SUMMARY
BACKGROUND
The pathogenesis of chronic tendinopathy is unclear. We have previously measured high intratendinous levels of glutamate in patients with tendinosis, suggesting potential roles of glutamate in the modulation of pain, vascular function, and degenerative changes including apoptosis of tenocytes. However, the origin of free glutamate found in tendon tissue is completely unknown.
METHODS
Surgical biopsies of pain-free normal tendons and tendinosis tendons (Achilles and patellar) were examined immunohistochemically using antibodies against vesicular glutamate transporters (VGluT1 and VGluT2), as indirect markers of glutamate release. In situ hybridization for VGluT2 mRNA was also conducted.
RESULTS
Specific immunoreactions for VGluT2, but not VGluT1, could be consistently detected in tenocytes. However, there were interindividual variations in the levels of immunoreactivity. The level of immunoreaction for VGluT2 was higher in tendinosis tendons compared to normal tendon (p<0.05). In situ hybridization of VGluT2 demonstrated that mRNA was localized in a similar pattern as the protein, with marked expression by certain tenocytes, particularly those showing abnormal appearances. Reactivity for VGluT1 and -2 was absent from nerves and vessel structures in both normal and painful tendons.
CONCLUSIONS
The current data demonstrate that tenocytes may be involved in the regulation of extracellular glutamate levels in tendons. Specifically, the observations suggest that free glutamate may be locally produced and released by tenocytes, rather than by peripheral neurons. Excessive free glutamate is expected to impact a variety of autocrine and paracrine functions important in the development of tendinosis, including tenocyte proliferation and apoptosis, extracellular matrix metabolism, nociception and blood flow.
INTRODUCTION
Glutamate is a pervasive amino acid with a well known role in excitatory synaptic transmission in the central nervous system (CNS), where it is considered responsible for transmitting most fast synaptic potentials 1, 2. The transmission of cellular signals among neurons by glutamate requires a number of genes including dedicated glutamate receptors, receptor interacting proteins, plasma membrane transporters, and vesicular transporters 3. There are two main classes of glutamate receptors, including metabotropic receptors (signaling via IP3, diacylglycerol, and cyclic AMP) and ionotropic receptors (capable of altering permeability to specific cations). Ionotropic receptors are further classed as N-methyl-D-aspartate (NMDA) receptors, DL-α-amino-3-hydroxy-5-methylisoxasole-4-propionate (AMPA) receptors, and kainate (KA) receptors 3. In addition to receptors, glutamate transporters in the plasma membrane (GLAST, GLT-1, EAAC1, EAAT4, -5) or in vesicles (VGluT1-3) play key roles in the regulation of extracellular glutamate concentrations 4.
It has become increasingly evident that genes encoding elements of the glutamate signaling machinery are also expressed by non-neuronal cells in a variety of peripheral tissues, and that glutamate functions as a regulatory cytokine with autocrine and paracrine functions in diverse physiologic processes such as bone turnover, insulin and growth hormone secretion, platelet formation and function, and keratinocyte development and differentiation 1, 5, 6. In addition to diverse roles in physiologic cell signaling, glutamate also plays known or emerging roles in a range of pathologies including inflammation and soft tissue repair 7–11. During acute inflammation of the knee, glutamate is released into the knee joint from peripheral glutamatergic nerves, resulting in a nitric oxide-dependent increase in local blood flow 8. Furthermore, glutamate receptor antagonism can reduce nociception and sensitization in response to a variety of inflammatory stimuli 12.
Painful overuse tendon injuries are a common medical problem seen by general and sports medicine practitioners, with a cumulative incidence of 52% in middle or long-distance runners (Achilles tendinopathy) and a prevalence of 32% to 44% with current symptoms in elite jumping athletes (patellar tendinopathy) 13, 14. The pathology considered to be responsible for many cases of chronic tendon overuse pain is often classified as “tendinosis.” Tendinosis is characterized morphologically by collagen fragmentation and disarray, tenocyte abnormalities (including apoptosis and necrosis, increased proliferation and excessive glycosoaminoglycan production) and by an increase in local intra- and peritendinous blood flow 15, 16. In this condition, high levels of glutamate have been measured from within tendinosis lesions using microdialysis in patients with Achilles, patellar and extensor carpi radialis brevis tendinosis 17–19. The observations thus suggest that glutamate may contribute to the symptoms of chronic tendon pain 17–19. Glutamate has also recently been suggested by Murrell and coworkers to be present within tendinosis lesions at levels sufficiently high to induce tenocyte apoptosis 20, a pathological feature of some advanced tendinosis lesions 21, 22.
Beyond the high levels of glutamate detected by microdialysis in painful tendons, nothing is known regarding the glutamate signaling machinery which is present in normal or pathological human tendons, although we previously reported that NMDA receptors are present in nerve fascicles in the human Achilles and patellar tendons 17, 23. More recent studies conducted on rat tendons have documented the presence of mRNA in tenocytes for several key elements of glutamate signaling machinery, including receptors (metabotropic glutamate receptors mGluR5 and mGluR6, NMDARL1), glutamate receptor-interacting proteins (GRIP1 and GRIP2) and plasma membrane transporters (EAAT4)20, 24. To our knowledge, there is no information on elements of the signal transduction pathway required for vesicular release of glutamate in human tendons, namely the presence of vesicular glutamate transporters, either at the protein or mRNA levels. This lack of knowledge is a drawback when considering the fact that high extracellular glutamate levels occur in tendinosis. In contrast to plasma membrane transporters which play a role in signal termination by internalization of glutamate, VGluTs are required for the transport of glutamate into secretory vesicles, and may therefore be considered as indirect markers of potential sources of free glutamate. VGluT1 and 2 are widely expressed in the CNS and considered definitive markers for excitatory glutamatergic synapses, whereas VGluT3 has a more restricted pattern of CNS expression 25.
Therefore, the purpose of the current study was to determine whether VGluT1 or -2 are present in human tendons. Further, our purpose was to determine whether their expression levels or pattern of distribution would be influenced by tendinosis pathology. We hypothesized that at least one or more of the two main VGluT isoforms examined would be present to a greater degree in tendinosis, due to the higher levels of extracellular glutamate associated with this condition.
METHODS
Patients and tendon sampling
The current study included samples from 29 individuals and 30 patellar or Achilles tendons. There were 14 tendinosis patients (1 patellar, 13 Achilles) and 15 subjects with no history of current or past tendon pain (8 patellar and 7 Achilles). There were 19 men and 10 women, average age 40.7 years (range 18 – 54), with no significant difference in age between patient and control groups. Both Achilles and patellar tendons were included, as it has been reported that there is no significant difference in tendon pathology between the two sites, i.e. the morphology seen in patellar tendinosis closely resembles that seen in Achilles tendinosis 26. All tendinosis patients displayed tenderness and pain during loading, and exhibited tendon changes verified by ultrasonography (localized widening of the tendon, irregular structure, and focal hypoechoic areas) or MRI (increased signal intensity and localized widening). All subjects were otherwise healthy and on no medications.
In the case of tendinosis patients, samples (2–3 cubic millimeters in size) were obtained from the area of the tendon corresponding to structural changes observed on ultrasound or during surgery. Samples were transported on ice in sterile conditions to the laboratory. Some samples were fixed for 24–48 hours in 4% formaldehyde buffered in 0.1 M phosphate buffer at 4° C, followed by embedding in OCT (Miles Laboratories, Naperville, IL, USA) and freezing. Other samples were directly snap frozen in isopentane chilled in liquid nitrogen. All samples were then stored at −80°.
The study protocol was approved by the Committee of Ethics at the Faculty of Medicine and Odontology, Umeå University, and the Regional Ethical Review Board in Umeå, and the experiments were conducted according to the principles expressed in the Declaration of Helsinki.
Morphological analysis
The presence of any significant artifacts was examined in longitudinal haematoxylin-eosin sections from normal and painful tendons using previously published morphological features of tendinosis 27. All samples included were free from significant artifact, and the tendinosis samples demonstrated, to varying degrees, typical features of the pathology including tenocyte hypercellularity, pyknotic or necrotic nuclei, tenocyte rounding, vascular hyperplasia, and collagen fibre disarray.
Immunofluorescence for VGluT1 and -2
Immunohistochemical staining was carried out on biopsies from all patients using goat polyclonal antibodies obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), namely sc26026 and sc46569 for VGluT1 and -2 respectively. Control staining included omission of the primary antibody, replacement of primary antibody with normal goat serum, or pre-absorption of the antibody with the corresponding blocking peptide at 150–200 μg/ml (sc26026P for VGluT1 and sc46569P for VGluT2). Both fixed and unfixed samples were included, as fixation appeared to have a minimal influence on the strength or distribution of observed reactions for VGluT1 or 2. Specimens of fixed and unfixed human colitis tissue were used as a reference tissue 28. After air drying for 30 minutes at room temperature, 7μm thick crysections were treated with acid potassium permanganate to reduce fixation-induced autofluorescence (in fixed samples), then washed in PBS and placed in 1% Triton in PBS for 20 minutes, rinsed in PBS and blocked for 15 minutes with normal 5% Donkey serum. Primary antibodies were applied overnight at 4° at 1:100 dilution, rinsed in PBS, then incubated with the secondary antibody (1:100 donkey anti-goat conjugated to FITC, Jackson ImmunoResearch Europe, Newmarket, UK) for 30 minutes in the dark. Sections were washed and coverslipped with Vectashield hardset mounting medium (Vector Laboratories, Burlingame, CA, USA) and viewed with a fluorescence microscope (Axioskop2, Carl Zeiss, Stockholm, Sweden) to determine the location and intensity of specific reactions. All sections, including those with and without peptide block, were viewed independently and then together by two observers (AS and SF) to achieve consensus regarding the extent and specificity of reactions. Parallel sections of some biopsies were stained identically on separate occasions to ensure repeatability of the procedure, and each round of staining included both normal and tendinosis tendons to ensure validity of comparisons.
In Situ Hybridization for VGLUT-2
In situ hybridization was carried out on a subset of tendon samples (n=3) to examine whether the mRNA would be localized in a similar or different pattern to the protein. A digoxigenin (DIG)-hyperlabeled oligonucleotide probe (ssDNA) for detection of human VGluT2 mRNA was used on sections from frozen tendinosis specimens. The probe sequence (CCTTG TACAA ATTCC TCTTT CTTTT CCCAA CCACT AGGCC AACCT CCA) was complementary to nucleotides 2066 - 2133 located within the coding sequence of human VGluT2 (GeneDetect, Auckland, New Zealand). In situ hybridization was performed according to an established protocol 29, using an alkaline phosphatase (AP)-labeled anti-DIG antibody (GeneDetect, Auckland, New Zealand) for detection,. Series of 10 mm thick cryosections were air-dried at room temperature (RT) for 30 min, then fixed in sterile 4 % paraformaldehyde (PFA) in 0.1 M PBS for 60 min at RT. The slides were then washed with 2x saline sodium citrate (SSC) for 2x10 min. The sections were thereafter incubated in 0.2 M HCl for 8 min at RT to inhibit endogenous alkaline phosphatase activity. After this, the sections were acetylated by incubation of slides for 15 min at RT in a mixture of 195 ml DEPC- H2O, 2.7 ml tiethanolamide, 0.355 ml HCl, and 0.5 ml acetic anhydride. Slides were then again rinsed in 2xSSC. After that, 25–50 ng of the ssDNA probe was put in 15 ml of hybridization solution in a 1.5 ml Eppendorf tube, denaturated for 5 min in 80°C and then put on ice. The probe-containing hybridization solution (500 ml formamide, 200 ml 20xSSC, 50 ml of 20x Denhardt’s solution, 50 ml herring sperm DNA (10 mg/ml) heat-denatured, 25 ml bakers yeast RNA (10 mg/ml), 175 ml dextran sulfate (50%)) was then applied to each section and incubated at 56°C overnight. The slides were then washed for 2x10 min at RT in 2xSSC and for 5 min at RT in STE-buffer (STE-buffer: 500 mM NaCl, 20 mM Tris-HCl [pH 7.5], 1 mM EDTA) And incubated in 100 ml RNase A (40 mg/ml in STE) for 30 min at 37°C. After this, the slides were washed for 20 min at 56°C in 2xSSC, 50% formamide (25 mL 100% and 25 ml 2xSSC buffer), then placed for 2x5 min at RT in 1xSSC, and for 2x5 min at RT in 0.5xSSC. Then the slides were washed for 5 min in buffer 1 (100 mM Tris-HCl [pH 7.5] + 150 mM NaCl) and incubated in buffer 1 containing 4% normal horse serum (NHS) for 60 min at RT in a humid chamber. Sections were then incubated in 100 mL of the AP-labeled anti-DIG antibody (diluted 1:500 in buffer 1 with 4 % NHS) for 60 min at RT in a humid chamber. The slides were then washed for 2x10 min in buffer 1, and for 2x5 min in buffer 2 (100 mM Tris-HCl [pH 9.5] + 100 mM NaCl + 50 mM MgCl2). After this, the enzyme (AP) substrate solution (20 ml NBT/BCIP in 1 ml buffer 2 with 10 ml levamisole) was sterile filtered (22 μm) and added to the sections, and slides were incubated upside down in the dark at 4°C overnight. Color reaction was stopped by placing the slides in buffer 3 (10 mM Tris-HCl [pH 8.0] + 1 mM EDTA). Slides were then dehydrated and counter-stained in 0.5% methyl green and mounted in Pertex microscopy mounting medium. The corresponding sense DIG-hyperlabeled ssDNA probes were used as negative controls. As positive control, a β-actin probe (GD5000-OP) was used (GeneDetect, Auckland, New Zealand).
Statistical analysis
The extent of immunofluorescence was graded semi-quantitatively from 0 (absent expression) to 3 (intense and widespread expression) concerning specific tenocyte immunofluorescence, and a Mann-Whitney U test was conducted on the resultant data (n=30) with statistical significance predetermined at p=0.05.
RESULTS
Immunofluorescence
Specific VGluT1 immunofluorescence could not be observed in either tendon or colon. By contrast, VGluT2 immunofluorescence was observed in both tendon and colon. VGluT2 immunoreactions were not present in tissue exposed to the preabsorbed antibody processed in parallel at the same concentration (Figure 1). In tendon, VGluT2 expression was observed in tenocytes and not in other structures (nerves or blood vessels). In colon specimens, VGluT2 expression was detected in the myenteric plexus, as previously described 28.
Figure 1.
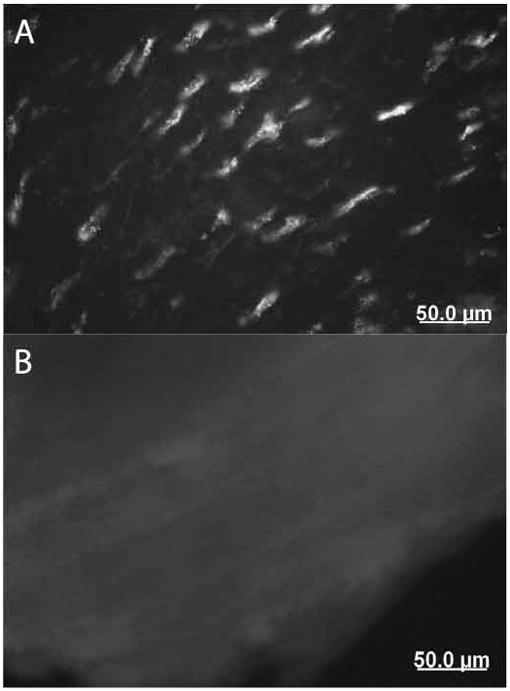
VGluT2 immunofluorescence in tendinosis tendon. A. Numerous tenocytes demonstrating positive immunoreactions (40x original magnification). B. Adjacent section demonstrating no reactions with preabsorbed antibody
VGluT2 immunoreaction was detected in 38% of normal tendons and in 71% of painful tendons. Accordingly, semiquantitative grading revealed a significantly greater expression of VGluT2 in tenocytes from tendinosis patients than in those of controls (p=0.005, Figure 2). The expression of VGluT2 in tendinosis tendons was thus both more widespread (i.e. large numbers of tendons showed were immunoreactive tenocytes) and more intense than in normal tendons (Figure 2, 3).
Figure 2.
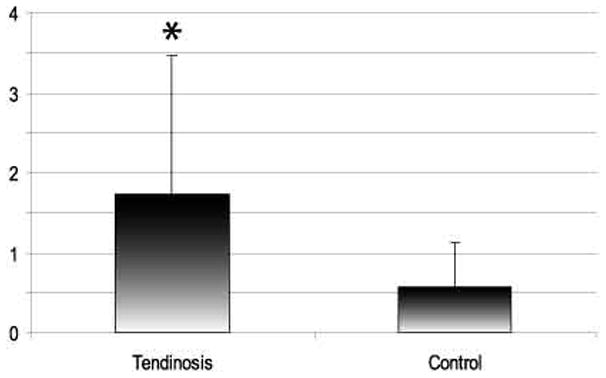
Results of VGluT2 immunofluorescence in tenocytes using semiquantitative grading. * significantly different, p=0.005 (Mann-Whitney U). Error bars represent standard deviation. 0= no immunoreaction, 3= intense and widespread immunoreaction.
Figure 3.
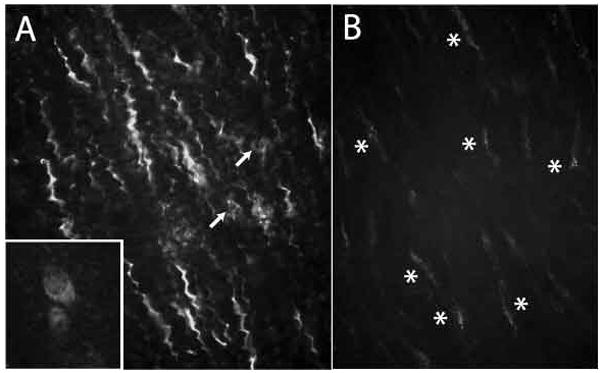
Marked VGluT2 immunofluorescence in abnormal tenocytes. A. Many tenocytes with an exaggerated crimp (waviness) are present in regions of tendinosis samples. Rounded tenocytes (arrows) are also present in the same field. Inset: Higher power view of a rounded tenocyte, demonstrating evident cytoplasmic localization of VGluT2. B. Tenocytes in healthy tendon, demonstrating faint punctuate reactions (asterisks).
VGluT2 immunoreactivitity in tenocytes was often seen in a punctuate cytoplasmic pattern (Figure 1). Reactivity was most intense in abnormally appearing tenocytes, including those showing rounded (as opposed to spindle shaped) or crimped (i.e. wavy) appearances (Figure 4). VGluT2 immunoreactivity in control tendons showed a similar pattern, but was often less intense and less widespread (Figure 4).
Figure 4.
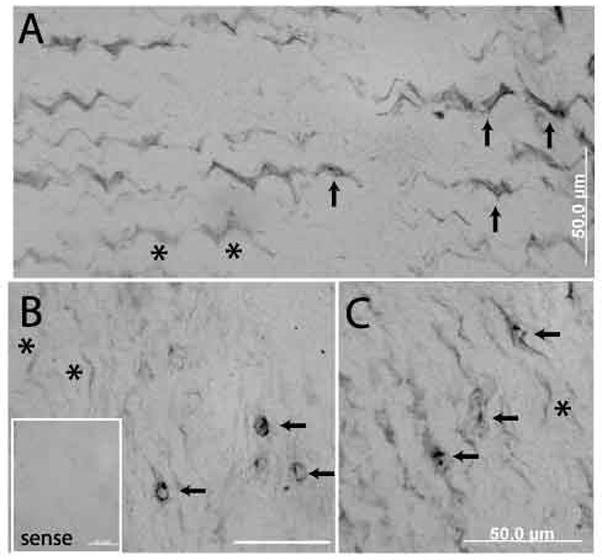
VGluT2 in situ hybridization. A. VGluT2 expression shows variability, with some tenocytes showing marked expression (arrows) and others only faint or absent (asterisks). B, C. Abnormal (rounded or broadened) tenocytes demonstrating prominent expression of VGluT2, with closely adjacent tenocytes demonstrating minimal or no expression (asterisks). Inset – sense control in a section adjacent to B demonstrates only background reactions.
There was no apparent difference in the extent of expression among men or women, nor any apparent relationship with age.
VGluT2 mRNA in tenocytes
VGluT2 mRNA expression was observed in the majority of tenocytes in both normal and abnormal regions of tendon, suggesting a more sensitive detection of VGluT2 mRNA compared to VGluT2 immunofluorescence (Figure 4). Nevertheless, the expression levels were somewhat variable within and between specimens, being most marked in tenocytes with abnormal appearance (rounded, broadened or crimped). Expression was not seen in any nerve or blood vessel structures.
Parallel in situ hybridization with the sense (control) probe verified that the observed AP end-reaction product resulted from the specific hybridization of the DNA probe to its complementary RNA sequence rather than due to a non-specific interaction (Figure 4B, inset). The β-actin probe labeled both tenocytes and vascular cells.
DISCUSSION
The current study documents the presence of vesicular glutamate transporters (VGluT2) in a novel cell type – the tendon fibroblast (tenocyte). This is completely new information for human tendons. However, along with other elements of glutamate signaling machinery which have recently been reported in rat tenocytes (glutamate receptors, glutamate receptor interacting proteins, and plasma membrane transporters) 20, 24, the current report concerning VGluT2 mRNA and protein supports a hypothesis that a peripheral glutamatergic system may be present in tendons. Moreover, the expression of VGluT2 at the protein level was most prominent in patients with chronic tendon pain (p=0.005), which strengthens previous suggestions that glutamate may be involved in the pathology of tendinosis 17, 23.
Non-neuronal expression of a glutamatergic marker in tendons
In the CNS, VGluT1 and 2 are expressed specifically at excitatory, glutamatergic synapses and play key roles in synaptogenesis as well as in memory and learning 2, 3. In the peripheral nervous system, the expression of vesicular glutamate transporters has been documented at a variety of locations including nerve ganglia in the colonic mucosa, the extrinsic and intrinsic innervation of the rat esophagus, in nerve terminals contacting neuroepithelial bodies in the lung, and in free nerve endings in the palatine mucosa 28, 30, 31. Expression has also recently been revealed in mechanosensitive nerve-associated structures such as Merkel cells and muscle spindles, implying that glutamate signaling in these locations may play a role in modulating touch sensitivity and muscle tone 32, 33. It is therefore of interest to note that in the present study, VGluT1 or 2 expression was not observed in nerve elements. During inflammation of joint structures (i.e. the rat knee synovium), glutamate has been suggested to be locally released by glutamatergic nerves, as the increase in glutamate could be blocked by blockade of peripheral nerves with lidocaine or dorsal rhizotomy 8. Conversely, the current study suggests that free glutamate within painful tendons probably derives from locally located cells (the tenocytes), particularly in tendinosis tendons.
Evidence for peripheral glutamatergic cell signaling
VGluTs have been proposed as molecular markers that may define the existence of a peripheral, locally acting glutamatergic signaling system with autocrine and paracrine functions. In the pineal gland, VGluT1 and 2 are expressed in synaptic-like-microvessels, and functional glutamate signaling (via metabotropic receptors) was shown to be involved in an important pineal gland function, the regulation of melatonin synthesis 1. VGluT1 and 2 are also expressed in glucagon-containing secretory granules in α cells in the Islets of Langherhans, where neighbouring β cells express functional AMPA-type receptors 34. Even more established is a physiologic role of glutamate signaling in the regulation of bone turnover, a process regulated in part by mechanotransduction (the cellular response to mechanical force). In bone, VGluT1 is expressed by mature osteoclasts and is directly responsible for their ability to release glutamate via transcytotic vesicles 35. Furthermore, VGluT1 knock-out mice develop an osteoporotic phenotype, strengthening the suggestion that glutamate plays a key role in suppressing bone resorption 35. Another bone cell type, osteoblasts, do not express VGluTs 35 but demonstrate all classes of glutamate receptors as well as plasma membrane transporters (reviewed in 36). The expression levels of several ionotropic and metabotropic glutamate receptors on osteoblasts was shown to be reduced by mechanical loading of bone segments in vitro 37. Likewise, in osteocytes and osteoblasts, GLAST expression appears to be reduced in areas of load-induced bone formation in vivo 38. Thus, paracrine glutamate signaling amongst osteoclasts, osteoblasts and osteocytes is heavily implicated in the process of mechanotransduction in bone. Preliminary evidence also points to a requirement for glutamate signaling in chondrocyte mechanotransduction 39, 40.
Potential role of glutamate in tendon mechanotransduction
Like bone and cartilage, tendon is a tissue in which the content and structure of the extracellular matrix is dynamically regulated in response to altered loading conditions. Mechanotransduction in tendon is relatively understudied, however. Tenocytes form elaborate gap-junction-linked arrays and, in response to mechanical load, release a variety of cytokines which are thought to regulate the activation of calcium-dependent signal pathways leading to proliferation and increased turnover and production of the collagen-proteoglycan extracellular matrix 41. Like chondrocytes and osteoblasts, human tenocytes express both voltage gated calcium channels as well as the mechanosensitive tandem pore domain potassium channel TREK-1 42. Considering that the hyperpolarization response to mechanical stimulation in chondrocytes requires NMDA receptor activation 39, the current study supports the hypothesis that tendons may exhibit a similar dependence on glutamate signaling for certain mechanotransduction signaling events. Thus, the regulation of extracellular glutamate concentrations by tenocytes via VGlut2 and via plasma membrane transporters requires further study.
Role of glutamate in tendinopathy
Current evidence from physiologic studies in humans and animals suggests that activation of AMPA and NMDA receptors can result in peripheral nociception 9, 10, 12, 43. We have previously shown that peripheral nerves in human tendons demonstrate the existence of NMDA receptors, which have also been observed in the nerve fibres of skin 44. A suggestion which is more directly supported by the current observations is that glutamate could modulate tenocyte behaviour within the tendinosis lesion. Glutamate is a well known toxic stimulus when present in excess, leading both to necrosis or apoptosis (or both) in a variety of cell types including neurons, fibroblasts, and chondrocytes 20, 45, 46. Murrell and coworkers have demonstrated that primary tenocyte cell cultures undergo a significant increase in the rate of cell death when exposed to 500μM glutamate for 24 hours. A strength in the study by Murrell is that the glutamate concentration to which tenocytes were exposed is on the same scale as has been measured in tendinpathy patients with microdialysis (250 μM) 19, 20. Advanced tendinosis lesions (ruptured supraspinatus tendon or long-standing severe jumper’s knee) also demonstrate increased numbers of apoptotic and necrotic tenocytes 16, 21, 22. Therefore, increased glutamate levels may contribute to cell death and subsequent local tissue degeneration in tendinopathy. Large numbers of apoptotic tenocytes have been reported in equine tendinosis lesions, but relatively few in the standard rat laboratory model, making it a challenge to study potential roles of glutamate and its relation to apoptosis in tendon overuse injury 47, 48. Glutamate toxicity and cell death are also thought to play a role in disorders such as Alzheimer’s disease 49.
Although tenocyte death occurs in some tendinosis lesions, chronically painful tendons are in general hypercellular mainly due to increased numbers of tenocytes, with a variety of other reparative cell types also found 50. Thus, another highly relevant feature of tendinosis pathology which glutamate could influence is cellular proliferation. Glutamate NMDA and AMPA antagonists inhibit cellular proliferation in a variety of studied cell cultures, including human colon adenocarcinoma, astrocytoma, breast and lung carcinoma, and neuroblastoma 21. Furthermore, the addition of 500μM to synovial fibroblasts resulted in a doubling of BrdU incorporation 11. Interestingly, this stimulation of fibroblast proliferation by glutamate only occurred in fibroblast cultures that had been derived from arthritic animals (collagen-induced rat arthritis model), suggesting that the influence of glutamate on cell behavior involves as-yet unspecified interactions with other molecular aspects of the pathology. Nonetheless, elevated glutamate levels in tendinosis lesions could potentially contribute to the simultaneous presence of increased apoptosis, necrosis, and proliferation which is observed in tendinosis 16. Glutamate may also modulate pro-apoptotic or proliferative effects of other conditions thought to be present in tendon such as hypoxia, oxidative stress, cytokines or growth factors.
In conclusion, the current study demonstrates the existence of VGluT2 in human tendon. The expression of VGluT2 mRNA and protein was upregulated in association with tendinosis, suggesting that locally derived glutamate may play roles in the pathology or in an attempted (i.e. failed) repair response. Local regulation of glutamate levels may influence key tenocyte functions modulated in response to injury and/or mechanical loading, including proliferation, apoptosis and extracellular matrix metabolism. Further studies of the role of glutamate signaling in tendon may shed light on the processes of mechanotransduction and overuse injury.
Acknowledgments
The authors would like to thank Ms Ulla Hedlund for excellent technical services. Funding was received from the Umeå University Faculty of Medicine (HA, SF), Swedish National Centre for Research in Sports (HA, SF), and the Canadian-Scandinavian Foundation and Canadian Institute of Health Research (AS).
References
- 1.Moriyama Y, Yamamoto A. Glutamatergic chemical transmission: Look! Here, there, and anywhere. J Biochem (Tokyo) 2004;135:155–163. doi: 10.1093/jb/mvh018. [DOI] [PubMed] [Google Scholar]
- 2.Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 3.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 4.Hinoi E, Takarada T, Tsuchihashi Y, Yoneda Y. Glutamate transporters as drug targets. Curr Drug Targets CNS Neurol Disord. 2005;4:211–220. doi: 10.2174/1568007053544093. [DOI] [PubMed] [Google Scholar]
- 5.Kalariti N, Pissimissis N, Koutsilieris M. The glutamatergic system outside the CNS and in cancer biology. Expert Opin Investig Drugs. 2005;14:1487–1496. doi: 10.1517/13543784.14.12.1487. [DOI] [PubMed] [Google Scholar]
- 6.Skerry TM, Genever PG. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 7.Jean YH, Wen ZH, Chang YC, et al. Increased concentrations of neuro-excitatory amino acids in rat anterior cruciate ligament-transected knee joint dialysates: a microdialysis study. J Orthop Res. 2005;23:569–575. doi: 10.1016/j.orthres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Lawand NB, Reddig WJ, Cashin AE, et al. NMDA receptors and associated signaling pathways: a role in knee joint blood flow regulation. Eur J Pharmacol. 2004;499:155–161. doi: 10.1016/j.ejphar.2004.07.110. [DOI] [PubMed] [Google Scholar]
- 9.Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-d-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 10.Gazerani P, Wang K, Cairns BE, et al. Effects of subcutaneous administration of glutamate on pain, sensitization and vasomotor responses in healthy men and women. Pain. 2006;124:338–348. doi: 10.1016/j.pain.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Hinoi E, Ohashi R, Miyata S, et al. Excitatory amino acid transporters expressed by synovial fibroblasts in rats with collagen-induced arthritis. Biochem Pharmacol. 2005;70:1744–1755. doi: 10.1016/j.bcp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of n-methyl-d-aspartate (NMDA) receptors in pain: a review. Anesth Analg. 2003;97:1108–1116. doi: 10.1213/01.ANE.0000081061.12235.55. [DOI] [PubMed] [Google Scholar]
- 13.Kujala UM, Sarna S, Kaprio J. Cumulative incidence of Achilles tendon rupture and tendinopathy in male former elite athletes. Clin J Sport Med. 2005;15:133–135. doi: 10.1097/01.jsm.0000165347.55638.23. [DOI] [PubMed] [Google Scholar]
- 14.Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561–567. doi: 10.1177/0363546504270454. [DOI] [PubMed] [Google Scholar]
- 15.Fenwick SA, Hazleman BL, Riley GP. The vasculature and its role in the damaged and healing tendon. Arthritis Res. 2002;4:252–260. doi: 10.1186/ar416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott A, Khan KM, Cook J, Duronio V. Human tendon overuse pathology: histopathologic and biochemical findings. In: Woo SL, Arnoczky SP, Renstrom P, editors. Tendinopathy in athletes; International Olympic Committee Encyclopedia of Sports Medicine; Malden, Massachusetts: Blackwell Publishing Ltd; 2007. pp. 69–84. [Google Scholar]
- 17.Alfredson H, Forsgren S, Thorsen K, Lorentzon R. In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in jumper’s knee. J Orthop Res. 2001;19:881–886. doi: 10.1016/S0736-0266(01)00016-X. [DOI] [PubMed] [Google Scholar]
- 18.Alfredson H, Ljung BO, Thorsen K, Lorentzon R. In vivo investigation of ECRB tendons with microdialysis technique--no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand. 2000;71:475–479. doi: 10.1080/000164700317381162. [DOI] [PubMed] [Google Scholar]
- 19.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: High levels of glutamate, but not prostaglandin E2 in chronic Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc. 1999;7:378–381. doi: 10.1007/s001670050184. [DOI] [PubMed] [Google Scholar]
- 20.Molloy TJ, Kemp MW, Wang Y, Murrell GA. Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration. J Appl Physiol. 2006;101:1702–1709. doi: 10.1152/japplphysiol.00386.2006. [DOI] [PubMed] [Google Scholar]
- 21.Lian O, Scott A, Engebretsen L, et al. Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med. 2007;35:605–611. doi: 10.1177/0363546506295702. [DOI] [PubMed] [Google Scholar]
- 22.Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002;20:1372–1379. doi: 10.1016/S0736-0266(02)00075-X. [DOI] [PubMed] [Google Scholar]
- 23.Alfredson H, Forsgren S, Thorsen K, et al. Glutamate NMDAR1 receptors localised to nerves in human Achilles tendons. Implications for treatment? Knee Surg Sports Traumatol Arthrosc. 2001;9:123–126. doi: 10.1007/s001670000188. [DOI] [PubMed] [Google Scholar]
- 24.Molloy TJ, Wang Y, Horner A, et al. Microarray analysis of healing rat Achilles tendon: evidence for glutamate signaling mechanisms and embryonic gene expression in healing tendon tissue. J Orthop Res. 2006;24:842–855. doi: 10.1002/jor.20093. [DOI] [PubMed] [Google Scholar]
- 25.McCullumsmith RE, Meador-Woodruff JH. Expression of transcripts for the vesicular glutamate transporters in the human medial temporal lobe. Ann N Y Acad Sci. 2003;1003:438–442. doi: 10.1196/annals.1300.046. [DOI] [PubMed] [Google Scholar]
- 26.Maffulli N, Testa V, Capasso G, et al. Similar histopathological picture in males with Achilles and patellar tendinopathy. Med Sci Sports Exerc. 2004;36:1470–1475. doi: 10.1249/01.mss.0000139895.94846.8d. [DOI] [PubMed] [Google Scholar]
- 27.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J Orthop Res. 2004;22:334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Tong Q, Ma J, Kirchgessner AL. Vesicular glutamate transporter 2 in the brain-gut axis. Neuroreport. 2001;12:3929–3934. doi: 10.1097/00001756-200112210-00015. [DOI] [PubMed] [Google Scholar]
- 29.Panoskaltsis-Mortari A, Bucy RP. In situ hybridization with digoxigenin-labeled RNA probes: facts and artifacts. Biotechniques. 1995;18:300–307. [PubMed] [Google Scholar]
- 30.Brouns I, Pintelon I, Van Genechten J, et al. Vesicular glutamate transporter 2 is expressed in different nerve fibre populations that selectively contact pulmonary neuroepithelial bodies. Histochem Cell Biol. 2004;121:1–12. doi: 10.1007/s00418-003-0609-1. [DOI] [PubMed] [Google Scholar]
- 31.Ewald P, Neuhuber WL, Raab M. Vesicular glutamate transporter 1 immunoreactivity in extrinsic and intrinsic innervation of the rat esophagus. Histochem Cell Biol. 2006;125:377–395. doi: 10.1007/s00418-005-0083-z. [DOI] [PubMed] [Google Scholar]
- 32.Wu SX, Koshimizu Y, Feng YP, et al. Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res. 2004;1011:247–251. doi: 10.1016/j.brainres.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 33.Haeberle H, Fujiwara M, Chuang J, et al. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci U S A. 2004;101:14503–14508. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi M, Yamada H, Uehara S, et al. Secretory granule-mediated co-secretion of l-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem. 2003;278:1966–1974. doi: 10.1074/jbc.M206758200. [DOI] [PubMed] [Google Scholar]
- 35.Morimoto R, Uehara S, Yatsushiro S, et al. Secretion of l-glutamate from osteoclasts through transcytosis. Embo J. 2006;25:4175–4186. doi: 10.1038/sj.emboj.7601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chenu C. Glutamatergic innervation in bone. Microsc Res Tech. 2002;58:70–76. doi: 10.1002/jemt.10120. [DOI] [PubMed] [Google Scholar]
- 37.Szczesniak AM, Gilbert RW, Mukhida M, Anderson GI. Mechanical loading modulates glutamate receptor subunit expression in bone. Bone. 2005;37:63–73. doi: 10.1016/j.bone.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Mason DJ, Suva LJ, Genever PG, et al. Mechanically regulated expression of a neural glutamate transporter in bone: a role for excitatory amino acids as osteotropic agents? Bone. 1997;20:199–205. doi: 10.1016/s8756-3282(96)00386-9. [DOI] [PubMed] [Google Scholar]
- 39.Salter DM, Wright MO, Millward-Sadler SJ. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology. 2004;41:273–281. [PubMed] [Google Scholar]
- 40.Wang L, Hinoi E, Takemori A, Yoneda Y. Release of endogenous glutamate by AMPA receptors expressed in cultured rat costal chondrocytes. Biol Pharm Bull. 2005;28:990–993. doi: 10.1248/bpb.28.990. [DOI] [PubMed] [Google Scholar]
- 41.Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact. 2005;5:70–84. [PubMed] [Google Scholar]
- 42.Magra M, Hughes S, El Haj AJ, Maffulli N. VOCCs and TREK-1 ion channel expression in human tenocytes. Am J Physiol Cell Physiol. 2007;292:C1053–1060. doi: 10.1152/ajpcell.00053.2006. [DOI] [PubMed] [Google Scholar]
- 43.Karim F, Bhave G, Gereau RWt. Metabotropic glutamate receptors on peripheral sensory neuron terminals as targets for the development of novel analgesics. Mol Psychiatry. 2001;6:615–617. doi: 10.1038/sj.mp.4000961. [DOI] [PubMed] [Google Scholar]
- 44.Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Hinoi E, Takemori A, et al. Glutamate inhibits chondral mineralization through apoptotic cell death mediated by retrograde operation of the cystine/glutamate antiporter. J Biol Chem. 2006;281:24553–24565. doi: 10.1074/jbc.M600939200. [DOI] [PubMed] [Google Scholar]
- 46.Olney JW. Excitotoxicity: An overview. Can Dis Wkly Rep. 1990;16(Suppl 1E):47–57. discussion 57–48. [PubMed] [Google Scholar]
- 47.Hosaka Y, Teraoka H, Yamamoto E, et al. Mechanism of cell death in inflamed superficial digital flexor tendon in the horse. J Comp Pathol. 2005;132:51–58. doi: 10.1016/j.jcpa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Scott A, Cook JL, Hart DA, et al. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor I signaling in early tendinosis in rats. Arthritis Rheum. 2007;56:871–881. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi T, Shoji M, Abe K. Molecular mechanisms of ischemic neuronal cell death--with relevance to Alzheimer’s disease. Curr Alzheimer Res. 2006;3:351–358. doi: 10.2174/156720506778249498. [DOI] [PubMed] [Google Scholar]
- 50.Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259–278. [PubMed] [Google Scholar]


