Abstract
Background aims
Adipose-derived mesenchymal stromal cells (ASCs) are promising tools for delivery of cytotherapy against cancer. However, ASCs can exert profound effects on biological behavior of tumor cells. Our study aimed to examine the influence of ASCs on gene expression and epigenetic methylation profiles of prostate cancer cells as well as the impact of expressing a therapeutic gene on modifying the interaction between ASCs and prostate cancer cells.
Methods
ASCs were modified by lentiviral transduction to express either green fluorescent protein as a control or pigment epithelium–derived factor (PEDF) as a therapeutic molecule. PC3 prostate cancer cells were cultured in the presence of ASC culture–conditioned media (CCM), and effects on PC3 or DU145. Ras cells were examined by means of real-time quantitative polymerase chain reaction, EpiTect methyl prostate cancer–focused real-time quantitative polymerase chain reaction arrays, and luciferase reporter assays.
Results
ASCs transduced with lentiviral vectors were able to mediate expression of several tumor-inhibitory genes, some of which correlated with epigenetic methylation changes on cocultured PC3 prostate cancer cells. When PC3 cells were cultured with ASC-PEDF CCM, we observed a shift in the balance of gene expression toward tumor inhibition, which suggests that PEDF reduces the potential tumor-promoting activity of unmodified ASCs.
Conclusions
These results suggest that ASC-PEDF CCM can promote reprogramming of tumor cells in a paracrine manner. An improved understanding of genetic and epigenetic events in prostate cancer growth in response to PEDF paracrine therapy would enable a more effective use of ASC-PEDF, with the goal of achieving safer yet more potent anti-tumor effects.
Keywords: adipose-derived mesenchymal stromal cells, epigenetics, gene expression, pigment epithelium—derived factor, prostate cancer
Introduction
Adipose-derived mesenchymal stromal cells (ASCs) are promising tools for delivery of cancer therapy because they can be obtained with relative ease and are a comparable reservoir of mesenchymal progenitor cells to bone marrow mesenchymal stromal cells (BM-MSCs) (1,2). ASCs are well suited as cellular gene therapy vectors because they can be expanded rapidly, allowing use at low passage numbers, and can be transduced by several viral and nonviral means. ASCs and BM-MSCs share many biological characteristics, including differentiation potential and surface marker expression (1). Interestingly, some evidence has emerged of BM-MSCs ability to exert anti-tumor potential (3–7), a property now also demonstrated for ASC-based therapies. Studies that used ASCs for reducing tumor growth have included the use of wild-type ASCs to inhibit hepatocellular carcinoma, breast and pancreatic tumor growth (8–10) as well as studies examining the potential of ASCs to deliver cytotoxic or secreted therapeutics to tumors. ASCs have been used to deliver tumor necrosis factor ligand member 10 (TRAIL), cytosine deaminase (CD), pigment epithelium–derived factor (PEDF) and melanoma differentiation-associated gene 7 (MDA-7), inhibiting growth of several tumor types (11–17).
The use of stem cells to deliver therapeutics remains controversial because the mechanisms underlying the anti-tumor effects are not clear, and, in some cases, stem cells can act to promote tumor growth if used at high concentrations. Therefore, in the present study, our goal was to examine the potential of ASCs to exert either a tumor-promoting or tumor-inhibitory effect in a prostate tumor model system. We examined whether ASCs or ASCs expressing the therapeutic molecule PEDF can modify gene expression and epigenetic methylation profiles of PC3 prostate cancer cells in a coculture system. We selected PEDF because it is downregulated as prostate cancers progress (18), and its loss reduces the ability of prostate cancer cells to respond to inhibitory growth signals. Also, we have shown previously that PEDF is an effective therapeutic against prostate tumors in vivo (17). Our results suggest that PEDF modifies the interaction of ASCs with prostate tumor cells, ameliorating and enhancing the intrinsic anti-tumor potential of ASCs, thus comprising a promising therapeutic modality.
Methods
Cell culture, viral transductions and transfections
Human prostate cancer cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) (PC3) or generated by us by means of Ras transduction (DU145. Ras) as described (19). Human ASCs of low passage numbers (P1–2) were used for viral transduction (17) and then were expanded in culture as described (1) after being isolated from from lipoaspirate tissue donated by three donor patients undergoing elective procedures (with informed consent under a protocol reviewed and approved by the Institutional Review Board, Pennington Biomedical Research Center). Briefly, after ASCs were collected, they were washed three to four times in 1 × Dulbecco’s phosphate buffered saline and suspended in 1 × phosphate-buffered saline/1% bovine serum/0.1% collagenase type I (20), with shaking at 37°C for 60 min, spun for 5 min at 300g (room temperature) and the pelleted stromal vascular fraction resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/F12 Ham’s/10% fetal bovine serum (FBS)/1% pennicilin/streptomycin, stromal vascular fraction will be plated to 80—90% confluence (∼6 d/∼30,000 cells/cm2) to yield the initial passage (P0) and seeded at 30,000 cells/cm2 and cultured to ∼80—90% confluence (∼6 d) for P1—2 to ∼5 × 105—106. Early-passage (P1—2) ASCs are typically >90%+ CD90/CD105 and <5%+ CD45/CD31 (20) and maintain clonogenic and differentiation capacity (21). Lentiviruses for expressing green fluorescent protein (GFP) or GFP plus human PEDF complementary DNAs were cloned (17) and produced as described (22). We and others have previously shown that lentivirus gives stable transduction through several passages (17,23); furthermore, transduction efficiency as examined by fluorescence-activated cell sorting for GFP expression was ∼60—70%+GFP (unsorted), as is typical for our transduction protocol (24). We used ASC of passage 1—2 (P1—2) to transduce with lentivirus and then used P2—3 after lentiviral transduction for in vitro studies. Infection of ASCs was performed in 8 µg/mL of polybrene at lentiviral multiplicity of infection (MOI) of 1. For preparing culture-conditioned media from ASCs (CCM), we plated 5 × 105 ASCs in a six-well format in 2.5 mL/well 10% FBS/DMEM:F12 media (Gibco/Life Technologies, Grand Island, NY, USA). The CCM allowed us to examine the paracrine effects of ASCs (control versus PEDF) on prostate cancer cells. The next day, we changed the media to 2% FBS/DMEM:F12 and collected the resulting ASC CCM 48 h later. This CCM was used to treat PC3 or DU145-Ras cells for 48 h for examining the effect of ASC-CM (native, GFP-transduced or GFP-PEDF—transduced) on gene expression or signaling changes compared with PC3 or DU145-Ras cells treated with control 2% FBS/DMEM:F12 media (no ASCs).
Clonogenic assay and cell differentiation
For colony formation assay, 250 cells were seeded in a 12-well format and allowed to form colonies over a period of 14d, after which wells were fixed in 10% buffered formalin and stained with the use of 0.5% crystal violet in water. Colonies were counted by means of an NIH ImageJ program. For the differentiation assays, 1.5 × 105 cells were seeded in a 12-well format in triplicate and cultured with differentiation supplements according to the manufacturer’s instructions, with the use of Mesenchymal Stem Cell Osteogenesis or Mesenchymal Adipo-genesis Kits (Millipore, Billerica, MA, USA). On day 21 after seeding and culturing in differentiation supplements, cells were stained for either alizarin S red or oil red O stains (Millipore). For soft agar assay, we used a colorimetric Cytoselect cell transformation assay (CellBiolabs Inc, San Diego, CA, USA) in a 96-well format.
Real-time quantitative polymerase chain reaction and EpiTect methyl quantitative real-time polymerase chain reaction array analyses
Total RNA from 5—10 × 105 cells was extracted with the use of a SurePrep kit (Fisher Scientific). Realtime polymerase chain reaction (RT-PCR) was performed by means of RT2 SYBR Green ROX qPCR Mastermix (Qiagen, Valencia, CA, USA) with the use of the Realplex 2S cycler (Eppendorf). The PAHS-135Z human prostate cancer—focused quantitative PCR (qPCR) array was used (Qiagen) with 4 µg RNA, according to the manufacturer’s protocols. Reactions were carried out by means of 1 × 10 min at 95°C and 40 × 15 seconds at 95°C, 1 min at 60°C. Results for each assay were normalized to the average of all five housekeeping genes. Gene expression changes are reported if a 2.0-fold threshold and a t test value of P < 0.05 were achieved. For EpiTect methyl qPCR, 1 µg of genomic DNA from PC3 cells was digested according to the manufacturer’s instructions. RT-PCR was performed as above, with the use of cycle threshold values to calculate the proportion of hypermethylated (HM), unmethylated (UM) and intermediately methylated (IM) DNA, through the use of the Human Prostate Cancer DNA Methylation PCR Array Signature Panel (EAHS-051Z, Qiagen).
Luciferase assays
Identification of signaling pathway(s) affected by ASC-expressing PEDF and/or rPEDF administration through incubation of prostate cancer cell lines with CCM was performed with the use of Lipofect-amine 2000 (Invitrogen, Carlsbad, CA, USA) transfections in a 96-well format with several plasmid DNAs containing reporter genes downstream of transcription factor—binding elements to help identify the key signaling pathways modulated by treatments. Pathways examined included luciferase-responsive TGF-β (FBEISBE), STAT3, NF-kB (NFkB) and Elk1 signaling reporters. Plasmids were obtained from Addgene (Cambridge, MA, USA), Clontech or Stratagene (La Jolla, CA, USA). For experiments comparing ASC-PEDF CCM with rPEDF, 288 pg/mL of rPEDF was used in 2% DMEM:F12 media to mimic average PEDF amounts secreted by ASCs into the CCM, as detected by enzyme-linked immunoassay (Figure 1A).
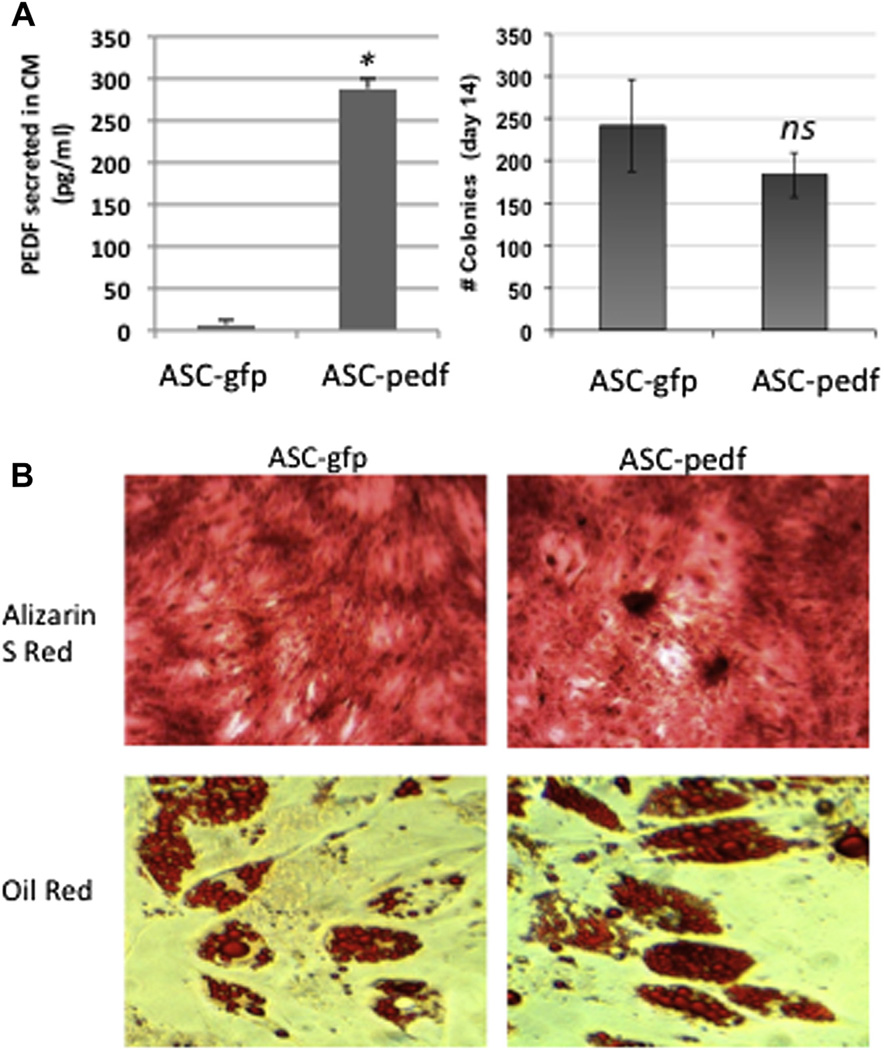
Figure 1.
Biological characteristics of ASCs modified with lentiviruses. (A) ASCs modified with either control lentivirus (CMV-ires.GFP; ASC-GFP) or CMV-PEDF.ires.GFP lentivirus (ASC-PEDF) at MOI = 1 still form equivalent numbers of clones in a clonogenic assay (P = 0.1). (B) Differentiation capacity does not appear to be impaired by transduction with PEDF as assessed by ability to differentiate into osteogenic cell lineage (alizarin S red stain) or adipogenic cell lineage (oil red O).
Statistical analysis
Assays were performed in triplicate, and values are provided as mean ± standard error of the mean or 95% confidence intervals. Comparisons were performed by means of an unpaired t test, and a value of P < 0.05 was considered to indicate a significant difference.
Results
ASC modification with lentiviral vectors
We assessed PEDF expression in the CCM of ASCs in vitro after transduction with a pigment-derived epithelial growth factor/GFP therapeutic vector (CMV-PEDF.ires.GFP) as compared with control expressing GFP (CMV-ires.GFP) (P < 0.001, Figure 1A). We determined that modification by lentiviral (Mountain View, CA, USA) transduction (MOI =1) did not appear to alter biological characteristics of ASCs examined because both ASC-GFP and ASC-PEDF still were able to form colonies in vitro by day 14 of culture (Figure 1A) without any significant difference in colony numbers between ASC types (P > 0.1). Additionally, ASCs modified with GFP or PEDF still were able to differentiate into osteogeneic cell lineage as determined by alizarin S red stain (day 21 of culture) as well as the adipogenic lineage, as determined by oil red O stain (day 14 of culture) (Figure 1B).
Several genes are uniquely modulated in PC3 cells by paracrine ASC mechanisms
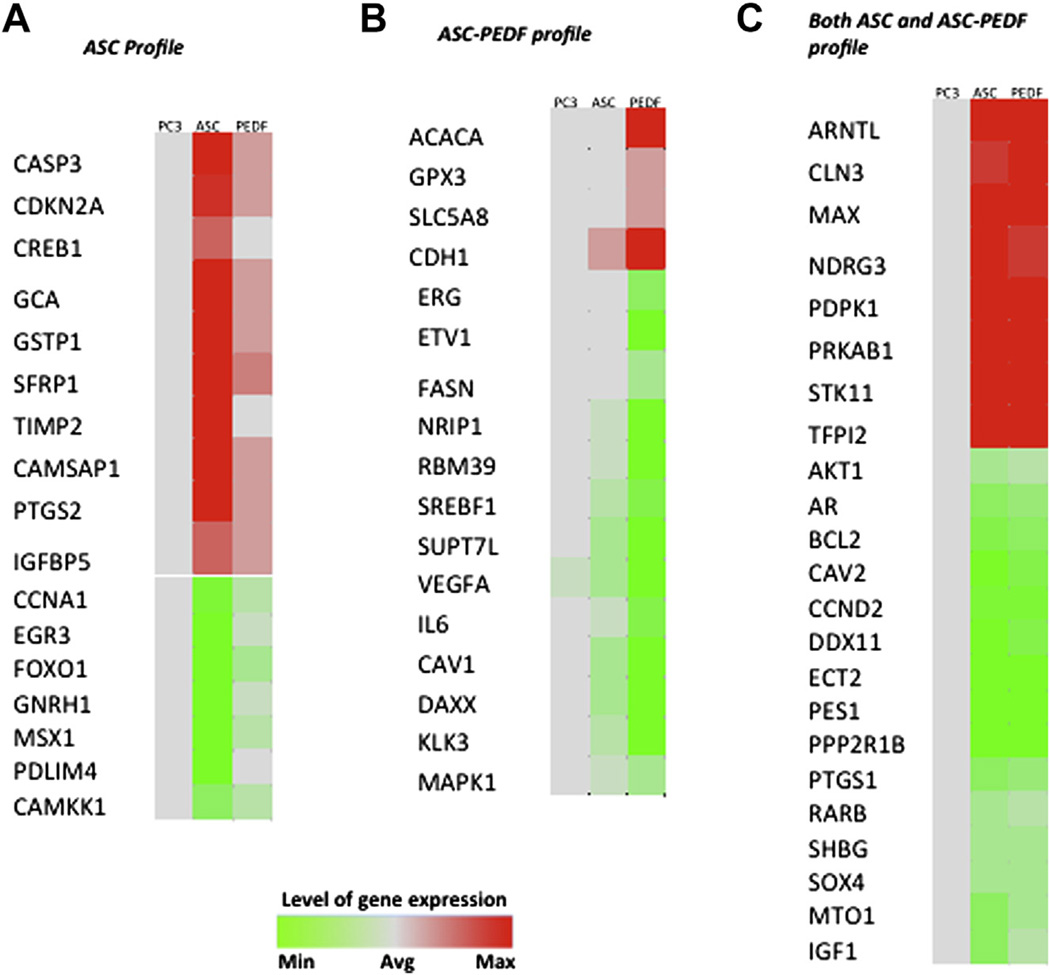
We observed several genes with modified expression in PC3 prostate cancer cells when cultured in the presence of CCM from ASC-GFP (ASCs) or ASC-PEDF (paracrine induction). First, we detailed the gene expression changes reflecting the effect of ASC CCM on PC3 prostate cancer cells. We used a prostate cancer—focused qPCR assay, which enabled us to query expression changes in 84 genes involved in prostate cancer. The gene expression profiles indicated that ASC coculture induced more prominent upregulation of 10 genes in PC3 cells as compared with the effect of ASC-PEDF (Figure 2A; P < 0.01). These genes included two apoptosis-promoting genes, CASP3 and cell cycle regulator CDKN2A (pl6INK4a), three genes downregulated in prostate cancer (GCA, GSTP1, and SFRP1) and one gene encoding a tissue inhibitor of matrix metal-oproteases (TIMP2). These changes would be consistent with a potential reduction in PC3 malignancy; however, ASCs also induced four expression changes that could promote malignant potential such as upregulation of a metastatic gene (CREB1), upregulation of CAMSAP1, a fatty acid metabolism gene typically upregulated in prostate cancer, PTGS2 (COX2), an inflammatory gene associated with prostate cancer risk, and IGFBP5, a gene upregulated in prostate cancer. Genes downregulated in PC3 cells on coculture with ASCs included a gene implicated in prostate cancer invasion (CCNA1), one gene encoding a transcription factor typically overexpressed in prostate cancer (EGR3), a gene overexpressed in tumors including prostate cancer (GNRH1) and a fatty acid metabolism gene (CAMKK1) (Figure 2B). Although these changes were consistent with a potential reduction in PC3 malignancy, other changes that could potentially enhance cell proliferation also were observed during incubation with ASC CCM, including down-regulation of tumor suppressor FOXO1 and transcription factor MSX1, both associated with tumor growth inhibition, and downregulation of PDLIM4, encoding an actin-binding protein, typically down-regulated during prostate tumorigenesis.
Figure 2.
Heat map shows paracrine effect of ASCs or ASC-PEDF on PC3 gene expression changes. (A) Gene changes effected on PC3 cells by ASCs. (B) Gene changes effected on PC3 cells by ASC-PEDF. (C) Gene changes effected on PC3 cells both by ASC and ASC-PEDF. Color bar indicates the level of gene expression for each gene depicted ranging from green (minimum) to red (maximum) (cutoff of P < 0.01 for gene expression changes).
Several genes are uniquely modified in PC3 cells when cultured in the presence of ASC-PEDF therapeutic CCM
We observed several genes with modified expression in PC3 cells when cultured in the presence of ASC-PEDF CCM, as compared with control ASC (Figure 2B; P < 0.01). These genes included upre-gulation of a tumor-suppressing glutatione peroxidase gene (GPX3) and upregulation of SLC5A8, a gene typically downregulated in prostate cancer. Also upregulated was CDH1 (E-cadherin), a change that likely decreases cancer cell invasiveness. These expression changes, probably mediated through paracrine mechanisms by ASC-PEDF, would be consistent with a potential reduction in PC3 malignancy. However, one gene was upregulated that could promote growth, the fatty acid metabolism gene acetyl-CoA carboxylase-α (ACACA). Genes down-regulated in PC3 on coculture with ASC-PEDF included reductions in two genes typically upregulated in prostate cancer (KLK3 and SUPT7L), several androgen or PI3KIAkt signaling genes (DAXX, IL6, NRIP1 and VEGFA). Transcription factors downregulated included ERG, whose upregulation is a poor prognosis indicator, RBM39, implicated in colorectal carcinoma progression, SREBF1, whose inhibition sensitizes cells to death ligands, and one fatty acid metabolism gene (FASN) highly upregulated in prostate cancer. Downregulation of the ETV1 and MAPK1 genes also should reduce the aggressive phenotype of prostate cancer cells. These expression changes would be consistent with a potential reduction in malignancy of PC3 cells.
Gene expression changes in PC3 cells modulated by both ASC and ASC-PEDF CCM
Several gene expression changes were commonly modulated by both ASC types on PC3 cells, consistent with a reduction in prostate cancer aggressiveness, including upregulation of two genes typically downregulated in prostate cancer (CLN3, TFPI2) (Figure 2C; P < 0.01). The commonly down-regulated genes modulated by both ASC CCMs on PC3 included some upregulated in prostate cancer (DDX11, ECT2, SOX4, CAV2, MTO1), one metastatic potential gene (PES1), genes encoding members of the androgen (AR, SHBG) and P13KIAkt signaling pathways (AKT1, BCL2, CCND2), inflammatory gene PTGS1 (COX3) and survival gene IGF1. These expression changes might reduce the growth and invasiveness potential of PC3. Changes that would be consistent with enhancing PC3 cell growth by both ASCs could include downregulation of genes already downregulated in prostate cancer (PPP2R1B), transcription factor (SOX4), and downregulation of a tumor suppressor hyper-methylated in prostate cancer (RARB). Additional changes consistent with growth promotion might include two genes encoding for enhanced metastatic potential (MAX, NDRG3), a gene belonging to PBKIAKT signaling (PDPK1), a gene upregulated in prostate cancer (ARNTL) and two genes involved in fatty acid metabolism (PRKAB1, STK11).
Methylation changes are detectable in PC3 cells in response to ASC or ASC-PEDF CCM
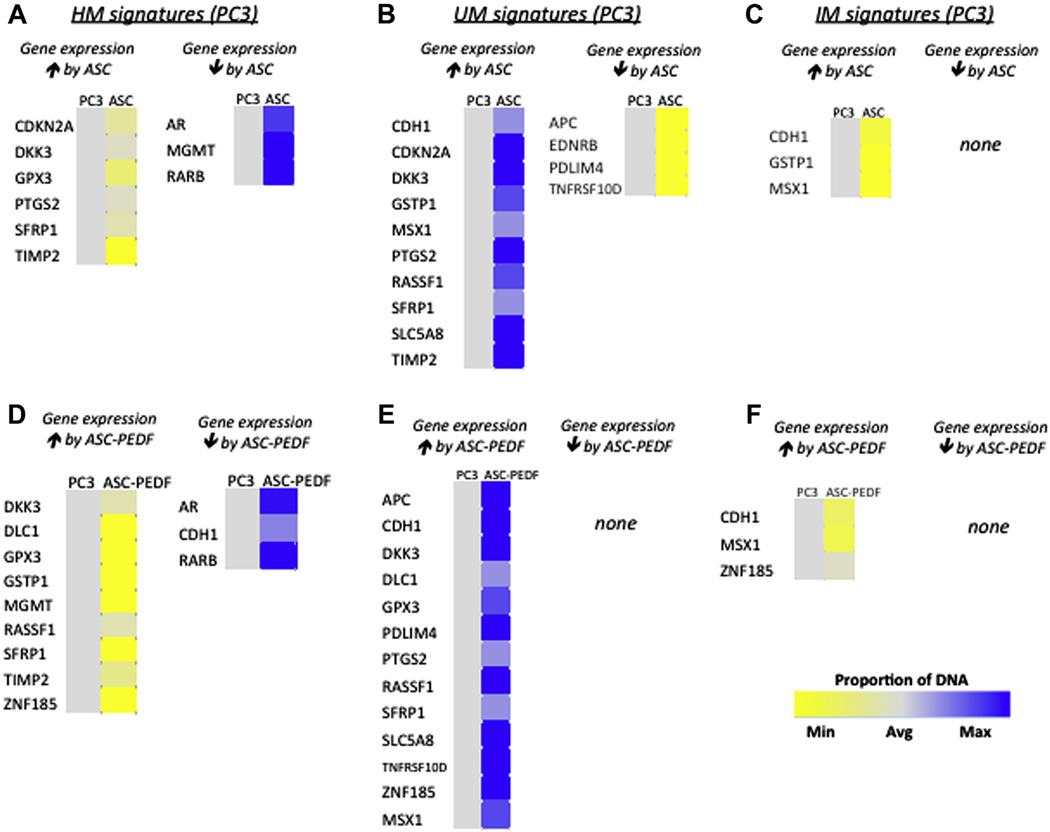
We examined methylation changes in focused prostate cancer—related genes with the use of the EpiTect Methyl II PCR array (Qiagen), which allowed profiling of HM, UM or IM epigenotypes. The epi-genotypes were examined simultaneously for a panel of 22 gene promoters selected on the basis of their reported methylation status in prostate cancer progression. We observed several changes when PC3 cells were cocultured with either ASC or ASC-PEDF (Figure 3). The coculture with ASCs induced detectable upregulation of several genes in PC3 cells, including CDKN2A, DKK3, PTGS2, SFRP1 and TIMP2 in HM (Figure 3A, yellow) and UM (Figure 3B, blue) methylation profile subsets. The UM profile displayed additional changes undetected in the HM subset, including upregulation of CDH1, GSTP1, MSX1, RASSF1 and SLC5A8 (Figure 3B, blue). ASCs induced detectable upregulation of GPX3 and downregulation of several genes in the PC3 HM profile including AR, MGMT and RARB (Figure 3A, blue). ASCs induced detectable downregulation also of APC, EDNRB, PDLIM4 and TNFRSF10D in the UM methylation subset (Figure 3B, yellow). A third methylation profile that we also examined was the IM group, in which CDH1, GSTP1 and MSX1 were upregulated as an effect of ASC on PC3 cells (Figure 3C, yellow). The culture with ASC-PEDF CCM induced detectable upregulation of several genes in PC3 prostate cancer cells, including DKK3, DLC1, GPX3, RASSF1, SFRP1 and ZNF185, as detected in both HM (Figure 3D, yellow) and UM (Figure 3E, blue) methylation profile subsets. The HM subset also presented upregulation of GSTP1, MGMT and TIMP2 (Figure 3D, yellow), whereas the UM also presented upregulation of APC, CDH1, SLC5A8, TNFRSF10D and MSX1 (Figure 3E, blue). ASC-PEDF may induce downregulation of AR, CDH1 and RARB as detected in the HM subset (Figure 3D, blue). The IM subset suggested that ASC-PEDF may induce upregulation of CDH1, MSX1 and ZNF185 (Figure 3F, yellow).
Figure 3.
Heat map shows paracrine effects of ASC or ASC-PEDF on PC3 methhylation profiles. HM signatures detected in PC3 cells after coculture, with the predictions of which changes might reduce or enhance gene expression by ASC (A) or ASC-PEDF (D). UM signatures detected in PC3 cells after coculture, with the predictions of which changes might reduce or enhance gene expression by ASC (B) or ASC-PEDF (E). IM signatures detected in PC3 cells after coculture, with the predictions of which changes might reduce or enhance gene expression by ASC (C) or ASC-PEDF (F). Color bar indicates the proportion of DNA detected in each category for each gene depicted ranging from yellow (minimum) to blue (maximum) (cutoff of P < 0.01 for gene expression changes).
Native ASCs and lentiviral-transduced ASCs: similarities and differences
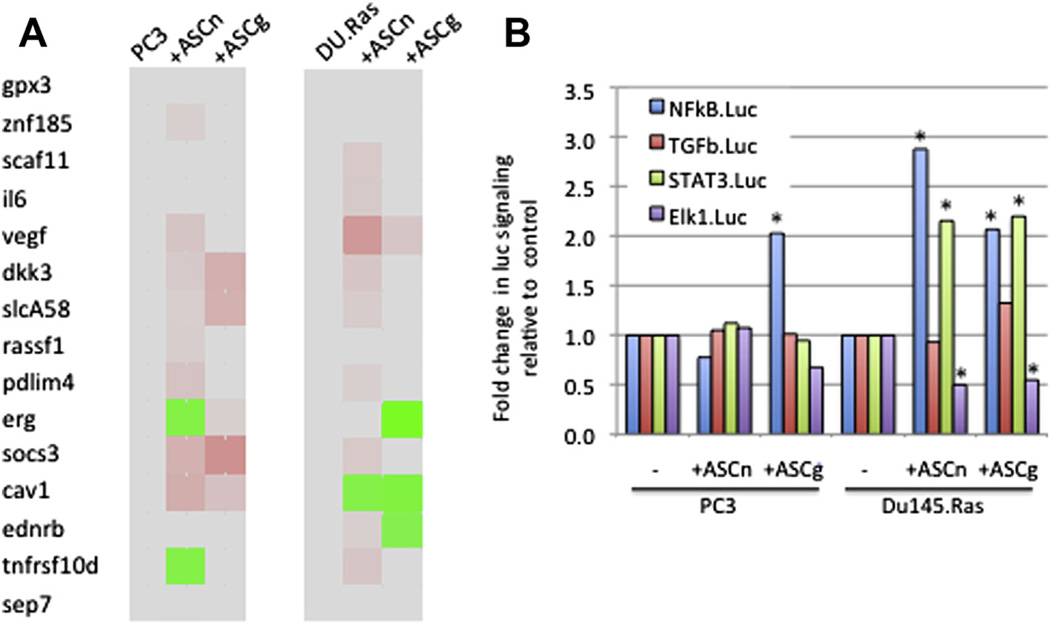
We examined whether lentiviral transduction of ASCs, albeit at a low MOI (= 1), could alter ASC paracrine interactions with prostate cancer cells PC3 or DU145.Ras. We selected 27 genes from the gene expression and methylation profiles observed in PC3 cells for the next analyses. Fifteen of these 27 genes were further validated by qPCR in PC3 cells in independent experiments to test the gene expression effect of native, untransduced ASC (ASCn) native versus ASC-GFP (ASCg). We observed that ASCn CCM mediated upregulation of VEGF, SOCS3 and PDLIM4 in both prostate cancer cell lines, upregulation of CAV1 and downregulation of TNFRSF10d in PC3 cells. ASCg mediated upregulation of DKK3, SLCA58, SOCS3 and CAV1 in PC3 cells and upregulation of VEGF and downregulation of ERG, CAV1 and EDNRB in DU145.Ras cells (Figure 4A). We also examined several signaling pathways potentially affected in prostate cancer cell lines on exposure to ASC CCM. Similar effects included upregulation of NFkB signaling by ASCg in both prostate cancer cell lines and by ASCn on DU145.Ras (Figure 5B). Also, ASCn and ASCg both upregulated STAT3 signaling and down-regulated Elk1 signaling in DU145.Ras.
Figure 4.
Comparison between ASC native and GFP-transduced ASC (AS-GFP). (A) Gene expression profiles for PC3 or DU145-Ras cells treated for 48 h with either control CCM (no ASC), CCM from ASC-native (ASCn) or ASC transduced with MOI = 1 of Lv-GFP (ASCg). (B) Luciferase reporter assays for PC3 or DU145-Ras cells treated as in (A). *P < 0.01.
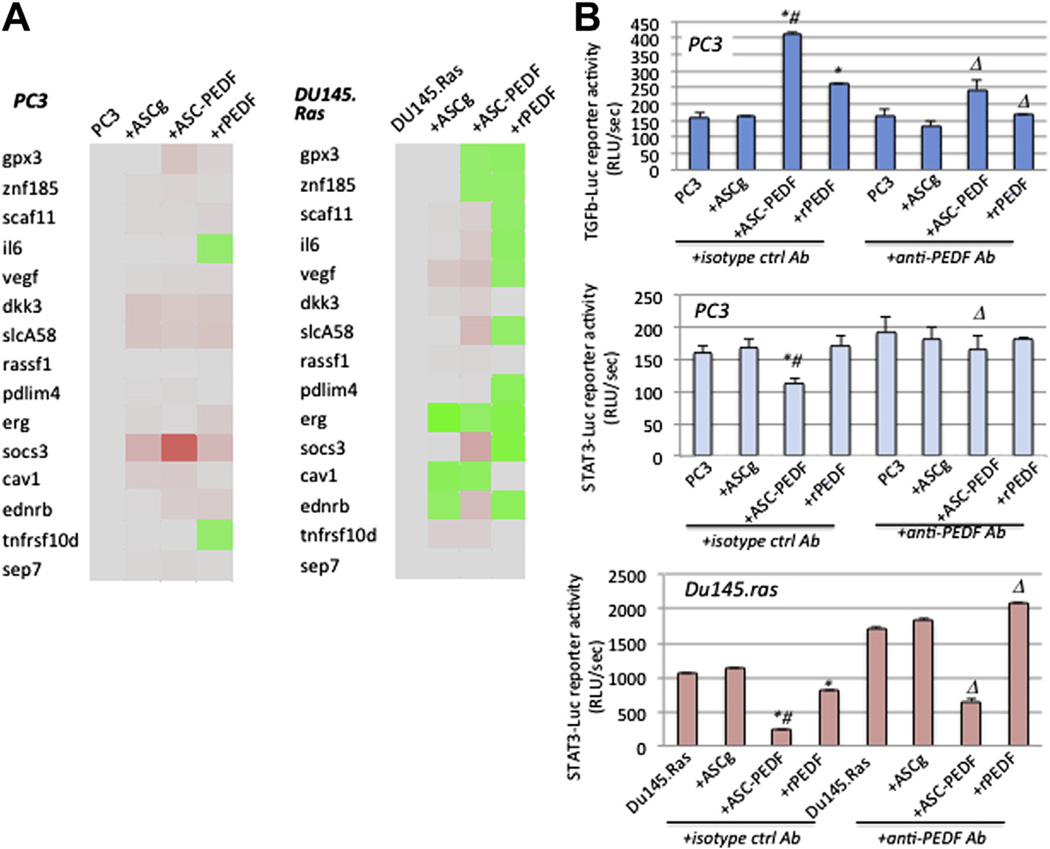
Figure 5.
Comparison between PEDF therapy derived from ASC-PEDF CCM and recombinant PEDF. (A) Gene expression profiles for PC3 or DU145-Ras cells with either control CCM (no ASC), CCM from ASC-GFP (MOI = 1), ASC-PEDF-GFP (ASC-PEDF, MOI = 1) or recombinant PEDF (rPEDF) as described in the Methods section. (B) Select luciferase reporter assays for PC3 (TGF-β-luc) or DU145-Ras (STAT3-luc) cells treated as in (A). *P < 0.05 compared with controls (prostate cancer cells alone or ASCg); #P < 0.05 for ASC-PEDF compared with rPEDF and ΔP < 0.05 for anti-PEDF antibody effect as compared with isotype control-treated.
ASC-PEDF and rPEDF therapies
We examined the effects of PEDF derived from ASC CCM and recombinant PEDF to determine whether there is a specific effect when PEDF is delivered from ASCs in the CCM (paracrine manner). We used the same gene panel from Figure 4 to examine the gene expression profiles modulated by ASC-PEDF versus rPEDF on PC3 or DU145.Ras prostate cancer cells (Figure 5A). In PC3 cells, ASC-PEDF upregulated SOCS3 at a higher magnitude (6-fold) than did rPEDF or controls. Unique changes modulated by rPEDF were downregulation of IL6 and TNFRSFlOd genes in PC3 cells. In DU145.Ras cells, ASC-PEDF uniquely upregulated VEGF, SLCA58 and SOCS3, whereas rPEDF uniquely downregulated SCAF11, IL6, VEGF, DKK3, SLCA58, PDLIM4, SOCS3 and EDNRB. We tested the whole panel of luciferase reporters from Figure 4B; however, only one to two signaling pathways showed showed significant changes in each prostate cancer cell line. For PC3, both ASC-PEDF CCM and rPEDF showed upregulation of TGF-β signaling (P < 0.01), although ASC-PEDF modulated a significantly higher TGF-β signaling upregulation compared with rPEDF (Figure 5B; P < 0.03). In PC3, ASC-PEDF CCM (but not rPEDF) downregulated STAT3 signaling. In DU145.Ras, both ASC-PEDF CCM and rPEDF induced reduced STAT3 signaling in prostate cancer cells (Figure 5B, P < 0.02), and the effect of ASC-PEDF was greater than that of rPEDF (P < 0.04). In both cell lines, the effect of PEDF was confirmed by use of a specific antibody, anti-PEDF, which at least partially reversed the effects of PEDF on signaling on both cancer cell lines as compared with isotype control (Figure 5B).
Discussion
The use of a cellular carrier can be enhanced when secreted gene therapy molecules are used, and we have shown that ASCs are suitable vehicles for delivery of PEDF to treat prostate tumors (17,25). Our previous work has shown that ASCs are amenable to stable or transient genetic modification, enabling delivery of secreted therapeutic molecules into the culture-conditioned media without adverse effects on ASC viability or differentiation. One major consideration for potentially translating ASC therapies is that they are non-tumorigenic once introduced into the recipient. Although we have not found ASC to be tumorigenic (17,25), this subject still is controversial. Other reports either support (11,15,26) or oppose our findings (12,27,28). Interestingly, some recent studies proposed that BM-MSCs or ASCs might have a potent tumor-inhibitory effect, acting to inhibit tumor growth, as reported for pancreatic models through induction of apoptosis (8), in hepatocellular carcinoma and in Kaposi sarcoma models through down-regulation of AKT signaling (10,29) and in colorectal cancer through induction of pro-apoptotic genes (30). Therefore, our present study was designed to examine gene expression, epigenetic profiles, and signaling pathways modified in prostate cancer cells on culture with ASC CCM. Our goal was to gain insight into whether ASCs (and/or therapeutically modified ASC-PEDF cells) are capable of inducing a profile balanced toward tumor-inhibition in a paracrine manner.
We observed several interesting paracrine effects on PC3 prostate tumor cells on incubation with ASC CCM that would be consistent with a tumor-inhibitory effect of ASC, including upregulation of apoptosis/cell cycle regulators CASP3 and pl6INK4a and reductions in metastasis/invasion gene CCNA1. Also, genes typically downregulated in prostate cancer were activated (GCA, SFRP1), along with an inhibitor of MMPs (TIMP2). Other expression reductions included two genes overexpressed in prostate cancer (EGR3 and GNRH1) and CAMKK1, a gene involved in fatty acid metabolism. ASC CCM also induced some changes that could promote prostate tumors such as upregulation of metastasis/growth gene CREB1 and downregulation of tumor suppressors FOXO1 and MSX1, and the actin-binding protein PDLIM4, all associated with tumor growth inhibition. Genes usually upregulated in prostate cancer were further upregulated, including CAMSAP1, PTGS2 and IGFBP5. Several of these gene expression changes correlated with altered methylation profiles as well, which suggests that regulation occurs at least in part through epigenetic interactions.
ASC-PEDF CCM induced a much more clear anti-tumor profile of gene expression changes in PC3 cells, with downregulation of four ARIPI3KIAKT signaling genes (including IL6), whereas four genes usually downregulated in prostate cancer or associated with a poor prognosis were upregulated. Furthermore, four genes typically upregulated in prostate cancer (KLK3 and SUPT7L) or associated with aggressiveness (ETV1, MAPK1) were downregulated. Despite a majority (∼93% of changes observed) of gene expression changes being consistent with tumor-inhibitory mechanisms by ASC-PEDF CCM, three gene expression changes observed still could potentially promote prostate tumor growth, including enhanced expression of the fatty acid metabolism gene ACACA, upregulation of SLC5A8, a gene typically downregulated in prostate cancer, as well as further downregulation of CAV1, typically downregulated in prostate cancer (CAV1). Given this overall gene expression response, ASC-PEDF CCM might be promoting regulatory changes at the level of the Gl/S transition (10) or could be stimulating expression of anabolic enzymes to coordinately regulate energy production and biosynthesis at multiple levels.
Common expression changes modulated by both ASC and ASC-PEDF include 15 tumor-inhibitory and 9 tumor-promoting genes. Of note are tumor-inhibitory changes mediated by both ASC or ASC-PEDF, including reductions in PI3KIAKT and AR signaling and inflammatory or survival pathways (COX3, IGF1), and reduced expression of the metastasis gene PES1. Of the potential tumor-promoting changes, metastasis and fatty acid metabolism genes were upregulated, and genes usually downregulated in prostate cancer were further reduced; one (RARB) correlated with methylation profiling. Also, genes usually upregulated in prostate cancer were upregulated such as ARNTL. Another ASC-PEDF—specific anti-tumorigenic expression pattern that correlated with methylation status was upregulation of GPX3, a proapoptosis/tumor suppressor gene. GPX3 (glutathione peroxidase 3) is a selenium-dependent enzyme that plays a critical role in detoxifying reactive oxidative species and maintaining the genetic integrity of mammalian cells. GPX3 is widely inactivated in prostate cancers and has been shown to be a potent tumor suppressor. PC3 xenografts expressing GPX3 show a reduction in tumor volume by ∼ 5-fold, elimination of metastasis and improved survival (31). This suggests that ASC-PEDF CCM could mediate the paracrine antitumor effects in vivo through epigenetic reactivation of GPX3. Additional gene expression changes mediated on PC3 included SOCS3 upregulation in PC3 cells with a higher magnitude (∼ 6-fold) by ASC-PEDF CCM as compared with rPEDF or controls. A unique change modulated by rPEDF included downregulation of IL6 in PC3 and DU145. Ras cells and downregulation of SOCS3 in DU145. Ras cells. These changes, in combination with reporter gene activity data, suggest that SOCS3 upregulation may render the ASC-PEDF CCM therapy more effective in its suppression of STAT3 activity as compared with rPEDF. SOCS3 is a negative regulator of the IL6ISTAT3 signaling axis (32) and can be subject to reactivation from epigenetic silencing; future studies will examine this interesting hypothesis. Reactivation could be a plausible explanation for why, even though IL6 message levels are low, SOCS3 levels are elevated, enabling SOCS3 to inhibit JAK activity and thus reduce STAT3 signaling in prostate cancer cells treated with ASC-PEDF CCM. The mechanisms of anti-tumor activity of ASC-PEDF CCM, however, appear to be complex and distinct for different prostate tumor cell lines. For example, in DU145-Ras, the effects of ASC-PEDF CCM included only STAT3 down-regulation, whereas in PC3 cells, the effect combined STAT3 downregulation and TGF-β signaling upregulation.
We also examined whether lentiviral transduction of ASCs could alter their ability to interact with prostate cancer cells in a paracrine manner. Overall, it appeared that both native ASCs (untransduced, ASCn) and ASC-GFP (ASCg) CCM could upregulate protumorigenic factors VEGF and ERG while upregulating the anti-tumor gene SOCS3. Also, even though ASCn upregulated PDLIM4, ASCg upregulated DKK3, SLCA58, CAV1 and EDNRB, and all of these are anti-tumor changes that may serve to regulate prostate cancer growth. The signaling pathways examined by reporter gene expression included upregulation of NFkB and STAT3 and down-regulation of Elk 1 signaling by both ASCn and ASCg in prostate cancer cells. These results suggest that lentiviral transduction might alter how ASCs mediate expression of tumor-promoting genes in tumor cells, but the expression and reporter gene activity profiles were considered to be generally equivalent.
Overall, this study is particularly interesting because in our previous work, we did not detect any tumorigenic potential for either ASC-GFP or ASC-PEDF in vivo (17). However, in the present (in vitro) study, some potential protumorigenic changes are detected in gene expression profiles of prostate cancer cells when incubated with ASC CCM. This apparent discrepancy would suggest that in vivo, the genetic and epigenetic changes presented here tilt the balance toward apoptosis and growth inhibition of prostate cancer cells. The relationship between the mediators identified here may be related to GPX3 through its ability to control the release of inflammatory mediators/adipokines through reduced reactive oxygen species and DNA damage (33,34). The potential GPX3 reactivation by ASC-PEDF CCM might act as a tumor suppressor to modify IL6 or other inflammatory cytokine signals as well as other adipokine (TGF-β) signals to affect tumorigenesis. Functioning as a transcription factor, STAT3 participates in the signaling pathways for many cytokines in various cells and organs that are regulated by the suppressor of cytokine signaling family, including SOCS3. STAT3 and SOCS3 can be potential targets for regulating inflammatory responses. Another signaling pathway of interest may include TGF-β, which might under some conditions act as a growth-inhibitory signal. Studies that used MSC CCM for treating experimental colitis support this hypothesis (35). MSCs upregulated VEGF and MCP1 but also modulated molecules related to the TGF-β signaling pathway (upregulated activin A, TGF-β and down-regulated inhibin bA). MSC CCM also mediated paracrine changes in RAW264.1 cells and reduced secretion of proinflammatory TNF and IL6 cytokines in the same colitis model. Another interesting difference between studies is that in vivo, ASCs secreting PEDF engraft into the tumor, and this action might maximize their therapeutic effect compared with in vitro studies with CCM. The colitis model cited above would support this hypothesis because it reported that CCM therapy required a 9-fold increase in the number of MSCs compared with cellular therapy (35).
Conclusions
An interesting aspect that we have thus observed for ASC or ASC-PEDF therapeutics is their potential ability to alter gene expression and to potentially reverse certain epigenetic marks with relevance to prostate cancer. A better understanding of the linkage between genetic and epigenetic events in tumor growth probably will result in a more effective use of ASC or ASC-PEDF for treating prostate cancer. We anticipate that delivery of immunostimulatory cytokines (36), anti-angiogenic molecules, apoptosis-inducing genes, nanoparticles and/or therapeutic exosomes (37) by ASCs or other stem cells to tumors will comprise very promising approaches for antitumor therapy. Future successful therapies may combine traditional targeted and stem cell—directed gene therapies to enable significant advances in the treatment of prostate and other cancers.
Acknowledgments
This work was supported by funding from the Pennington Biomedical Research Foundation (JMG), R21CA153165 (MLF), T32ES007254 QS), and the UTMB Department of Pharmacology & Toxicology (MLF).
Footnotes
Disclosure of interests: The authors have no commercial, proprietary, or financial interest in th products or companies described in this article. JMG has leadership roles at LaCell LLC to declare.
References
- 1.Pachon-Pena G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell BA, et al. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F3rd. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 5.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 6.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Secchiero P, Zorzet S, Tripodo C, Corallini F, Melloni E, Caruso L, et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin’s lymphoma xenografts. PLoS One. 2010;5:elll40. doi: 10.1371/journal.pone.0011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cousin B, Ravet E, Poglio S, De Toni F, Bertuzzi M, Lulka H, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;47:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, et al. Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy. 2009;113:289–298. doi: 10.1080/14653240902807026. [DOI] [PubMed] [Google Scholar]
- 10.Zhao W, Ren G, Zhang L, Zhang Z, Liu J, Kuang P, et al. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radiopharm. 2012;279:606–613. doi: 10.1089/cbr.2011.1150. [DOI] [PubMed] [Google Scholar]
- 11.Altanerova V, Horvathova E, Matuskova M, Kucerova L, Altaner C. Genotoxic damage of human adipose-tissue derived mesenchymal stem cells triggers their terminal differentiation. Neoplasma. 2009;566:542–547. doi: 10.4149/neo_2009_06_542. [DOI] [PubMed] [Google Scholar]
- 12.Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18:223–231. doi: 10.1038/mt.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SA, Hwang SK, Wang KC, Cho BK, Phi JH, Lee JY, et al. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. NeuroOncol. 2011;131:61–69. doi: 10.1093/neuonc/noq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;6713:6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 15.Kucerova L, Matuskova M, Pastorakova A, Tyciakova S, Jakubikova J, Bohovic R, et al. Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med. 2008;1010:1071–1082. doi: 10.1002/jgm.1239. [DOI] [PubMed] [Google Scholar]
- 16.Vilalta M, Degano IR, Bago J, Aguilar E, Gambhir SS, Rubio N, et al. Human adipose tissue-derived mesenchymal stromal cells as vehicles for tumor bystander effect: a model based on bioluminescence imaging. Gene Ther. 2009;164:547–557. doi: 10.1038/gt.2008.176. [DOI] [PubMed] [Google Scholar]
- 17.Zolochevska O, Yu G, Gimble JM, Figueiredo ML. PEDF and MDA-7 cytokine gene therapies delivered by adipose-derived mesenchymal stem cells are effective in reducing prostate cancer cell growth. Stem Cells Dev. 2012;21:1112–1123. doi: 10.1089/scd.2011.0247. [DOI] [PubMed] [Google Scholar]
- 18.Qingyi Z, Lin Y, Junhong W, Jian S, Weizhou H, Long M, et al. Unfavorable prognostic value of human PEDF decreased in high-grade prostatic intraepithelial neoplasia: a differential proteomics approach. Cancer Invest. 2009;277:794–801. doi: 10.1080/07357900802175617. [DOI] [PubMed] [Google Scholar]
- 19.Guan M, Jiang H, Xu C, Xu R, Chen Z, Lu Y. Adenovirus-mediated PEDF expression inhibits prostate cancer cell growth and results in augmented expression of PAI-2. Cancer Biol Ther. 2007;6:419–425. doi: 10.4161/cbt.6.3.3757. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell JB, Mcintosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 21.Guilak F, Lott KE, Awad HA, Cao Q, Hicok KC, Fermor B, et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol. 2006;206:229–237. doi: 10.1002/jcp.20463. [DOI] [PubMed] [Google Scholar]
- 22.Zolochevska O, Figueiredo ML. Cell cycle regulator cdk2apl inhibits prostate cancer cell growth and modifies androgen-responsive pathway function. Prostate. 2009;69:1586–1597. doi: 10.1002/pros.21007. [DOI] [PubMed] [Google Scholar]
- 23.McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, et al. Lentiviral vector mediated modification of mesenchymal stem cells and enhanced survival in an in vitro model of ischaemia. Stem Cell Res Ther. 2011;2:12. doi: 10.1186/scrt53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson P, Ngoc Tran TD, Zhang H, Zolochevska O, Figueiredo M, Feng JM, et al. Transient receptor potential melastatin 4 channel controls calcium signals and dental follicle stem cell differentiation. Stem Cells. 2013;31:167–177. doi: 10.1002/stem.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zolochevska O, Bergstrom SH, Comeaux B, Emrick T, Figueiredo ML. Novel antitumor strategies using cytokine PEDF for prostate cancer therapy. Curr Angiogenesis. 2012;14:277–298. [Google Scholar]
- 26.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;208:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 27.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 28.Shinagawa K, Kitadai Y, Tanaka M, Sumida T, Kodama M, Higashi Y, et al. Mesenchymal stem cells enhance growth and metastasis of colon cancer. Int J Cancer. 2010;127:2323–2333. doi: 10.1002/ijc.25440. [DOI] [PubMed] [Google Scholar]
- 29.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–1247. doi: 10.1084/jem.20051921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cihova M, Altanerova V, Altaner C. Stem cell based cancer gene therapy. Mol Pharm. 2011;85:1480–1487. doi: 10.1021/mp200151a. [DOI] [PubMed] [Google Scholar]
- 31.Yu YP, Yu G, Tseng G, Cieply K, Nelson J, Defrances M, et al. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–8050. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
- 32.Isomoto H. Epigenetic alterations in cholangiocarcinoma-sustained IL-6/STAT3 signaling in cholangio-carcinoma due to SOCS3 epigenetic silencing. Digestion. 2009;79(Suppl 1):2–8. doi: 10.1159/000167859. [DOI] [PubMed] [Google Scholar]
- 33.Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm. 2010;2010:513948. doi: 10.1155/2010/513948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE, et al. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res. 2013;73:1245–1255. doi: 10.1158/0008-5472.CAN-12-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe S, Arimura Y, Nagaishi K, Isshiki H, Onodera K, Nasuno M, et al. Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors. J Gastroenterol. 2013 Nov 12; doi: 10.1007/s00535-013-0901-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Zolochevska O, Ellis J, Parelkar S, Chan-Seng D, Emrick T, Wei J, et al. Interleukin-27 Gene delivery for modifying malignant interactions between prostate tumor and bone. Hum Gene Ther. 2013;24:970–981. doi: 10.1089/hum.2013.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]