Abstract
Background
There is increasing evidence that autoimmune disease is associated with development of chronic obstructive pulmonary disease (COPD). We aim to assess the relationship between systemic lupus erythematosus (SLE) and COPD risk in a nationwide population.
Methods
We conducted a retrospective cohort study using the catastrophic illness registry of the Taiwan National Health Insurance Research Database (NHIRD). We identified 10,623 patients with SLE newly diagnosed between 2000 and 2010. Each patient was randomly frequency-matched with four people without SLE on age, sex, and index year from the general population. Both cohorts were followed up until the end of 2010 to measure the incidence of COPD. The risk of COPD was analyzed using Cox proportional hazards regression models including age, sex, index year and comorbidities.
Results
The overall incidence rate of COPD was 1.73–fold higher in the SLE cohort than in the control cohort (17.4 vs. 10.1 per 10,000 person-years, 95% CI = 1.62–1.84). Age related analysis showed increased incidence of COPD with age in both SLE and control cohorts. However, adjusted HR maximum was observed in the youngest age group (adjusted HR: 4.33, 95% CI, 2.39–7.85) while adjusted HR minimum was witnessed in the oldest age group (adjusted HR: 1.19, 95% CI, 0.85–1.22).
Conclusion
Patients with SLE have a significant risk of developing COPD than the control population. Based on the findings from this study, it can be hypothesized that in addition to cigarette smoke SLE may be a determining factor for COPD incidence. However, further investigation is needed to corroborate this hypothesis.
Introduction
Systemic lupus erythematosus (SLE), an autoimmune disease that affects multiple organ systems, is more prevalent in women, particularly of child-bearing age. Although the exact mechanism of SLE initiation and progression is unknown, several risk factors, such as heredity, hormonal abnormalities, environmental pollutants, and viral infections, have been recognized as important contributors toward SLE development. An important clinical evidence of this disease is the presence of anti-double-stranded DNA (anti-ds DNA) autoantibodies, which are highly specific and can be used as diagnostic markers for disease activity and progression [1]. The annual incidence of SLE in adults ranges from 2.0 to 7.6 cases per 100,000 person-years in developed countries [2]. A recent population-based study from Taiwan found that the average incidence of SLE cases was 4.87 per 100,000 person-years between 2003 and 2008 [3].
Chronic obstructive pulmonary disease (COPD) is a slow progressive disease characterized by obstruction of airflow in the lungs due to chronic inflammation on the lining of airways. It is the 4th leading cause of death worldwide, with reported prevalence rates between 5% and 13% [4], [5]. Although exposure to cigarette smoke plays a pivotal role in COPD development, a substantial group of patients with COPD has been identified as nonsmokers [6], [7]. Therefore, in addition to tobacco smoke, relevance of other contributing factors in COPD development cannot be ignored; thus, other contributing factors exist and one of them may be autoimmunity. The role of autoimmune pathology in the development and progression of COPD is becoming increasingly appreciated [8].
Smoking is a common environmental risk factor for COPD, but COPD development in patients with SLE may not be attributed to cigarette smoke alone. SLE may play an independent role in COPD incidence. Even smokers with SLE may display a different time course in COPD development compared with non-SLE individuals who were addicted to smoking. A recent epidemiological study from Israel has reported that rheumatoid arthritis, also an autoimmune disease, is significantly associated with COPD; the study was adjusted by controlling confounders including smoking [9]. Other study sources have revealed that the majority of patients with COPD contain increased levels of serum antibodies capable of interacting with self-antigens [10]–[16], and occurrence of antibodies to specific autoantigens correlates with disease severity [12]–[14], [16]. The mechanism of interactions between autoimmunity and COPD is still inconclusive.
The primary aim of our study is to determine whether a differential risk of developing COPD for adults exists with and without SLE by examining a relatively large population cohort in Taiwan. The results in this study were generated from a population-based retrospective cohort obtained from the National Health Insurance (NHI) system's database. To the best of our knowledge, this is the first nationwide population-based study evaluating the relationship between SLE and the risk of developing COPD.
Methods
Data Source
The National Health Insurance (NHI) program in Taiwan was first established in 1995, since then it has covered approximately 99% of Taiwan's population (23.74 million) [17]. The National Health Research Institute (NHRI) of Taiwan, in co-operation with the National Health Insurance Bureau (NHIB), has established a National Health Insurance Research Database (NHIRD), from which data were pooled for our study [18], [19]. The International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) was used for SLE diagnosis. Following regulations implemented by the Department of Health, the identity of each patient was encrypted for privacy and data security. This study was exempted from full ethical review by the International Review Board, China Medical University and Hospital Research Ethics Committee (IRB permit number: CMU-REC-101-012).
Study Population
Patients certified with catastrophic illnesses including SLE were exempted from paying a copayment and therefore could be easily identified from the Registry of Catastrophic Illness Patient Database (RCIPD). A catastrophic illness is defined as a severe illness requiring advanced health care. Catastrophic illnesses usually involve high health care costs and may incapacitate the person from working, creating a financial hardship. All patients with SLE are categorized to have a catastrophic illness in the NHI system of Taiwan [3]. Patients certified with SLE catastrophic illness certification (ICD-9-CM code 710.0) as identified from the RCIPD covering a period of 10 years (2000–2010) were selected for this study. The index date for patients with SLE was assigned to be the date on which symptoms of SLE were first revealed. For comparison study, a non-SLE cohort control was randomly selected (4 for every patient in the SLE cohort) from the list of insured persons without a history of SLE and was matched for age, sex, and index year of SLE diagnosis. Patients in both cohorts with prior incidence of COPD (ICD-9 code 491, 492, and 496) or with missing information related to age and/or sex were excluded from this study.
Outcome and Comorbidities
All study patients were followed until they were diagnosed with COPD as evident from the medical records. To measure the incidence of COPD, the SLE and non-SLE cohort were followed until COPD was diagnosed or death of the subject occurred or patients failed to follow-up or 31 December 2010, whichever came first. Comorbidities including hypertension (ICD-9-CM codes 401–405), diabetes (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), coronary artery disease (CAD) (ICD-9-codes 410-414), cerebrovascular accident (CVA) (ICD-9-codes 430-438), and end-stage renal disease (ESRD) (ICD-9-CM code 585) were defined as diseases diagnosed before the index date.
Statistical analysis
All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute, Cary, NC, USA). A two-sided statistical test with p<0.05 was considered statistically significant. The SLE cohort and non-SLE cohort data were compared using the Chi-square test for categorical variables and unpaired Student t-test for continuous variables. For comparisons between the SLE cohort and the non-SLE cohort, the incidence rate ratio (IRR) of COPD and 95% confidence interval (CI) were measured using the Poisson regression model. The univariable and multivariable Cox proportional hazard regression models were applied to measure the hazard ratio (HR) and 95% confidence interval (CI) of COPD incidence for the SLE cohort compared with that of the non-SLE cohort. Variables in the multivariable model included age, sex, hypertension, diabetes, hyperlipidemia, CAD, CVA, and ESRD. The Cox model was also used to estimate age, sex, and comorbidity specific HR. The Kaplan–Meier method was used to estimate cumulative incidence and the differences between the curves were tested with two-tailed log-rank test.
Results
The demographics and medical comorbidities of patients enrolled in the study program are depicted in (Table 1). A total of 53,115 patients were enrolled in this study. The mean age (±SD) was 37.3±11.5 years for the SLE cohort and 37.1±11.9 years for the non-SLE control cohort.
Table 1. Demographic characteristics and comorbidities in patients with and without systemic lupus erythematosus.
| Variable | SLE, n(%) | p value | |
| No | Yes | ||
| N = 42492 | N = 10623 | ||
| Sex | |||
| Female | 37508(88.3) | 9377(88.3) | 0.99 |
| Male | 4984(11.7) | 1246(11.7) | |
| Age, mean(SD) | 37.1(11.9) | 37.3(11.5) | 0.07# |
| Age | |||
| 20–34 years | 18596(43.8) | 4649(43.8) | 0.99 |
| 35–49 years | 14012(33.0) | 3503(33.0) | |
| 50–64 years | 6480(15.3) | 1620(15.3) | |
| 65+ years | 3404(8.01) | 851(8.01) | |
| Comorbidities | |||
| Hypertension | 2853(6.71) | 2017(19.0) | <0.0001 |
| Diabetes | 1687(3.97) | 540(5.08) | <0.0001 |
| Hyperlipidemia | 765(1.80) | 646(6.08) | <0.0001 |
| CAD | 1023(2.41) | 514(4.84) | <0.0001 |
| CVA | 1071(2.52) | 635(5.98) | <0.0001 |
| ESRD | 197(0.46) | 350(3.29) | <0.0001 |
Chi-square test, #: Two sample t-test.
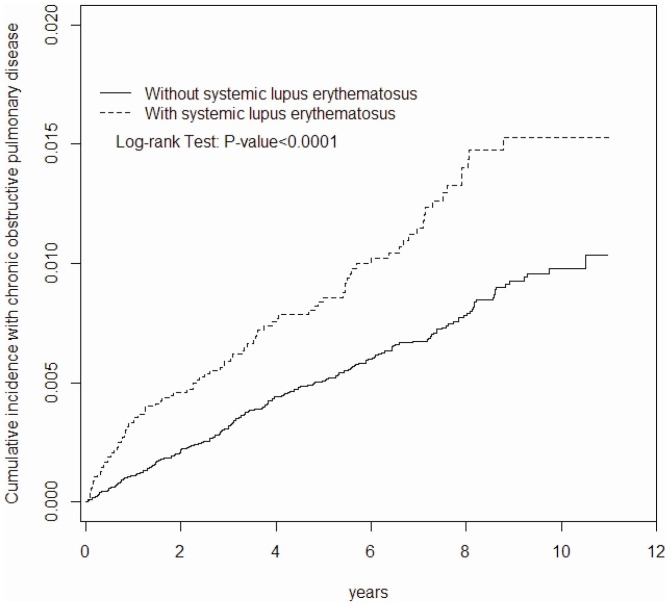
The majority of the subjects selected for this study were female (88.3%) and nearly (73.8%) of the subjects were aged 20–49 years (43.8%). When compared with the non-SLE control cohort, patients with SLE displayed higher proportion of comorbidities including hypertension (19.0% vs. 6.71%), diabetes (5.08% vs. 3.97%), hyperlipidemia (6.08% vs. 1.80%), CAD (4.84% vs. 2.41%), CVA (5.98% vs. 2.52%), and ESRD (3.29% vs. 0.46%). The mean follow up duration was 5.13±3.29 years for the SLE cohort and 5.32±3.24 years for the non-SLE cohort. The SLE cohort group reported higher rate of COPD incidence compared with the control cohort (17.4 vs. 10.1 per 10,000 person-years, 95% CI, 1.62–1.84, adjusted HR: 1.92, 95% CI, 1.50–2.44) (Table 2). The cumulative incidence of COPD by the end of follow-up period was approximately 0.55% higher in the SLE cohort than the non-SLE cohort (1.53% vs. 0.98%; P<0.001) (Figure 1). The sex specific risk analysis for both the cohorts revealed that both genders were equally susceptible in developing COPD (adjusted HR for women: 2.10, 95% CI, 1.55–2.83 vs. adjusted HR for men: 1.88, 95% CI, 1.24–2.86). Age related analysis showed increased incidence of COPD with age in both SLE and non-SLE cohorts. However, adjusted HR maximum was witnessed in the age range 20–49 years (adjusted HR: 4.33, 95% CI, 2.39–7.85) while adjusted HR minimum was observed in the oldest age group (>65 years) (Table 2). The multivariable Cox proportional hazard regression model showed an increased risk of COPD in patients with SLE with one of following characteristics: men (adjusted HR: 2.33, 95% CI, 1.84–2.94), older age (adjusted HR: 61.9, 95% CI, 34.7–110.7), diabetes (adjusted HR: 1.52, 95% CI, 1.16–1.99), CAD (adjusted HR: 1.86, 95% CI, 1.41–2.45), and CVA (adjusted HR: 1.99, 95% CI, 1.51–1.36) (Table 3).
Table 2. Sex- and age-specific incidence rates of COPD in subjects with and without systemic lupus erythematosus (SLE) and Cox model estimated hazard ratios for patients with SLE.
| SLE | ||||||||
| No | Yes | |||||||
| Variables (smoking rate %)‡ | Event | PY | Rate# | Event | PY | Rate# | IRR*(95% CI) | Adjusted HR† (95% CI) |
| All | 228 | 226007 | 10.1 | 95 | 54533 | 17.4 | 1.73(1.62, 1.84)*** | 1.92(1.50, 2.44)*** |
| 20–49 (25.0%) | 21 | 176590 | 1.19 | 30 | 43844 | 6.84 | 5.75(5.34, 6.20)*** | 4.33(2.39, 7.85)*** |
| 50–64 (14.9%) | 33 | 34499 | 9.57 | 21 | 7763 | 27.1 | 2.83(2.43, 3.29)*** | 2.38(1.37, 4.13)*** |
| 65+ (11.0%) | 174 | 14918 | 116.6 | 44 | 2926 | 150.4 | 1.29(1.05, 1.58)* | 1.19(0.85, 1.66) |
| Women | 146 | 200870 | 7.27 | 63 | 48708 | 12.9 | 1.78(1.66, 1.91)*** | 2.10(1.55, 2.83)*** |
| 20–49 (5.6%) | 17 | 159543 | 1.07 | 20 | 39726 | 5.03 | 4.72(4.37, 5.11)*** | 3.30(1.66, 6.58)*** |
| 50–64 (2.2%) | 21 | 29555 | 7.11 | 13 | 6698 | 19.4 | 2.73(2.30, 3.24)*** | 2.20(1.09, 4.45)* |
| 65+ (1.0%) | 108 | 11773 | 91.7 | 30 | 2283 | 131.4 | 1.43(1.14, 1.81)** | 1.45(0.96, 2.17) |
| Men | 82 | 25137 | 32.6 | 32 | 5825 | 54.9 | 1.68(1.42, 2.00)*** | 1.88(1.24, 2.86)** |
| 20–49 (42.8%) | 4 | 17047 | 2.35 | 10 | 4117 | 24.3 | 10.4(8.17, 13.1)*** | 9.45(2.81, 31.9)*** |
| 50–64 (30.1%) | 12 | 4944 | 24.3 | 8 | 1065 | 75.1 | 3.09(2.19, 4.38)*** | 2.81(1.14, 6.95)* |
| 65+ (19.4%) | 66 | 3145 | 209.9 | 14 | 643 | 217.7 | 1.04(0.68, 1.58) | 1.01(0.56, 1.82) |
Rate#, incidence rate per 10,000 person-years.
IRR*, incidence rate ratio.
Model was adjusted for age, sex, and comorbidities.
Current smoking rate of general population in Taiwan (%).
* p<0.05, ** p<0.01, *** p<0.001.
Figure 1. Cummulative incidence of COPD for subjects with and without systemic lupus erythematosus using the Kaplan–Meir method.
Table 3. Cox model with hazard ratios and 95% confidence intervals of COPD associated with systemic lupus erythematosus and covariates.
| Crude | Adjusted† | |||
| Variable | HR | (95% CI) | HR | (95% CI) |
| Age | ||||
| 20–34 years | 1 | (Reference) | 1 | (Reference) |
| 35–49 years | 3.74 | (1.99, 7.03)*** | 3.62 | (1.93, 6.79)*** |
| 50–64 years | 12.1 | (6.63, 22.2)*** | 9.03 | (4.90, 16.6)*** |
| 65+ years | 115.6 | (66.0, 202.3)*** | 61.9 | (34.7, 110.7)*** |
| Sex | ||||
| Male | 4.38 | (3.48, 5.50)*** | 2.33 | (1.84, 2.94)*** |
| Female | 1 | (Reference) | 1 | (Reference) |
| Baseline comorbidities (yes vs no) | ||||
| SLE | 1.72 | (1.36, 2.19)*** | 1.82 | (1.43, 2.31)*** |
| Hypertension | 9.53 | (7.66, 11.8)*** | 1.28 | (0.97, 1.68) |
| Diabetes | 9.17 | (7.20, 11.7)*** | 1.52 | (1.16, 1.99)** |
| Hyperlipidemia | 4.34 | (3.06, 6.17)*** | 0.87 | (0.60, 1.26) |
| CAD | 13.1 | (10.3, 16.7)*** | 1.86 | (1.41, 2.45)*** |
| CVA | 12.2 | (9.56, 15.6)*** | 1.99 | (1.51, 2.620*** |
| ESRD | 2.20 | (1.09, 4.43)* | 0.67 | (0.33, 1.36) |
Adjusted HR: multivariable analysis including for age, sex,
hypertension, diabetes, hyperlipidemia, CAD, CVA, and ESRD.
*p<0.05, ** p<0.01, *** p<0.001.
Discussion
To the best of our knowledge, this is the first nationwide population-based study evaluating the relationship between SLE and COPD risk. In this study, there was a significantly higher incidence of COPD among patients with COPD than that in the general population. Further age related risk analysis indicated higher COPD incidence in aged patients although adjusted HR progressively decreased with age. This apparent contradictory finding could be explained by assuming aged patients may be exposed to cigarette smoke for a prolonged period of time or they could present with a large number of comorbid diseases. Therefore, the adjusted HR of oldest age group was not statistically relevant.
Another remarkable finding from this study revealed that the HR of developing COPD was comparatively higher (adjusted HR: 9.45, 95% CI, 2.81–31.9) in men aged 21–49 years with SLE than in men with similar age range in the control cohort. The differences in smoking rate might account for this observation. The smoking rate in men of the SLE cohort may be higher than that of the non-SLE cohort. Alternatively, SLE could be considered as an independent risk factor for COPD or smokers with SLE developed COPD within a short time frame. A recent public health report released from the Ministry of Health and Welfare of Taiwan, has reported that about 42.8% of males with age ranging from 20 to 50 years are smokers [20]. Although the NHI database does not contain information about personal smoking habits, we reasoned this value could be attributed to male smokers aged 20–50-years old in the non-SLE cohort. Therefore, to explain the occurrence of such a high HR, we hypothesized that either SLE directly causes COPD incidence or facilitates shortening of the disease course. However, further investigation is needed to corroborate this hypothesis.
For our study purpose, exposure to tobacco smoke is still considered as the most important confounding factor for COPD. Unfortunately, the NHI database does not provide any information on personal smoking habits. Freemer et al note that exposure to tobacco smoke has been associated with several autoimmune diseases [21]. Majka et al report the relationship between the development of SLE and cigarette smoking is less affirmative [22]. We have searched for previous publications which involved smoking rate among SLE patients and general population. We found that some studies with no significant difference between the two groups [23]–[26]. It is inconclusive that patients with SLE have a significant high smoking rate in all races. We roughly estimated the current smoking rate of non-SLE cohorts by considering data from public health reports obtained from the Ministry of Health and Welfare of Taiwan (Table 2). The overall smoking rate in the general population is 19.8% (males, 35.0%, females, 4.1%). Therefore, we considered combined effects of SLE and smoking for evaluation of increased incidence of COPD in this study.
Based on disease definition in the registry system as established by NHIRD of Taiwan, SLE is categorized as a “catastrophic illness” and patients diagnosed with SLE are entitled to receive the “catastrophic illness certification” issued by the government. The catastrophic illness certified patient is eligible for a great deal of discount benefits with regard to medical expenses. The certification process requires critical evaluation of medical records, serological, and/or pathological reports by experts specialized in the disease field [27]. The diagnosis of COPD is based on target history and requires comprehensive pulmonary function assessment. The criteria set forth by the GOLD guideline are usually followed in concluding the diagnosis of COPD [28]. A spot check is also performed regularly. Therefore, NHIRD provides a reliable data source for SLE and COPD occurrences. Moreover, any pre-existing condition of COPD would be detected by rheumatologists during comprehensive evaluation of patient's medical history while confirming SLE diagnosis. Therefore, patients diagnosed with COPD afterwards could be considered as new cases.
The exact link between inflammation and autoimmune disorders is not known. However, smoking serves as an important bridging link between the two diseases. Smoking is a well-known risk factor associated with several autoimmune diseases. Chronic exposure to cigarette smoke may be detrimental to the regular functioning of the body at the intracellular level. For example, smoking induces production of intracellular antigens [29]–[31], augments B cell autoreactivity and stimulates the proliferation of peripheral T-lymphocytes, and actively promotes generation of endogenous oxidative free radicals [29], [32]–[33]. Harmful toxins present in cigarette smoke may interact with DNA and cause genetic mutations, which ultimately lead to altered gene expressions resulting in autoimmune diseases [9]. COPD is a representative example of inflammatory disease. Although the exact underlying mechanism causing COPD has not been established, several mechanisms have been implicated in the pathogenesis of smoking induced COPD. Sirtuin 1 (SIRT1) is a deacetylase anti-inflammatory protein, the important functions of which are mediated by several different transcription factors (e.g., NF-kappaB). Decreased levels of SIRT1 and NF-kappaB activity have been reported in smokers exhibiting COPD conditions [34]. Vascular endothelial growth factor (VEGF) signaling is a crucial event associated with lung structure maintenance. Bronchiolar expressions of VEGF and VEGF2 receptors are significantly lower in smokers with COPD [35]. The toll-like receptors in the lung epithelium plays a pivotal role in host defense mechanism. Tobacco smoke has been identified as a potential modulator of expression of toll-like receptor 4 (TLR4) in respiratory epithelium [36].
The strength of our study lies in the use of population-based data that are highly representative of the general population. However, our study has several limitations. First, the National Health Insurance Research Database (NHIRD) does not contain detailed information regarding smoking habits, body weight index (BMI), diet preference, drug use, and family history of systemic diseases, all of which may be associated risk factors for COPD development. Second, the evidence derived from a retrospective cohort study is statistically less relevant than information obtained from randomized trials because of potential biases related to adjustments made for confounding variables. Despite our meticulous study design, biases resulting from unknown confounding factors may have affected our results. Third, all data in the NHIRD are anonymous. Thus, relevant clinical variables, such as serum laboratory data, pulmonary function tests, imaging results, and pathology findings were unavailable for patients in our study.
Conclusion
To the best of our knowledge, our study is the first nationwide population-based study evaluating the risk of developing COPD in patients with SLE. Patients with SLE have a significant risk of developing COPD than the control population. Based on the findings from this study, it can be hypothesized that in addition to cigarette smoke SLE may be a determining factor for COPD incidence. However, further investigation is needed to corroborate this hypothesis.
Funding Statement
This work was supported by Taiwan Department of Health Clinical Trial and Research Center and for Excellence (DOH102-TD-B-111-004) and Taiwan Department of Health Cancer Research Center for Excellence (DOH102-TD-C-111-005). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
References
- 1. Pons-Estel GJ, Alarco'n GS, Scofield L, Reinlib L, Cooper GS (2010) Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthr Rheum 39: 257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fessel WJ (1988) Epidemiology of systemic lupus erythematosus. Rheum Dis Clin N Am 14: 15–23. [PubMed] [Google Scholar]
- 3.Yeh KW, Yu CH, Chan PC, Horng JT, Huang JL (2013) Burden of systemic lupus erythematosus in Taiwan: a population-based survey. Rheumatol Int DOI: 10.1007/s00296-012-2643-6. [DOI] [PubMed]
- 4. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–65. [DOI] [PubMed] [Google Scholar]
- 5. Mannino DM, Buist AS (2007) Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370: 765–73. [DOI] [PubMed] [Google Scholar]
- 6. Birring SS, Brightling CE, Bradding P, Entwisle JJ, Vara DD, et al. (2002) Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive study. Am J Respir Crit Care Med 166: 1078–83. [DOI] [PubMed] [Google Scholar]
- 7. Hagstad S, Ekerljung L, Lindberg A, Backman H, Rönmark E, et al. (2012) COPD among non-smokers—Report from the Obstructive Lung Disease in Northern Sweden (OLIN) studies. Respir Med 106: 980–8. [DOI] [PubMed] [Google Scholar]
- 8. Packard TA, Li QZ, Cosgrove GP, Bowler RP, Cambier JC (2013) COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol Res 55: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bieber V, Cohen AD, Freud T, Agmon-Levin N, Gertel S, et al. (2013) Autoimmune smoke and fire—coexisting rheumatoid arthritis and chronic obstructive pulmonary disease: a cross-sectional analysis. Immunol Res 56: 261–6. [DOI] [PubMed] [Google Scholar]
- 10. Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, et al. (2008) Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karayama M, Inui N, Suda T, Nakamura Y, Nakamura H, et al. (2010) Antiendothelial Cell Antibodies in Patients With COPD. Chest 138: 1303–8. [DOI] [PubMed] [Google Scholar]
- 12. Kirkham PA, Caramori G, Casolari P, Papi AA, Edwards M, et al. (2011) Oxidative stress-induced antibodies to carbonyl-modified protein correlate with severity of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 184: 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuo YB, Chang CA, Wu YK, Hsieh MJ, Tsai CH, et al. (2010) Identification and clinical association of anti-cytokeratin 18 autoantibody in COPD. Immunol Lett 128: 131–6. [DOI] [PubMed] [Google Scholar]
- 14. Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, et al. (2007) Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med 13: 567–9. [DOI] [PubMed] [Google Scholar]
- 15. Leidinger P, Keller A, Heisel S, Ludwig N, Rheinheimer S, et al. (2009) Novel autoantigens immunogenic in COPD patients. Respir Res 10: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Núñez B, Sauleda J, Antó JM, Julià MR, Orozco M, et al. (2011) Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 183: 1025–31. [DOI] [PubMed] [Google Scholar]
- 17.Cheng TM (2009) Taiwan's national health insurance system: high value for the dollar. Six Countries, Six Reform Models: Their Healthcare Reform: Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. Hackensack, NJ: World Scientific 171–204.
- 18. Shen TC, Tu CY, Lin CL, Wei CC, Li YF (2014) Increased risk of asthma in patients with systemic lupus erythematosus. Am J Respir Crit Care Med 189: 496–9. [DOI] [PubMed] [Google Scholar]
- 19.Shen TC, Lin CL, Wei CC, Tu CY, Li YF (2014) The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM Jan 20. [DOI] [PubMed]
- 20.Health Promotion Administration, Ministry of Health and Welfare of Taiwan website. Available: http://www.hpa.gov.tw/BHPNet/Web/HealthTopic/Topic.aspx?id=200712250024. Accessed 2014 Feb 18.
- 21. Freemer MM, King TE Jr, Criswell LA (2006) Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis 65: 581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Majka DS, Holers VM (2006) Cigarette smoking and the risk of systemic lupus erythematosus and rheumatoid arthritis. Ann Rheum Dis 65: 561–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drenkard C, Rask KJ, Easley KA, Bao G, Lim SS (2013) Primary preventive services in patients with systemic lupus erythematosus: study from a population-based sample in Southeast U.S. Semin Arthritis Rheum 43: 209–16. [DOI] [PubMed] [Google Scholar]
- 24. Petri M, Thompson E, Abusuwwa R, Huang J, Garrctt E (2001) BALES: the Baltimore Lupus Environmental Study. Arthritis Rheum 44 Suppl 9S331. [Google Scholar]
- 25. Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS (2001) Smoking and use of hair treatments in relation to risk of developing systemic lupus erythematosus. J Rheumatol 28: 2653–6. [PubMed] [Google Scholar]
- 26. Reidenberg MM, Drayer DE, Lorenzo B, Strom BL, West SL, et al. (1993) Acetylation phenotypes and environmental chemical exposure of people with idiopathic systemic lupus erythematosus. Arthritis Rheum 36: 971–3. [DOI] [PubMed] [Google Scholar]
- 27. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, et al. (1982) Special article: the 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25: 1271–7. [DOI] [PubMed] [Google Scholar]
- 28.The Global Initiative for Chronic Obstructive Lung Disease website. Available: http://www.goldcopd.org/. Accessed 2013 Nov 20.
- 29. Arnson Y, Shoenfeld Y, Amital H (2010) Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 34: J258–65. [DOI] [PubMed] [Google Scholar]
- 30. Zifman E, Amital H, Gilburd B, Shoenfeld Y (2008) Antioxidants and smoking in autoimmune disease–opposing sides of the seesaw? Autoimmun Rev 8: 165–9. [DOI] [PubMed] [Google Scholar]
- 31. Carmi G, Amital H (2011) The geoepidemiology of autoimmunity: capsules from the 7th International Congress on Autoimmunity, Ljubljana, Slovenia, May 2010. Isr Med Assoc J 13: 121–7. [PubMed] [Google Scholar]
- 32. Bengtsson AA, Rylander L, Hagmar L, Nived O, Sturfelt G (2002) Risk factors for developing systemic lupus erythematosus: a casecontrol study in southern Sweden. Rheumatology [Oxford] 41: 563–71. [DOI] [PubMed] [Google Scholar]
- 33. Vermeulen A (1993) Environment, human reproduction, menopause, and andropause. Environ Health Perspect 101 (Suppl2)91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, et al. (2008) Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal 10: 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suzuki M, Betsuyaku T, Nagai K, Fuke S, Nasuhara Y, et al. (2008) Decreased airway expression of vascular endothelial growth factor in cigarette smoke-induced emphysema in mice and COPD patients. Inhal Toxicol 20: 349–59. [DOI] [PubMed] [Google Scholar]
- 36. MacRedmond RE, Greene CM, Dorscheid DR, McElvaney NG, O'Neill SJ (2007) Epithelial expression of TLR4 is modulated in COPD and by steroids, salmeterol and cigarette smoke. Respir Res 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]