | 3600 (31%) | 101 (25%) | 4·3 |
| 150·1–864·0 | 267·5 (106·7) | 2889 (25%) | 135 (33%) | 7·1 |
|
| ||||
|
Arsenic dose (μg per day)
| ||||
| 0·041–35·0 | 6·4 (22·4) | 2922 (25%) | 92 (23%) | 4·7 |
| 35·1–163·0 | 44·3 (39·7) | 2937 (25%) | 101 (25%) | 5·3 |
| 163·1–401·0 | 113·0 (70·9) | 2941 (25%) | 94 (23%) | 4·9 |
| 401·1–4898·0 | 242·2 (117·4) | 2940 (25%) | 120 (29%) | 6·2 |
|
| ||||
|
Total arsenic in urine (μg/g)
| ||||
| 7·0–105·0 | 30·8 (77·0) | 2793 (24%) | 83 (20%) | 4·4 |
| 105·1–199·0 | 65·7 (86·0) | 2829 (24%) | 99 (24%) | 5·4 |
| 199·1–352·0 | 108·3 (95·4) | 2805 (24%) | 102 (25%) | 5·6 |
| 352·1–5000·0 | 198·5 (124·5) | 2797 (24%) | 106 (26%) | 5·8 |
Data are mean (SD) or number (%), unless otherwise indicated.
Spot urine samples were provided by 11 224 (96%) of 11 746 participants interviewed at baseline, 11 109 (98%) of 11 323 interviewed at follow-up 1, and 10 726 (98%) of 10 934 interviewed at follow-up 2. Arsenic exposure (baseline concentration of arsenic in well water, arsenic dose per day, and total arsenic concentration in urine) was associated with all-cause mortality (table 2). Sexadjusted and age-adjusted estimates did not differ much from multivariate-adjusted estimates (data not shown). The mortality rate increased at all concentrations of arsenic in well water, indicating an increasing risk rather than a threshold effect (figure 2). Similar results were noted with arsenic dose per day and total arsenic concentration in urine (table 2). With the data for ordinal exposure in the multivariate models, a one-quartile increase in arsenic concentration in well water was associated with a 15% increase in all-cause mortality (95% CI 1·05–1·26), with corresponding increases of 14% (1·04–1·25) for arsenic dose per day and 13% (1·03–1·24) for total arsenic concentration in urine. Similar results were noted for the associations between arsenic exposure and mortality associated with chronic disease (table 2). After adjustment for potential confounding, we estimated the summary attributable proportion based on the arsenic concentration in well water for all-cause and chronic-disease mortalities to be 21% and 24%, respectively.
Table 2.
Hazard ratio (HR) for mortality in participants in relation to baseline arsenic exposure
| All-cause mortality*
|
Chronic-disease mortality*
|
|||
|---|---|---|---|---|
| Deaths | HR (95% CI) | Deaths | HR (95% CI) | |
|
Arsenic (μg/L) in well water
| ||||
| 0·1–10·0 | 74 | 1·00 | 58 | 1·00 |
| 10·1–50·0 | 90 | 1·34 (0·99–1·82) | 69 | 1·33 (0·94–1·87) |
| 50·1–150·0 | 98 | 1·09 (0·81–1·47) | 83 | 1·22 (0·87–1·70) |
| 150·1–864·0 | 131 | 1·68 (1·26–2·23) | 101 | 1·68 (1·21–2·33) |
|
| ||||
|
Arsenic dose (μg per day)
| ||||
| 0·041–35·0 | 87 | 1·00 | 66 | 1·00 |
| 35·1–163·0 | 97 | 1·10 (0·83–1·47) | 80 | 1·21 (0·88–1·67) |
| 163·1–401·0 | 91 | 1·09 (0·81–1·46) | 76 | 1·22 (0·88–1·71) |
| 401·1–4898·0 | 118 | 1·54 (1·17–2·04) | 89 | 1·58 (1·15–2·18) |
|
| ||||
|
Total arsenic in urine (μg/g)
| ||||
| 7·0–105·0 | 83 | 1·00 | 64 | 1·00 |
| 105·1–199·0 | 96 | 1·07 (0·80–1·43) | 80 | 1·17 (0·84–1·62) |
| 199·1–352·0 | 100 | 1·22 (0·91–1·63) | 83 | 1·37 (0·98–1·90) |
| 352·1–5000·0 | 105 | 1·45 (1·09–1·94) | 77 | 1·47 (1·05–2·06) |
Data are number or HR (95% CI).
Multivariate estimates were adjusted for age, sex, body-mass index, systolic blood pressure, education, and smoking status.
Figure 2. Cumulative hazard function estimate of mortality plotted against time for arsenic in drinking water exposure category.
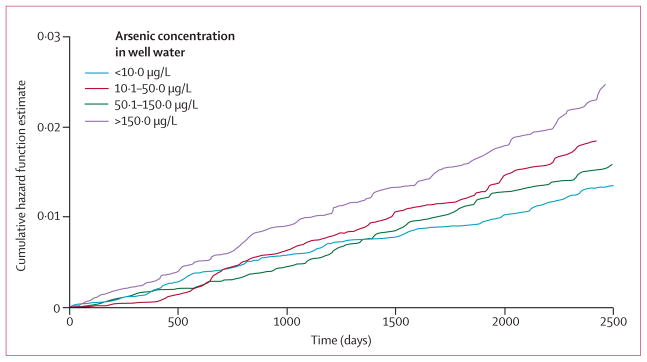
Data were adjusted for age, sex, smoking status, body-mass index, systolic blood pressure, and education.
Inclusion of cumulative arsenic index in mortality models and sensitivity analyses did not show additional predictive power beyond that shown with other arsenic exposure measures (which did not include duration of well use; data not shown). Arsenic dose per day was highly correlated with cumulative arsenic index (data not shown).
We assessed 2-year and 4-year changes in arsenic exposure (measured as repeated total arsenic concentrations in urine) after enrolment of the baseline cohort (table 3). The multivariate-adjusted HR for comparison of high baseline exposure to low baseline exposure was 1·46 (95% CI 1·14–1·86) for deaths occurring after follow-up 1. Compared with individuals with low exposure at baseline and follow-up 1, those with high exposure at baseline and low exposure at follow-up 1 or high exposure at baseline and follow-up 1 had similar increased risks of mortality (table 3). The multivariate-adjusted HR for comparison of high exposure at baseline with low exposure at baseline was 1·34 (0·98–1·84) for deaths occurring after follow-up 2. Further stratification of exposure status at baseline by exposure levels at follow-up 2 also did not seem to have a significantly differential effect on mortality risk (table 3).
Table 3.
Hazard ratio (HR) of all-cause mortality in participants in relation to change in total arsenic concentration in urine
| Follow-up exposure | Events | Patients at risk | All-cause mortality* | |
|---|---|---|---|---|
|
Baseline and follow-up 1
| ||||
| Low | Low | 103 | 4453 | 1·00 |
| Low | High | 13 | 765 | 0·88 (0·49–1·57†) |
| High | Low | 70 | 1937 | 1·56 (1·14–2·13) |
| High | High | 82 | 3373 | 1·33 (0·99–1·80‡) |
|
| ||||
|
Baseline and follow-up 2
| ||||
| Low | Low | 61 | 4226 | 1·00 |
| Low | High | 12 | 833 | 1·37 (0·75–2·50§) |
| High | Low | 47 | 2072 | 1·67 (1·14–2·44) |
| High | High | 38 | 3064 | 1·17 (0·77–1·77¶) |
Data are number or HR (95% CI).
Multivariate estimates were adjusted for age, sex, body mass-index, systolic blood pressure, education, and smoking status.
p=0·67 versus low-low category.
p=0·34 versus high-low category.
p=0·30 versus low-low category.
p=0·11 versus high-low category.
Discussion
The risk of all-cause mortality and chronic-disease mortality increased with increasing arsenic exposure. Moreover, the data indicate that there is a trend in risk within the arsenic range to which this population was exposed. With repeated measurements of total arsenic concentrations in urine from all cohort members, long-term exposure to arsenic (captured by use of the baseline ascertainment of exposure) was a more important predictor of mortality than were subsequent short-term changes of exposure (derived from the 2-year and 4-year follow-up assessments of total arsenic concentrations in urine). Based on the risk estimates, an estimated 21·4% of all deaths and 23·5% of deaths associated with chronic disease in this population could be attributed to arsenic exposure (>10 μg/L) in drinking water.
Whereas in previous studies, associations were shown between arsenic exposure and cause-specific mortality,4,5,7,9,11,16,18,27,28 in this population-based study we prospectively investigated the association between arsenic exposure in drinking water and all-cause mortality in a Bangladeshi population. Ecological measurements of arsenic exposure and other potential confounders were used in the analyses of other studies, making them more susceptible to measurement error in exposure assessment and ecological bias. For example, Wu and colleagues9 showed significant dose-response patterns in mortality rates associated with cancers of the lung, skin, bladder, and kidney at the village level, but stated that their findings might not apply at the individual level. One of the main strengths of this analysis is that associations between arsenic exposure and mortality were measured at the individual level, reducing to a minimum the consequences of confounding and exposure measurement error, and strengthening causal inference at the individual level. Furthermore, previous studies were largely done in populations exposed to high concentrations of arsenic. A wide range of concentrations of arsenic exposure was present in our study area (arsenic concentrations in well water were 0·1–864·0 μg/L); therefore, we had the opportunity to evaluate arsenic-associated mortality rate at the low exposure range for which there was evidence of increased mortality risk.
With repeated measurements of total arsenic concentration in urine with time, we noted that once chronically exposed, decreasing exposure for a short amount of time did not reduce an individual’s risk of mortality. However, we will continue to assess the modification of risk as the cohort is followed up for longer than in this study. In other studies, mortality rate attributed to cancers and heart disease did not begin to decline until about two decades after prevention of exposure to high concentrations of arsenic in well water.28,29 Therefore, evidence from these studies and our data suggest that other health strategies for prevention and promotion with remediation for arsenic-exposed populations are important and should be considered.
To assess potential confounding by prevalent medical disorders, deaths that occurred in the first 2 years after enrolment into the cohort were excluded from analyses. Associations between arsenic concentrations in well water and mortality did not change much (after exclusions), suggesting that medical disorders prevalent at baseline presented minimum confounding effects. A possible limitation of this analysis was that comorbid disorders were not included in the model of total arsenic concentration in urine since some of them could affect total arsenic concentrations in urine and mortality rate; however, since the effect estimates noted with total arsenic concentration in urine were similar to the results noted with arsenic concentrations in well water, we do not judge this limitation to be a major source of bias in this analysis. Additionally, the association between arsenic exposure and mortality associated with chronic disease (excluding deaths unlikely to be related to arsenic exposure) was similar to results of the associations noted for all-cause mortality. Moreover, analyses of mortality associated with chronic disease produced more apparent trends in the low-to-moderate arsenic exposure ranges compared with all-cause mortality.
We used several measures of arsenic exposure in this analysis derived at the individual level from water-based and urine-based ascertainments of exposure. The associations noted with each of the assessments of exposure were similar, suggesting that measurement error or misclassification of chronic arsenic exposure was kept to a minimum in this study. The slightly attenuated effect estimates for the associations between daily arsenic dose and mortality rate compared with arsenic in well water might be explained by measurement error in the assessment of self-reported daily water consumption.
The major strengths of this study were the prospective design, large size of the study cohort, wide range of arsenic exposures, several measurements of baseline arsenic exposure, the repeated prospective assessment of total arsenic concentration in urine, and nearly complete follow-up for vital status. The results of this study have important public health implications for arsenic in drinking water. Roughly 24% of the people in the cohort had arsenic concentrations in well water less than 10 μg/L, and 45% had less than 50 μg/L, which makes the exposure levels similar to other populations that have low-level arsenic exposure.
This study also had limitations. Whereas total arsenic concentration in urine for the cohort measured by use of graphite furnace atomic absorption is the most cost-efficient and feasible method for measurement of arsenic concentrations in a large-scale cohort, it does not allow for the specific estimation of the fractions of arsenobetaine or arsenocholine. Detailed speciation studies of random subsets from our study cohort have shown a very small percentage (3%) of total arsenic concentration in urine to be arsenobetaine and arsenocholine.30 Therefore, we do not believe that our results would differ much with the exclusion of this fraction.
We noted significant associations between arsenic exposure through drinking water and mortality rate, emphasising the public health challenge for millions of Bangladeshis due to this environmental exposure. Although initiatives to reduce exposure to arsenic in drinking water are in progress, investigation into solutions to mitigate the resulting health effects of this catastrophe deserve urgent attention and resources. Future research with prospectively gathered data for changes at the individual level in arsenic exposure will strengthen our understanding of the effect that changes in exposure have on long-term mortality risk.
Acknowledgments
The research is supported by National Institute of Health, Bethesda, MD, USA (grants P42ES010349, R01CA102484, R01CA107431, P30ES009089, and P30CA014599).
Footnotes
Contributors
MA, TK, PJR, BP, and HA participated in the data analysis, interpretation of results, and writing of the report with input and editing from all authors. YC, FP, TI, AA, MR-Z, RH, GS, and VS participated in the study implementation. AVG, JG, and HA contributed to the conception of the study.
Conflicts of interests
We declare that we have no conflicts of interest.
References
- 1.WHO. Water sanitation and health. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Ahsan H, Perrin M, Rahman A, et al. Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med. 2000;42:1195–201. doi: 10.1097/00043764-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Smith AH, Lingas EO, Rahman M. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ. 2000;78:1093–103. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang CY, Chang CC, Ho SC, Chiu HF. Is colon cancer mortality related to arsenic exposure? J Toxicol Environ Health A. 2008;71:533–38. doi: 10.1080/15287390801907509. [DOI] [PubMed] [Google Scholar]
- 5.Chen CJ, Chuang YC, You SL, Lin TM, Wu HY. A retrospective study on malignant neoplasms of bladder, lung and liver in blackfoot disease endemic area in Taiwan. Br J Cancer. 1986;53:399–405. doi: 10.1038/bjc.1986.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11:673–79. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol. 1998;27:561–69. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda T, Babazono A, Yamamoto E, et al. Ingested arsenic and internal cancer: a historical cohort study followed for 33 years. Am J Epidemiol. 1995;141:198–209. doi: 10.1093/oxfordjournals.aje.a117421. [DOI] [PubMed] [Google Scholar]
- 9.Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–32. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 10.Morales KH, Ryan L, Kuo TL, Wu MM, Chen CJ. Risk of internal cancers from arsenic in drinking water. Environ Health Perspect. 2000;108:655–61. doi: 10.1289/ehp.00108655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, Calderon RL. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107:359–65. doi: 10.1289/ehp.99107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng CH. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology. 2002;53:529–37. doi: 10.1177/000331970205300505. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH, Chong CK, Chen CJ, Tai TY. Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis. 1996;120:125–33. doi: 10.1016/0021-9150(95)05693-9. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Y, Marshall G, Ferreccio C, et al. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166:1381–91. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- 15.Yang CY, Chiu HF, Wu TN, Chuang HY, Ho SC. Reduction in kidney cancer mortality following installation of a tap water supply system in an arsenic-endemic area of Taiwan. Arch Environ Health. 2004;59:484–88. doi: 10.1080/00039890409603430. [DOI] [PubMed] [Google Scholar]
- 16.Taeger D, Pesch B. Arsenic in drinking water and bladder cancer mortality in the United States: an analysis based on 133 U.S. counties and 30 years of observation. J Occup Environ Med. 2004;46:1007–08. doi: 10.1097/01.jom.0000141655.00168.0d. [DOI] [PubMed] [Google Scholar]
- 17.Harbut MR, Kamel NS. Arsenic body burden and morbidity and mortality. Arch Environ Health. 2000;55:285–86. [PubMed] [Google Scholar]
- 18.Sohel N, Persson LA, Rahman M, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20:824–30. doi: 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- 19.Ahsan H, Chen Y, Parvez F, et al. Health Effects of Arsenic Longitudinal Study (HEALS): description of a multidisciplinary epidemiologic investigation. J Expo Sci Environ Epidemiol. 2006;16:191–205. doi: 10.1038/sj.jea.7500449. [DOI] [PubMed] [Google Scholar]
- 20.Ronsmans C, Vanneste AM, Chakraborty J, Van Ginneken J. A comparison of three verbal autopsy methods to ascertain levels and causes of maternal deaths in Matlab, Bangladesh. Int J Epidemiol. 1998;27:660–66. doi: 10.1093/ije/27.4.660. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z, Zheng Y, Mortlock R, Van Geen A. Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2004;379:512–18. doi: 10.1007/s00216-004-2618-x. [DOI] [PubMed] [Google Scholar]
- 22.Nixon DE, Mussmann GV, Eckdahl SJ, Moyer TP. Total arsenic in urine: palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin Chem. 1991;37:1575–79. [PubMed] [Google Scholar]
- 23.Nermell B, Lindberg AL, Rahman M, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106:212–18. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–78. [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 26.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122:904–14. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 27.Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health. 1999;54:186–93. doi: 10.1080/00039899909602258. [DOI] [PubMed] [Google Scholar]
- 28.Yang CY, Chiu HF, Chang CC, Ho SC, Wu TN. Bladder cancer mortality reduction after installation of a tap-water supply system in an arsenious-endemic area in southwestern Taiwan. Environ Res. 2005;98:127–32. doi: 10.1016/j.envres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Chang CC, Ho SC, Tsai SS, Yang CY. Ischemic heart disease mortality reduction in an arseniasis-endemic area in southwestern Taiwan after a switch in the tap-water supply system. J Toxicol Environ Health A. 2004;67:1353–61. doi: 10.1080/15287390490471451. [DOI] [PubMed] [Google Scholar]
- 30.Ahsan H, Chen Y, Kibriya MG, et al. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Biomarkers Prev. 2007;16:1270–78. doi: 10.1158/1055-9965.EPI-06-0676. [DOI] [PubMed] [Google Scholar]