Fish mycobacteriosis is a common bacterial disease in many species of freshwater and marine fish (Decostere, Hermans & Haesebrouck 2004). Infections are common in wild fish, aquaculture and ornamental fish (Jacobs, Stine, Baya & Kent 2009). This chronic progressive disease is commonly caused by aquatic Mycobacterium spp. such as M. marinum, M. fortuitum and M. chelonae (Decostere et al. 2004), although some previously recognized human mycobacteria such as M. haemophilum and M. peregrinum have also been found in zebrafish during mycobacteriosis outbreaks (Kent, Whipps, Matthews, Florio, Watral, Bishop-Stewart, Poort & Bermudez 2004). Fish mycobacteriosis usually leads to systemic infections, which have reportedly caused massive mortalities among fish grown in intensive aquaculture systems (Bruno, Griffiths, Mitchell, Wood, Fletcher, Drobniewski & Hastings 1998; Diamant, Banet, Ucko, Colorni, Knibb & Kvitt 2000). With the remarkable increase in fin fish culture, there has been a concurrent increased concern about mycobacteriosis in cultured species (Ostland, Watral, Whipps, Austin, St-Hilaire, Westerman & Kent 2008). In addition to acute mortalities, chronic mycobacterium infections may result not only in low level mortality but also in reduced growth or marketability of fish due to macroscopic lesions. In addition, Mycobacterium spp. infecting fish are all potentially zoonotic (Tchornobay, Claudy, Perrot, Levigne & Denis 1992; Vazquez & Sobel 1992; Parent, Salam, Appelbaum & Dossett 1995; Lehane & Rawlin 2000). There are a few reports of treatment of infections with antibiotics (Santacana, Conroy, Mujica, Marin & Lopez 1982; Lawhavinit, Hatai, Kubota, Toda & Suzuki 1988; Conroy & Conroy 1999), but these have been limited and mostly at the experimental level. Presently, there are no effective drugs for treating food fish on a commercial scale.
Vaccination for mycobacteria has limited success in mammalian systems. The BCG vaccine prepared from Mycobacterium bovis bacillus Calmette-Guerin is the most widely used vaccine worldwide for human and bovine tuberculosis. It is a live attenuated vaccine, which is effective against severe forms of childhood tuberculosis, but appears to have limited effects against adult pulmonary disease (Martin 2006; Liu, Tran, Leung, Alexander & Zhu 2009). Many alternative vaccine candidates against tuberculosis have been developed and actively evaluated in clinical trials, including plasmid DNA, subunit and alternative live attenuated vaccines (Hernandez Pando, Aguilar, Infante, Cataldi, Bigi, Martin & Gicquel 2006; Huygen 2006; Martin 2006; Gupta, Katoch & McMurray 2007; Liu et al. 2009). Vaccine candidates based on rational attenuated mutants of Mycobacterium tuberculosis seem very promising (Hernandez Pando et al. 2006; Liu et al. 2009). While there are no reports of live vaccines for mycobacteriosis in fish, a DNA vaccine encoding a major secreted fibronectin-binding protein of aquatic Mycobacterium spp. was shown to provide protection to hybrid-striped bass against an M. marinum challenge (Pasnik & Smith 2005). However, it is unclear whether such a DNA vaccine will become commercially viable for fish immunization because it is based on a single antigen. In addition, DNA vaccines in general are still in the experimental stage despite of years of intensive research (Cui 2005). Similarly, killed mycobacteria and the extracellular products of Mycobacterium spp. were shown to induce cell-mediated responses and antibody responses, respectively, when injected into rainbow trout, but there are no data showing whether the immune responses afforded the trout protection against a mycobacterial challenge (Bartos & Sommer 1981; Chen, Yoshida, Adams, Thompson & Richards 1996). Therefore, there continues to be a need to develop an efficacious vaccine against fish mycobacteriosis.
In the present study, we preliminarily evaluated the feasibility of prophylaxis against fish mycobacteriosis by immunizing zebrafish, Danio rerio (Hamilton), with a candidate vaccine based on a live M. marinum mutant (L1D) that had impaired ability to replicate in macrophages (Ramakrishnan, Federspiel & Falkow 2000). As controls, fish were also immunized with the extracellular culture filtrate proteins (CFPs) from M. marinum culture or a heat-killed M. marinum strain, both adjuvanted with polyinosinic-polycytidylic acid [(poly(I:C)], a synthetic double-stranded RNA (dsRNA). Poly(I:C) is a ligand of Toll-like receptor 3 (TLR3) (Alexopoulou, Holt, Medzhitov & Flavell 2001) and has been confirmed to be present in fish (Bilodeau & Waldbieser 2005; Phelan, Mellon & Kim 2005; Novoa, Romero, Mulero, Rodriguez, Fernandez & Figueras 2006). The interaction between dsRNA and TLR3 promotes hosts to initiate innate immune responses and orchestrates the transition from innate to adaptive immune responses (van Duin, Medzhitov & Shaw 2006). The poly(I:C) was used in this study as an adjuvant to enhance the immune responses induced by the CFPs and heat-killed M. marinum strain. The commercial applicability of the poly(I:C) for massive vaccinations is likely to be limited due to its potential toxicity (Giantonio, Hochster, Blum, Wiernik, Hudes, Kirkwood, Trump & Oken 2001). Highly virulent M. marinum OSU-214 was cultured in Middlebrook broth (7H9 with ADC) (Becton-Dickson) in a shaker incubator at room temperature for 5 days. To prepare the CFPs, the culture was centrifuged (3500 g for 20 min at 4 °C) (Wedlock, Denis, Skinner, Koach, De Lisle, Vordermeier, Hewinson, Van Drunen Littel-Van Den Hurk, Babiuk, Hecker & Buddle 2005). The supernatant was sterile-filtered twice through a 200 nm filter and dialysed against distilled water using a 5-kDa molecular weight dialysis tube (Spectrum Chemicals and Laboratory Products). The CFPs were lyophilized and stored at −80 °C. The protein content in the CFP preparation was determined using a Bio-Rad Quick Start Bradford Protein Assay Kit (Hercules). To prepare the killed OSU-214 bacteria, the pellets after centrifugation were re-suspended in distilled water, dialysed overnight against water and incubated in a 90 °C oven for 1 h. The killed bacteria were then freeze-dried and stored at −80 °C. Again, the total protein content in the killed bacterial preparation was quantified. Both the CFP and the killed bacterial preparations were confirmed to be free of live bacteria when cultured on 7H9 agar plates. The M. marinum mutant L1D was grown in 7H9 broth similarly (Ramakrishnan et al. 2000). The bacteria were harvested by centrifugation and washed with sterile phosphate buffered saline (PBS, 10 mm, pH 7.4) three times, re-suspended in PBS, and used immediately.
Zebrafish were from the Zebrafish International Resource Center (ZIRC) (Eugene, OR) and maintained in a BSL-2 fish facility in the Microbiology Department in Oregon State University. Fish were randomly assigned into four groups. They were injected intraperitoneally with sterile PBS, the mixture of CFPs and poly(I:C) dissolved into PBS, killed OSU-214 suspended together with poly(I:C) into PBS, or the live L1D M. marinum mutant (3.5 × 104 per fish). The dose of poly(I:C) was 0.5 lg per fish. The zebrafish were around 0.5 g. Fish were immunized on days 0 and 10. Water temperature in tanks was maintained at 27–28 °C. Ammonia and nitrite levels were monitored daily using test kits and water changes were performed periodically. In addition, box-type aquarium filters with porous lava rock were placed into each tank for biological filtration. Fish were fed twice daily (Zeigler adult zebrafish diet, Zeigler Bros Inc.). About 10 fish from each group were euthanized and bled on day 25. On day 26, the remaining fish were intraperitoneally injected with 5 × 104 of live M. marinum OSU-214 and monitored for 62 more days. Dead fish were carefully examined, and their liver homogenate was occasionally cultured to confirm the presence of M. marinum. Specific antibodies (Abs) against the CFPs or the killed OSU-214 bacteria were determined using ELISA as described previously (Sloat & Cui 2006), except that the plates were coated with either the CFPs or the killed OSU-214 (100 ng per well), and that the secondary Ab was replaced with a protein A-peroxidase conjugate (1:500-fold dilution, Sigma-Aldrich).
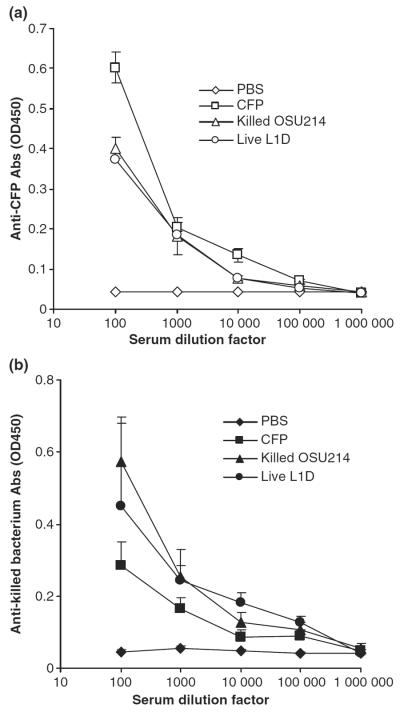
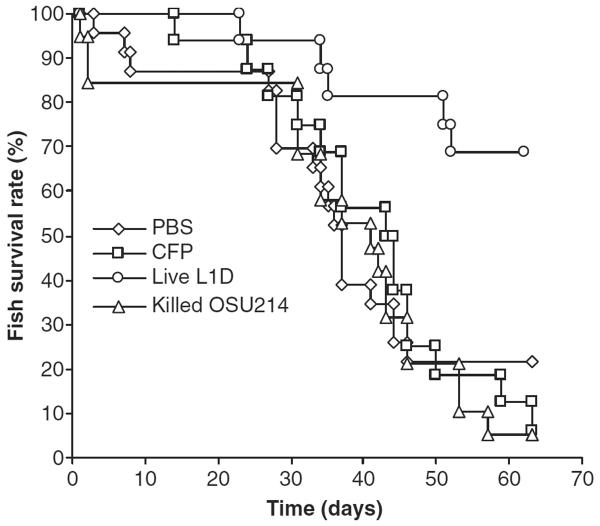
As shown in Fig. 1, strong Ab responses against both the CFPs and the killed OSU-214 bacteria were induced by all three vaccine candidates, suggesting that there were antigens that were presented in both the CFPs and the bacteria. However, our preliminary data showed that only those fish that were immunized with the live attenuated L1D M. marinum mutant were protected against a challenge using the virulent M. marinum OSU-214 strain (Fig. 2, P = 0.003, Live L1D vs. PBS). Apparently, the strength of the Ab response against mycobacterial CFPs and the killed bacteria was not correlated with the protection against M. marinum infection. This was probably because mycobacteria reside intracellularly in macrophages and cellular immune responses were required to control them. In fact, efforts on identifying correlates to protective immunity against mycobacteria are mainly focused on cellular immune responses, with whole blood interferon-gamma being the best available correlate (Ellner, Hirsch & Whalen 2000; Buddle, Wedlock, Denis & Skinner 2005; Maglione & Chan 2009). Alternatively, it may be because fish produce only IgM-like antibodies, which are of intrinsic low avidity and lack affinity maturation (Bengtén, Clem, Miller, Warr & Wilson 2006). Fish circulating antibodies are often non-protective and even high levels of specific antibodies often do not correlate with protection or disease status (Alvarez-Pellitero 2008). Finally, it should be noted that the L1D M. marinum strain has a kanamycin insertion mutation in its mag 24–1 gene (macrophage activated gene) (Ramakrishnan et al. 2000). It is possible that the mutant strain will reacquire virulence once introduced back into a live host. The re-acquisition of virulence is a recurrent phenomenon that has often prevented the application of vaccines based on attenuated pathogens (Hofmann-Lehmann, Vlasak, Williams, Chenine, Mcclure, Anderson, O'Neil & Ruprecht 2003). Thus, an alternative, more stable mag 24–1 mutant strain may need to be created in the future.
Figure 1.
(a) Anti-CFP Abs and (b) anti-killed bacterial Abs induced in zebrafish by all three vaccine candidates, CFPs/poly(I:C), killed OSU214/poly(I:C) and live L1D. Blood samples from 10 fish in every group were pooled and assayed three times. Data shown are the mean ± SD.
Figure 2.
Only the live attenuated L1D vaccine candidate significantly protected zebrafish from a challenge using the virulent Mycobacterium marinum OSU-214 strain. The initial number of fish in each group was around 20. Log Rank analysis of Kaplan–Meier survival curves indicated that the Live L1D curve was significantly different from that of the other groups (Live L1D vs. PBS, P = 0.003; L1D vs. CFP, P = 0.001; L1D vs. killed OSU-214, P = 0.0004).
In conclusion, our preliminary data showed that immunization of zebrafish with an attenuated live M. marinum vaccine reduced mycobacteriosis. Further studies with other fish species are needed to determine whether this phenomenon can be extended to commercially important aquaculture species.
Acknowledgements
This report was partially prepared by Oregon Sea Grant under award number NA16RG1039 (project number R/BT-43-PD) from the National Oceanic and Atmospheric Administration's National Sea Grant College Program, U.S. Department of Commerce and by appropriations made by the Oregon State legislature. The statements, findings, conclusions and recommendations are those of the authors and do not necessarily reflect the views of these funders. This study was also supported in part by a grant from the National Institutes of Health (NIH NCRR 5R24RR017386-02). We would like to thank Dr Lalita Ramakrishnan in the University of Washington for the M. marinum mutant L1D. We would also like to thank Dr Luiz E. Bermudez for fruitful discussions. D.S.S. was supported by a fellowship from the Scientific Mission Department, High Education Ministry of Egypt and the Helwan University.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Alvarez-Pellitero P. Fish immunity and parasite infections: from innate immunity to immunoprophylactic prospects. Veterinary Immunology and Immunopathology. 2008;126:171–198. doi: 10.1016/j.vetimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Bartos JM, Sommer CV. In vivo cell mediated immune response to M. tuberculosis and M. salmoniphilum in rainbow trout (Salmo gairdneri) Development & Comparative Immunology. 1981;5:75–83. doi: 10.1016/s0145-305x(81)80009-2. [DOI] [PubMed] [Google Scholar]
- Bengtén E, Clem LW, Miller NW, Warr GW, Wilson M. Channel catfish immunoglobulins: repertoire and expression. Developmental & Comparative Immunology. 2006;30:77–92. doi: 10.1016/j.dci.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Bilodeau AL, Waldbieser GC. Activation of TLR3 and TLR5 in channel catfish exposed to virulent Edwardsiella ictaluri. Development & Comparative Immunology. 2005;29:713–721. doi: 10.1016/j.dci.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Bruno DW, Griffiths J, Mitchell CG, Wood BP, Fletcher ZJ, Drobniewski FA, Hastings TS. Pathology attributed to Mycobacterium chelonae infection among farmed and laboratory-infected Atlantic salmon Salmo salar. Diseases of Aquatic Organisms. 1998;33:101–109. doi: 10.3354/dao033101. [DOI] [PubMed] [Google Scholar]
- Buddle BM, Wedlock DN, Denis M, Skinner MA. Identification of immune response correlates for protection against bovine tuberculosis. Veterinary Immunology and Immunopathology. 2005;108:45–51. doi: 10.1016/j.vetimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Chen SC, Yoshida T, Adams A, Thompson KD, Richards RH. Immune response of rainbow trout to extracellular products of Mycobacterium spp. Journal of Aquatic Animal Health. 1996;8:216–222. [Google Scholar]
- Conroy G, Conroy DA. Acid-fast bacterial infection and its control in guppies (Lebistes reticulatus) reared on an ornamental fish farm in Venezuela. Veterinary Record. 1999;144:177–178. doi: 10.1136/vr.144.7.177. [DOI] [PubMed] [Google Scholar]
- Cui Z. DNA vaccine. Advances in Genetics. 2005;54:257–289. doi: 10.1016/S0065-2660(05)54011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decostere A, Hermans K, Haesebrouck F. Piscine mycobacteriosis: a literature review covering the agent and the disease it causes in fish and humans. Veterinary Microbiology. 2004;99:159–166. doi: 10.1016/j.vetmic.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Diamant A, Banet A, Ucko M, Colorni A, Knibb W, Kvitt H. Mycobacteriosis in wild rabbitfish Siganus rivulatus associated with cage farming in the Gulf of Eilat, Red Sea. Diseases Of Aquatic Organisms. 2000;39:211–219. doi: 10.3354/dao039211. [DOI] [PubMed] [Google Scholar]
- van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends in Immunology. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ellner JJ, Hirsch CS, Whalen CC. Correlates of protective immunity to Mycobacterium tuberculosis in humans. Clinical Infectious Diseases. 2000;30(Suppl. 3):S279–S282. doi: 10.1086/313874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giantonio BJ, Hochster H, Blum R, Wiernik PH, Hudes GR, Kirkwood J, Trump D, Oken MM. Toxicity and response evaluation of the interferon inducer poly ICLC administered at low dose in advanced renal carcinoma and relapsed or refractory lymphoma: a report of two clinical trials of the Eastern Cooperative Oncology Group. Investigational New Drugs. 2001;19:89–92. doi: 10.1023/a:1006458232384. [DOI] [PubMed] [Google Scholar]
- Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine. 2007;25:3742–3751. doi: 10.1016/j.vaccine.2007.01.112. [DOI] [PubMed] [Google Scholar]
- Hernandez Pando R, Aguilar LD, Infante E, Cataldi A, Bigi F, Martin C, Gicquel B. The use of mutant mycobacteria as new vaccines to prevent tuberculosis. Tuberculosis (Edinburgh, Scotland) 2006;86:203–210. doi: 10.1016/j.tube.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Vlasak J, Williams AL, Chenine AL, Mcclure HM, Anderson DC, O'Neil S, Ruprecht RM. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS. 2003;17:157–166. doi: 10.1097/00002030-200301240-00004. [DOI] [PubMed] [Google Scholar]
- Huygen K. DNA vaccines against mycobacterial diseases. Future Microbiology. 2006;1:63–73. doi: 10.2217/17460913.1.1.63. [DOI] [PubMed] [Google Scholar]
- Jacobs JM, Stine CB, Baya AM, Kent ML. A review of mycobacteriosis in marine fish. Journal of Fish Diseases. 2009;32:119–130. doi: 10.1111/j.1365-2761.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comparative Biochemistry and Physiology – Part C Toxicology and Pharmacology. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Lawhavinit O, Hatai K, Kubota S, Toda K, Suzuki N. Treatment of Mycobacterium infection in pejerrey, Odonthestes bonariensis C & V. The Bulletin of The Nippon Veterinary and Zootechnical College. 1988;37:35–38. [Google Scholar]
- Lehane L, Rawlin GT. Topically acquired bacterial zoonoses from fish: a review. Medical Journal of Australia. 2000;173:256–259. doi: 10.5694/j.1326-5377.2000.tb125632.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Tran V, Leung AS, Alexander DC, Zhu B. BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Human Vaccine. 2009;5:70–78. doi: 10.4161/hv.5.2.7210. [DOI] [PubMed] [Google Scholar]
- Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. European Journal of Immunology. 2009;39:676–686. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. Tuberculosis vaccines: past, present and future. Current Opinion in Pulmonary Medicine. 2006;12:186–191. doi: 10.1097/01.mcp.0000219267.27439.1b. [DOI] [PubMed] [Google Scholar]
- Novoa B, Romero A, Mulero V, Rodriguez I, Fernandez I, Figueras A. Zebrafish (Danio rerio) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV) Vaccine. 2006;24:5806–5816. doi: 10.1016/j.vaccine.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Ostland VE, Watral V, Whipps CM, Austin FW, St-Hilaire S, Westerman ME, Kent ML. Biochemical, molecular, and virulence characteristics of select Mycobacterium marinum isolates in hybrid striped bass Morone chrysops × M. saxatilis and zebrafish Danio rerio. Diseases of Aquatic Organisms. 2008;79:107–118. doi: 10.3354/dao01891. [DOI] [PubMed] [Google Scholar]
- Parent LJ, Salam MM, Appelbaum PC, Dossett JH. Disseminated Mycobacterium marinum infection and bacteremia in a child with severe combined immunodeficiency. Clinical Infectious Diseases. 1995;21:1325–1327. doi: 10.1093/clinids/21.5.1325. [DOI] [PubMed] [Google Scholar]
- Pasnik DJ, Smith SA. Immunogenic and protective effects of a DNA vaccine for Mycobacterium marinum in fish. Veterinary Immunology and Immunopathology. 2005;103:195–206. doi: 10.1016/j.vetimm.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Phelan PE, Mellon MT, Kim CH. Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio) Molecular Immunology. 2005;42:1057–1071. doi: 10.1016/j.molimm.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Federspiel NA, Falkow S. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science. 2000;288:1436–1439. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- Santacana JA, Conroy DA, Mujica ME, Marin C, Lopez N. Acid-fast bacterial infection and its control in three-spot gouramies, Trichogaster trichopterus Palllas. Journal of Fish Diseases. 1982;5:545–547. [Google Scholar]
- Sloat BR, Cui Z. Nasal immunization with a dual antigen anthrax vaccine induced strong mucosal and systemic immune responses against toxins and bacilli. Vaccine. 2006;24:6405–6413. doi: 10.1016/j.vaccine.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Tchornobay AM, Claudy AL, Perrot JL, Levigne V, Denis M. Fatal disseminated Mycobacterium marinum infection. International Journal of Dermatology. 1992;31:286–287. doi: 10.1111/j.1365-4362.1992.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Sobel JD. A case of disseminated Mycobacterium marinum infection in an immunocompetent patient. European Journal of Clinical Microbiology and Infectious Diseases. 1992;11:908–911. doi: 10.1007/BF01962371. [DOI] [PubMed] [Google Scholar]
- Wedlock DN, Denis M, Skinner MA, Koach J, De Lisle GW, Vordermeier HM, Hewinson RG, Van Drunen Littel-Van Den Hurk S, Babiuk LA, Hecker R, Buddle BM. Vaccination of cattle with a CpG oligodeoxynucleotide-formulated mycobacterial protein vaccine and Mycobacterium bovis BCG induces levels of protection against bovine tuberculosis superior to those induced by vaccination with BCG alone. Infection and Immunity. 2005;73:3540–3546. doi: 10.1128/IAI.73.6.3540-3546.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]